- 1Centre for Host-Microbiome Interactions, Faculty of Dentistry, Oral and Craniofacial Sciences, Kings College London, London, United Kingdom
- 2Department of Periodontology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
- 3Department of Cardiology, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom
- 4Department of Anaesthesia, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 5Ealing Hospital, London North West University Healthcare NHS Trust, London, United Kingdom
- 6Unilever Oral Care, Bebington, United Kingdom
Infective endocarditis (IE) is a bacterial infection of the heart's inner lining. A low incidence rate combined with a high mortality rate mean that IE can be difficult to treat effectively. There is currently substantial evidence supporting a link between oral health and IE with the oral microbiome impacting various aspects of IE, including pathogenesis, diagnosis, treatment, and mortality rates. The oral microbiome is highly diverse and plays a crucial role in maintaining oral health by providing protective functions. However, when dysbiosis occurs, conditions such as periodontal or peri-implant disease can arise, offering a pathway for bacteraemia to develop. The role of the oral microbiome as a coloniser, facilitator and driver of IE remains to be uncovered by next-generation sequencing techniques. Understanding the dysbiosis and ecology of the oral microbiome of IE patients will allow improvements into the diagnosis, treatment, and prognosis of the disease. Furthermore, an increased awareness amongst those at high-risk of developing IE may encourage improved oral hygiene methods and lower incidence rates. This narrative review examines current findings on the relationship between oral health and IE. It draws from key studies on both topics, with manuscripts selected for their pertinence to the subject. It highlights the link between the oral microbiome and IE by exploring diagnostic techniques and treatments for IE caused by oral commensals.
Overview of infective endocarditis and the oral microbiome
Infective endocarditis (IE) is a disease characterised by inflammation or infection of the endocardial surface of the heart. It can affect native and prosthetic heart valves as well as cardiac implantable electronic devices (CIEDs) such as pacemakers, defibrillators and cardiac resynchronisation therapy devices. Although relatively rare, with an annual age- and gender-standardised incidence of 3–10 cases per 100,000 inhabitants (1), it is recognised as a major worldwide health challenge with a mortality of up to 30% at 30 days. Patients with IE are predominantly male and generally older, with a peak incidence between the ages of 70 and 80 years (2). The bacterial port of entry is usually via the transcutaneous, oral or respiratory routes, and less commonly through the gastrointestinal or genitourinary tracts. Staphylococcus aureus is now the predominant causative microorganism of IE in the developed world, accounting for 30% of all cases, followed by Streptococci and Enterococci (3). In all cases of suspected IE, careful clinical and cardiac imaging evaluation is required to establish an early diagnosis and to enable prompt treatment with antimicrobial therapy and/or cardiac surgery before progressive cardiac involvement or systemic complications ensue.
For both native valve IE and prosthetic valve IE cases, bacteraemia is a prerequisite (4). Under healthy conditions, the valvular endothelium is resistant to bacterial colonisation, and thus a disruption of the valvular surface is necessary for bacterial attachment (5, 6). Once bacterial colonisation occurs, the bacteria form biofilms —complex communities embedded in a matrix of secreted macromolecules (7). Biofilm formation not only facilitates immune evasion but also contributes to suboptimal antibiotic therapy by creating a protective barrier that impedes antibiotic penetration. Pathogens from the oral microbiome can enter the bloodstream, attach to damaged cardiac tissue, and initiate an inflammatory response, ultimately leading to the characteristic lesions of IE (8).
Good oral health contributes to both quality of life and systemic health. The oral microbiome has co-evolved with humans, forming an ecosystem which is important for maintaining health when in homeostasis (9). Within the spectrum of IE, the oral microbiome has been heavily implicated in the causation of IE (10, 11). Recent studies indicate that common dental procedures (including non-surgical) may result in bacteraemia of typical oral commensals in 60%–80% of patients (12). Mechanical plaque control through toothbrushing is well established (13). However, given that the milder forms of periodontitis occur in up to 50% of the general population (14), even simple toothbrushing may increase the risk of transient bacteraemia (15, 16). The healthy myocardial endocardium is generally resistant to the bacteraemia arising from daily activities such as chewing and tooth brushing (17). However, endothelial injury arising from almost any type of congenital or degenerative structural heart disease can increase the risk of IE (8). Other factors that may be associated with endothelial bacterial adherence and IE include systemic inflammatory diseases, such as diabetes mellitus, human immunodeficiency virus (HIV), rheumatoid arthritis, systemic lupus erythematosus, intravenous drug use, previous IE and rheumatic heart disease (18). Widespread medical advances during the 20th and 21st centuries have played a substantial role in shaping the current patient demographics and microbiology of IE. For example, an increased prevalence of prosthetic valves, CIEDs, long-term intravenous lines and invasive procedures now form the principal risk factors. As a result, healthcare-related IE (hospital-acquired and outpatient-acquired) accounts for up to one-third of all cases of IE in contemporary cohorts (19).
Despite advances in diagnostic imaging, antibiotic therapies and surgical or percutaneous valvular treatment options, the prognosis for IE remains poor. Even with optimal therapy, IE is associated with high in-hospital mortality rate of up to 25% (20, 21). Complications of IE related to haemodynamic instability, embolisation, septic abscesses and neurological events are amongst the most devastating, and have a significant effect on increasing mortality (22, 23). Early surgery in high-risk patients with Staphylococcus aureus IE (24) can be further complicated with IE relapses and postoperative valvular dysfunction (25). As a result, even after the acute phase, the risk of mortality for IE remains high, with survivors affected by increased morbidity and reduced quality of life (26). Therefore, it is imperative that clinicians have a high index of suspicion for the diagnosis of IE. This is particularly pertinent in patients that present with relevant symptoms, risk factors and typical microorganisms. The European Society of Cardiology (ESC) guidelines provide an evidence-based framework for the best diagnostic and therapeutic approach to managing patients with IE (27).
Current diagnostic methods for infective endocarditis
The modified Duke criteria
Reaching an early and accurate diagnosis of IE requires a combination of clinical suspicion, microbiology analysis and cardiac imaging with evidence of IE-related damage. Evaluation of the patients with suspected IE relies on the modified Duke criteria (MDC) (28), which integrates these clinical domains and classifies them into minor and major criteria. A definite diagnosis of IE requires two major, one major and three minor, or five minor criteria to be met (29). It should be noted that the MDC has lower sensitivity for patients with CIED-related IE, right-sided IE, prosthetic valve endocarditis (PVE) and blood culture negative endocarditis (BCNE) (30). For this reason, the MDC should be used as a guide for assimilating the clinical and laboratory findings to confirm the diagnosis, rather than a replacement for clinical judgement (31).
Microbiology
Blood cultures (BC) are the first and essential diagnostic tool for identifying continuous bacteraemia in IE (32, 33). Three sets of BCs should be drawn at 30-minute intervals from separate sites and using aseptic technique before patients are started on antibiotics (32). The standard practice of incubation for BC is 5–7 days at 35–37°C, which achieves bacteraemia detection rate of 96%–98% (32, 34). Around 10%–20% of patients with IE have negative BC results, creating diagnostic uncertainty (10). This can arise due to antibiotic treatment before BCs, infection with fastidious fungi or characteristically slow-growing bacteria (Haemophilus species; Aggregatibacter species; Cardiobacterium hominis; Eiknella corrodens; and Kingella species—HACEK organisms) (32) or an alternative diagnosis. Improved practices for BC sampling, modification of loading delay, BC preincubation and extended BC incubation can help to improve the diagnostic yield (32, 35, 36). Direct identification of specific organisms can be achieved using other techniques, including serological testing, broad-range polymerase chain reaction (PCR) and mass spectroscopy (37). Recently, metagenomic techniques have been considered to offer non-invasive diagnosis and biomarker discovery (38). Metagenomic diagnosis for IE could provide a faster alternative diagnostic tool, improving outcomes for patients in the future. A recent systematic review highlighted that current metagenomic methods have satisfactory diagnostic performance and yield an overall detection rate higher than some conventional methods (39).
Cardiac imaging
The MDC defines three major imaging criteria for IE: abscess, vegetation and new dehiscence of prosthetic valve (28). Transthoracic echocardiography (TTE) remains the cornerstone of imaging for the initial assessment of suspected IE (27). Transoesophageal echocardiography (TEE) is recommended when TTE is equivocal or non-diagnostic, when complications are suspected, or when intracardiac device leads are present. Cardiac computed tomography (CT) is an important adjunctive imaging modality for use when the diagnostic performance of echocardiography is affected by artifacts or when TEE is contraindicated altogether (40). Cardiac CT has high spatial resolution, which can help to delineate paravalvular anatomy for complications (the extend of abscesses, fistulae or mycotic aneurysms) and plan surgical interventions. Compared with CT, TEE has improved temporal resolution and is therefore more useful for identifying small IE vegetations (<10 mm) and valvular incompetence (41). Additional diagnostic value can be provided by combining CT with metabolic imaging using 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) or leukocyte scintigraphy [radiolabelled leukocyte single-photon emission computed tomography (SPECT)], which can help to show areas of increased metabolic activity or inflammation in the regions of suspected IE (42). Awareness of the available imaging modalities and the relative strengths and weaknesses of each technique facilitates assessment of patients with suspected IE.
Management of infective endocarditis
Effective management of IE requires a multidisciplinary input from infectious disease specialists, cardiologists and cardiothoracic surgeons. Empirical antimicrobial therapy can be started after the BCs are taken, with further targeted amendments based on BC results, resistance patterns, infection severity and the presence of prosthetic material. A combination of antibiotics is usually needed for up to 6 weeks to eradicate the culprit organism (43). Prolonged regimens are required to obtain a sustained bactericidal effect given that the infections of the valvular apparatus are usually difficult to reach by the host immune system and antibiotics. Stable patients who are responding well to treatment may be considered for an early shift from intravenous to oral antibiotics for the remainder of the course (44). Emerging evidence also suggests that effective courses of antibiotics with the duration as short as two weeks can be given to some IE patients with no complications and highly susceptible microbial strains (45). Prompt referral for surgery is indicated for significant valve dysfunction with heart failure, embolic complications and persistent infection (46–48). Surgery aims to restore normal valve function and to resect all infected tissue. Valvular repair or replacement using either bioprosthetic or mechanical valves may be considered (49).
Discussion
Oral microbiome, oral health and infective endocarditis
The oral microbiome is one of the most complex ecosystems within the human body and plays an important role in physiological, metabolic, immunological and systemic health (50). The oral cavity itself is comprised of a multitude of distinct habitats created by the periodontal pocket, gingivae, tongue, teeth, cheek, palate and saliva. These environments provide an ideal medium for more than 700 known bacterial species to reside, with an ever increasing number being continuously discovered using metagenomic profiling techniques (9, 51). As a consequence, the oral cavity remains an important portal of entry for microorganisms into the blood stream that are heavily implicated in the causation of infective endocarditis (50).
Recent studies indicate that common dental procedures such as flossing, toothbrushing, and chewing may result in transient bacteraemia involving common oral commensals (15, 52). More invasive procedures, such as endodontic treatments, tooth extractions, caries removal, and periodontal or apical surgery, can also induce gingival or mucosal trauma and subsequent bacteraemia (53). Several factors, including diet, smoking, alcohol consumption, stress, and antibiotic therapy, are associated with changes in the oral microbiota, potentially disrupting the balance between commensal and pathogenic organisms (54). This imbalance, known as dysbiosis, is a major driver of periodontal disease and tooth loss (55, 56). Chronic inflammation and pathophysiological destruction of the gingival tissue and bone allows oral pathogens to cause bacteraemia with less resistance, contributing to systemic inflammation (57).
The relationship between oral dysbiosis, periodontal disease, and IE remains incompletely understood. However, monitoring changes in the oral microbial composition of IE patients is essential for developing diagnostic tools aimed at preventing periodontal disease and mitigating the systemic sequelae associated with increased bacteraemia. Conventional periodontal diagnostic methods, such as clinical examinations and imaging, are effective in detecting periodontal disease, which, if left untreated, can increase the risk of cardiovascular disease (58). Although these methods do not directly diagnose IE, they play a crucial role in managing oral health and reducing the risk of bacteraemia. Advanced techniques, such as microbial testing, are increasingly valuable in assessing the risk of systemic infections caused by specific oral pathogens. Early management of periodontal and peri-implant diseases using these diagnostic tools can significantly reduce the risk of bacteraemia and systemic complications, including IE, particularly in high-risk patients. The treatment of periodontitis and management of peri-implant diseases (59, 60) including professional mechanical plaque removal, are essential for reducing microbial load and controlling inflammation. By maintaining the integrity of the oral epithelium, these interventions help to reduce gingival inflammation and bleeding, prevent periodontal disease progression, and lower the risk of systemic infections.
It is important to note that current guidelines, including those from the American Heart Association (AHA) and the European Society of Cardiology (ESC), recommend antibiotic prophylaxis (AP) only for high-risk patients undergoing invasive dental procedures (43). In contrast, the National Institute for Health and Care Excellence (NICE) in the United Kingdom has advised the discontinuation of routine AP for IE prevention in patients with heart conditions undergoing dental procedures [NICE guideline CG64, 2008; updated in 2015 (61)]. Despite these differing recommendations, uncertainty remains about the precise relationship between the oral microbiome and IE, highlighting the need for further research (11). This underscores the importance of good oral hygiene and regular periodontal care as primary preventive measures in managing patients at risk of IE.
Common causative oral transient bacteria of IE
Saliva is abundant with bacteria, containing up to 108 microbes per millilitre (62). Under poor oral health conditions, the concentration of certain periodontal pathogens can increase. Several of these microorganisms have been identified to be causative of IE (Table 1). Oral Streptococci are historically associated with the development of IE and are found in around 20% of all cases (21, 118). Viridian Group Streptococci (VGS) represent the most common species linked with IE. Patients affected by periodontitis have twice the mortality rate when IE is caused by VGS rather than other bacteria (119). Periodontitis has been shown to enhance bacteraemia caused by VGS (120). Although there is a clear link, taxonomical classification between VGS species has proven difficult due to their ability to take up free DNA from the external environment. VGS are categorised into 5 groups: the S. salivarius group, the S. mitis group, the S. mutans group, the S. sanguinis group and the S. anginosus group. The most common causative pathogen of the VGS species is S. sanguinis, making up over 30% of all VGS IE cases (121, 122). Under normal conditions it is possible for S. sanguinis to form a monospecies oral biofilm. However, in the bloodstream S. sanguinis can adhere to the circulating platelets and submucosal collagens at the sites of valvular damage, which can predispose to the formation of IE vegetations (123).
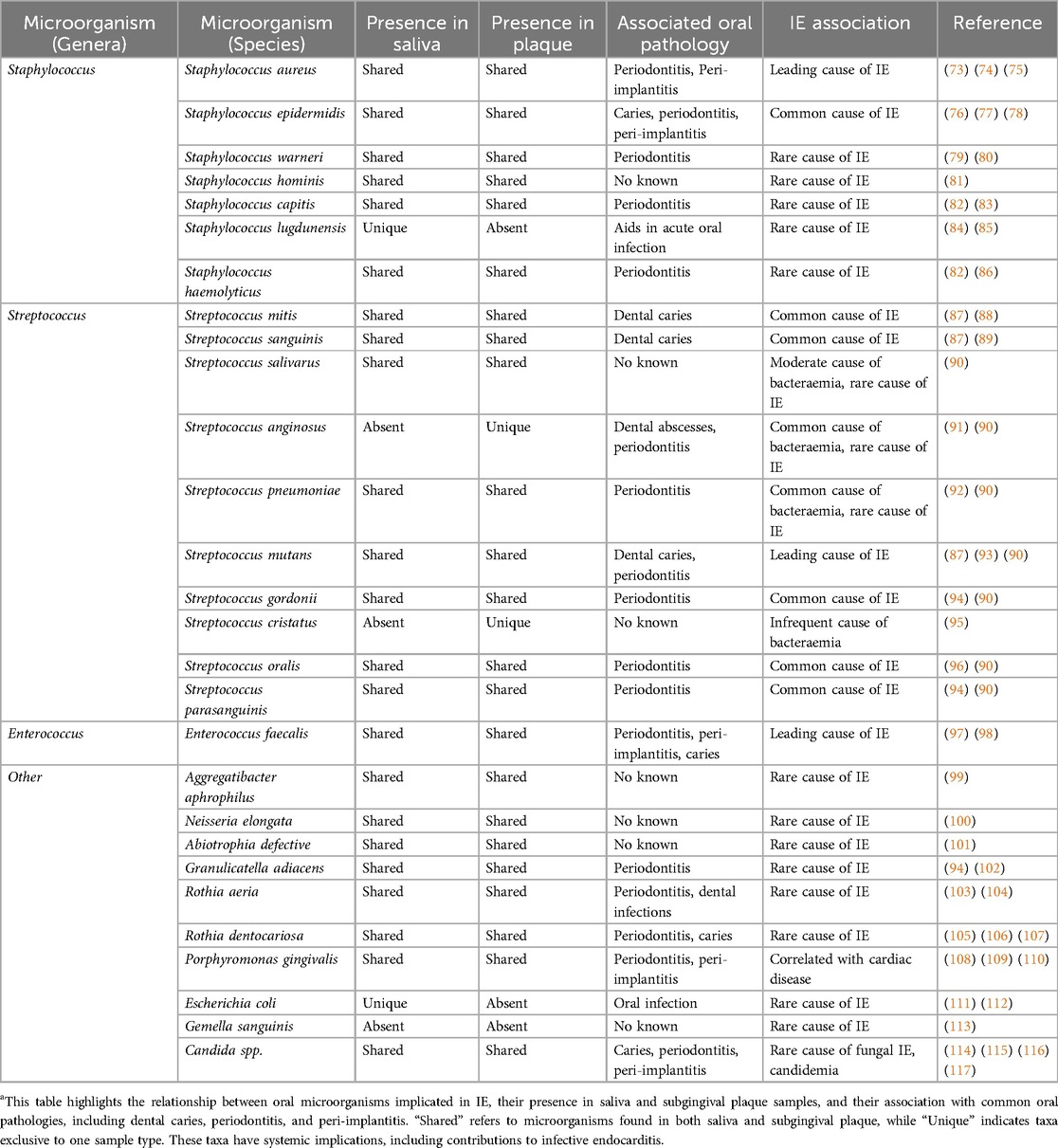
Table 1. IE-associated microorganisms and oral pathogens in human saliva and subgingival plaque samples (11, 63–72).
IE caused by S. pneumonia, which has ubiquitous pneumolysin genes with the S. mitis group, is rare and accounts for less than 3% of all cases, but can follow a severe clinical course (124). Risk factors for developing S. pneumonia IE include immunosuppression in the context of solid organ transplantation, malignancy or HIV infection, and chronic disorders, such as liver cirrhosis, COPD and diabetes mellitus (125). S. pneumonia releases pneumolysin and creates microlesions by forming large pores in cholesterol-containing membranes of eukaryotic cells (126). These microlesions can allow the formation of biofilms, which perpetuate further secretion of pneumolysins and deactivation of cardiac macrophages (125). It is currently unclear whether members of the S. mitis group possess this property, but this is likely based on multiple studies demonstrating that S. mitis species transfer genes in a similar manner to S. pneumonia (127, 128).
Periodontopathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum have also been implicated in the development of IE (129, 130). These organisms can enter the bloodstream through bleeding gums and damaged periodontal tissues, potentially contributing to endocardial infections. P. gingivalis, in particular, has been strongly associated with cardiovascular diseases, including IE (131), due to its ability to evade the immune system and induce systemic inflammation. This pathogen is found in 85.75% of subgingival plaque samples from periodontal patients (132) and has even been isolated from cardiac valve specimens (133). However, other studies suggest that P. gingivalis may not directly influence the degeneration of aortic or mitral valves (131). Another bacterium, Rothia dentocariosa, commonly resides within the oral and respiratory tracts and is typically associated with dental caries or periodontal disease. Although R. dentocariosa is a rare cause of IE, it has been observed in patients with predisposing cardiac conditions and, in rare cases, in previously healthy individuals (134, 135).
Oral microbiome interactions and its effect on periodontal and dental health in IE patients
The oral microbiome forms dental biofilms, which are a leading cause for the development of dental caries and periodontal diseases. Early phase colonisers, formed of predominantly Streptococcus species, can establish the right conditions for the population of disease-causing bacteria to grow at a later point (136). These later phase colonisers are usually anaerobic and, therefore, require early colonisers to extract oxygen from periodontal pockets to allow for their proliferation (137). The resulting biofilm is made up of proteins, polysaccharides and extracellular DNA, which promote interaction and population shifts between microbial species (138). Biofilm-associated microbes can survive in various stress conditions, being less susceptible to antibiotics and more likely to develop antibiotic resistance (139). Maintaining oral health based on regular oral hygiene measures, such as tooth brushing and flossing, helps to disturb these biofilms and reduce infections (140). However, if left undisturbed, the extracellular matrix characterising biofilms with multiple, surface-adherent microbial communities could favour the proliferation of more virulent species. This microbial shift could escalate into dysbiosis, a precursor of periodontal disease (141). Further research into the development of the biofilms initiated by Streptococcal species could have an impact on the antibiotic prophylaxis strategies in high-risk patients, which is increasingly important in the era of antimicrobial resistance.
Successful antibiotic prophylaxis is based on the principle that reducing bacteraemia during interventional procedures lowers the risk of IE (142). Early pre-clinical studies and observational data supported this strategy, leading to recommendations for the use of prophylactic antibiotics in high-risk cardiac patients undergoing bacteraemia-inducing procedures (143). However, the systematic use of prophylactic antibiotics has been contested due to the lack of randomised controlled trials (61). Previous meta-analyses demonstrated that antibiotic prophylaxis did not significantly reduce the risk of IE after dental procedures and that limiting its use to high-risk individuals did not increase the incidence of IE (144, 145). More recent evidence, however, indicates that antibiotic prophylaxis in high-risk patients undergoing invasive dental procedures, such as extractions and oral surgeries, significantly reduces the risk of IE (146–148). This conflicting evidence highlights the complex relationship between interventional procedures, bacteraemia, and IE, suggesting that successful prophylaxis depends on the clear identification of high-risk patients (149, 150).
Patients with a history of IE, prosthetic heart valves, and congenital heart disease have been defined as high-risk (according to (61)) and are recommended for antibiotic prophylaxis before invasive oral procedures (27). Some cardiac patients harbour significantly more oral Streptococci than non-cardiac patients (151), making them more susceptible to periodontal, peri-implant and other dental diseases, such as gingivitis and caries (152). Therefore, combining antibiotic prophylaxis with professional non-surgical periodontal therapy can reduce microbial load and inflammation, lowering the predisposition to IE. However, this approach must be balanced carefully, as antibiotics can disrupt the oral microbiota, leading to dysbiosis and promoting antibiotic resistance with long-term use (153). While short-term antibiotic use can eliminate pathogens, prolonged exposure may result in altered microbial diversity, negatively impacting both oral and systemic health (154). Furthermore, through resistome analyses, our group has identified a number of antibiotic resistance genes in saliva samples taken from IE patients (155).
Over the past years efforts have been made at Guy's and St. Thomas Trust (GSTT) to improve the understanding of the link between IE and oral health. A specific inpatient cardiac dental clinic has been set up to better inform patients of all ages who have, or are at significant risk of developing, IE. This has been coupled with an innovative oral health education programme for outpatients being seen in specialised valve outpatient clinics. It is possible that the physical and psychological burden of IE contributes to poor oral hygiene. Patients suffering from such chronic diseases may experience reduced motivation for self-care. Providing support through professional oral hygiene measures and behavioural interventions can improve outcomes. Our preliminary observations from these initiatives suggest that patient awareness as to the risks of IE with valvular heart disease is poor and that patients who present with IE tend to have suboptimal dental hygiene and higher rates of active periodontitis. Patients attending these initiatives are keen to improve oral hygiene after receiving professional oral hygiene instructions suggesting that the main barrier to improved oral health is a lack of awareness around the link to systemic disease. Observations such as these serve not only to reinforce the links between the oral microbiome and IE, but also to map out new collaborative pathways between cardiologists and dental teams with the aim of reducing the incidence and recurrence of IE in patients.
Conclusion
The oral microbiome has been heavily implicated in the causation of IE. The current literature has identified key causative bacteria of IE, such as S. aureus and a series of Streptococcus species, as well as providing ideas for diagnostic improvements using metagenomic techniques. However, gaps still appear when looking at antibiotic strains and specific preventative steps that can be employed by high-risk individuals. Future studies should look to emulate past successes of metagenomic studies in order to identify biomarkers and microbial networks related to IE.
Author contributions
JF: Conceptualization, Project administration, Writing – original draft, Writing – review & editing, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Visualization. RR: Conceptualization, Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology, Resources, Supervision. VA: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. AY: Writing – original draft, Writing – review & editing, Conceptualization, Formal Analysis, Investigation, Methodology. RG: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. AI: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. NO: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. LN: Writing – original draft, Writing – review & editing, Formal Analysis, Investigation, Methodology. ED’A: Writing – original draft, Writing – review & editing, Conceptualization. VS: Conceptualization, Writing – original draft, Writing – review & editing, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the London Interdisciplinary Biosciences Consortium, the Biotechnology and Biological Sciences Research Council and Unilever.
Acknowledgments
This manuscript benefited from multiple discussions with several clinicians and researchers, and we are grateful to: A.E. Scott and R. Kundu (Unilever); L. Nanayakkara (Barts and the London School of Medicine and Dentistry); A. Ayman, P. Cheong, S. Kaka, D. Moyes and S. Shoaie (King's College London); R. Dworakowski and J Breeze (King's College Hospital). We would like to thank King's British Heart Foundation Centre of Research Excellence, King’s College Hospital NHS Foundation Trust and Guy's and St Thomas’ NHS Foundation Trust, for facilitating discussions with scientists that greatly enriched this manuscript.
Conflict of interest
ED’A is an employee of Unilever PLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rajani R, Klein JL. Infective endocarditis: a contemporary update. Clin Med (Lond). (2020) 20(1):31–5. doi: 10.7861/clinmed.cme.20.1.1
2. Correa de Sa DD, Tleyjeh IM, Anavekar NS, Schultz JC, Thomas JM, Lahr BD, et al. Epidemiological trends of infective endocarditis: a population-based study in Olmsted county, Minnesota. Mayo Clin Proc. (2010) 85(5):422–6. doi: 10.4065/mcp.2009.0585
3. Federspiel JJ, Stearns SC, Peppercorn AF. Increasing US rates of endocarditis with Staphylococcus aureus: 1998–2008. JAMA Intern Med. (2012) 172(4):363–5. doi: 10.1001/archinternmed.2011.1027
4. Cabell CH, Abrutyn E, Karchmer AE. Bacterial endocarditis: the disease, treatment, and prevention. Circulation. (2003) 107(20):e185–7. doi: 10.1161/01.CIR.0000071082.36561.F1
5. Durack DT, Beeson PB, Petersdorf RG. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. (1973) 54:142–51.4700697
6. Kamde SP, Anjankar A. Pathogenesis, diagnosis, antimicrobial therapy, and management of infective endocarditis, and its complications. Cureus. (2022) 14(9):e29182. doi: 10.7759/cureus.29182
7. Elgharably H, Hussain ST, Shrestha NK, Blackstone EH, Pettersson GB. Current hypotheses in cardiac surgery: biofilm in infective endocarditis. Semin Thorac Cardiovasc Surg. (2016) 28(1):56–9. doi: 10.1053/j.semtcvs.2015.12.005
8. Ashley EA, Niebauer J. Cardiology Explained. London: Remedica (2004). Chapter 10, Infective Endocarditis.
9. Baker JL, Mark Welch JL, Kauffman KM, McLean JS, He X. The oral microbiome: diversity, biogeography and human health. Nat Rev Microbiol. (2024) 22(2):89–104. doi: 10.1038/s41579-023-00963-6
10. Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG Jr. Infective endocarditis. Nat Rev Dis Primers. (2016) 2:16059–16059. doi: 10.1038/nrdp.2016.59
11. Ismail A, Yogarajah A, Falconer JL, Dworakowski R, Watson S, Breeze J, et al. Insights into microorganisms, associated factors, and the oral microbiome in infective endocarditis patients. Front Oral Health. (2024) 5:1270492. doi: 10.3389/froh.2024.1270492
12. Marttila E, Grönholm L, Saloniemi M, Rautemaa-Richardson R. Prevalence of bacteraemia following dental extraction—efficacy of the prophylactic use of amoxicillin and clindamycin. Acta Ondontol Scand. (2021) 79(1):25–30. doi: 10.1080/00016357.2020.1768285
13. Axelsson P, Lindhe J. Effect of controlled oral hygieneprocedures on caries and periodontaldisease in adults. J Clin Periodontol. (1978) 5(2):133–151. doi: 10.1111/j.1600-051X.1978.tb01914.x
14. Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA. Age-dependent distribution of periodontitis in two countries: findings from NHANES 2009 to 2014 and SHIP-TREND 2008–2012. J Clin Periodontol. (2018) 45(Suppl 20):S130–48. doi: 10.1111/jcpe.12944
15. Lockhart PB, Brennan MT, Thornhill M, Michalowicz BS, Noll J, Bahrani-Mougeot FK, et al. Poor oral hygeine as a risk factor for infective endocarditis-related bacteraemia. J. Am. Dent. Assoc. (2009) 140:1238–44. doi: 10.14219/jada.archive.2009.0046
16. Thornhill MH, Gibson TB, Yoon F, Dayer MJ, Prendergast BD, Lockhart PB, et al. Endocarditis, invasive dental procedures, and antibiotic prophylaxis efficacy in US medicaid patients. Oral Dis. (2023a) 30(3):1–15. doi: 10.1111/odi.14585
17. Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation. (2008) 117(24):3118–25. doi: 10.1161/CIRCULATIONAHA.107.758524
18. Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus Endocarditis. JAMA. (2005) 293(24):3012–21. doi: 10.1001/jama.293.24.3012
19. Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. (2012) 54(9):1230–9. doi: 10.1093/cid/cis199
20. Mostaghim AS, Lo HYA, Khardori NA. A retrospective epidemiology study to define risk factors, microbiology, and clinical outcomes of infective endocarditis in a large tertiary-care teaching hospital. SAGE Open Med. (2017) 5:2050312117741772. doi: 10.1177/2050312117741772
21. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. Arch Intern Med. (2009) 169:463–73. doi: 10.1001/archinternmed.2008.603
22. Rizzi M, Ravasio V, Carobbio A, Mattucci I, Crapis M, Stellini R, et al. Predicting the occurrence of embolic events: an analysis of 1456 episodes of infective endocarditis from the Italian study on endocarditis (SEI). BMC Infect Dis. (2014) 14(14):230. doi: 10.1186/1471-2334-14-230
23. Abdallah L, Habib G, Remadi J-P, Salaun E, Casalta J-P, Tribouilloy C. Comparison of prognoses of Staphylococcus aureus left-sided prosthetic endocarditis and prosthetic endocarditis caused by other pathogens. Arch Cardiovasc Dis. (2016) 109:542–9. doi: 10.1016/j.acvd.2016.02.010
24. Ferrera C, Vilacosta I, Fernández C, López J, Sarriá C, Olmos C, et al. Early surgery for acute-onset infective endocarditis. Eur J Cardiothorac Surg. (2018) 54(6):1060–6. doi: 10.1093/ejcts/ezy208
25. Thuny F, Beurtheret S, Mancini J, Gariboldi V, Casalta J-P, Riberi A, et al. The timing of surgery influences mortality and morbidity in adults with severe complicated infective endocarditis: a propensity analysis. Eur Heart J. (2011) 32(16):2027–33. doi: 10.1093/eurheartj/ehp089
26. Suárez Bagnasco M, Núñez-Gil IJ. Infective endocarditis and thoracic aortic disease: a review on forgotten psychological aspects. World J Cardiol. (2017) 9(7):620–8. doi: 10.4330/wjc.v9.i7.620
27. Delgado V, Marsan NA, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. (2023) 44(39):3948–4042. doi: 10.1093/eurheartj/ehad193
28. Fowler VG Jr, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-international society for cardiovascular infectious diseases criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis. (2023) 77(4):518–26. doi: 10.1093/cid/ciad271
29. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. (2000) 30(4):633–8. doi: 10.1086/313753
30. Percoraro AJK. Modified Duke/European society of cardiology. Clujul Med. (2015) 88:321–6. doi: 10.1136/openhrt-2021-001856
31. Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, Lupse M. Assessment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Clujul Med. (2015) 88:321–6. doi: 10.15386/cjmed-469
33. Stefani S. Diagnostic techniques in bloodstream infections: where are we going? Int J Antimicrob Agents. (2009) 34:S9–S12. doi: 10.1016/S0924-8579(09)70558-8
34. Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed? J Clin Microbiol. (2007) 45(11):3546–8. doi: 10.1128/JCM.01555-07
35. Lamy B, Dargère S, Arendrup MC, Parienti J-J, Tattevin P. How to optomise the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the-art. Front Microbiol. (2016) 12(7):697. doi: 10.3389/fmicb.2016.00697
36. Bengtsson J, Wahl M, Larsson P. Assessment of the BacT/alert blood culture system: rapid bacteraemia diagnosis with loading throughout the 24h. Clin Microbiol Infect. (1998) 4:33–7. doi: 10.1111/j.1469-0691.1998.tb00331.x
37. Seng P, Drancourt M, Gouriet F, La Scola B, Fournier P-E, Rolain JM, et al. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. (2009) 49(4):543–51. doi: 10.1086/600885
38. Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. (2012) 13:260–70. doi: 10.1038/nrg3182
39. Liu J, Zhang Q, Dong Y-Q, Yin J, Yun-Qing Q. Diagnostic accuracy of metagenomic next-generation sequencing in diagnosing infectious diseases: a meta-analysis. Sci Rep. (2022) 12:21032. doi: 10.1038/s41598-022-25314-y
40. Habets J, Tanis W, Reitsma JB. Are novel non-invasive imaging techniques needed in patients with suspected prosthetic heart valve endocarditis? A systematic review and meta-analysis. Eur Radiol. (2015) 25(7):2125–33. doi: 10.1007/s00330-015-3605-7
41. Saeedan MB, Wang TKM, Cremer P, Wahadat AR, Budde RPJ, Unai S, et al. Role of cardiac CT in infective endocarditis: current evidence, opportunities, and challenges. Radiol Cardiothorac Imaging. (2021) 3(1):e200378. doi: 10.1148/ryct.2021200378
42. García-Arribas D, Vilacosta I, Candil AO, Rey CR, Olmos C, Pérez Castejón MJ, et al. Usefulness of positron emission tomography/computed tomography in patients with valve-tube graft infection. Heart. (2018) 104:1447–54. doi: 10.1136/heartjnl-2017-312918
43. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J. (2015) 36(44):3075. doi: 10.1093/eurheartj/ehv319
44. Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. (2019) 380(5):415–24. doi: 10.1056/NEJMoa1808312
45. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation. (2015) 132(15):1435–86. doi: 10.1161/CIR.0000000000000296
46. Morris NA, Matiello M, Lyons JL, Samuels MA. Neurologic complications in infective endocarditis. Neurohospitalist. (2014) 4(4):213–22. doi: 10.1177/1941874414537077
47. Hess A, Klein I, Iung B, Lavallée P, Ilic-Habensus E, Dornic Q, et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. Am J Neuroradiol. (2013) 34(8):1579–84. doi: 10.3174/ajnr.A3582
48. Hoen B, Duval X. Infective endocarditis. N Engl J Med. (2013) 368:1425–33. doi: 10.1056/NEJMcp1206782
49. Iung B, Duval X. Infective endocarditis: innovations in the management of an old disease. Nat Rev Cardiol. (2019) 16(10):623–35. doi: 10.1038/s41569-019-0215-0
50. Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, et al. The oral microbiome—an update for oral healthcare professionals. Br Dent J. (2016) 221:657–66. doi: 10.1038/sj.bdj.2016.865
51. Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems. (2018) 3(6):e00187–18. doi: 10.1128/msystems.00187-18
52. Martins CC, Lockhart PB, Firmino RT, Kilmartin C, Cahill TJ, Dayer M, et al. Bacteremia following different oral procedures: systematic review and meta-analysis. Oral Dis. (2023) 30(3):1–9. doi: 10.1111/odi.14531
53. Cotti E, Mercuro G. Apical periodontitis and cardiovascular diseases: previous findings and ongoing research. Int Endod J. (2015) 48(10):926–32. doi: 10.1111/iej.12506
54. Buduneli N. Environmental factors and periodontal microbiome. Periodontol 2000. (2021) 85(1):112–25. doi: 10.1111/prd.12355
55. Maitre Y, Micheneau P, Delpierre A, Mahalli R, Guerin M, Amador G, et al. Did the brain and oral microbiota talk to each other? A review of the literature. J Clin Med. (2020) 9:3876. doi: 10.3390/jcm9123876
56. Carrizales-Sepulveda EF, Ordaz-Farias A, Vera-Pineda R, Flores-Ramirez R. Periodontal disease, systemic inflammation and the risk of cardiovascular disease. Heart Lung Circ. (2018) 27:1327–34. doi: 10.1016/j.hlc.2018.05.102
57. Khor B, Snow M, Herrman E, Ray N, Mansukhani K, Patel KA, et al. Interconnections between the oral and gut microbiomes: reversal of microbial dysbiosis and the balance between systemic health and disease. Microorganisms. (2021) 9:496. doi: 10.3390/microorganisms9030496
58. Sia S-K, Jan M-S, Wang Y-H, Huang Y-F, Wei JC-C. Periodontitis is associated with incidental valvular heart disease: a nationwide population-based cohort study. J Clin Periodontol. (2021) 48(8):1085–92. doi: 10.1111/jcpe.13478
59. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I–III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47(Suppl 22):4–60. doi: 10.1111/jcpe.13290
60. Herrera D, Berglundh T, Schwarz F, Chapple I, Jepsen S, Sculean A, et al. Prevention and treatment of peri-implant diseases-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2023) 50(Suppl 26):4–76. doi: 10.1111/jcpe.13823
61. National Institute for Health and Care Excellence. Prophylaxis Against Infective Endocarditis [NICE Guideline No. CG064] (2015). Available online at: http://www.nice.org.uk/CG064 (accessed November 14, 2023)
62. Marsh PD, Do T, Beighton D, Devine DA. Influence of saliva on the oral microbiota. Periodontol 2000. (2016) 70:80–92. doi: 10.1111/prd.12098
63. Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto TK. Occurrence of staphylococci in the oral cavities of healthy adults and nasal–oral trafficking of the bacteria. J Med Microbiol. (2008) 57(pt. 1):95–9. doi: 10.1099/jmm.0.47561-0
64. da Silva ESC, Feres M, Figueiredo LC, Shibli JA, Ramiro FS, Faveri M. Microbiological diversity of peri-implantitis biofilm by sanger sequencing. Clin Oral Impants Res. (2014) 25(10):1192–9. doi: 10.1111/clr.12231
65. Al-Ahmad A, Muzafferiy F, Anderson AC, Wölber JP, Ratka-Krüger P, Fretwurst T, et al. Shift of microbial composition of peri-implantitis-associated oral biofilm as revealed by 16S rRNA gene cloning. J Med Microbiol. (2018) 67(3):332–40. doi: 10.1099/jmm.0.000682
66. Sanz-Martin I, Doolittle-Hall J, Teles RP, Patel M, Belibasakis GN, Hämmerle CHF, et al. Exploring the microbiome of healthy and diseased peri-implant sites using illumina sequencing. J Clin Periodontol. (2017) 44(12):1274–84. doi: 10.1111/jcpe.12788
67. Shi C, Cai L, Xun Z, Zheng S, Shao F, Wang B, et al. Metagenomic analysis of the salivary microbiota in patients with caries, periodontitis and comorbid diseases. J Dent Sci. (2021) 16(4):1264–73. doi: 10.1016/j.jds.2020.12.002
68. Pallos D, Sousa V, Feres M, Retamal-Valdes B, Chen T, Curtis M, et al. Salivary microbial dysbiosis is associated with peri-implantitis: a case-control study in a Brazilian population. Front Cell Infect Microbiol. (2022) 11(11):696432. doi: 10.3389/fcimb.2021.696432
69. Duan X-B, Wu T-X, Guo Y-C, Zhou X-D, Lei Y-L, Xu X, et al. Marginal bone loss around non-submerged implants is associated with salivary microbiome during bone healing. Int J Oral Sci. (2017) 9(2):95–103. doi: 10.1038/ijos.2017.18
70. Sousa V, Nibali L, Spratt D, Dopico J, Mardas N, Petrie A, et al. Peri-implant and periodontal microbiome diversity in aggressive periodontitis patients: a pilot study. Clin Oral Implants Res. (2017) 28(5):558–70. doi: 10.1111/clr.12834
71. Nibali L, Sousa V, Davrandi M, Liu LS, Spratt D, Donos N. Patterns of subgingival microbiota in different periodontal phenotypes. J Dent. (2022) 117:103912. doi: 10.1016/j.jdent.2021.103912
72. Nibali L, Sousa V, Davrandi M, Spratt D, Alyahya Q, Dopico J, et al. Differences in the periodontal microbiome of successfully treated and persistent aggressive periodontitis. J Clin Periodontol. (2020) 47(8):980–90. doi: 10.1111/jcpe.13330
73. Passariello C, Puttini M, Iebba V, Pera P, Gigola P. Influence of oral conditions on colonization by highly toxigenic Staphylococcus aureus strains. Oral Ddisease. (2012) 18(4):402–9. doi: 10.1111/j.1601-0825.2011.01889.x
74. Mangalekar SB, Sultana M, Mulay A, Vaddalapu H, Newaskar DP, Bacha S, et al. Analysis of microbiological profiles of Indian patients with peri-implantitis and periodontitis. Bioinformation. (2024) 20(6):615–9. doi: 10.6026/973206300200615
75. Barnett R. Infective endocarditis. Lancet. (2016) 388(10050):1148. doi: 10.1016/S0140-6736(16)31602-6
76. Divakar DD, Muzaheed , Aldeyab SS, Alfawaz SA, AlKheraif AA, Khan AA. High proportions of Staphylococcus epidermidis in dental caries harbor multiple classes of antibiotics resistance, significantly increase inflammatory interleukins in dental pulps. Microb Pathog. (2017) 109:29–34. doi: 10.1016/j.micpath.2017.05.017
77. Sahrmann P, Gilli F, Wiedemeier DB, Attin T, Schmidlin PR, Karygianni L. The microbiome of peri-implantitis: a systematic review and meta-analysis. Microorganisms. (2020) 8(5):661. doi: 10.3390/microorganisms8050661
79. Fritoli A, Lobão E, Soares G, Retamal-Valdes B, Feres M. Evaluation of Enterococcus faecalis, Staphylococcus warneri and Staphylococcus aureus species in adults with generalized chronic periodontitis. Rev Gaúcha Odontol. (2017) 65(2):121–7. doi: 10.1590/1981-863720170002000043137
80. Alawad MJ, Ali GA, Goravey W. Underrecognized pathogen; Staphylococcus warneri-associated native mitral valve endocarditis in an immunocompetent host: a case report and literature review. Clin Case Rep. (2022) 10(4):e05591. doi: 10.1002/ccr3.5591
81. Vasconcellos D, Weng B, Wu P, Thompson G, Sutjita M. Staphylococcus hominis infective endocarditis presenting with embolic splenic and renal infarcts and spinal discitis. Case Rep Infect Dis. (2022) 14:7183049. doi: 10.1155/2022/7183049
82. Colombo APV, do Souto RM, Araújo LL, Espíndola LCP, Hartenbach FARR, Magalhães CB, et al. Antimicrobial resistance and virulence of subgingival staphylococci isolated from periodontal health and diseases. Sci Rep. (2023) 13:11613. doi: 10.1038/s41598-023-38599-4
83. Douedi S, Odak M, Ravin A, Campbell N. Staphylococcus capitis endocarditis of a native valve. Cureus. (2021) 13(6):e15738. doi: 10.7759/cureus.15738
84. You YO, Kim KJ, Min BM, Chung CP. Staphylococcus lugdunensis–a potential pathogen in oral infection. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (1999) 88(3):297–302. doi: 10.1016/S1079-2104(99)70031-4
85. Leis BT, Parekh DD, Macknak BF, Kogilwaimath S. Staphylococcus lugdunensis endocarditis: lower mortality in the contemporary era? CJC Open. (2022) 4(5):474–8. doi: 10.1016/j.cjco.2022.01.009
86. Falcone M, Campanile F, Giannella M, Borbone S, Stefani S, Venditti M. Staphylococcus haemolyticus endocarditis: clinical and microbiologic analysis of 4 cases. Diagn Microbiol Infect Dis. (2007) 57(3):325–31. doi: 10.1016/j.diagmicrobio.2006.08.019
87. Zhu T, Huang Z, Shu X, Zhang C, Dong Z, Peng Q. Functional nanomaterials and their potentials in antibacterial treatment of dental caries. Colloids Surf B Biointerfaces. (2022) 218:112761. doi: 10.1016/j.colsurfb.2022.112761
88. Shelburne SA, Sahasrabhojane P, Saldana M, Yao H, Su X, Horstmann N, et al. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerg Infect Dis. (2014) 20(5):762–71. doi: 10.3201/eid2005.130953
89. Soh BWT, Salim A, O'Riordan R, Owens P, Matiullah S. Unveiling the devastations of Streptococcus sanguinis infective endocarditis masquerading as iron deficiency anaemia: a case report. Eur Heart J Case Rep. (2024) 8(8):ytae388. doi: 10.1093/ehjcr/ytae388
90. Chamat S, Dahl A, Oestergaard L, Arpi M, Fosboel E, Boel J, et al. Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal species. Eur Heart J. (2020) 142(8):ehaa946.2015. doi: 10.1093/ehjci/ehaa946.2015
91. Furuholm J, Uittamo J, Rautaporras N, Välimaa H, Snäll J. Streptococcus anginosus: a stealthy villain in deep odontogenic abscesses. Ondontology. (2023) 111(2):522–30. doi: 10.1007/s10266-022-00763-z
92. Allicock OM, York A, Waghela P, Yolda-Carr D, Weinberger DM, Wyllie AL. Impact of temporary storage conditions on the viability of Streptococcus pneumoniae in saliva. MSphere. (2022) 7(6):e0033122. doi: 10.1128/msphere.00331-22
93. Dani S, Prabhu A, Chaitra KR, Desai NC, Patil SR, Rajeev R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: a clinico-microbiological study. Contemp Clin Dent. (2016) 7(4):529–34. doi: 10.4103/0976-237X.194114
94. Belstrøm D, Fiehn N-E, Nielsen CH, Kirkby N, Twetman S, Klepac-Ceraj V, et al. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J Clin Periodontol. (2014) 41(2):104–12. doi: 10.1111/jcpe.12190
95. Vecilla DF, Gutierrez MJU, del Arco JLDdT. Streptococcus cristatus, an infrequent cause of bacteremia and infective endocarditis. Case report and literature review. Rev Esp Quimioter. (2023) 36(1):110–3. doi: 10.37201/req/090.2022
96. Chahal G, Quintana-Hayashi MP, Gaytán MO, Benktander J, Padra M, King SJ, et al. Streptococcus oralis employs multiple mechanisms of salivary mucin binding that differ between strains. Front Cell Infect Microbiol. (2022) 12:889711. doi: 10.3389/fcimb.2022.889711
97. Souto R, Colombo APV. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch Oral Biol. (2008) 53(2):155–60. doi: 10.1016/j.archoralbio.2007.08.004
98. Megran DW. Enterococcal endocarditis. Clin Infect Dis. (1992) 15(1):63–71. doi: 10.1093/clinids/15.1.63
99. Mendoza NVZ, Valverde NNG, Velarde VJR. Challenges and insights in Aggregatibacter aphrophilus endocarditis: a review of literature. Arch Peru Cardiol Cir Cardiovasc. (2023) 4(3):102–8. doi: 10.47487/apcyccv.v4i3.306
100. Youssef D, Marroush TS, Levine MT, Sharma M. Endocarditis due to Neisseria elongata: a case report and review of the literature. Germs. (2019) 9(4):188–92. doi: 10.18683/germs.2019.1176
101. Lancaster I, Patel D, Tamboli C, Chun P, Sethi V, Namey J. Abiotrophia defectiva infective endocarditis: a rare and dangerous cause of endocarditis. Case Rep Infect Dis. (2022) 2022:1. doi: 10.1155/2022/7050257
102. Shailaja TS, Sathiavathy KA, Unni G. Infective endocarditis caused by Granulicatella adiacens. Indian Heart J. (2013) 65(4):447–9. doi: 10.1016/j.ihj.2013.06.014
103. Franconieri F, Join-Lambert O, Creveuil C, Auzou M, Labombarda F, Aouba A, et al. Rothia spp. infective endocarditis: a systematic literature review. Infect Dis Now. (2021) 51(3):228–35. doi: 10.1016/j.medmal.2020.10.021
104. Aoyagi S, Tobinaga S, Wada K, Nata S-I, Yasunaga H. Rothia aeria endocarditis complicated with multiple systemic embolisms. Kurume Med J. (2021) 68(3.4):259. doi: 10.2739/kurumemedj.MS6834009
105. Aguilar-Luis MA, Casas Apayco L, Tinco Valdez C, De Lama-Odría MDC, Weilg C, Mazulis F, et al. Screening and assessment of antimicrobial susceptibility of periodontopathic Bacteria in Peruvian patients with periodontitis: a pilot study. Int J Dent. (2021) 2021:2695793. doi: 10.1155/2021/2695793
106. Georg LK, Brown JM. Rothia, gen. nov. an aerobic genus of the family Actinomycetaceae. Int J Syst Evol Microbiol. (1967) 17(1):79–88. doi: 10.1099/00207713-17-1-79
107. Elkattawy S, Alyacoub R, Younes I, Mowafy A, Noori M, Mirza M. A rare report of Rothia dentocariosa endocarditis. J Community Hosp Intern Med Perspect. (2021) 11(3):413–5. doi: 10.1080/20009666.2021.1880539
108. Murugaiyan V, Utreja S, Hovey KM, Sun Y, LaMonte MJ, Wactawski-Wende J, et al. Defining Porphyromonas gingivalis strains associated with periodontal disease. Sci Rep. (2024) 14:6222. doi: 10.1038/s41598-024-56849-x
109. Savčić N, Henjaš D, Jezdić M, Đinić Krasavčević A, Milinković I. Porphyromonas gingivalis in different peri-implant conditions: a pilot cross—sectional study. Acta Stomatol Croat. (2022) 56(4):387–94. doi: 10.15644/asc56/4/5
110. Xie M, Tang Q, Yu S, Sun J, Mei F, Zhao J, et al. Porphyromonas gingivalis disrupts vascular endothelial homeostasis in a TLR-NF-κB axis dependent manner. Int J Oral Sci. (2020) 12:28. doi: 10.1038/s41368-020-00096-z
111. Vulcano AB, Tino-De-Franco M, Amaral JA, Ribeiro OG, Cabrera WH, Bordenalli MA, et al. Oral infection with enteropathogenic Escherichia coli triggers immune response and intestinal histological alterations in mice selected for their minimal acute inflammatory responses. Microbiol Immunol. (2014) 58(6):352–9. doi: 10.1111/1348-0421.12153
112. Anjum Z, Tariq Z. Escherichia coli-associated infective endocarditis in a patient with septic abortion: a rare culprit in a unique presentation. Cureus. (2019) 11(9):e5632. doi: 10.7759/cureus.5632
113. Muguntan M, Bhalla S, Shete V, Grover N. Gemella sanguinis: a rare cause of native valve endocarditis in a child. Med J Armed Forces India. (2015) 72(Suppl 1):S84–6. doi: 10.1016/j.mjafi.2015.08.008
114. Xiang Z, Wakade RS, Ribeiro AA, Hu W, Bittinger K, Simon-Soro A, et al. Human tooth as a fungal niche: candida albicans traits in dental plaque isolates. mBio. (2023) 14:e02769–22. doi: 10.1128/mbio.02769-22
115. Unniachan AS, Jayakumari NK, Sethuraman S. Association between candida species and periodontal disease: a systematic review. Current Medical Mycology. (2020) 6(2):63–8. doi: 10.18502/CMM.6.2.3420
116. de Mendoza IL-I, Cayero-Garay A, Quindos-Andres G, Aguirre-Urizar JM. A systematic review on the implication of candida in peri-implantitis. Int J Implant Dent. (2021) 7:73. doi: 10.1186/s40729-021-00338-7
117. Jamil Y, Akinleye A, Mirzaei M, Lempel M, Farhat K, Pan S. Candida endocarditis: update on management considerations. World J Cardiol. (2023) 15(10):469–78. doi: 10.4330/wjc.v15.i10.469
118. Shah ASV, McAllister DA, Gallacher P, Astengo F, Rodríguez Pérez JA, Hall J, et al. Incidence, microbiology, and outcomes in patients hospitalised with infective endocarditis. Circulation. (2020) 141:2067–77. doi: 10.1161/CIRCULATIONAHA.119.044913
119. Thoresen T, Jordal S, Lie S-A, Wünsche F, Jacobsen MR, Lund B. Infective endocarditis: association between origin of causing bacteria and findings during oral infection screening. BMC Oral Health. (2022) 22:491. doi: 10.1186/s12903-022-02509-3
120. Dhotre S, Jahagirdar V, Suryawanshi N, Davane M, Patil R, Nagoba B. Assessment of periodontitis and its role in viridans streptococcal bacteremia and infective endocarditis. Indian Heart J. (2018) 70(2):225–32. doi: 10.1016/j.ihj.2017.06.019
121. Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridians streptococci isolated from cases of infective endocarditis. J Med Microbiol. (1993) 39:179–82. doi: 10.1099/00222615-39-3-179
122. Horaud T, Delbos F. Viridians streptococci in infective endocarditis: species distribution and susceptibility to antibiotics. Eur Heart J. (1984) 5(Suppl C):39–44. doi: 10.1093/eurheartj/5.suppl_C.39
123. Martini AM, Moricz BS, Ripperger AK, Tran PM, Sharp ME, Forsythe AN, et al. Association of novel Streptococcus sanguinis virulence factors with pathogenesis in a native valve infective endocarditis model. Front Microbiol. (2020) 11(11):10. doi: 10.3389/fmicb.2020.00010
124. Choi M, Mailman TL. Pneumococcal endocarditis in infants and children. Pediatr Infect Dis J. (2004) 23(2):166–71. doi: 10.1097/01.inf.0000109290.91866.93
125. Brown AO, Garsin DA. The pathogenesis of cardiac microlesion formation during severe bateraemia infection. PLoS Pathog. (2020) 16:e1009021. doi: 10.1371/journal.ppat.1009021
126. Rossjohn J, Gilbert RJ, Crane D, Morgan PJ, Mitchell TJ, Rowe AJ, et al. The molecular mechanism of pseumolysin, a virulence factor from Streptococcus pneumoniae. J Mol Biol. (1998) 284:449–61. doi: 10.1006/jmbi.1998.2167
127. Kilian M, Riley DR, Jensen A, Brüggemann H, Tettelin H. Parallel evolution of Streptococcus pneumoniae and Streptococcus mitis to pathogenic and mutualistic lifestyles. mBio. (2014) 5(4):e01490–14. doi: 10.1128/mBio.01490-14
128. Salvadori G, Junges R, Morrison DA, Petersen FC. Competence in Streptococcus pneumoniae and close commensal relatives: mechanisms and implications. Front Cell Infec. Microbiol. (2019) 9:94. doi: 10.3389/fcimb.2019.00094
129. Oliveira FAF, Forte CPF, Silva PGB, Lopes CB, Montenegro RC, Santos ÂKCRD, et al. Molecular analysis of oral bacteria in heart valve of patients with cardiovascular disease by real-time polymerase chain reaction. Medicine. (2015) 94(47):e2067. doi: 10.1097/MD.0000000000002067
130. Dhaliwal D, Bhargava R, Movahed MR. Fusobacterium nucleatum endocarditis: a case report and literature review. Am J Cardiovasc Dis. (2023) 13(1):29–31.36938520
131. Radwan-Oczko M, Jaworski A, Duś I, Plonek T, Szulc M, Kustrzycki W. Porphyromonas gingivalis in periodontal pockets and heart valves. Virulence. (2014) 5(4):575–80. doi: 10.4161/viru.28657
132. Datta HK, Ng WF, Walker JA, Tuck SP, Varanasi SS. The cell biology of bone metabolism. J Clin Pathol. (2008) 61:577–87. doi: 10.1136/jcp.2007.048868
133. Hokamura K, Inaba H, Nakano K, Nomura R, Yoshioka H, Taniguchi K, et al. Molecular analysis of aortic intimal hyperplasia caused by Porphyromonas gingivalis infection in mice with endothelial damage. J Periodontal Res. (2010) 45:337–44. doi: 10.1111/j.1600-0765.2009.01242.x
134. Fridman D, Chaudhry A, Makaryus J, Black K, Makaryus AN. Rothia dentocariosa endocarditis: an especially rare case in a previously healthy man. The Tex Heart Inst J. (2016) 43(3):255–7. doi: 10.14503/THIJ-15-5068
135. Boudewijns M, Magerman K, Verhaegen J, Debrock G, Peetermans WE, Donkersloot P, et al. Rothia dentocariosa, endocarditis and mycotic aneurysms: case report and review of the literature. Clin Microbiol Infect. (2003) 9(3):222–9. doi: 10.1046/j.1469-0691.2003.00503.x
136. Larsen T, Fiehn NE. Dental biofilm infections—an update. APMIS. (2017) 125:376–84. doi: 10.1111/apm.12688
137. Li J, Helmerhorst EJ, Leone CW, Troxler RF, Yaskell T, Haffajee AD, et al. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. (2004) 97(6):1311–8. doi: 10.1111/j.1365-2672.2004.02420.x
138. Huang R, Li M, Gregory RL. Bacterial interactions in dental biofilm. Virulence. (2011) 2:435–44. doi: 10.4161/viru.2.5.16140
139. Verderosa AD, Totsika M, Fairfull-Smith KE. Bacterial biofilm eradication agents: a current review. Front Chem. (2019) 7:824. doi: 10.3389/fchem.2019.00824
140. Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. (2012) 33(26):5967–82. doi: 10.1016/j.biomaterials.2012.05.031
141. Dahlen G, Basic A, Bylund J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J Clin Med. (2019) 8(9):1339. doi: 10.3390/jcm8091339
142. Durack DT. Prevention of infective endocarditis. N Engl J Med. (1995) 332(1):38–44. doi: 10.1056/NEJM199501053320107
143. Jones TD, Baumgartner L, Bellows MT, Breese BB, Kuttner AG, McCarty M, et al. Prevention of rheumatic fever and bacterial endocarditis through control of streptococcal infections. Circulation. (1955) 11:317–20.
144. Cahill TJ, Dayer M, Prendergast B, Thornhill M. Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ. (2017) 358:j3942. doi: 10.1136/bmj.j3942
145. Williams ML, Doyle MP, McNamara N, Tardo D, Mathew M, Robinson B. Epidemiology of infective endocarditis before versus after change of international guidelines: a systematic review. Ther Adv Cardiovasc Dis. (2021) 15:17539447211002687. doi: 10.1177/17539447211002687
146. Thornhill MH, Gibson TB, Yoon F, Dayer MJ, Prendergast BD, Lockhart PB, et al. Antibiotic prophylaxis against infective endocarditis before invasive dental procedures. J Am Coll Cardiol. (2022) 80(11):1029–41. doi: 10.1016/j.jacc.2022.06.030
147. Thornhill MH, Crum A, Campbell R, Stone T, Lee EC, Bradburn M, et al. Temporal association between invasive procedures and infective endocarditis. Heart. (2023) 109(3):223–31. doi: 10.1136/heartjnl-2022-321519
148. Thornhill M, Prendergast B, Dayer M, Frisby A, Lockhart P, Baddour LM. New evidence calls into question NICE’s endocarditis prevention guidance. Br Dent J. (2024) 236:702–8. doi: 10.1038/s41415-024-7344-5
149. Lockhart PB, Chu V, Zhao J, Gohs F, Thornhill MH, Pihlstrom B, et al. Oral hygiene and infective endocarditis: a case control study. Oral Surg Oral Med Oral Pathol Oral Radiol. (2023) 136(3):333–42. doi: 10.1016/j.oooo.2023.02.020
150. Thornhill M, Prendergast B, Dayer M, Frisby A, Lockhart P, Baddour LM. Prevention of infective endocarditis in at-risk patients: how should dentists proceed in 2024? Br Dent J. (2024) 236:709–16. doi: 10.1038/s41415-024-7355-2
151. Blochowiak KJ. Dental treatment and recommended management in patients at risk of infective endocarditis. Kardiochir Torakochirurgia Pol. (2019) 16:37–41. doi: 10.5114/kitp.2019.83944
152. Ali HM, Mustafa M, Hasabalrasol S, Elshazali OH, Nasir EF, Ali RW, et al. Presence of plaque, gingivitis and caries in Sudanese children with congenital heart defects. Clin Oral Investig. (2017) 21:1299–307. doi: 10.1007/s00784-016-1884-2
153. Cheng X, He F, Si M, Sun P, Cheng Q. Effects of antibiotic use on saliva antibody content and oral microbiota in sprague dawley rats. Front Cell Infect Microbiol. (2022) 12:721691. doi: 10.3389/fcimb.2022.721691
154. Yuan X, Zhou F, Wang H, Xu X, Xu S, Zhang C, et al. Systemic antibiotics increase microbiota pathogenicity and oral bone loss. Int J Oral Sci. (2023) 15:4. doi: 10.1038/s41368-022-00212-1
Keywords: oral health, endocarditis, diagnostics, microbiome, periodontal
Citation: Falconer JL, Rajani R, Androshchuk V, Yogarajah A, Greenbury RA, Ismail A, Oh N, Nibali L, D’Agostino EM and Sousa V (2024) Exploring links between oral health and infective endocarditis. Front. Oral. Health 5:1426903. doi: 10.3389/froh.2024.1426903
Received: 2 May 2024; Accepted: 18 October 2024;
Published: 2 December 2024.
Edited by:
Thuy Do, University of Leeds, United KingdomCopyright: © 2024 Falconer, Rajani, Androshchuk, Yogarajah, Greenbury, Ismail, Oh, Nibali, D'Agostino and Sousa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa Sousa, dmFuZXNzYS5zb3VzYUBrY2wuYWMudWs=
 Joseph Luke Falconer
Joseph Luke Falconer Ronak Rajani
Ronak Rajani Vitaliy Androshchuk3
Vitaliy Androshchuk3 Amieth Yogarajah
Amieth Yogarajah Ayden Ismail
Ayden Ismail Natasha Oh
Natasha Oh Luigi Nibali
Luigi Nibali Eleanor M. D’Agostino
Eleanor M. D’Agostino Vanessa Sousa
Vanessa Sousa