
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health , 02 December 2024
Sec. Oral Epidemiology
Volume 5 - 2024 | https://doi.org/10.3389/froh.2024.1486182
This article is part of the Research Topic Oral Health Care for Vulnerable and Underserved Populations View all 7 articles
Odontogenic infections have a high prevalence and can lead to severe complications. Due to demographic changes, the number of geriatric patients has increased in recent years. The aim of this study was to analyse odontogenic abscesses in elderly patients and to differentiate them from non–elderly patients regarding clinical presentation, bacterial analysis and therapy. We retrospectively reviewed 1,173 inpatients with odontogenic abscesses from 2014 to 2020. Patients were divided into elderly patients (≥70 years, n = 240) and non-elderly patients (<70 years, n = 933). Demographics, clinical parameters, laboratory values and treatment parameters were analysed. Overall, elderly patients had a longer hospital stay (LOS) (median 4 [range 28] vs. 3 [range 22] days) and more complications (9.6% vs. 7.9%) than non-elderly patients, although these differences were not statistically significant. Peri-/submandibular (p = 0.015), parapharyngeal (p < 0.001) and oral base infections (p = 0.036) were associated with significantly longer LOS in the elderly. Chronic renal failure (CRF) was associated with LOS (p = 0.010) and complications (p = 0.006). In the elderly, c-reactive protein (CRP) correlated significantly with LOS (p < 0.001) and more complications (p = 0.036). This study identifies anatomical spaces and CRF as outcome predictors of odontogenic abscesses in the elderly. In addition, CRP level may serve as a predictor of complicated course in elderly patients.
Infections of the oral and maxillofacial region have a high incidence and can cause severe complications (1–3), such as mediastinitis, airway obstruction or sepsis (4, 5).
Odontogenic infections are the most common cause of oral and maxillofacial abscesses (2, 6–8).
Several general and local predisposing factors are known to increase the risk of severe odontogenic infections. These include for example unstable diabetes mellitus (DM), immunosuppression, history of radiation and/or chemotherapy (9–21).
Orofacial odontogenic infections are frequently mixed aerobic and anaerobic infections (6, 22). The bacterial spectrum is diverse, and the microbiology result often excogitates the existence of commensal oral flora (6, 23).
Treatment of odontogenic infection is based on surgical drainage, focus remediation and antibiotic therapy (6, 24, 25). However, if the abscess is treated incorrectly or late, the infection may spread in deeper facial spaces, where it becomes difficult to treat and may be fatal (26–29). In addition to surgery, the choice of empirical antibiotics is also critical (26, 30). Widespread indifferent use of antibiotics has led to the emergence of resistant bacteria (31).
According to the joint definition of the German Society of Geriatric Medicine (DGG), the German Society of Gerontology and Geriatrics (DGGG), the Federal Association of Clinical geriatric Institutions (BAG) and the Professional Association of German Internists Section geriatrics, the geriatric patient is characterised by advanced age, mostly 70 years and older, combined with a geriatric–typical multimorbidity (32–34). Elderly patients are considered high-risk patients with a higher probability of prolonged hospitalisation (33, 35).
Odontogenic infections affect people of all ages (28). However, age-associated decline in reserve and function may reduce the ability to cope with acute external stressors, typically defined as frailty (36, 37). Frailty is associated with a higher risk of poor outcomes such as disability and mortality (38). Inflammation may be closely associated with frailty (39). In the elderly, their reduced immunological reaction and comorbidities may be associated with an increase in severe infections (40). In addition, diagnosis and treatment of odontogenic infections in the elderly may be more complex than in younger patients because of more comorbid conditions (41).
The number of individuals aged 60 and over worldwide is expected to increase to more than 2 billion by 2050 (42).
Thus, there will be more elderly patients with oral and maxillofacial infections (1). Only a few studies consider age as an important factor in head and neck infections, but without specification in odontogenic infections (1, 3).
We aimed to analyse the differences in clinical features, treatment modalities, and bacterial analysis of odontogenic infections between elderly and non-elderly patients. We hypothesise that we will identify variables associated with worse outcome to improve the clinical risk stratification of elderly patients.
All patients with upper and lower jaw infections requiring admission to our hospital between January 2014 and April 2020 were included in this retrospective study. Patients with International Statistical Classification of Diseases and Related Health Problems (ICD) codes K 10.3, K10.2, K 12.2, K 14.0 were primarily included. ICD-criteria were fulfilled by 2,902 patients.
With reference to the valid definition of the DGG, the DGGG and other professional societies, we divided the patients into two subgroups: older than or equal to 70 years (n = 711) and younger than 70 years (n = 2,191).
To study only infections with an odontogenic focus, exclusion criteria were: Patients with antiresorptive-associated necrosis of the jaw (ARONJ), or patients with an antiresorptive medication (n = 311). Furthermore, in order to achieve improved comparability the results, we excluded patients with head and neck cancer (n = 146), infection without a dental cause (n = 192), patients who had received radiation therapy to the head and neck (n = 19), primary osteomyelitis (n = 32), foreign body infections (n = 30), patients who had undergone tooth extraction without a surgical incision and drainage (n = 99) and patients who had received antibiotic therapy without surgical intervention (n = 80). Furthermore, we excluded outpatients (n = 819) because of insufficient data. Overall, a total of 1,173 patients (elderly n = 240 patients and non-elderly n = 933 patients) were included.
The institutional ethics committee of the Carl von Ossietzky University Oldenburg approved the study.
Data were collected from the clinical information system (CGM Medico, Release 27.01.04.01, CGM Clinical Europe GmbH, Koblenz, Germany).
All patients underwent a full diagnostic workup, including medical history, physical examination, laboratory tests and radiological imaging.
To obtain further information, we analysed the doctor's letter, premedication, operative report, medication list, laboratory values, microbiological report, radiological imaging and clinical history of each patient.
Factors included in the analysis were age, sex, selected systemic diseases, dental aetiology, treatment modalities, leukocytes, C-reactive protein levels, microbiology results and living situation.
Based on a literature review, DM, immunosuppression, history of malignancy or chemotherapy, cardiovascular disease, chronic respiratory disease, CRF [GFR < 30 ml/min, stage G4 Kidney Disease: Improving Global Outcome Classification (KDIGO)], dementia, cerebrovascular disease were chosen as relevant systemic diseases.
The White blood cell count (WBC) in Tsd./μl and CRP in g/dl, taken at the time of first contact with the emergency department were analysed.
All patients received surgical abscess incisions and intravenous antibiotic treatment (except eight patients) with different antibiotics. We included patients with intraoral and extraoral incisions or both. The sample for microbiological pathogens and resistance was obtained from the first purulent secretion that appeared. The submandibular approach was chosen as standard for drainage of larger mandibular abscesses. For other sites (e.g., temporal or in the cheek), an appropriately established surgical approach was chosen.
After incision and access to the abscess pocket swabs were taken if deemed necessary by the surgeon. To avoid contamination, samples were taken directly from the abscess secretion carefully avoiding any contact to the surrounding tissue. However, possible unconscious or unintentional contamination cannot be ruled out completely due to the often small surgical incision, especially in case of intraoral incisions. Samples were inoculated on BD Columbia agar + 5% sheep blood, BD Chocolate agar, BD Schaedler agar and BD Schaedler KV agar (all BD, Heidelberg, Germany). Columbia and chocolate agar were incubated for 48 h at 35 ± 2°C and 5% CO2 atmosphere. Schaedler agar and Schaedler KV agar were incubated for 72 h at 35 ± 2°C under anaerobic conditions.
Plates were read after 24, 48 and 72 h. Bacteria that were considered relevant were further identified (Table 2). No growth or a mixture of bacteria of the common oral flora was considered as “no growth of pathogenic bacteria”.
Identification of bacteria was performed biochemically with the Vitek 2 system (bioMérieux, Nuertingen, Germany) or with MALDI-TOF MS using the MALDI Biotyper microflex system (Bruker Daltonics, Bremen, Germany). Susceptibility testing results were excluded from the analysis due to inconsistent testing strategies during the study period.
After pseudonymisation, a database was constructed using Microsoft Excel (Version 16.59, Microsoft, Redmond, WA, USA).
Continuous variables were presented as median and range [in the tables also as mean ± standard deviation (SD)] and analysed using the Mann–Whitney U-test. Categorial variables were evaluated using the Chi–squared test.
Regression analysis was also performed. First univariate linear regression analysis was used for numerical outcomes and univariate binary logistic regression for categorial outcomes.
Thereafter, statistically significant and historically reported important variables were analysed by multivariate regression analysis.
Biometric advice was provided by the Institute of Biometrics and Clinical Research Münster, Germany.
All statistical analyses were performed with SPSS version 21 (SPSS, Chicago, Illinois). A p-value < 0.05 was considered statistically significant.
We analysed 1,173 patients with odontogenic infections. Of these 240 (20.5%) were elderly patients with an age equal to or greater than 70 years and 933 (79.5%) were non-elderly patients with an age less than 70 years.
Five hundred forty-nine (46.8%) were females and 624 (53.2%) males. The median age of all patients was 48.79 [range 77.08] years. In the elderly, median age was 78.98 [range 25.25] years. In the non-elderly the median age was 41.92 [range 51.64] years.
A total of 1,131 patients (96.4%) lived in their own home, 42 patients (3.6%) in a nursing home. In the elderly, 30 patients (12.5%) lived in a nursing home compared to only 12 non-elderly patients (1.3%), (p < 0.001).
Overall, the most common co-morbidity was cardiovascular disease (n = 170; 14.5%), followed by DM (n = 99; 8.4%) and chronic respiratory disease (n = 84; 7.2%). In the elderly, the most common co–morbidity was also cardiovascular disease, (n = 97; 40.4%), followed by DM, (n = 47; 19.6%), and cerebrovascular disease, (n = 37; 15.4%).
There was a significant difference between the elderly and non-elderly groups in all systemic diseases examined, except for immunosuppressive medication (Table 1).
Overall, the peri/submandibular lodge was most frequently involved (n = 395; 33.7%), followed by the paramandibular/vestibular space, (n = 342; 29.2%) and the fossa canina space (n = 130; 11.1%). Older patients had significantly more infections in the paramandibular/vestibular space and in the fossa canina space. In contrast younger patients had significantly more infections in the peri/submandibular and parapharyngeal area (Table 1).
The most causative tooth for infection was one of the lower molars, (n = 544; 46.4%). Two hundred twenty-seven patients (19.4%) had an infection after tooth extraction, 115 patients (50.7%) after extraction of wisdom teeth and 112 (49.3%) after extraction of other teeth.
In younger patients, an infection after tooth extraction was the second leading cause of odontogenic abscess (n = 190; 20.4%) (Table 1).
In general, 617 patients (52.6%) had an intraoral incision and 500 patients (42.6%) an extraoral incision. Fifty-six patients (4.8%) had a combined incision (Table 1).
Leukocyte count was performed on admission in 798 patients, CRP was determined in 710 patients. The median value of WBC was 11.80 [range 32.3] Tsd./μl, the median value of CRP was 6.80 [range 57.4] g/dl (Table 1).
Microbiological analysis was performed in 616 patients (52.5%). No pathogenic bacteria were found in n = 203 (33.0%); in the remaining 413 cases, 81 different species were found, which we grouped into 14 bacterial groups.
Overall, the most frequently isolated bacteria were members of the Streptococcus anginosus group (S. anginosus, S.constellatus and S. intermedius), (n = 146; 23.7%), followed by Prevotella species (spp.) in 135 patients (21.9%) and coagulase negative staphylococci (CNS), (n = 110; 17.9%).
In older patients, the most frequently isolated bacteria also belong to the Streptococcus anginosus group, (n = 19; 20.7%), followed by Prevotella spp., (n = 17; 18.5%) and CNS, (n = 15; 16.3%). This order can also be observed in the younger patients.
The only significant difference in isolation frequency between younger and older patients was found in the viridans group streptococci (n = 6/75; p = 0.044) and Candida spp. (n = 9/15; p = 0.005) (Table 2).
Eight patients (0.7%) received no antibiotic treatment. Eight hundred and eighty-six patients (75.5%) received ampicillin/sulbactam (Unacid®) and 161 patients (13.7%) received clindamycin according to local and national guidelines (43). In addition, 85 patients (7.2%) received a combination of ampicillin/sulbactam and metronidazole and ten patients (0.9%) received a combination of clindamycin and metronidazole.
Overall, 97 patients (8.3%) had complications. Elderly patients had more complications, (n = 23; 9.6%) than non-elderly patients, (n = 74; 7.9%), (p = 0.407).
The most frequently complication was intensive care treatment (n = 60; 5.1%), mainly due to upper airway obstruction, (n = 38/60; 63.3%).
Furthermore, ICU admission in cause of upper airway obstruction mainly occured in patients with infection in the large and deep spaces, like the parapharyngeal or perimandibular/submandibular space.
Younger patients were more frequently admitted to an intensive care unit (ICU) (p = 0.038), also regarding to upper airway obstruction (p = 0.029).
Furthermore, patients with an abscess in the parapharyngeal space were significantly more admitted to the ICU (p = 0.001) due to upper airway obstruction (p < 0.001) (Table 3).
Performing binary logistic regression analysis, the following parameters showed a significant impact on complications: pre-existing CRF (Odds ratio (OR):3.14 [CI:1.325–7.430], p = 0.009), parapharyngeal infection [OR:3,50 (CI:2.006–6.110), p < 0.001], base of the mouth infection [OR:2.88 (CI:1.620–5.109), p < 0.001] and origin of the infection in the lower molars [OR:1.72 (CI:1.130–2.628), p = 0.011].
In elderly patients, the impact of CRF [OR:4.06 (CI:1.501–10.996), p = 0.006] on complications increases.
Performing multivariate regression analysis, infection in the perimandibular (OR 1.01 [CI:0.977–1.024], p < 0.001, oral base [OR:6.65 (CI:3.012–14.701), p < 0.001] and parapharyngeal space [OR:7.16 (CI:3.121–16.432), p < 0.001] had a significant impact on complications.
Regression analysis also showed that the likelihood of complications increases with a higher WBC [OR:1.10 (CI:1.054–1.150), p < 0.001] and CRP [OR:1.08 (CI:1.057–1.104), p < 0.001], however the multivariate regression analysis showed only a significant prognostic impact of CRP [OR:1.06 (CI1.034–1.088), p < 0.001] (Table 4).
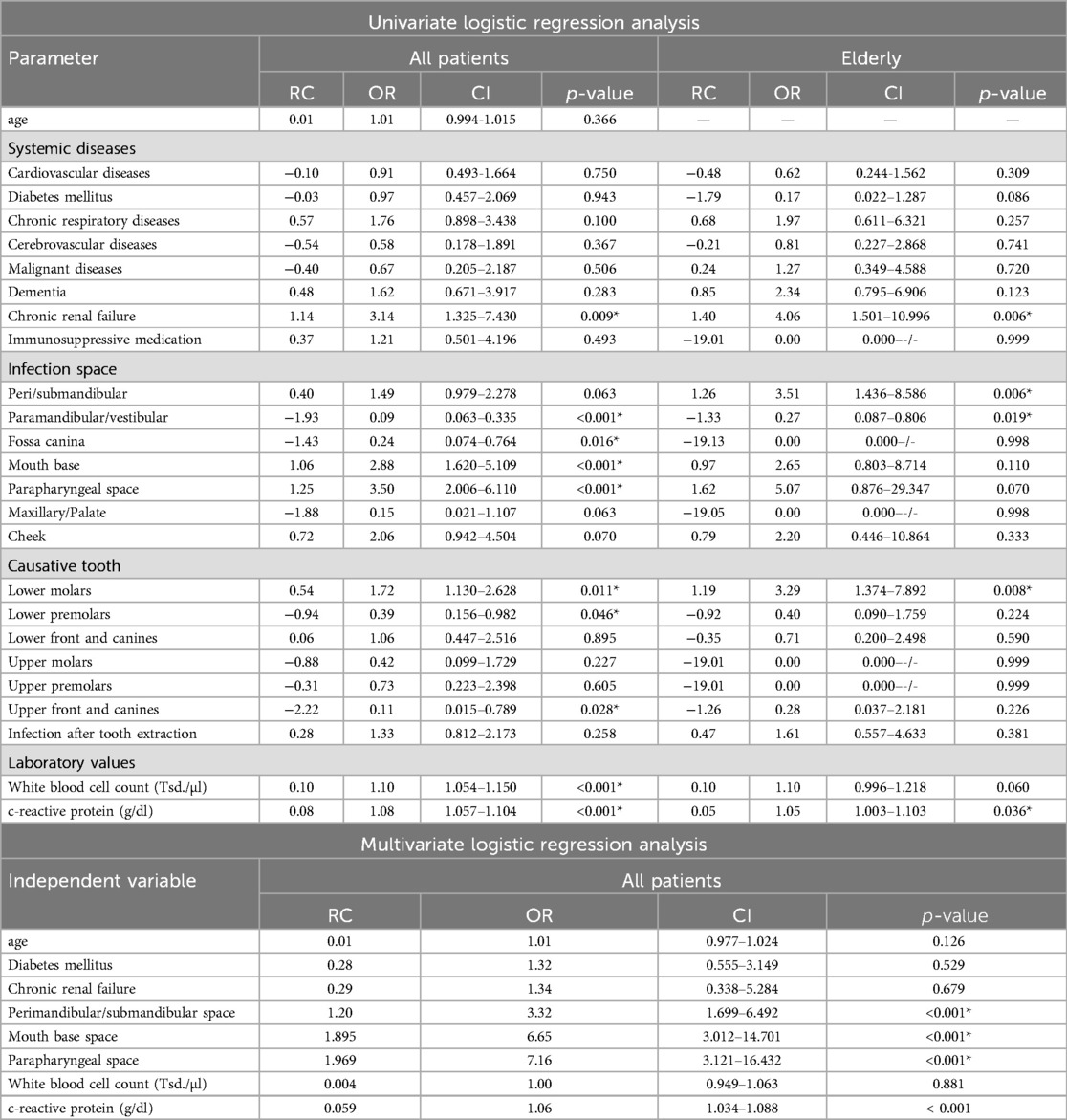
Table 4. Binary logistic regression analysis for complications (*p < 0.05), (RC, regression coefficient, OR, odds ratio, CI, 95% confidence interval).
Performing ROC-analysis we also saw a higher diagnostic accuracy for CRP (Area under the curve (AUC):0.692 [CI:0.625–0.758], p < 0.001) than WBC [AUC: 0.611 (CI:0.541–0.681), p = 0.001] (Table 5).
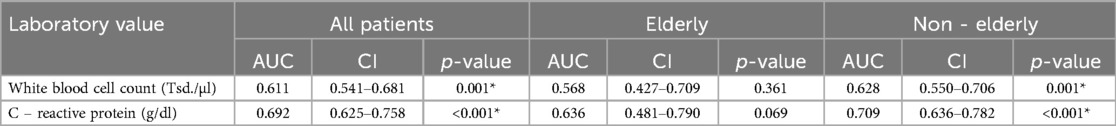
Table 5. ROC analysis for WBC and CRP as predictor for complications (*p < 0.05), (AUC, area under the curve, CI, 95% confidence interval).
The median hospital stay time was 3 [range 28] days.
Elderly patients had a longer LOS 4 [range 28] days than non-elderly patients 3 [range 22] days, (p = 0.129).
Elderly patients with an infection in the peri/submandibular (p = 0.038), parapharyngeal (p = 0.018) oral base (p = 0.017) and cheek space (p = 0.004) as well as elderly patients with post-extraction infections (p = 0.001) had a significantly more extended LOS than non-elderly patients (Table 6).
Univariate linear regression analysis showed that patient age had a small effect on LOS [RC:0.02 (CI:0.008–0.023), p < 0.001].
Regarding systemic diseases, patients with a CRF had a significantly longer LOS [RC:1.48 (CI:0.552–2.411), p = 0.002] with a higher impact in older patients [RC:1.76 (CI: 0.420–3.099), p = 0.010].
Peri/submandibular [RC:0.99 (CI:0.671–1.314), p < 0.001], parapharyngeal [RC:2.00 (CI:1.432–2.574), p < 0.001] and oral base [RC:1.00 (CI:0.426–1.573), p = 0.001] infections had a significant impact on LOS, again with an increasing impact in the elderly.
In the regression analysis, a higher WBC [RC:0.08 (CI:0.038–0.125), p < 0.001] and CRP-level [RC:0.12 (CI:0.094–0.140), p < 0.001] at the time of admission leads to a significantly longer LOS. For CRP, these effect increases in older patients [RC:0.21 (CI:0.142–0.278), p < 0.001].
Performing multivariate regression analysis pre-existing CRF [RC:1.507 (CI:0.304–2.709), p = 0.014], infections in the peri/submandibular [RC:1.364 (CI:0.890–1.838), p < 0.001], parapharyngeal [RC:2.783 (CI:2.046–3.520), p < 0.001] and oral base space [RC:1.655 (CI:0.929–2.381), p < 0.001] are significant predictors for longer LOS. In elderly patients, infections in the parapharyngeal space [RC:6.074 (CI:2.203–9.945), p = 0.002] and oral base space [RC:1.958 (CI:0.106–3.810), p = 0038] showed a significant association with prolonged LOS.
Furthermore CRP is a significant prognostic marker for longer LOS [RC:0.092 (CI:0.066–0.117), p < 0.001] with an increasing impact in the elderly [RC:0.166 (CI:0.081–0.251), p < 0.001] (Table 7).
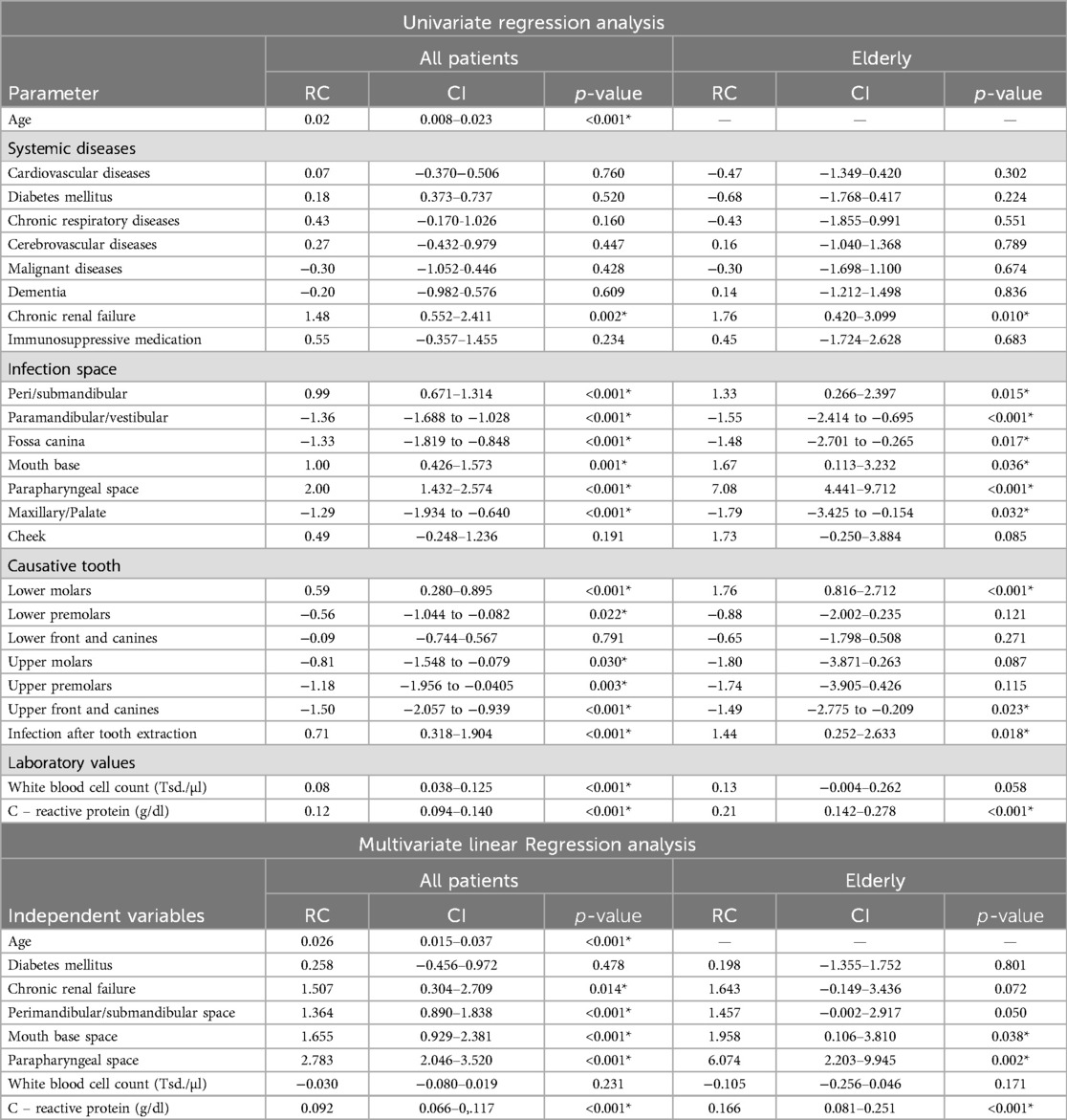
Table 7. Linear regression analysis for LOS (*p < 0.05), (RC, regression coefficient, CI, 95% confidence interval).
The mean age of all patients in our study was 50.0 ± 19.9 years, similar to other studies (7, 44, 45). Also similarly, the incidence of deep neck infections was higher in the elderly (41, 44, 46).
Equal to other studies, males were predominant (53.2%), with a ratio of 1,13:1 (10, 47–49),. In the present study, older patients were predominantly female (54.2%), similar to the study of Chi et al. (41), but in contrast to the findings by Zheng et al. (1).
We found a significant association between CRF and complications. Furthermore, regression analysis showed that CRF was the strongest predictor for complications and a prolonged LOS.
Patients with CRF, especially in end-stage renal diseases (ESRD), have been reported to have a higher risk of infectious complications (50, 51) due to multifactorial mechanisms such as neutrophil dysfunction, uremic toxicity, biological incompatibility, anaemia, iron overload and a dialysis access and procedure (52–54). As described by Dalrymple et al., infection is the second leading cause of death in patients with an ESRD (54). The authors also found that patients with CRF have longer LOS for infection-related admissions than those without (54, 55).
Several factors such as advanced age, high burden of co-morbidities, hypoalbuminemia (56, 57), immunosuppressive therapy (58), nephrotic syndrome (59), uraemia, anaemia or malnutrition (60, 61) may additionally increase the risk of infection in patients with CRF.
We identified two studies that investigated ESRD in relation to head and neck infections.
Chang et al. found a higher incidence rate and cumulative incidence in patients with ESRD than in those without. They also described a higher mortality rate and poor survival in patients with ESRD, although LOS was not significantly longer (62). A limitation is that only a quarter of patients underwent surgical treatment, whereas in our study all patients did. Another limitation is the definition of ESRD. Chang et al. defined the target group based on ESRD-related ICD-9 without specific GFR, whereas our study defined CRF as GFR < 30 ml/min (62).
Tsai et al. reported that patients with deep neck infections and ESRD had a significantly longer LOS, more ICU admissions and a higher mortality rate than patients without. They also found a higher incidence of methicillin-resistant Staphylococcus aureus (MRSA) in the ERSD group, which was not investigated in this study (63).
Regarding the top, the studies by Chang et al. and Tsai et al. looked at all types of deep neck infections without specifying odontogenic origin. However, Chang et al. mentioned the odontogenic origin as the most common cause of head and neck infections (62).
Both studies examined the age of the patients in a subanalysis. Tsai et al. described a higher incidence of ESRD in patients younger than 65 years, whereas Chang et al. described no difference between those younger and older than 65 years (62, 63). Both findings contrast with our study, in which older patients had a significantly higher incidence of CRF than non-elderly patients.
Many studies also reported an association between DM and a complicated course of treatment for head and neck infections up to life—threatening complications (11, 64–66).
However, we found no significant correlations between DM and complications or prolonged LOS.
In this study, the most common site of infection in all patients was the peri/submandibular space, which is similar to many other studies (18, 19, 49, 66).
Contrary to the expected accelerated spreading of the infection in compromised elderly patients, these group had significantly more infections in the paramandibular/vestibular, fossa canina or maxillary spaces. Since the study excluded outpatients, we suspect that the number of younger patients with infections in this space may not necessarily be lower but was not included the analysis due to the criteria.
Flynn et al. described these cavities as “low-risk spaces”, with a low incidence of complications (67). Nevertheless, these patients were admitted to the hospital in the present study. We hypothesise that hospitalisation was due to pre-existing systemic disease and possible concomitant (anticoagulant) medication.
Chi et al. reported the parapharyngeal cavity as the most affected space, in older as well as in younger patients with a cut-off age of 65 years. However, this study also included non—odontogenic causes (41).
The lower molar was the most common focus of infection, which is consistent with many other studies (19, 22, 44, 49, 68, 69). Particularly in younger patients, the lower molars were by far the most common focus (51.2%), resulting in many infections in the submandibular space.
In older patients, the distribution of the odontogenic focus was more even. Since the apex of the tooth of origin determines the path of dissemination (22), the results are consistent with the distribution of infection space in the older group.
In our study, infections at the base of the mouth (OR:2.88; p < 0.001) and in the parapharyngeal cavity (OR:3.50; p < 0.001) lead to significantly more complications. In older patients, infections in the peri/submandibular space significantly increase the likelihood of complications (OR:3.51; p = 0.006).
In addition, infections in the lower molars lead to a significantly higher risk of complications (OR:1.72, p = 0.011). These results are in line with the studies of Alotaibi et al. and Ylijoki et al. (9, 10).
Due to modern diagnostics and therapy, serious complications after odontogenic infection have a low incidence and mainly occur in the presence of predisposing factors (14, 19, 70).
In our study 97 patients (8.3%) experienced complications, the most commonly of which were ICU admission (5.1%) mainly for upper airway obstruction (3.2%). The percentage of patients requiring ICU admission was lower than previously reported (18, 66, 71, 72). For example, Barber et al. described a percentage of 21.4% requiring ICU admission, with no significant difference in age (21). Gams et al. even reported an incidence of ICU admission up to 45% (66).
Severe odontogenic infections are known to cause upper airway obstruction (1, 8). Adovica et al. reported a complication rate of 11.4%, mainly caused by upper airway obstruction (18). Suehara et al. even reported a complication rate of 50.3%, mainly caused by airway compromise (72).
As widely expected, and in conclusion with our results, the risk of upper airway obstruction depends on the infection space which in turn often depends on the dental origin. Branstetter et al. described the “mylohyoid line” as a significant border in the communication between the mouth base or sublingual space and the submandibular space of the neck. Furthermore, they described that infections with their dental origin in the anterior part of the mandible first affect the sublingual space, whereas infections from the second or third molar can directly spread into the deeper lodges (73). Nevertheless, both spaces communicate via the unattached posterior margin of the mylohyoid muscle (74).
One possible explanation for this quantitative difference in complications and upper airway obstruction is the inclusion of infection sites in some study analyses. For example, infections in the submandibular and parapharyngeal spaces are much more common and require intubation and intensive care therapy to protect the airway.
In this study, 38 patients (3.2%) underwent a second operation to drain all collections, which is consistent with the findings of Gholami et al. (69). The rate was higher in the elderly (5.0%) compared to the non-elderly (2.8%), but not significantly different. Adovica et al. reported a significantly higher reoperation rate in elderly patients compared to younger patients (18).
In the present study the rate of complications was slightly higher in older patients (9.6% vs. 7.9%), but the difference was not significant. Similarly, regression analysis didn't show a significant effect of age on complications. This contrasts with other studies. Zhang et al. reported a higher incidence of life-threatening complications in patients over 65 years of age. The average age of patients with life-threatening complications was 9.6 years higher than those without (7). Adovica et al. describe a higher complication rate, ICU admission and LOS in older patients compared to younger patients, but without precise information on the age structure (18). In addition, Suehara et al. reported age as s significant variable associated with complications (72).
Furthermore, when looking at the subgroups, the incidence of ICU therapy and upper airway obstruction was higher in younger patients. In addition, Riekert et al. also described, that age was not significantly correlated with an ICU admission (75).
LOS is widely accepted as a surrogate marker of complicated course and adverse outcome in hospitalised patients (76, 77).
The mean LOS in our study was 3.92(3) ± 2.69 days, which was shorter than in other studies (10, 14, 66), but within the range of 3–10 days reported in previous studies (25, 30, 48, 68, 71, 78, 79). Park et al. reported a mean LOS of 12.43 days (44), Suehara et al. of 12.6 ± 14.4 days (72) and Seppänen et al. even reported a mean LOS of 14.8 days; however they only included 35 patients (14).
In our study, age as an isolated risk factor was associated with a longer LOS, but the difference was not significant (p = 0.129). Regression analysis showed a weak association between age and longer LOS (p = 0.001).
Wang et al. reported that older patients had a significantly longer LOS (80). Gams et al. described age as a significant predictor of longer LOS (66).
Park et al. identified age as a predictor for a LOS longer than 12 days. Among the other risk factors, age had the highest ratio for a longer LOS (44).
Regression analysis showed a significant association between peri/submandibular, parapharyngeal and oral base infections and prolonged LOS (p < 0.05). In older patients, the regression coefficient increased in all areas. As a result, we saw a stronger association between older patients with infections in these areas and prolonged LOS (p < 0.05). In contrast, Flynn at el. didn't find a significant association between prolonged LOS and involvement of the specific deep fascial spaces (67).
We also observed a significant association between lower molar infection and longer LOS, which also increased in the older population. In line with this, Alotaibi et al. described a longer LOS in patients with mandibular odontogenic infections compared to those with maxillary odontogenic infections (10).
In our study, regression analysis showed, that WBC and CRP levels on admission were positively correlated with LOS (p < 0.001 and complications (p < 0.001). Park et al. also described a CRP level > 10 mg/dl as a risk factor for prolonged LOS (44).
Flynn et al. described the WBC level as a significant predictor of reoperation and prolonged LOS (67). Mathew et al. reported a WBC count greater than 15 × 109/L as a risk factor for life-threatening complications (11).
Many other studies have also shown that CRP levels can be a valuable marker for determining the severity of an odontogenic infections (9, 75, 76, 81–83). Sharma et al. reported a strong correlation between CRP levels and the severity of infection (81).
Ylijoki et al. and Riekert et al. reported a significantly higher WBC and CRP levels on admission in patients requiring intensive care compared to those not requiring intensive care (9, 75).
Riekert et al. conclude that higher CRP levels appear to be associated with the severity of deep cavity infections of odontogenic origin (75).
Regression analysis showed a significant correlation between higher WBC and CRP levels and complications as well as prolonged LOS, which is consistent with the findings of Gholami et al. (69). Wang et al. reported a CRP level > 100 µg/ml as a significant predictor of prolonged LOS, whereas a WBC level > 15,000/mm3 was not significantly associated with LOS (80). Consistent with this, Stathopoulos et al. also confirmed the CRP level as the only significant predictor of prolonged LOS.
Regarding the multivariate regression analysis, only higher CRP levels had a significant correlation with prolonged LOS (p < 0.001) and complications (p < 0.001). Therefore, we suggest that CRP is the most predictable laboratory marker of complicated courses in the elderly.
The three most frequency isolated bacteria by culture-based methods in this study were members of the Streptococcus anginosus group, Prevotella spp. and CNS, similar to the findings of Gams et al. (66) and other authors (78, 84).
Many studies have investigated the microbiology of odontogenic infections. Common to all these studies is the diversity of bacteria observed (22).
However, the literature is mixed regarding the predominant bacterial species in odontogenic infections (30, 78, 85). According to Gams et al., there are several possible explanations for this finding, including the laboratory culture technique, the protocol for transport to the laboratory and the method of sample collection (66). Previous reports show that standard culture methods of odontogenic infections yield an average of 2–8 species. When molecular techniques are used, the average is around 18 species (22, 86, 87). In addition, Walia et al. reported that the swab technique is associated with the isolation of gram-positive aerobes, whereas the aspiration technique results in more gram-negative anaerobes (30).
Furthermore, data from whole genome sequencing suggest that the role of aerobic gram-positive bacteria is overestimated when using culture-based diagnostics and that odontogenic infections are dominated by anaerobic Prevotella, Porphyromonas and Fusobacterium spp (88).
These limitations apply to our study as well. We used a swab technique followed by subsequent culture. Additionally, we had to group the results of cultures with no growth and those with a mixture of oral flora bacteria, where no further identification was conducted. This partly explains the relatively high percentage of cases (33%) in which no pathogenic bacteria were identified. Nevertheless, our findings regarding predominant bacteria are consistent with those of other authors using culture-based techniques (66, 78, 84).
Candida spp. was more frequently isolated from samples from elderly patients than from younger patients, whereas viridans group streptococci were more frequently identified in non-elderly patients. Candida spp. and viridans group streptococci are not known to be the predominant species in odontogenic infections. Therefore, the absence of viridans group streptococci, which are part of the normal oral flora, and a higher abundance of Candida spp. in the elderly may be a surrogate for an altered composition of the oral microbiome in the elderly patients included in this study. The prevalence of Candida spp. carriage has previously been shown to increase with age (89). This could be induced by previous antimicrobial therapy or denture prosthesis, which could explain the higher prevalence of Candida spp. in the elderly (90, 91).
The study has a few limitations, and some study parameters are debatable:
First, the study is a monocentric, retrospective study, all patients came from the catchment area of the Klinikum Oldenburg AöR, which represents a subpopulation in Germany.
To ensure better comparability of the study groups and to avoid possible alterations in the results, we defined a lot of stringent exclusion criteria. Nevertheless, these facts could influence the results in the sense of a selection bias and can limit the transferability of the results to other countries and ethnic groups.
Referring to the joint statement of the significant German Geriatric Societies we defined 70 years as cutoff age for elderly patients. Referring to the literature, some authors prefer 65 years as cutoff age (41, 63) again other authors use 60 years for cutoff definition (1). Nevertheless, there is no generally cutoff value for the calendared age and the resulting is a “grey area” regarding the allocation of the age group between 60 and 70 years. Despite this problem to define geriatric patients based on their calendar age, other important factors like multimorbidity, frailty and decrease of functional reserve had to be considered for the definition of the geriatric patient. An improvement for further studies could be the selection by a geriatric assessment tool.
The data from this study show, that age alone is not a risk factor for complications and longer LOS. However, in combination with specific anatomical spaces, in particular the parapharyngeal and oral base space, older patients have a higher likelihood of complications and longer LOS.
In addition, we found a significant effect of CRF on complications and LOS with an increase especially in the older population.
The present study shows that high WBC and especially high CRP levels on admission can serve as sensitive and reliable predictive markers for complications and prolonged LOS. Furthermore, in older patients, CRP is the more predictable marker of a complicated course and prolonged LOS.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Medizinische Ethikkommission, Carl von Ossietzky Universität Oldenburg. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
DK: Writing – original draft, Writing – review & editing. PT: Writing – review & editing. MR: Methodology, Writing – review & editing. LL: Resources, Supervision, Writing – review & editing. AH: Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships cthat could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2024.1486182/full#supplementary-material
1. Zheng L, Yang C, Zhang W, Cai X, Jiang B, Wang B, et al. Comparison of multi-space infections of the head and neck in the elderly and non-elderly: part I the descriptive data. J Craniomaxillofac Surg. (2013) 41(8):e208–212. doi: 10.1016/j.jcms.2013.01.020
2. Igoumenakis D, Gkinis G, Kostakis G, Mezitis M, Rallis G. Severe odontogenic infections: causes of spread and their management. Surg Infect (Larchmt). 2014;15(1):64–8. doi: 10.1089/sur.2012.178
3. Zheng L, Yang C, Zhang W, Cai X, Jiang B, Wang B, et al. Comparison of multi-space infections of the head and neck in the elderly and non-elderly people, part II: the influencing factors of the outcomes. J Craniofac Surg. (2015) 26(2):581–4. doi: 10.1097/SCS.0000000000001465
4. Ottaviani G, Costantinides F, Perinetti G, Luzzati R, Contardo L, Visintini E, et al. Epidemiology and variables involved in dental abscess: survey of dental emergency unit in trieste. Oral Dis. (2014) 20(5):499–504. doi: 10.1111/odi.12164
5. Jiménez Y, Bagán JV, Murillo J, Poveda R. Odontogenic infections. Complications. Systemic manifestations. Med Oral Patol Oral Cir Bucal. (2004) (9 Suppl):143–7. 139–143.
6. Shakya N, Sharma D, Newaskar V, Agrawal D, Shrivastava S, Yadav R. Epidemiology, microbiology and antibiotic sensitivity of odontogenic space infections in central India. J Maxillofac Oral Surg. (2018) 17(3):324–31. doi: 10.1007/s12663-017-1014-y
7. Zhang C, Tang Y, Zheng M, Yang J, Zhu G, Zhou H, et al. Maxillofacial space infection experience in west China: a retrospective study of 212 cases. Int J Infect Dis. (2010) 14(5):e414–417. doi: 10.1016/j.ijid.2009.08.002
8. Uluibau IC, Jaunay T, Goss AN. Severe odontogenic infections. Aust Dent J. (2005) 50(4 Suppl 2):S74–81.16416722
9. Ylijoki S, Suuronen R, Jousimies-Somer H, Meurman JH, Lindqvist C. Differences between patients with or without the need for intensive care due to severe odontogenic infections. J Oral Maxillofac Surg. (2001) 59(8):867–72. discussion 872-863. doi: 10.1053/joms.2001.25017
10. Alotaibi N, Cloutier L, Khaldoun E, Bois E, Chirat M, Salvan D. Criteria for admission of odontogenic infections at high risk of deep neck space infection. Eur Ann Otorhinolaryngol Head Neck Dis. (2015) 132(5):261–4. doi: 10.1016/j.anorl.2015.08.007
11. Mathew GC, Ranganathan LK, Gandhi S, Jacob ME, Singh I, Solanki M, et al. Odontogenic maxillofacial space infections at a tertiary care center in north India: a five-year retrospective study. Int J Infect Dis. (2012) 16(4):e296–302. doi: 10.1016/j.ijid.2011.12.014
12. Rao DD, Desai A, Kulkarni RD, Gopalkrishnan K, Rao CB. Comparison of maxillofacial space infection in diabetic and nondiabetic patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2010) 110(4):e7–12.20656528
13. Wang J, Ahani A, Pogrel MA. A five-year retrospective study of odontogenic maxillofacial infections in a large urban public hospital. Int J Oral Maxillofac Surg. (2005) 34(6):646–9. doi: 10.1016/j.ijom.2005.03.001
14. Seppänen L, Lauhio A, Lindqvist C, Suuronen R, Rautemaa R. Analysis of systemic and local odontogenic infection complications requiring hospital care. J Infect. (2008) 57(2):116–22. doi: 10.1016/j.jinf.2008.06.002
15. Han X, An J, Zhang Y, Gong X, He Y. Risk factors for life-threatening complications of maxillofacial space infection. J Craniofac Surg. (2016) 27(2):385–90. doi: 10.1097/SCS.0000000000002416
16. Mirochnik R, Araida S, Yaffe V, Abu El-Naaj I. C-reactive protein concentration as a prognostic factor for inflammation in the management of odontogenic infections. Br J Oral Maxillofac Surg. (2017) 55(10):1013–7. doi: 10.1016/j.bjoms.2017.10.006
17. Bali RK, Sharma P, Gaba S, Kaur A, Ghanghas P. A review of complications of odontogenic infections. Natl J Maxillofac Surg. (2015) 6(2):136–43. doi: 10.4103/0975-5950.183867
18. Adoviča A, Veidere L, Ronis M, Sumeraga G. Deep neck infections: review of 263 cases. Otolaryngol Pol. (2017) 71(5):37–42. doi: 10.5604/01.3001.0010.5315
19. Opitz D, Camerer C, Camerer DM, Raguse JD, Menneking H, Hoffmeister B, et al. Incidence and management of severe odontogenic infections-a retrospective analysis from 2004 to 2011. J Craniomaxillofac Surg. (2015) 43(2):285–9. doi: 10.1016/j.jcms.2014.12.002
20. Vieira F, Allen SM, Stocks RM, Thompson JW. Deep neck infection. Otolaryngol Clin North Am. (2008) 41(3):459–83., vii. doi: 10.1016/j.otc.2008.01.002
21. Barber BR, Dziegielewski PT, Biron VL, Ma A, Seikaly H. Factors associated with severe deep neck space infections: targeting multiple fronts. J Otolaryngol Head Neck Surg. (2014) 43(1):35. doi: 10.1186/s40463-014-0035-5
22. Neal TW, Schlieve T. Complications of severe odontogenic infections: a review. Biology (Basel). (2022) 11(12).36552293
23. Robertson D, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. (2009) 58(Pt 2):155–62. doi: 10.1099/jmm.0.003517-0
24. Shah A, Ramola V, Nautiyal V. Aerobic microbiology and culture sensitivity of head and neck space infection of odontogenic origin. Natl J Maxillofac Surg. (2016) 7(1):56–61. doi: 10.4103/0975-5950.196126
25. Jundt JS, Gutta R. Characteristics and cost impact of severe odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol. (2012) 114(5):558–66. doi: 10.1016/j.oooo.2011.10.044
26. Kang SH, Kim MK. Antibiotic sensitivity and resistance of bacteria from odontogenic maxillofacial abscesses. J Korean Assoc Oral Maxillofac Surg. (2019) 45(6):324–31. doi: 10.5125/jkaoms.2019.45.6.324
27. Sato FR, Hajala FA, Freire Filho FW, Moreira RW, de Moraes M. Eight-year retrospective study of odontogenic origin infections in a postgraduation program on oral and maxillofacial surgery. J Oral Maxillofac Surg. (2009) 67(5):1092–7. doi: 10.1016/j.joms.2008.09.008
28. Sánchez R, Mirada E, Arias J, Paño JR, Burgueño M. Severe odontogenic infections: epidemiological, microbiological and therapeutic factors. Med Oral Patol Oral Cir Bucal. (2011) 16(5):e670–676. doi: 10.4317/medoral.16995
29. Cunha TF, Soares Melancia TA, Zagalo Fernandes Ribeiro CM, Almeida de Brito JA, Abreu Miguel SS, André Abreu Esteves Bogalhão do Casal D. Risk factors for surgical site infection in cervico-facial oncological surgery. J Craniomaxillofac Surg. (2012) 40(5):443–8. doi: 10.1016/j.jcms.2011.07.019
30. Walia IS, Borle RM, Mehendiratta D, Yadav AO. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J Maxillofac Oral Surg. (2014) 13(1):16–21. doi: 10.1007/s12663-012-0455-6
31. Sweeney LC, Dave J, Chambers PA, Heritage J. Antibiotic resistance in general dental practice–a cause for concern? J Antimicrob Chemother. (2004) 53(4):567–76. doi: 10.1093/jac/dkh137
32. Geriatrics GSf. What is geriatrics? Available online at: https://www.dggeriatrie.de/nachwuchs/91-was-ist-geriatrie.html (accessed September 23, 2024).
33. Gerhard T, Mayer K, Braisch U, Dallmeier D, Jamour M, Klaus J, et al. Validierung des Geriatrie-Checks zur Identifikation geriatrischer Patienten in der Notaufnahme. Zeitschrift für Gerontologie und Geriatrie. (2021) 54(2):106–12. doi: 10.1007/s00391-020-01699-1
34. Singler K, Dormann H, Dodt C, Heppner HJ, Püllen R, Burkhardt M, et al. Der geriatrische patient in der Notaufnahme. Notfall+Rettungsmedizin. (2016) 19(6):496–9. doi: 10.1007/s10049-016-0216-z
35. Biber R, Bail HJ, Sieber C, Weis P, Christ M, Singler K. Correlation between age, emergency department length of stay and hospital admission rate in emergency department patients aged ≥70 years. Gerontology. (2013) 59(1):17–22. doi: 10.1159/000342202
36. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9
37. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. (2016) 31:1–8. doi: 10.1016/j.arr.2016.08.006
38. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56(3):M146–156. doi: 10.1093/gerona/56.3.M146
39. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. (2014) 9:433–41.24672230
40. Yamaoka M, Ono Y, Takahashi M, Ishizuka M, Uchihashi T, Yasuda K, et al. Acute inflammation in horizontal incompletely impacted third molar with radiolucency in the elderly. Clin Interv Aging. (2009) 4:337–42. doi: 10.2147/CIA.S6052
41. Chi TH, Tsao YH, Yuan CH. Influences of patient age on deep neck infection: clinical etiology and treatment outcome. Otolaryngol Head Neck Surg. (2014) 151(4):586–90. doi: 10.1177/0194599814542589
42. Liang SY. Sepsis and other infectious disease emergencies in the elderly. Emerg Med Clin North Am. (2016) 34(3):501–22. doi: 10.1016/j.emc.2016.04.005
43. Deutsche Gesellschaft für Mund- K-uG. Odontogene Infektionen [Guideline]. 2016 [updated 08.09.2016; cited 2024 16.09.2024]. 1.0:Available online at: AWMF Registernummer: 007-006, http://www.awmf.org
44. Park J, Lee JY, Hwang DS, Kim YD, Shin SH, Kim UK, et al. A retrospective analysis of risk factors of oromaxillofacial infection in patients presenting to a hospital emergency ward. Maxillofac Plast Reconstr Surg. (2019) 41(1):49. doi: 10.1186/s40902-019-0238-9
45. Huang TT, Liu TC, Chen PR, Tseng FY, Yeh TH, Chen YS. Deep neck infection: analysis of 185 cases. Head Neck. (2004) 26(10):854–60. doi: 10.1002/hed.20014
46. Marioni G, Staffieri A, Parisi S, Marchese-Ragona R, Zuccon A, Staffieri C, et al. Rational diagnostic and therapeutic management of deep neck infections: analysis of 233 consecutive cases. Ann Otol Rhinol Laryngol. (2010) 119(3):181–7. doi: 10.1177/000348941011900306
47. Dang N P, Delbet-Dupas C, Mulliez A, Devoize L, Dallel R, Barthélémy I. Five predictors affecting the prognosis of patients with severe odontogenic infections. Int J Environ Res Public Health. (2020) 17(23).
48. Doležalová H, Zemek J, Tuček L. Deep neck infections of odontogenic origin and their clinical significance. A retrospective study from hradec králové, Czech republic. Acta Medica (Hradec Kralove). (2015) 58(3):86–91. doi: 10.14712/18059694.2015.98
49. Keswani ES, Venkateshwar G. Odontogenic maxillofacial space infections: a 5-year retrospective review in navi mumbai. J Maxillofac Oral Surg. (2019) 18(3):345–53. doi: 10.1007/s12663-018-1152-x
50. Cahuayme-Zuniga LJ, Brust KB. Mycobacterial infections in patients with chronic kidney disease and kidney transplantation. Adv Chronic Kidney Dis. (2019) 26(1):35–40. doi: 10.1053/j.ackd.2018.09.004
51. Ndlovu KCZ, Swe Swe-Han K, Assounga A. Association of Staphylococcus nasal colonization and HIV in end-stage renal failure patients undergoing peritoneal dialysis. Ren Fail. (2019) 41(1):303–13. doi: 10.1080/0886022X.2019.1598433
52. Chang GH, Lu A, Yang YH, Liu CY, Chang PJ, Lee CP, et al. High risk of peritonsillar abscess in End-stage renal disease patients: a nationwide real-world cohort study. Int J Environ Res Public Health. (2021) 18(13). doi: 10.3390/ijerph18136775
53. Chonchol M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial. (2006) 19(4):291–6. doi: 10.1111/j.1525-139X.2006.00175.x
54. Dalrymple LS, Go AS. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. (2008) 3(5):1487–93. doi: 10.2215/CJN.01290308
55. Naqvi SB, Collins AJ. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. (2006) 13(3):199–204. doi: 10.1053/j.ackd.2006.04.004
56. Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO study. J Am Soc Nephrol. (2003) 14(7):1863–70. doi: 10.1097/01.ASN.0000074237.78764.D1
57. Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol. (2004) 15(4):1038–45. doi: 10.1097/01.ASN.0000119144.95922.C4
58. Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. (1998) 9(5):869–76. doi: 10.1681/ASN.V95869
59. McIntyre P, Craig JC. Prevention of serious bacterial infection in children with nephrotic syndrome. J Paediatr Child Health. (1998) 34(4):314–7. doi: 10.1046/j.1440-1754.1998.00232.x
60. Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol. (1993) 3(9):1541–54. doi: 10.1681/ASN.V391541
61. Vanholder R, Van Loo A, Dhondt AM, De Smet R, Ringoir S. Influence of uraemia and haemodialysis on host defence and infection. Nephrol Dial Transplant. (1996) 11(4):593–8. doi: 10.1093/oxfordjournals.ndt.a027346
62. Chang GH, Tsai MS, Liu CY, Lin MH, Tsai YT, Hsu CM, et al. End-stage renal disease: a risk factor of deep neck infection—a nationwide follow-up study in Taiwan. BMC Infect Dis. (2017) 17(1):424. doi: 10.1186/s12879-017-2531-5
63. Tsai MS, Yang YH, Huang TY, Tsai YT, Lu A, Wu CY, et al. Pathogens and prognosis of deep neck infection in end-stage renal disease patients. Laryngoscope. (2022) 132(7):1403–9. doi: 10.1002/lary.29955
64. Rahimi-Nedjat RK, Sagheb K, Sagheb K, Hormes M, Walter C, Al-Nawas B. The role of diabetes mellitus on the formation of severe odontogenic abscesses-a retrospective study. Clin Oral Investig. (2021) 25(11):6279–85. doi: 10.1007/s00784-021-03926-4
65. Kamat RD, Dhupar V, Akkara F, Shetye O. A comparative analysis of odontogenic maxillofacial infections in diabetic and nondiabetic patients: an institutional study. J Korean Assoc Oral Maxillofac Surg. (2015) 41(4):176–80. doi: 10.5125/jkaoms.2015.41.4.176
66. Gams K, Shewale J, Demian N, Khalil K, Banki F. Characteristics, length of stay, and hospital bills associated with severe odontogenic infections in Houston, TX. J Am Dent Assoc. (2017) 148(4):221–9. doi: 10.1016/j.adaj.2016.11.033
67. Flynn TR, Shanti RM, Hayes C. Severe odontogenic infections, part 2: prospective outcomes study. J Oral Maxillofac Surg. (2006) 64(7):1104–13. doi: 10.1016/j.joms.2006.03.031
68. Zawiślak E, Nowak R. Odontogenic head and neck region infections requiring hospitalization: an 18-month retrospective analysis. Biomed Res Int. (2021) 2021:7086763. doi: 10.1155/2021/7086763
69. Gholami M, Mohammadi H, Amiri N, Khalife H. Key factors of odontogenic infections requiring hospitalization: a retrospective study of 102 cases. J Oral Maxillofac Surg Med Pathol. (2017) 29(5):395–9. doi: 10.1016/j.ajoms.2017.03.016
70. Lorenzini G, Picciotti M, Di Vece L, Pepponi E, Brindisi L, Vessio V, et al. Cervical necrotizing fasciitis of odontogenic origin involving the temporal region–a case report. J Craniomaxillofac Surg. (2011) 39(8):570–3. doi: 10.1016/j.jcms.2010.05.002
71. Christensen B, Han M, Dillon JK. The cause of cost in the management of odontogenic infections 2: multivariate outcome analyses. J Oral Maxillofac Surg. (2013) 71(12):2068–76. doi: 10.1016/j.joms.2013.05.027
72. Suehara AB, Rodrigues AAN, Kavabata NK, Menezes MB, Ramos EA, Kawamukai JN, et al. Predictive factors of lethality and complications of deep fascial space infections of the neck. Rev Col Bras Cir. (2020) 47:e20202524. doi: 10.1590/0100-6991e-20202524
73. Branstetter BF, Weissman JL. Infection of the facial area, oral cavity, oropharynx, and retropharynx. Neuroimaging Clin N Am. (2003) 13(3):393–410. ix. doi: 10.1016/S1052-5149(03)00034-0
74. Branstetter BF, Weissman JL. Normal anatomy of the neck with CT and MR imaging correlation. Radiol Clin North Am. (2000) 38(5):925–40. ix. doi: 10.1016/S0033-8389(05)70213-X
75. Riekert M, Kreppel M, Zöller JE, Zirk M, Annecke T, Schick VC. Severe odontogenic deep neck space infections: risk factors for difficult airways and ICU admissions. Oral Maxillofac Surg. (2019) 23(3):331–6. doi: 10.1007/s10006-019-00770-5
76. Stathopoulos P, Igoumenakis D, Shuttleworth J, Smith W, Ameerally P. Predictive factors of hospital stay in patients with odontogenic maxillofacial infections: the role of C-reactive protein. Br J Oral Maxillofac Surg. (2017) 55(4):367–70. doi: 10.1016/j.bjoms.2016.11.004
77. Dodson TB, Barton JA, Kaban LB. Predictors of outcome in children hospitalized with maxillofacial infections: a linear logistic model. J Oral Maxillofac Surg. (1991) 49(8):838–42. doi: 10.1016/0278-2391(91)90012-B
78. Farmahan S, Tuopar D, Ameerally PJ, Kotecha R, Sisodia B. Microbiological examination and antibiotic sensitivity of infections in the head and neck. Has anything changed? Br J Oral Maxillofac Surg. (2014) 52(7):632–5. doi: 10.1016/j.bjoms.2014.02.028
79. Shewale JB, Correa AM, Baker CM, Villafane-Ferriol N, Hofstetter WL, Jordan VS, et al. Impact of a fast-track esophagectomy protocol on esophageal cancer patient outcomes and hospital charges. Ann Surg. (2015) 261(6):1114–23. doi: 10.1097/SLA.0000000000000971
80. Wang LF, Kuo WR, Tsai SM, Huang KJ. Characterizations of life-threatening deep cervical space infections: a review of one hundred ninety-six cases. Am J Otolaryngol. (2003) 24(2):111–7. doi: 10.1053/ajot.2003.31
81. Sharma A, Giraddi G, Krishnan G, Shahi AK. Efficacy of serum prealbumin and CRP levels as monitoring tools for patients with fascial space infections of odontogenic origin: a clinicobiochemical study. J Maxillofac Oral Surg. (2014) 13(1):1–9. doi: 10.1007/s12663-012-0376-4
82. Ren YF, Malmstrom HS. Rapid quantitative determination of C-reactive protein at chair side in dental emergency patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2007) 104(1):49–55. doi: 10.1016/j.tripleo.2007.01.007
83. Bagul R, Chandan S, Sane VD, Patil S, Yadav D. Comparative evaluation of C-reactive protein and WBC count in fascial space infections of odontogenic origin. J Maxillofac Oral Surg. (2017) 16(2):238–42. doi: 10.1007/s12663-016-0953-z
84. Morey EF, Moule AJ, Higgins TJ. Pyogenic dental infections–a retrospective analysis. Aust Dent J. (1984) 29(3):150–3. doi: 10.1111/j.1834-7819.1984.tb01129.x
85. Flynn TR, Shanti RM, Levi MH, Adamo AK, Kraut RA, Trieger N. Severe odontogenic infections, part 1: prospective report. J Oral Maxillofac Surg. (2006) 64(7):1093–103. doi: 10.1016/j.joms.2006.03.015
86. de Sousa EL, Ferraz CC, Gomes BP, Pinheiro ET, Teixeira FB, de Souza-Filho FJ. Bacteriological study of root canals associated with periapical abscesses. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2003) 96(3):332–9. doi: 10.1016/S1079-2104(03)00261-0
87. Flynn TR, Paster BJ, Stokes LN, Susarla SM, Shanti RM. Molecular methods for diagnosis of odontogenic infections. J Oral Maxillofac Surg. (2012) 70(8):1854–9. doi: 10.1016/j.joms.2011.09.009
88. Böttger S, Zechel-Gran S, Schmermund D, Streckbein P, Wilbrand JF, Knitschke M, et al. Clinical relevance of the microbiome in odontogenic abscesses. Biology (Basel). (2021) 10(9).
89. Darwazeh AM, Hammad MM, Al-Jamaei AA. The relationship between oral hygiene and oral colonization with Candida species in healthy adult subjects*. Int J Dent Hyg. (2010) 8(2):128–33. doi: 10.1111/j.1601-5037.2009.00407.x
90. Baena-Monroy T, Moreno-Maldonado V, Franco-Martínez F, Aldape-Barrios B, Quindós G, Sánchez-Vargas LO. Candida albicans, Staphylococcus aureus and Streptococcus mutans colonization in patients wearing dental prosthesis. Med Oral Patol Oral Cir Bucal. (2005) 10(Suppl1):E27–39.15800465
Keywords: odontogenic abscess, elderly, geriatric patients, chronic renal failure, C-reactive protein
Citation: Kaercher D, Thelen P, Ruettermann M, Li L and Hamprecht A (2024) Outcome predictors of odontogenic abscesses in the elderly. Front. Oral. Health 5:1486182. doi: 10.3389/froh.2024.1486182
Received: 25 August 2024; Accepted: 30 October 2024;
Published: 2 December 2024.
Edited by:
Adriana Modesto Gomes Da Silva, University of Pittsburgh, United StatesReviewed by:
Oladimeji Adeniyi Akadiri, University of Port Harcourt, NigeriaCopyright: © 2024 Kärcher, Thelen, Ruettermann, Li and Hamprecht. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Kaercher, a2FlcmNoZXIuZGFuaWVsQGtsaW5pa3VtLW9sZGVuYnVyZy5kZQ==; ZGFuaWVsa2FlcmNoZXJAbWUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.