- 1Department of Public Health Dentistry, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India
- 2University of Michigan School of Dentistry, Ann Arbor, MI, United States
- 3Department of Biochemistry, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India
- 4Department of Community Medicine, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India
- 5Department of Pedodontics and Preventive Dentistry, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India
- 6Department of Periodontics, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India
Background: Tobacco use is one of the most important public health concerns, with approximately 8.7 million tobacco-related deaths each year, primarily in low- and middle-income countries. Even more concerning is the fact that 1.3 million of these deaths are seen in nonsmokers, including babies and children. This study was performed to determine whether a school-based “tobacco-free” educational intervention program among 12-year-old children would be effective in reducing their exposure to second-hand tobacco smoke (SHS) by improving their knowledge, attitude and behavior post intervention and estimating salivary cotinine levels as markers of SHS exposure.
Materials and method: A randomized controlled trial was performed by a cluster random sampling technique, with 30 participants each in the experimental and control arms. A knowledge, attitude, avoidance behavior and self-efficacy of avoidance questionnaire was administered, followed by estimation of salivary cotinine levels. The experimental arm received the “tobacco-free” intervention, which comprised a 40-min health education session, with the first follow-up at 15 days and the second at 30 days after the intervention. After the intervention, the questionnaire was readministered, followed by re-estimation of salivary cotinine levels.
Results: One month after the intervention, the number of participants who had a smoker who lived with them and the number of people who smoked inside the house were reduced in the experimental group compared to the control group. In the knowledge domain and the attitude domain, 80% and 60% of the items showed a statistically significant improvement in the experimental group compared to the control group. In the avoidance behavior domain and the Self-Efficacy of Avoidance Domain, all the items showed improvement in the experimental group compared to the control group. When the mean salivary cotinine levels were compared pre- and postintervention, it was found that although the mean postintervention salivary cotinine levels increased in both the experimental and control groups, the increase was less in the experimental group than in the control group.
Conclusion: The present study has been shown to be effective in improving the knowledge, attitude and avoidance behavior of adolescents toward exposure to secondhand smoke.
1. Introduction
“The scientific evidence is clear: there is no safe level of second-hand smoke (1)”
Tobacco use is one of the most important public health concerns, with approximately 8.7 million tobacco-related deaths each year, primarily in low- and middle-income countries. Even more concerning is the fact that 1.3 million of these deaths are seen in nonsmokers, including babies and children. Almost half of all children are exposed to tobacco-polluted air, and 65,000 children die each year as a result of illnesses induced by second-hand smoke (1).
It has been reported that in India, 11% of 13- to 15-year-olds are exposed to SHS at home, and 21% are exposed to SHS in enclosed public spaces (2). Second-hand smoke (SHS) is a mixture of compounds produced by tobacco ingested by an “active smoker” and is often referred to as “passive smoke”, “environmental tobacco smoke (ETS)”, or “tobacco air pollution”. At least 69 of the 7,000 chemicals emitted could cause cancer (1). SHS includes both side-stream smoke from the end of the cigarette and smoke exhaled by smokers. Smoke from one room flows to other rooms in the building regardless of whether doors are kept locked or windows are kept open. SHS can linger indoors for hours and become increasingly hazardous over time (3). Toxic components of SHS stick to rugs, draperies, clothing, food, and other surfaces and stay in the room for months after active smoking has stopped, resulting in “third-hand smoke” (THS), which is identified as an SHS side effect (4). THS, also known as “residual tobacco smoke” or “aged tobacco smoke”, can harm anyone who is exposed to it.
In adults, SHS exposure has been associated with stroke, coronary heart disease, cancer, chronic obstructive pulmonary disease, respiratory infections, and other illnesses, while in children, it has been linked to severe asthma, respiratory tract infections, ear infections, and sudden infant death syndrome (5–7).
Because their lungs and bodies are still developing and they breathe faster than adults, young children are more vulnerable. Furthermore, passive smoking is detrimental to mental health, may cause depressive symptoms (3, 8) and diminishes cognitive performance (9).
Although legislation banning smoking in enclosed public places has been widely introduced, the global progress on implementation remains uneven, with implementation rates ranging from 13% to 85% (10). Many laws have been enacted in India to discourage tobacco consumption, beginning with the Cigarettes Act of 1975. In 2003, the Cigarette and Other Tobacco Products Act was passed, and the Framework Convention on Tobacco Control came into effect in 2005. In 2008, the Government of India passed legislation prohibiting smoking in public places (11, 12). These laws have given the general public some optimism that the tobacco epidemic may one day come to an end.
However, none of these laws prohibit smoking inside households, and it has been discovered that children of smoking parents are especially vulnerable to SHS exposure at home since children spend so much time with their parents (13, 14).
Some studies (15, 16) have shown that interventions targeted at parents for bringing about smoking cessation/reduction, thereby reducing ETS at home, improved child health outcomes and reduced not only the risk of tobacco exposure among children but also reduced initiation of the smoking habit. However, systematic reviews have shown that the effectiveness of these interventions was not clearly demonstrated (17), and even if outcomes were present, they were not sustained for a long period of time (18, 19).
This brings us to our research question, i.e., can educational programs targeted at children bring about an improvement in their knowledge, attitude and behavior toward SHS, thus reducing their exposure to the same. A literature search revealed no evidence pertaining to targeted intervention programs affecting children. This led to the conceptualization of the present study, with the aim of assessing the effect of a “tobacco-free” educational intervention among 12-year-old children in reducing their exposure to second-hand tobacco smoke. The objective was to determine their knowledge, attitude and behavior pre- and postintervention along with estimating salivary cotinine levels as markers of SHS exposure. Since salivary cotinine is a sensitive biochemical marker that is highly linked to SHS exposure and has a half-life of 16 h, it has been extensively used as a marker for detecting both active and passive smoking (20).
2. Materials and methods
2.1. Study design & study setting
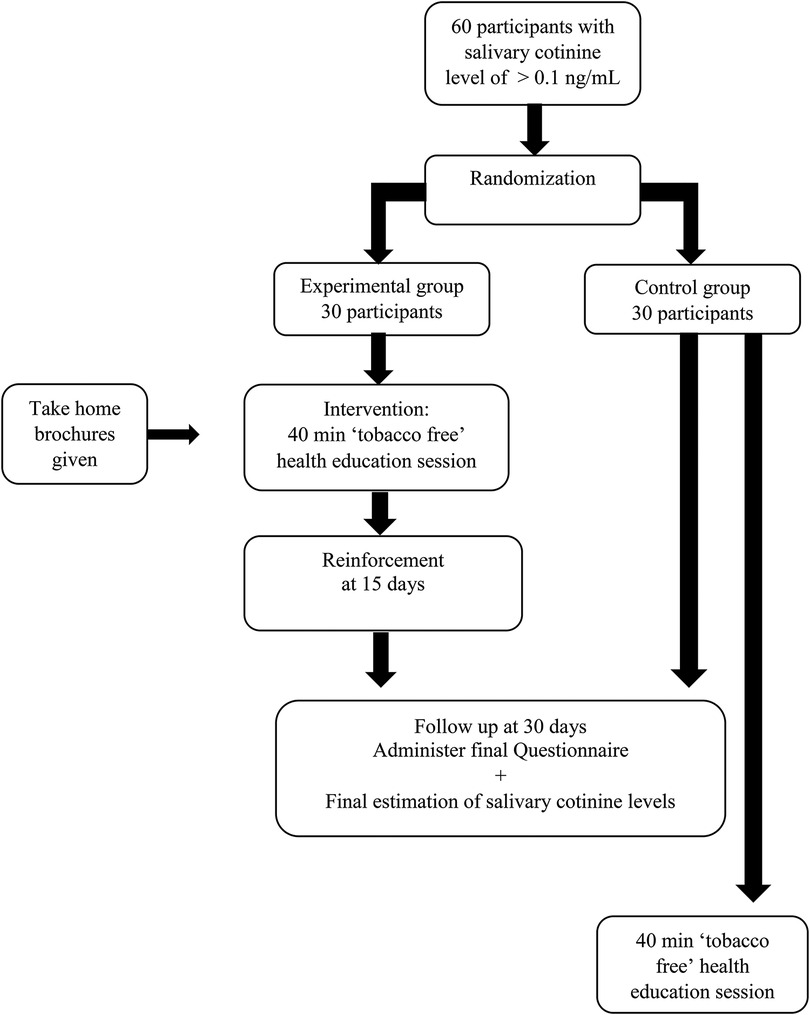
This study is a component of a multiphase study that was conducted among 12-year-old school children in Mangalore, India. The first phase was a descriptive cross-sectional study that used a cluster random sampling of all schools, which is described in detail elsewhere (21). The 12-year-olds in the selected schools who met the inclusion criteria, i.e., who consented to participate in the study and those participants whose parents gave written informed consent and they themselves give informed assent, were given a questionnaire. Participants who reported second-hand cigarette smoke exposure were chosen at random, and their salivary cotinine levels were measured. All participants with salivary cotinine levels greater than 0.1 ng/ml (22) were included for sampling.
2.2. Sample size calculation and random allocation
G Power 3.1.2 was used to calculate the sample size. We determined a total sample size of 60, with 30 in the control group and 30 in the experimental group, 90% power, an effect size of 0.3, a 95% confidence interval, and a 10% attrition rate. Since no studies were available in this age group to obtain the standardized mean difference in salivary cotinine values between the two groups, we assumed a Cohen's d of 0.3, since it is generally agreed that the means of the two groups should differ by at least 0.3 standard deviations to say that the difference is significant.
2.2.1. Criteria for inclusion
All participants with salivary cotinine levels greater than 0.1 ng/ml (20) were grouped school wise, and the school clusters were included in the sampling frame. Clusters of schools were chosen instead of individual participants to eliminate contamination bias that could occur if both experimental and control groups were present in the same school. Cluster random sampling was then performed for the current randomized controlled trial (Figure 1).
Random allocation: Allocation concealment was accomplished through the use of sequentially numbered, opaque, sealed envelopes (SNOSE), and clusters were divided into experimental and control groups by simple randomization. We chose one school to be the experimental group and another to be the control group. The outcome assessors were blinded in the trial, and the random allocation process was carried out by one of the authors (SM).
2.3. Data collection
2.3.1. Questionnaire
The questionnaire was in the English language and comprised three components: the first was demographic data, and the second was a 5-item assessment of children's exposure to SHS (23). The third component was the 25-item questionnaire (24) designed to assess children's secondhand smoke knowledge, attitude, avoidance behavior, and self-efficacy of avoidance. Out of the 25 items, ten items measured knowledge, five items assessed attitude, five items assessed avoidance behavior against secondhand smoke, and five items assessed self-efficacy of avoidance. More details are given elsewhere (21). The questionnaire assessment was performed twice, once at baseline and once at one month after the intervention.
Pilot study: Content validity was assessed by subject experts (UB and RS). The questionnaire was administered to ten twelve-year-old children who were not part of the study, and a Cronbach's alpha of 0.8 was obtained, showing high reliability.
2.3.2. Salivary cotinine estimation
Salivary cotinine is undetectable in people who do not smoke and in those who are not exposed to passive smoking. A saliva cotinine concentration of less than 10 ng/ml suggests potential passive exposure, while a concentration greater than 10 ng/ml indicates active nicotine usage (20).
Saliva collection: Saliva collection was performed twice during the course of the study, once at baseline before the intervention and the other at 30 days after the intervention. Saliva collection from participants was scheduled in the mornings, approximately one hour after their morning breakfast. They were asked to rinse their mouth thoroughly with water 10 min before saliva collection. Labelled saliva collection boxes were distributed. They were then asked to tilt their head forward to allow the saliva to pool in the floor of the mouth. Whole saliva was collected by unstimulated passive drool. Collected saliva samples were immediately placed in an ice chest and transported to the laboratory within 30 min. The pH of all the samples was noted using Fisher Scientific Indikrom Papers. Samples with a pH less than 3.5 or more than 9.0 were discarded and recollected the next day. The samples were refrigerated before analysis.
2.3.3. Laboratory analysis
The laboratory analysis was performed by an expert biochemist (NM). On the day of centrifugation, the samples were transferred into labelled centrifugation tubes and centrifuged at 5,200 rpm for 15 min. The clear supernatant was pipetted out, and the centrifuge was stored in Eppendorf tubes at −21°C until analysis. The samples were allowed to thaw completely before carrying out the assay. The Salimetrics® High Sensitivity Salivary Cotinine Quantitative Enzyme Immunoassay Kit, a competitive immunoassay kit, was used for the assay (25).
2.3.4. Reagent preparation
All reagents were brought to room temperature and mixed before use. The microtiter plate was also brought to room temperature while inside the foil pouch to keep it away from moisture contamination. A 1× wash buffer was prepared as per the kit insert. Serial dilutions of cotinine standards were prepared, and the final concentrations achieved were 200 ng/ml, 66.7 ng/ml, 22.2 ng/ml, 7.4 ng/ml, 2.5 ng/ml and 0.8 ng/ml. After the reagents were ready, the plate layout was determined. The microplate incubator/shaker was set to 37°C.
2.3.5. Sample preparation
Twenty microliters of standards, controls, and saliva samples were pipetted into appropriate wells. Twenty microliters of assay diluent was pipetted into 2 wells to serve as the zero. For nonspecific binding (NSB) wells, 120 μl of assay diluent was pipetted. The diluted enzyme conjugate solution (1:300) was immediately mixed, and 100 μl was added to each well using a multichannel pipette. One hundred microliters of cotinine antiserum was pipetted into all wells, except the NSB wells, using a multichannel pipette.
The plate was covered with an adhesive cover and was constantly mixed at 500 rpm in a preheated 37°C microplate incubator/shaker for 0.5 h at 37°C. The plate was then washed 4 times with 1× wash buffer by pipetting 300 μl of wash buffer into each well and then discarding the liquid. Before turning the plate upright, it was thoroughly blotted on paper towels. A multichannel pipette was used to add 200 μl of the TMB (3,3′,5,5′-tetramethylbenzidine) substrate solution to each well. Before incubating in the dark at room temperature for 25 min, the plate was mixed on a plate rotator for 5 min at 500 rpm. A multichannel pipette was used to add 50 μl of stop solution, which was then mixed on a plate rotator for 3 min at 500 rpm. At this stage, the green color turns to yellow in all the wells. The bottom of the plate was dried by wiping off with a water-moistened, lint-free cloth. An ELISA plate reader (Lisa plus microplate reader) was used to read the plate at 450 nm and 620 nm within 10 min of adding the stop solution.
The “High” and “Low” Salimetric controls were run with each assay. The concentrations of the controls and the samples were determined by interpolation using data reduction software (4-parameter nonlinear regression curve fit). The sensitivity of the assay was 0.15 ng/ml.
2.3.6. Intervention
The intervention was carried out by the principal investigator (RA) and consisted of a 40-min health education session addressing tobacco and its adverse effects, laws regarding tobacco in India and the world and information on how to prevent exposure to tobacco smoke. Children were also given posters that contained messages on the effects of tobacco and how to make their homes smoke-free, to be placed in their homes.
The health education intervention was delivered at baseline, and the children were recalled 15 days after the intervention to reinforce the health education session and to discuss their experiences and the challenges they faced.
Participants in the control arm received conventional standard health education once at the end of the study.
2.4. Statistical analysis
It was done based on the Intention to Treat analysis. The data were entered into SPSS software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and analysed. Descriptive statistics are presented, and subgroup analysis was performed wherever needed. The level of significance was kept at 0.05 with 95% confidence levels. The chi-square test was performed for categorical variables, and the paired t test was performed to compare means.
2.5. Ethical considerations
Ethical approval was obtained from the Institutional Ethics Committee (Ref No. 17021). Written informed consent from parents and informed assent from the participants was obtained prior to recruitment. There were no personal identifiers on the vials sent to the laboratory, and individual results have not been disclosed. Confidentiality of the participants was maintained at all times. The saliva collected was completely utilized for laboratory evaluation, and no biological specimens were stored for further use. This trial has been registered with the Clinical Trial Registry of India (CTRI—09/015706).
The article follows the CONSORT 2010 checklist when reporting a cluster randomized trial (Annexure 1).
3. Results
In the phase 1 part of the study (21), 236 participants reported that somebody who lived with them smoked tobacco. Among them, 142 were willing to participate in this phase of the study. The saliva samples of these 142 children were collected and analysed. Of them, 112 were found to have a salivary cotinine level of more than 0.1 ng/ml, and 30 had less than 0.1 ng/ml. We obtained 3 school clusters that had at least 30 participants who could participate in this study. Two schools were randomly allocated, one each into the experimental and control arms. From each school, 30 participants were randomly selected to obtain our sample size of 60 participants, which comprised a total of 33 boys and 27 girls. There was no attrition of study participants, and the originally assigned groups were analysed.
3.1. Exposure to SHS
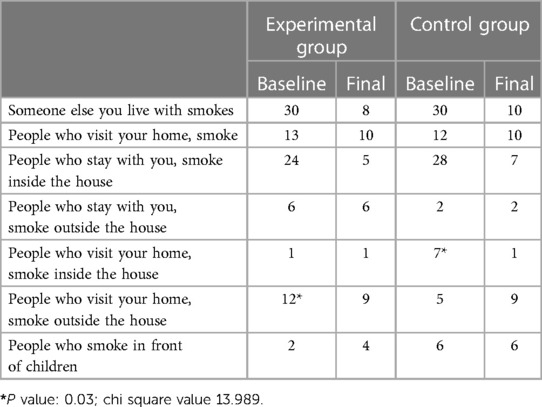
When we analysed the responses of the groups based on the correct responses for the questionnaire, at baseline, we found that all 60 participants reported being exposed to tobacco smoke at home. The children also reported that most of the smokers smoked inside the house, 80% in the experimental group and 93.3% in the control group. Thirteen children from the experimental group reported that people who visited their house smoked, although 12 of them said that they smoked outside, and only one reported that they smoked inside the house. In the control group, among the 12 students who reported that people who visited their house smoked, 7 of them said that they smoked outside, whereas 5 reported that they smoked inside the house. Among the experimental group, only 2 children reported that people who lived with them smoked in front of children, whereas in the control group, 6 did.
When we compared the baseline and final scores of the experimental and control groups, we found that at baseline, although all participants, both in the experimental and control groups, had a smoker who lived with them, one month after the intervention, we found that 22 participants in the experimental group and 20 participants in the control group reported that the smoker who lived with them had stopped smoking. Even the number of smokers who visited their house was reduced in both groups. We also found that 24 people smoked inside the house at baseline in the experimental group, which was reduced to 5 after the intervention (Table 1).
However, the numbers of those who smoked outside the house remained the same in both groups. After the intervention, the number of house guests who smoked outside decreased in the experimental group but increased in the control group. This was found to be statistically significant (P = 0.03). A bewildering finding was that more children reported that the number of people who smoked in front of children increased after the intervention in the experimental group but remained unchanged in the control group. This was also found to be statistically significant (Table 1).
3.2. Questionnaire
3.2.1. Knowledge domain
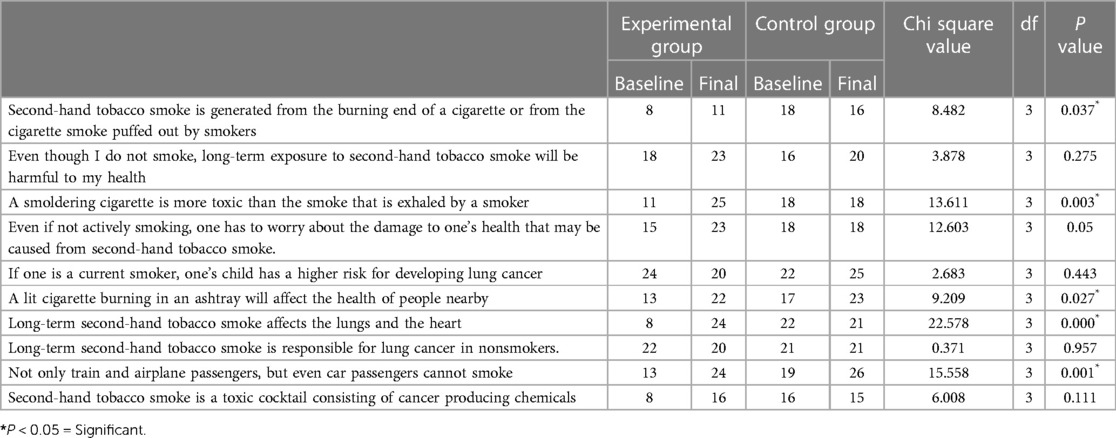
Both in the control group and in the experimental group, more participants had good knowledge that a current smoker's child has a higher risk for developing lung cancer than a nonsmoker. Many participants were also aware that long-term second-hand tobacco smoke was responsible for lung cancer in nonsmokers. When compared with baseline, the number of participants who understood that secondhand tobacco smoke was generated from the burning end of a cigarette or from the cigarette smoke puffed out by smokers increased in the experimental group and decreased in the control group. This was found to be statistically significant (P = 0.037).
The number of participants who knew that a smoldering cigarette was more toxic than the smoke exhaled by a smoker increased after intervention in the experimental group but remained the same in the control group (P = 0.003). The number of participants who knew that even if not actively smoking, they had to worry about the damage to their health that may be caused by secondhand tobacco smoke increased from 15 to 23 in the experimental group but remained unchanged at 18 in the control group, and this increase was found to be statistically significant (P = 0.05). However, the participants who knew that a lit cigarette burning in an ashtray would affect the health of people nearby increased statistically both in the experimental and in the control group (P = 0.027). Although only 8 participants in the experimental group at baseline knew that long-term secondhand tobacco smoke affected the lungs and the heart, it increased to 24 after the intervention. This was found to be statistically significant (P = 0.000) when compared to the control group. Similarly, the number of participants who knew that not only train and airplane passengers but also car passengers should not smoke was found to statistically increase after the intervention (P = 0.001) (Table 2).
3.2.2. Attitude domain
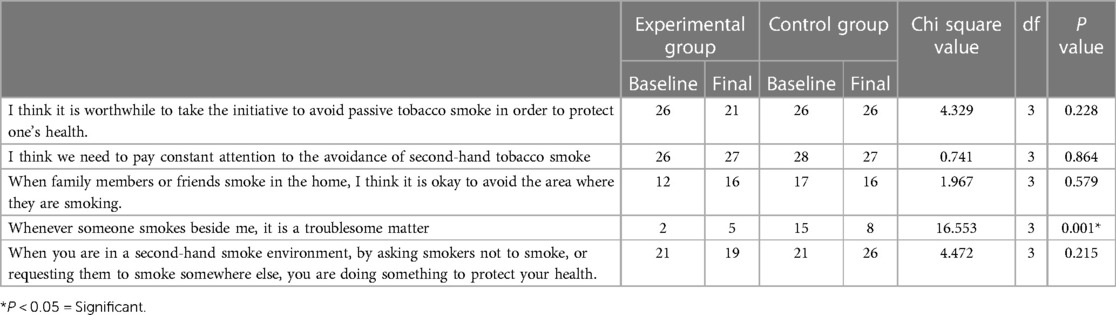
In both groups, at baseline, 26 out of 30 felt that it was worthwhile to take the initiative to avoid passive tobacco smoke to protect one's health. We also found that the majority of the participants felt that they needed to pay constant attention to the avoidance of secondhand tobacco smoke. However, very few participants in both groups felt that when family members or friends smoke in the home, it was okay to avoid the area where they are smoking. Only 17 participants felt that whenever someone smoked beside them, it was a troublesome matter, and among them, only 2 were in the experimental group, i.e., 28 participants in the experimental group did not find second-hand smoke a troublesome matter. However, 42 participants felt that in a second-hand smoke environment, by asking smokers not to smoke, they were doing something to protect their health.
The only statistically significant difference was found in that in the experimental group, participants who felt that whenever someone smoked beside them, it was a troublesome matter increased from 2 to 5 after intervention, whereas it decreased in the control group from 15 to 8 after a month (P = 0.001) (Table 3).
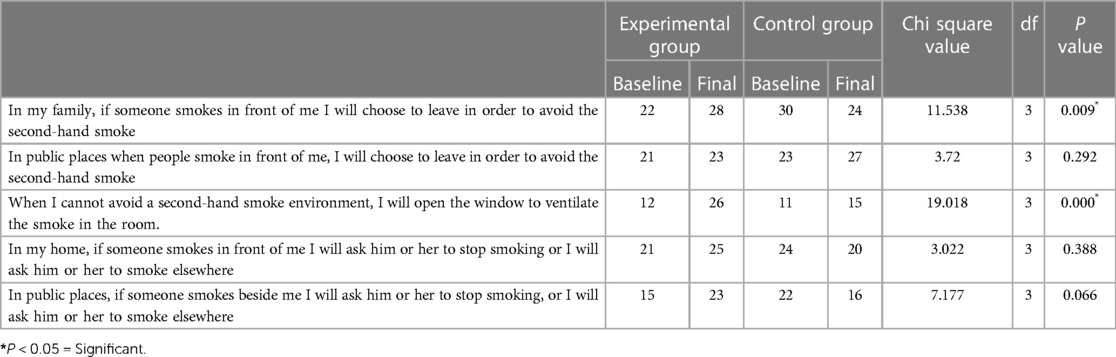
3.2.3. Avoidance behavior domain
When we analysed avoidance behavior with respect to second-hand smoke, all 30 participants in the control group and 22 in the experimental group reported that if someone from their family smoked in front of them, they would choose to leave to avoid second-hand smoke.
The number of participants who were confident of choosing to leave if someone from their family smoked in front of them to avoid secondhand smoke increased significantly in the experimental group but decreased in the control group (P = 0.009). The number of participants who reported that when they could not avoid a second-hand smoke environment, they would open the window to ventilate the smoke in the room also increased after intervention (P = 0.000) (Table 4).
3.2.4. The self-efficacy of avoidance domain
Although most of the participants had the confidence to request that their friends and family members stop smoking, only 58.3% had the confidence to ask strangers not to smoke in public spaces. Approximately 67% of the participants were confident that they could avoid secondhand smoke while with relatives or elders.
After the intervention, the number of participants who had the confidence to ask family members to stop smoking at home and strangers not to smoke in banned public spaces increased from 20 to 26 (P = 0.021) and 18 to 23 (P = 0.039), respectively. After the intervention, 24 participants were confident that they could avoid secondhand smoke while with relatives or elders, compared to 15 at baseline, and this difference was found to be statistically significant (P = 0.014) (Table 5).
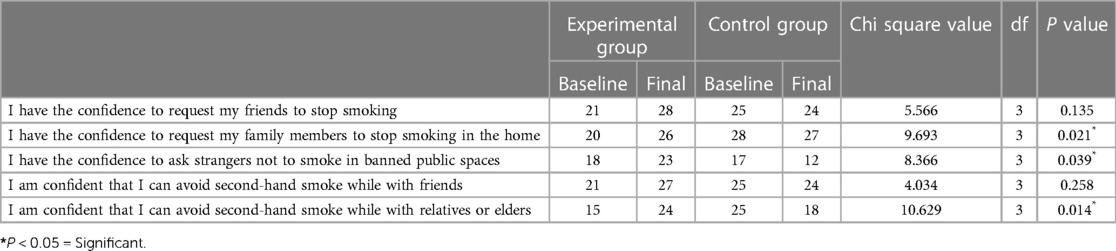
Table 5. Comparison of baseline and final scores based on self-efficacy of avoidance (weighted chi-square).
When we compared the baseline domain scores with postintervention scores, we found that out of the four domains, the domains of knowledge and avoidance behavior showed statistically significant improvement in the domain scores of the experimental group (Table 6).
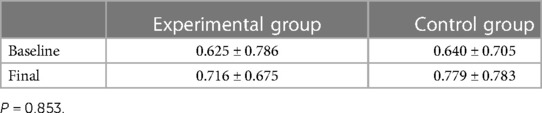
3.3. Salivary cotinine levels
The mean baseline salivary cotinine levels were 0.625 in the experimental group and 0.64 in the control group.
The mean salivary cotinine levels increased in both the experimental and control groups. This increase was found to be 0.091 in the experimental group compared to 0.139 in the control group. Although this difference was not found to be statistically significant, we consider this higher increase in the salivary cotinine levels in the control group to be clinically significant (Table 7).
4. Discussion
The purpose of this study was to determine whether a school-based “tobacco-free” educational intervention program among 12-year-old children would be effective in reducing their exposure to second-hand tobacco smoke by improving their knowledge, attitude and behavior post intervention and estimating salivary cotinine levels as markers of SHS exposure.
In the present study, when the mean salivary cotinine levels were compared pre- and postintervention, we found that although the mean postintervention salivary cotinine levels increased in both the experimental and control groups, the increase was less in the experimental group than in the control group. Although the difference was not statistically significant, it can be considered clinically significant since salivary cotinine levels greater than 0.1 ng/ml (20) demonstrate exposure to SHS. An increase in the mean salivary cotinine level of 0.13 ng/ml in the control group definitely points toward a significant increase in the exposure to SHS. This finding is also significant because this increase in the mean salivary cotinine levels was noticed in one month in the control group without the 40-min health education intervention.
Despite an extensive search of all the databases, we could not find studies comparing salivary cotinine levels before and after a health educational intervention among adolescents. However, studies (26, 27) among adults have shown that the percentage of participants with measurable concentrations of salivary cotinine decreased considerably after intervention in the form of legislation.
In the present study, all 60 participants reported exposure to SHS at home at baseline. When we analysed the pre- and postintervention responses of the groups regarding exposure to SHS, we found that at the start of the study, although all participants had a smoker who lived with them, one month after the intervention, we found that the number of participants who had a smoker who lived with them had reduced to 8 in the experimental group and to 10 in the control group. Even the number of guests who smoked in their house was reduced in both groups. We also found that the number of people who smoked inside the house decreased after the intervention. This was a very promising finding. However, an interesting finding was that after the intervention, there was a statistically significant increase in the number of people smoking in front of children at home in the experimental group compared to the control group. This could be attributed to the fact that with increasing awareness about the effects of SHS, the children could have begun noticing smoking behaviour at home.
When we analysed the responses of the groups based on the comparison of baseline and final scores of the questionnaire, we found that in the knowledge domain, there was a statistically significant increase in the knowledge of the participants in 8 out of the 10 items in the experimental group. Similar findings were reported by Raji et al. (28) in a study on Nigerian adolescents.
In the attitude domain, we found that out of the 5 items, 3 items showed an improvement in the experimental group compared to only one item in the control group. In the avoidance behavior domain, all 5 items showed improvement in the experimental group compared to only 2 items in the control group. A dearth of published studies pertaining to intervention programs targeted at children to reduce their exposure to SHS compelled us to discuss our findings only with the limited evidence available in the literature. Similar findings were reported by Raji et al. (28), who reported a postintervention increase in the percentage of adolescents who reported that they would leave a place where cigarettes were being smoked. In the self - efficacy of avoidance domain, all 5 items showed a statistically significant improvement in the experimental group compared to the control group.
5. Conclusions
The present study has been shown to be effective in improving the knowledge, attitude and avoidance behavior of adolescents toward exposure to secondhand smoke. When we compared the overall pre- and postintervention domain scores, we found that all the domains showed improvement in the experimental group compared to the control group. When the mean salivary cotinine levels were compared pre- and postintervention, we found that although the mean postintervention salivary cotinine levels increased in both the experimental and control groups, the increase was less in the experimental group than in the control group. This study shows that the educational intervention program is effective in improving the knowledge, attitude, avoidance behavior and self-efficacy of avoidance among adolescents toward exposure to secondhand smoke, and the findings of this randomized controlled trial can be generalized to the adolescent population worldwide.
6. Recommendations
Smoke-free air is a fundamental human right, and everyone has the right to breathe clean air. Children, unlike adults, are unable to control their exposure to tobacco smoke. They are compelled to live in the environment that has been established for them by adults. Although tobacco control measures that educate the public about the dangers of smoking around children are crucial in making houses smoke-free, this study has also shown the positive effect of targeting children themselves. Educating children about the harms of secondhand tobacco smoke would make them aware of the environmental tobacco smoke around them and empower them to try and avoid exposure to SHS. It could also motivate them to never indulge in tobacco habits in the future. A trickle effect of educating children could be that they could also play a role in educating their parents and motivating them to stop tobacco smoking at home. A dedicated hour every week incorporated into the school curriculum to educate children about the ill effects of tobacco would help them develop a healthy attitude and avoid exposure to SHS. The findings can also be applied in designing educational programs or policies contributing to broader efforts in public health and tobacco control. This could go a long way in not only bringing about awareness and improved health but also developing a healthy attitude and lifestyle in our future generation, converting our dream of a tobacco-free world into reality.
7. Limitations
Although salivary cotinine is one of the most reliable markers for SHS exposure, it has a half-life of 16 h and can be detected for a limited time period in saliva, which could be a limitation. This study analysed the baseline and postintervention findings over a period of 30 days, which can be considered a short-term effect. Continued analysis of the effects of periodic “Tobacco-free” intervention for a better understanding of the long-term impact on SHS exposure as well as knowledge, attitude and behaviour needs to be carried out in this group of children.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee, Manipal College of Dental Sciences, Mangalore. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AR: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. NR: Data curation, Methodology, Writing – original draft. NM: Methodology, Supervision, Writing – review & editing. BU: Supervision, Writing – review & editing, Methodology. RS: Supervision, Writing – review & editing, Methodology. AR: Supervision, Writing – review & editing, Funding acquisition. MS: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study is part of an extramural project funded by Public Health Research Initiative (PHRI) research grants managed by PHFI with the financial support of the Department of Science and Technology (DST) Government of India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. WHO report on the global tobacco epidemic, 2023: protect people from tobacco smoke. World Health Organization. (2023). ISBN 978-92-4-007716-4 (electronic version) Available at https://apps.who.int/iris/handle/10665/372043 (Accessed on 16 July 2023).
2. Global Youth Tobacco survey (GYTS) Factsheet India. (2019). Ministry of Health and Family Welfare, Government of India. Available at: https://ntcp.mohfw.gov.in/assets/document/National_Fact_Sheet_of_fourth_round_of_Global_Youth_Tobacco_Survey_GYTS-4.pdf (Accessed on 12 Sep 2023).
3. Arfaeinia H, Ghaemi M, Jahantigh A, Soleimani F, Hashemi H. Secondhand and thirdhand smoke: a review on chemical contents, exposure routes, and protective strategies. Environ Sci Pollut Res Int. (2023) 30(32):78017–29. doi: 10.1007/s11356-023-28128-1
4. Carreras G, Lachi A, Cortini B, Gallus S, López MJ, López-Nicolás Á, et al. Burden of disease from exposure to secondhand smoke in children in Europe. Pediatr Res. (2021) 90:216–22. doi: 10.1038/s41390-020-01223-6
5. Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. (2011) 377:139–46. doi: 10.1016/S0140-6736(10)61388-8
6. Carreras G, Lugo A, Gallus S, Cortini B, Fernandez E, Lopez MJ, et al. Burden of disease attributable to secondhand smoke exposure: a systematic review. Prev Med. (2019) 129:105833. doi: 10.1016/j.ypmed.2019.105833
7. Carreras G, Lachi A, Cortini B, Gallus S, López MJ, López-Nicolás Á, et al. Burden of disease from second-hand tobacco smoke exposure at home among adults from European union countries in 2017: an analysis using a review of recent meta-analyses. Prev Med. (2021) 145:106412. doi: 10.1016/j.ypmed.2020.106412
8. Jacob L, Smith L, Jackson SE, Haro JM, Shin JI. Secondhand smoking and depressive symptoms among in-school adolescents. Am J Prev Med. (2020) 58(5):613–21. doi: 10.1016/j.amepre.2019.12.008
9. Ellis-Suriani Z, Norsa’adah B, Othman A, Siti-Azrin AH. Association between secondhand smoke exposure at home and cognitive performance among rural primary school children in Malaysia. Tob Induc Dis. (2021) 19:27. doi: 10.18332/tid/133638
10. World Health Organization. Global progress report on implementation of the WHO Framework Convention on Tobacco Control. (2021). Available at: https://fctc.who.int/publications/i/item/9789240041769 (Accessed on 1 Aug 2023).
11. Government of India. COTPA 2003. Available at: https://ntcp.mohfw.gov.in/cigarettes_and_other_tobacco_products (Accessed on 1 Sep 2022).
12. Mehrotra R, Mehrotra V, Jandoo T. Tobacco control legislation in India: past and present. Indian J Cancer. (2010) 47(Suppl 1):75–80. doi: 10.4103/0019-509X.63870
13. Adgate JL, Church TR, Ryan AD, Ramachandran G, Fredrickson AL, Stock TH, et al. Outdoor, indoor, and personal exposure to VOCs in children. Environ Health Perspect. (2004) 112(14):1386–92. doi: 10.1289/ehp.7107
14. Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: an ecological measurement approach. J Pediatr Psychol. (2008) 33(2):156–75. doi: 10.1093/jpepsy/jsm123
15. Harutyunyan A, Movsisyan N, Petrosyan V, Petrosyan D, Stillman F. Reducing children's exposure to secondhand smoke at home: a randomized trial. Pediatrics. (2013) 132(6):1071–80. doi: 10.1542/peds.2012-2351
16. Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med. (2003) 157(3):295–302. doi: 10.1001/archpedi.157.3.295
17. Baxi R, Sharma M, Roseby R, Polnay A, Priest N, Waters E, et al. Family and carer smoking control programmes for reducing children's Exposure to environmental tobacco smoke. Cochrane Database Syst Rev. (2014) (3):CD001746. doi: 10.1002/14651858.CD001746.pub3
18. Zhou YH, Mak YW, Ho GWK. Effectiveness of interventions to reduce exposure to parental secondhand smoke at home among children in China: a systematic review. Int J Environ Res Public Health. (2019) 16(1):107. doi: 10.3390/ijerph16010107
19. Brown N, Luckett T, Davidson PM, Di Giacomo M. Interventions to reduce harm from smoking with families in infancy and early childhood: a systematic review. Int J Environ Res Public Health. (2015) 12(3):3091–119. doi: 10.3390/ijerph120303091
20. Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med. (1990) 19(2):190–7. doi: 10.1016/0091-7435(90)90020-K
21. Rao A, Unnikrishnan B, Rungta N, Nandini M, Shenoy R, Rao A, et al. Exposure to second hand tobacco smoke among 12-year-old adolescents in Mangalore, Karnataka—a descriptive study. Asian Pac J Cancer Prev. (2021) 22(3):827–35. doi: 10.31557/APJCP.2021.22.3.827
22. Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, et al. Assessing secondhand smoke using biological markers. Tob Control. (2013) 22(3):164–71. doi: 10.1136/tobaccocontrol-2011-050298
23. Lin PL, Huang HL, Lu KY, Chen T, Lin WT, Lee CH, et al. Second-hand smoke exposure and the factors associated with avoidance behavior among the mothers of preschool children: a school-based cross-sectional study. BMC Public Health. (2010) 10(1):606. doi: 10.1186/1471-2458-10-606
24. Gharaibeh H, Haddad L, Alzyoud S, El-Shahawy O, Baker NA, Umlauf M. Knowledge, attitudes, and behavior in avoiding secondhand smoke exposure among nonsmoking employed women with higher education in Jordan. Int J Environ Res Public Health. (2011) 8(11):4207–19. doi: 10.3390/ijerph8114207
25. Salivary Cotinine ELISA Kit—Salimetrics. Available at: https://salimetrics.com/assay-kit/salivary-cotinine-elisa-kit/ (Accessed on 1 Aug 2023).
26. Nichter M, Padmajam S, Nichter M, Sairu P, Aswathy S, Mini GK, et al. Developing a smoke free homes initiative in Kerala, India. BMC Public Health. (2015) 15:480. doi: 10.1186/s12889-015-1815-1
27. Lidón-Moyano C, Fu M, Perez-Ortuño R, Ballbè M, Sampedro-Vida M, Martín-Sánchez JC, et al. Assessment of salivary cotinine concentration among general nonsmokers population: before and after Spanish smoking legislations. Cancer Epidemiol. (2017) 51:87–91. doi: 10.1016/j.canep.2017.10.011
Keywords: adolescent, cotinine, environmental exposure, health education, randomized controlled trial, saliva, second-hand smoke, tobacco
Citation: Rao A, Rungta N, Nandini M, Unnikrishnan B, Shenoy R, Rao A and Shetty MK (2023) Effect of educational intervention in reducing exposure to second hand tobacco smoke among 12-year-old children as determined by their salivary cotinine levels and knowledge, attitude and behavior - a randomized controlled trial. Front. Oral. Health 4:1277307. doi: 10.3389/froh.2023.1277307
Received: 14 August 2023; Accepted: 12 September 2023;
Published: 28 September 2023.
Edited by:
Mamata Hebbal, Princess Nourah bint Abdulrahman University, Saudi ArabiaReviewed by:
Akshatha Gadiyar, Goa Dental College and Hospital, IndiaSyed Wali Peeran, Jazan University, Saudi Arabia
© 2023 Rao, Rungta, M, Unnikrishnan, Shenoy, Rao and Shetty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mranali K. Shetty bXJhbmFsaS5zaGV0dHlAbWFuaXBhbC5lZHU=
 Ashwini Rao
Ashwini Rao Nikita Rungta2
Nikita Rungta2 B. Unnikrishnan
B. Unnikrishnan Ramya Shenoy
Ramya Shenoy Mranali K. Shetty
Mranali K. Shetty