- 1Dental Public Health, Preventive Dental Science, College of Dentistry, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Centre, Riyadh, Saudi Arabia
- 2Department of Public Health Dentistry, Ragas Dental College and Hospital, Chennai, India
- 3Department of Oral and Maxillofacial Pathology, Ragas Dental College and Hospital, Chennai, India
- 4The Tamil Nadu Dr. M.G.R Medical University, Chennai, India
- 5Department of Oral and Dental Health Sciences in Ar Rass, Qassim University, Al Qassim, Saudi Arabia
- 6Oral Medicine and Maxillofacial Radiology, Maxillofacial Surgery and Diagnostic Sciences, College of Dentistry, King Saud bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Centre, Riyadh, Saudi Arabia
Background: There is a high incidence of oral cancer and oral potential malignant disorder observed in southeast Asian countries such as India. Our study aimed to assess the correlation between screening and histopathological diagnosis and to predict the specificity and sensitivity of chair-side/field-based assessment of the oral lesion.
Materials and methods: A total of 40,852 subjects aged between 20 and 60 years were screened in the 1st phase of the study, suspected lesions were stained with toluidine blue (Manufactured by Otto Chemicals private limited, India) at two time points, those who stained positively during the two points were taken up for biopsy. Provisional diagnosis was later correlated with histopathological diagnosis.
Results: Subjects who underwent biopsy had a mean age of (49.01 ± 9.8 years), Leukoplakia (1.5%) was the most common lesion observed among tobacco users, interestingly it had the least correlation (39.6%) in diagnosis, Overall sensitivity (88%) and a positive predictive value (80%) was high for clinical diagnosis of OPMD in our study.
Conclusion: Correlation of clinical and histopathological diagnosis observed in our study confirms higher yield of true positives while screening in remote and vulnerable populations, which would assure a better quality of life for these subjects.
1. Introduction
India witnesses one-third of the global oral cancer cases, with an incidence of one-fourth of the global mortality due to oral cancer. A high percentage of these cases are identified and diagnosed at the advanced stage of the disease. Early detection of potentially malignant lesions ensures a better prognosis and quality of life (1–3).
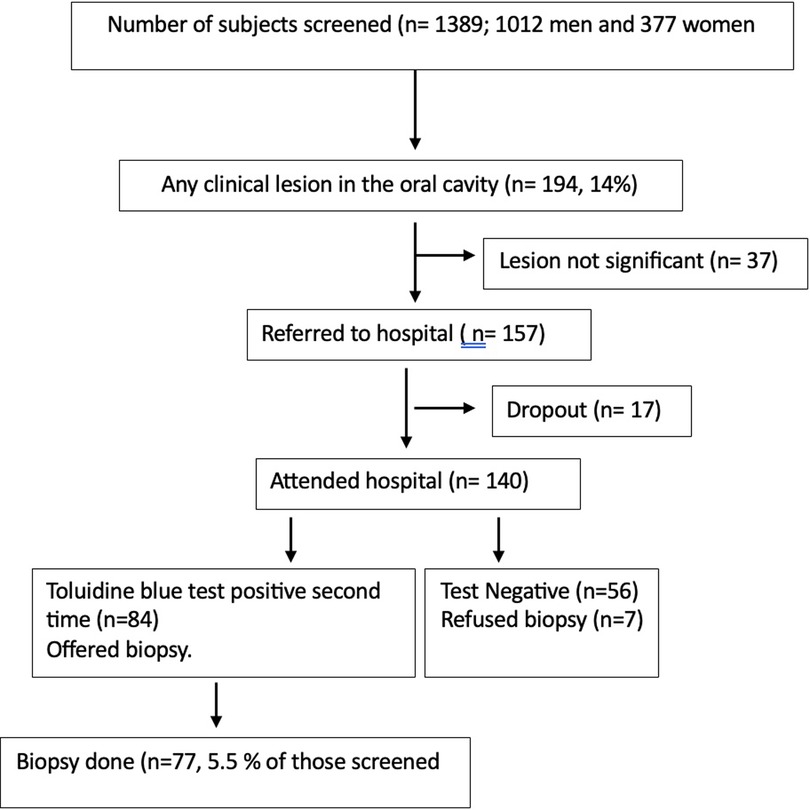
Figure 1. Flow chart on the details of participants screened, diagnosed clinically, and referred for biopsy and biopsied was presented.
The increased incidence of oral cancer is of alarming concern to public health and dental public health, as this has emerged over the years to be the most common type of cancer in the country, this is despite various regulatory measures undertaken by the country to create awareness and restrict exposure to the associated risk factors (4).
Screening has been an effective approach, to identifying asymptomatic cases, with varying degrees of success, leading to questioning of the feasibility (2). Risk-based screening has been proven to have greater efficiency in comparison with general population-based screening as evident from the literature. These screening procedures become more resourcefully feasible if healthcare workers (HCWs) are enrolled (after appropriate training and calibration) to carry out the initial screening of the oral cavity and refer the potential cases for investigation to hospitals (5, 6).
Though the histopathological investigation is the gold standard for confirming the diagnosis, the emphasis lies on appropriate referral based on thorough clinical assessment of the oral cavity and the lesion when provisionally identified, when executed with acumen by the clinician or trained health care workers saves resources and unwarranted invasive intervention among cases (7).
There is a lacuna in the literature concerning the correlation between the screening-based diagnosis of oral cancer or other potentially malignant lesions with the histopathological diagnosis, few studies in the past have attempted to ascertain this correlation with retrospective data (8, 9). This correlation based on the sensitivity and specificity of chair-side diagnosis becomes important to help standardize the diagnostic technique used for screening, leading to uniform reporting and appropriate use of limited resources. The study had various primary objectives which have been analyzed and published in the past, this paper was taken up with the intention to analyze and discuss the sensitivity and specificity of the visual oral screening when compared to the histological findings, which were considered the “gold standard” (10).
The present study was undertaken among a large population of “high-risk” individuals at Ranipet industrial town of Tamil Nadu, India. The study aimed to assess the correlation between screening and histopathological diagnosis, to predict the specificity and sensitivity of chair-side/field-based assessment of the oral lesion.
2. Materials and methods
Ranipet a district in the Tamil Nadu state of India, with the namesake town is a semi-urban region composed of a heterogeneous population. It serves as an industrial hub, with a thriving tannery industry. There is a high prevalence of tobacco and alcohol use observed in the population.
In the present population-based screening study a memorandum of understanding (MOU) was drafted and agreed upon between Thirumalai mission trust hospital located in Vanapadi village in Ranipet district and ragas dental college and hospital, Chennai. The trust hospital caters to the healthcare needs of approximately 142,150 people (315 villages and 35,000 families) and has been doing so for more than a decade.
Awareness programs are undertaken by the trust-based hospital in this population with an organized and trained workforce consisting of family care volunteers (FCV) for approximately every 50 households working under the supervision of multipurpose workers (MPWs) (one for about 500–1,000 households). FCV are well acquainted with the communities and are part of the community, hence involving them ensured better participation and understanding among the screened population. Based on this local manpower availability, and technical and infrastructural assistance of the trust-based hospital an oral cancer screening program was conceptualized between the hospital and the dental college. The screening (cross-sectional) of the whole population in the region was carried out between August 2018 and December 2019. Ethical clearance was obtained from the Institutional Review Board of the hospital and the dental college (project 20180703 approved on July 30, 2018) before the commencement of the screening program. The findings of this study have been reported by STROBE guidelines (11).
Written informed consent was obtained from all study participants prior to enrolling them in the study, they were made aware of all the aspects of participation and outcomes, and the information was conveyed in the vernacular language (Tamil) for better understanding and compliance. The study was conducted according to the ethical guidelines established by the Declaration of Helsinki and other guidelines like Good Clinical Practice Guidelines and those established by the Indian Council for Medical Research.
Adult participants irrespective of oral adverse habits were considered and were initially screened for the presence of oral lesions. Of the total population considered for the study 71,356 people aged between 21 and 60 years were deemed to be enrolled in the study. The coverage area was divided into two zones (ZONE I AND ZONE II) for the convenience of investigators, these zones had a population of 40,852 and 30,504 respectively. A 1:2 ratio of the case to control was adopted in the study. All known potential confounders such as age, gender, habits and occupation were matched between the cases and controls. Controls were selected from the same population without any history of oral adverse habits.
The intra-oral assessment was carried out by the dentists in the field setting (at anganwadis and schools in the vicinity of the population screened). American Dental Association (ADA) oral examination classification III was used for assessment in field settings with the artificial light source. While screening, if a potential lesion was identified, a subject expert's opinion was sorted by sending images through the mobile application (WhatsApp). Variations of the normal mucosa and certain non-significant pathological changes of mucosa were not further investigated (usual variants of mucosal keratosis, smokers' palate, denture stomatitis, etc.). Suspected lesions were taken up for staining during two time periods, firstly at the field setting, those lesions which positively stained with toluidine blue, these patients were asked to visit the dental clinic at the hospital for the second, those lesions which positively stained on both occasions were further taken up for biopsy (5 mm punch biopsy), biopsy sample collection was done by a trained dentist. The sample was stored in 10% formaldehyde, transported within 3–4 h, and was sent to oral pathology department of ragas dental college and hospital for histopathologic examination. Sutures were placed at the intra-oral site of biopsy collection in patients who underwent the procedure, and after a week suture removal was undertaken, ensuring proper healing at the site.
All subjects with a history of tobacco use underwent counseling at de-addiction center of the hospital. Nicotine replacement therapy to overcome tobacco use was given free of cost as part of the de-addiction and counseling process.
Those identified with established oral malignant lesions were referred to a regional cancer care center (Aringar Anna Cancer Treatment Center, Kanchipuram, Tamil Nadu, India) for appropriate treatment and follow-up. The data were cleaned and entered in Microsoft Excel for analysis. Descriptive analysis was carried out for to express the frequencies. Sensitivity and specificity of the diagnosis during initial screening was assessed against the histopathological gold standard. Statistical Package for Social Services (Version 20.0 for Windows, SPSS Inc., Chicago, IL, USA) and Microsoft Excel was used for statistical analysis.
3. Results
The screening program results have already been presented in an earlier publication by the authors (10). In this screening program, 77 biopsies were taken from subjects, among whom 74 were histologically examined. Three specimens were excluded as they were found to be inadequate by the pathologist.
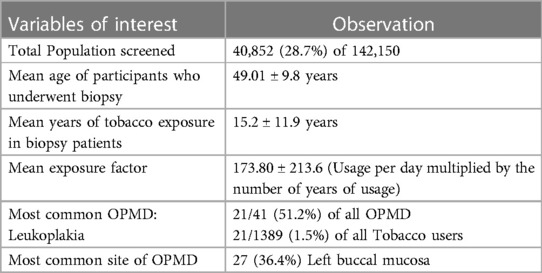
Table 1 depicts variables of interest such as total population screened 40,852 (28.7%), patients who underwent biopsy had a mean age of (49.01 ± 9.8 years), tobacco exposure (15.2 ± 11.9 years), and an exposure factor of (173.80 ± 213.6). Leukoplakia (1.5%) was the most common OPMD observed in the tobacco users and left buccal mucosa (36.4%) was the most common site of OPMD occurrence.
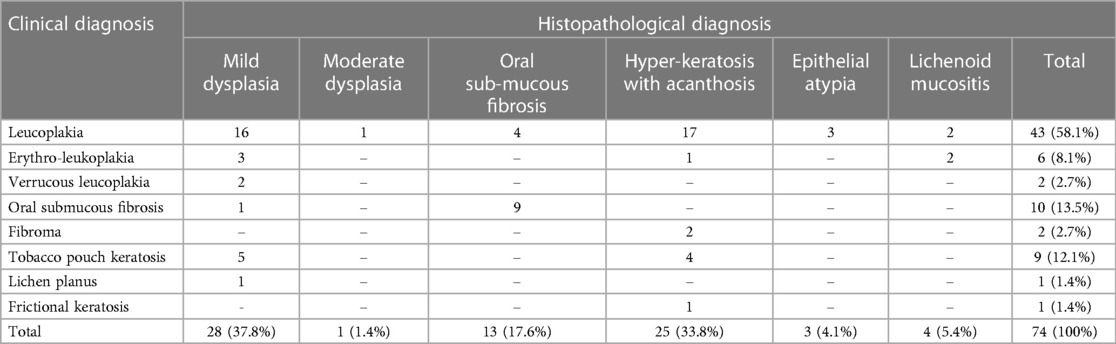
Table 2 demonstrates the clinical vs. the histological diagnosis for the biopsy specimen. Among 43 (58.1%) subjects with a clinical diagnosis of Leucoplakia, only 21 were confirmed to have dysplastic changes during the histological examination. Except for those diagnosed with fibroma and frictional keratosis (Histologically confirmed as hyperkeratosis with acanthosis), histologically dysplastic features were confirmed for those subjects with a clinical diagnosis of potentially malignant oral lesions.
Though the overall sensitivity of clinical diagnosis was 88%, the sensitivity values ranged from 39.6% for Leucoplakia to 100% for Verrucous leukoplakia and Lichen planus. However, the positive predictive value was over 80% for all the clinical conditions recorded in the screening program (Table 3).
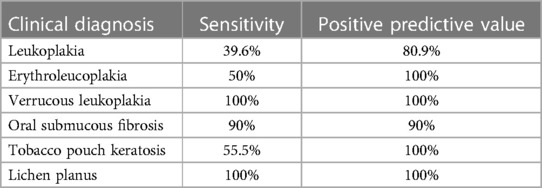
Table 3. Sensitivity and positive predictive value (PPV) in relation to clinical diagnosis of OPMD as observed in the study.
4. Discussion
Evidence suggests that early intervention among the identified true positive cases have a good prognosis, improved survival rate and better quality of life than otherwise (12, 13). A recent systematic review outlines the significance of high-risk population-based screening which could save “two to three times more lives than non-targeted screening” (14, 15). In the present study, a high-risk population in an industrial town was considered for screening.
The mean age of patients referred for biopsy in our study was 49.01 ± 9.8 which is in line with study by Torabi et al. (15), other studies on the similar topic have reported aa slightly higher mean age of 56 years and 55 years by Maia et al, and Mehrotra et al., respectively.
Leukoplakia was the most common OPMD observed in our screening population, similar finding has been reported on leukoplakia in studies by Chher et al., and Pentenero et al. (16, 17), who undertook oral mucosal lesion assessment in large population screening at Cambodia and Italy respectively. Contrastingly studies by Oivio et al. (18), and Feng et al. (19), in Finland and China have reported oral lichen planus to be the major OPMD diagnosed in their screening population.
Left buccal mucosa was the most common site of OPMD followed by right buccal mucosa and right vestibule in our study, the finding of buccal mucosa to be the most common site of OPMD is in line with studies by Torabi et al. (15), and M. Bokor-Bratic et al., (20). Recently the WHO Collaborating Centre for oral cancer comprising the expert group on oral cancer and OPMD revised the classification of OPMDs and considered Oral leukoplakia, oral lichen planus, oral lichenoid lesions, proliferative verrucous leukoplakia, and oral submucous fibrosis as OPMDs (15). These OPMDs have a differing percentage of malignant transformation based on the type of lesion from 5%–18%.
The present study observed an average 15.2 years of habit (smoke, smokeless tobacco, betel quid, and areca nut) prevalence among those identified for biopsy, and a steady increase in OPMD cases was observed when average usage per day was ascertained (from <5 to >20). These observations are in line with study by Shivakumar et al. (21), who reported 7.31 ± 6.94 years of habit (tobacco) prevalence in OPMD cases and average usage per day at 4.92 ± 4.02.
Exposure factor (usage per day multiplied by the number of years of usage) also indicated an increased prevalence of OPMD with increase in the exposure factor. The average exposure factor of 173.80 was observed in the present study. This variable in our study is in concordance with person years observation of subjects with adverse oral habits in a study by Sankaranarayanan et al. (22), who screened for oral cancer cases in Trivandrum district of Kerala, India and reported increased incidence and mortality as person years of habit observations increased between cases and controls.
There was an inverse correlation observed with respect to area of lesion in our study, most of the lesions which measured (1–1,000 sq mm) were non OPMD lesions. This finding is interesting and has not been reported previous in literature. As well as there was no significantly different consistency observed between OPMD (33 soft and 8 firm) and non-OPMD lesions (27 soft and 6 firm).
Screening related sensitivity for OPMD was found to be good in the present study at 88%, various studies have reported on the similar lines while assessing the concurrence of screening with histopathological diagnosis. Study on clinicopathologic correlation of white lesions by Abidullah et al. (23), reported a 78% correlation.
The highest correlation in clinicopathological diagnosis was observed for lichen planus (100%) and Verrucous Leukoplakia (100%) followed by oral submucous fibrosis (90%). Interestingly though leukoplakia was the most common OPMD only 39.6% sensitivity was observed while diagnosing it clinically. This may be since leukoplakia being a clinical term does not specifically identify with the histopathological presentation, the degree of lesion presentation with that of histopathology is low. In the present study, clinical and histopathological correlation were inconsistent with erythroleukoplakia cases too, these has been correlated to similarity in presentation of severe dysplasia and carcinoma in situ.
There is a need for sustained oral cancer screening as part of national health survey, Taiwan is the only country in the world that has implemented a sustained oral cancer screening program, and it should be noted that it currently offers screening to high-risk patients (betel chewers or former betel chewers and smokers) (2). These screening procedures would be beneficial especially in the south-east Asian regions, where OPMD prevalence is found to be very high.
Another aspect that needs attention to increase the correlation in diagnosis and the yield of screening is to standardize training of dentists and their examiners, this variability of training hinders clinical diagnosis.
This study has added strength to exiting data on correlation of clinical and histopathological diagnosis of OPMD, a high sensitivity was observed for visual screening of lesions and hence can serve as an efficient tool in initial screening especially at remote and high-risk population. The findings of these study have certain limitations such as attrition of patients when referred for biopsy, which might be because of anxiety, insufficient biopsy sample for certain cases which was eventually not included as part of the study. Further studies can be undertaken with caution on these limitations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB, Ragas Dental College and Hospital, Chennai IRB, Thirumalai Mission Hospital, Ranipet. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KI: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. MK: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. RK: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. AN: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. MM: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. SK: Conceptualization, Methodology, Supervision, Validation, Visualization, Writing – review & editing. LB: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Acknowledgments
The authors would like to thank Thirumalai Mission Trust Hospital, Ranipet and the population who were part of the screening.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Borse V, Konwar AN, Buragohain P. Oral cancer diagnosis and perspectives in India. Sens Int. (2020) 1:100046. doi: 10.1016/j.sintl.2020.100046
2. Warnakulasuriya S, Kerr AR. Oral cancer screening: past, present, and future. J Dent Res. (2021) 100(12):1313–20. doi: 10.1177/00220345211014795
3. Seoane J, Takkouche B, Varela-Centelles P, Tomás I, Seoane-Romero JM. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol. (2012) 37(2):99–106. doi: 10.1111/j.1749-4486.2012.02464.x
4. Sharma S, Satyanarayana L, Asthana S, Shivalingesh KK, Goutham BS, Ramachandra S. Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J Oral Maxillofac Pathol. (2018) 22(1):18–26. doi: 10.4103/jomfp.JOMFP_113_17
6. Mariño R, Haresaku S, McGrath R, Bailey D, Mccullough M, Musolino R, et al. Oral cancer screening practices of oral health professionals in Australia. BMC Oral Health. (2017) 17(1):151. doi: 10.1186/s12903-017-0439-5
7. Soyele OO, Aborisade A, Adesina OM, Olatunji A, Adedigba M, Ladeji AM, et al. Concordance between clinical and histopathologic diagnosis and an audit of oral histopathology service at a Nigerian tertiary hospital. Pan Afr Med J. (2019) 34:100. doi: 10.11604/pamj.2019.34.100.19388
8. Acharya S, Tayaar AS. Analysis of clinical and histopathological profiles of oral squamous cell carcinoma in young Indian adults: a retrospective study. J Dent Sci. (2012) 7(3):224–30. doi: 10.1016/j.jds.2012.05.005
9. Rahaman SMU, Mujib BA. Histopathological correlation of oral squamous cell carcinoma among younger and older patients. J Oral Maxillofac Pathol. (2014) 18(2):183–8. doi: 10.4103/0973-029X.140734
10. Madan Kumar PD, Iyer K, Soni S, Nagarajan L, Kumar K, Solomon S, et al. A simple screening program for oral cancer in a defined geographic area in southern India: a community-based cross-sectional study. Cancer Res Stat Treat. (2022) 5:226–31. doi: 10.4103/crst.crst_92_22
11. Available at: https://www.strobe-statement.org/checklists/ (Accessed Auguest 07, 2023).
12. Parak U, Lopes Carvalho A, Roitberg F, Mandrik O. Effectiveness of screening for oral cancer and oral potentially malignant disorders (OPMD): a systematic review. Prev Med Rep. (2022) 30:101987. doi: 10.1016/j.pmedr.2022.101987
13. Bouaoud J, Bossi P, Elkabets M, Schmitz S, Van Kempen LC, Martinez P, et al. Unmet needs and perspectives in oral cancer prevention. Cancers. (2022) 14(7):1815. doi: 10.3390/cancers14071815
14. González-Moles MÁ, Aguilar-Ruiz M, Ramos-García P. Challenges in the early diagnosis of oral cancer, evidence gaps and strategies for improvement: a scoping review of systematic reviews. Cancers. (2022) 14(19):4967. doi: 10.3390/cancers14194967
15. Torabi M, Afshar MK, Afshar HM, Mohammahzadeh I. Correlation between clinical and histopathologic diagnosis of oral potentially malignant disorder and oral squamous cell carcinoma. Pesqui Bras Odontopediatria Clín Integr. (2021) 21. doi: 10.1590/pboci.2021.068
16. Chher T, Hak S, Kallarakkal TG, Durward C, Ramanathan A, Ghani WMN, et al. Prevalence of oral cancer, oral potentially malignant disorders, and other oral mucosal lesions in Cambodia. Ethn Health. (2018) 23(1):1–15. doi: 10.1080/13557858.2016.1246431
17. Pentenero M, Broccoletti R, Carbone M, Conrotto D, Gandolfo S. The prevalence of oral mucosal lesions in adults from the Turin area. Oral Dis. (2008) 14(4):356–66. doi: 10.1111/j.1601-0825.2007.01391.x
18. Oivio UM, Pesonen P, Ylipalosaari M, Kullaa A, Salo T. Prevalence of oral mucosal normal variations and lesions in a middle-aged population: a northern Finland birth cohort 1966 study. BMC Oral Health. (2020) 20:357. doi: 10.1186/s12903-020-01351-9
19. Feng J, Zhou Z, Shen X, Wang Y, Shi L, Wang Y, et al. Prevalence and distribution of oral mucosal lesions: a cross-sectional study in Shanghai, China. J Oral Pathol Med. (2015) 44(7):490–4. doi: 10.1111/jop.12264
20. Bokor-Bratíc M, Vucković N, Mirković S. Correlation between clinical and histopathologic diagnosis of potentially malignant oral lesions. Arch Oncol. (2004) 12(3):145–7. doi: 10.2298/AOO0403145B
21. Shivakumar KM, Raje V, Kadashetti V. Prevalence of oral potentially malignant disorders (OPMD) in adults of Western Maharashtra, India: a cross-sectional study. J Cancer Res Ther. (2022) 18(Supplement):S239–43. doi: 10.4103/jcrt.JCRT_1444_20
22. Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. (2005) 365(9475):1927–33. doi: 10.1016/S0140-6736(05)66658-5
Keywords: high risk population, oral cancer, oral potentially malignant disorders, screening, tobacco
Citation: Iyer K, Kumar M, Kannan R, Narayanan A, Moothedath M, Khanagar SB and Bijai LK (2023) Clinical and histopathological correlation of oral malignancy and potentially malignant disorders based on a screening program at high-risk population in Tamil Nadu, India. Front. Oral. Health 4:1286780. doi: 10.3389/froh.2023.1286780
Received: 31 August 2023; Accepted: 10 October 2023;
Published: 3 November 2023.
Edited by:
Praveen S. Jodalli, Manipal College of Dental Sciences, IndiaReviewed by:
Deepti Vadavi, DAPM RV Dental College, IndiaRam Surath Kumar, KLE Academy of Higher Education and Research (KAHER), India
© 2023 Iyer, Kumar, Kannan, Narayanan, Moothedath, Khanagar and Bijai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kiran Iyer ZHJraXJhbml5ZXI4QGdtYWlsLmNvbQ==
 Kiran Iyer
Kiran Iyer Madan Kumar
Madan Kumar Ranganathan Kannan3
Ranganathan Kannan3