- Faculty of Dentistry, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
Non-communicable diseases (NCDs), which contribute significantly to global morbidity, are largely preventable through behavioral changes. As with other NCDs, periodontitis is associated with modifiable risk factors such as smoking and stress and is linked to multiple adverse health outcomes through a shared pathway of chronic systemic inflammation. While the health benefits of physical activity have been widely promoted in public health and extensively studied for other systemic conditions, its impact on periodontal health has only recently started to gain attention. This article critically evaluates the current literature on the relationship between physical activity and periodontitis. While cross-sectional studies have shown an inverse association between physical activity levels and periodontitis risk in the general population, clinical oral health surveys of elite athletes with high levels of physical activity have nonetheless revealed poor periodontal conditions. Although causality has not been determined, physical activity could positively impact periodontitis directly, by reducing inflammatory biomarkers, and indirectly, through its modulatory effects on insulin sensitivity, obesity, bone density, stress, and other health promoting behaviors. Given the importance of risk factor control during initial periodontal therapy, understanding the role of physical activity as a potential behavioral risk modifier is paramount. The findings of this review provide an evidence-based overview of how physical activity could influence periodontitis. There is a need for longitudinal cohort studies to verify the temporality of the reported associations and exclude confounders, while interventions are needed to assess the efficacy of physical activity on periodontal treatment outcomes.
1. Introduction
Physical activity has been widely promoted as a public health measure in the prevention and management of non-communicable diseases (NCDs) (1–3). Evidence supports the beneficial role of physical activity in reducing stress, adipose tissue and systemic inflammation (4), as well as improving circulation, insulin sensitivity and musculoskeletal health (5). For this reason, physical activity is listed by the World Health Organization (WHO) as one of the four modifiable behavioral risk factors in NCD prevention, together with diet, tobacco and alcohol use (6). Although the WHO recommends adults aged 18-64 to complete at least 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic physical activity weekly, more than a quarter of adults do not meet this target (7). At the same time, there has been a rapid rise in the number of NCDs worldwide, which account for nearly three-quarters of overall deaths despite being largely preventable (6). Physical inactivity is now the fourth leading risk factor for global mortality (8) and is estimated to be responsible for up to 10% of NCDs (9), including diabetes, heart disease and certain cancers, creating significant economic and societal burdens.
Periodontal disease is a preventable chronic disease that affects up to half of the global population and shares common risk factors with other NCDs (10). As with the majority of NCDs, inflammation underpins the progression of periodontal disease, which is characterized by an excessive inflammatory host response to microbial dysbiosis resulting in destruction of the supporting structures of the dentition. Tooth loss attributed to periodontitis profoundly reduces individual quality of life through impairments in speech, mastication and psychosocial wellbeing, resulting in a global estimated cost of US$54 billion in lost productivity per annum (11).
Behavioral modification, effective and sustained dental biofilm control, and the management of risk factors are considered primary components of periodontal disease prevention and therapy (12). While current oral health interventions have focused on tobacco cessation (13), oral hygiene instruction (14, 15), and dietary modification (16), physical activity has not been widely studied as a risk indicator for periodontitis. However, emerging evidence suggests that physical activity is associated with improved periodontal outcomes (17–19). Guidance from the Scottish Dental Clinical Effectiveness Programme on the Prevention and Treatment of Periodontal Diseases in Primary Care recommends discussions with patients regarding the benefits of regular exercise (20). The European Federation of Periodontology's S3 Clinical Practice Guidelines acknowledge the need for additional research to assess whether physical activity positively impacts periodontal treatment outcomes, but nonetheless include it as a potential risk indicator to be controlled in the first step of periodontitis therapy (12).
The aim of the present review is to summarize the available evidence on the relationship between physical activity and periodontal disease. Many of the studies that demonstrate an association between activity levels and periodontal disease severity are limited by their cross-sectional design, the influence of confounders, and differences in the measurement and definition of physical activity. It has also been found, paradoxically, that elite athletes have poor periodontal conditions despite good oral hygiene practices (21–23). Understanding the role of physical activity in periodontitis risk will not only help dentists deliver more comprehensive behavioral change interventions for the prevention and management of this multifactorial disease, but may influence future health promotion guidelines for both oral and general health.
2. Literature search
To prepare this narrative review, a literature search was conducted with the keywords [(exercise) OR (physical activity) AND (periodontitis) OR (periodontal disease)] in PubMed, Web of Science, and Google Scholar. Only articles from 2005 to 2023 published in English with full-text availability and clinical periodontal outcomes were included. After duplicate removal, titles and abstracts were screened. Studies were rejected if physical activity was not one of the main variables analysed, but only included to reduce the influence of external variables in measuring associations of periodontitis and other conditions, such hypertension (24) or dementia (25). The full texts of the remaining papers were reviewed, resulting in the inclusion of 12 studies investigating the association between physical activity and periodontal disease and 11 studies investigating the periodontal conditions of elite athletes.
3. Physical activity levels and periodontal disease
A recent meta-analysis found that regular physical activity reduced periodontitis risk by 23% (26). Physical activity has been defined by the WHO as “any bodily movement produced by skeletal muscles that requires energy expenditure” (27). An internationally validated measurement tool used to assess levels of physical activity is the International Physical Activity Questionnaire (IPAQ), a self-report questionnaire that measures frequency and duration of different intensities of physical activities across the last 7 days. Despite the recall bias associated with self-report measures, the use of the IPAQ with its clear scoring protocol enables greater standardization when describing physical activity levels across different adult populations. It also facilitates comparisons across studies, as the results from the IPAQ can be categorically scored as low, moderate or high physical activity levels based on preset definitions, or calculated into continuous data in the form of MET-minutes, which represent multiples of resting metabolic rate. The physical activities surveyed are recorded under different domains, such as leisure-time, occupational and transport-related physical activity, thus enabling the undertaking of more specific analysis. The Global Physical Activity Questionnaire developed by the WHO has also been used to survey physical activity levels across a typical week and has shown reliability and correlation with IPAQ (28).
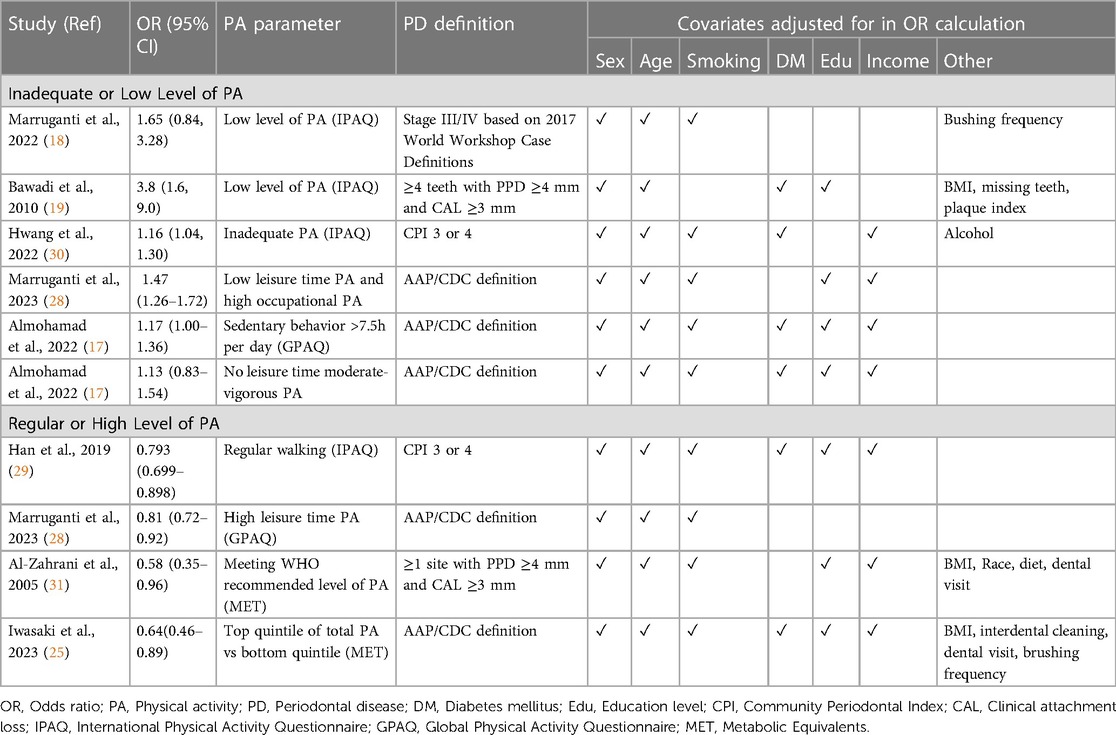
Across all eight of the included cross-sectional studies, lower levels of physical activity were associated with greater prevalence of periodontal disease. Four studies used the IPAQ to study the existence of an independent association between periodontitis severity and levels of physical activity in adults from Italy (18), Jordan (19) and South Korea (29, 30). Models were adjusted for covariates that could affect periodontitis such as smoking, diabetes mellitus, age, gender, occupation, education level, household income, and BMI, as shown in Table 1. High levels of physical activity were significantly associated with a lower prevalence of stage III and IV periodontitis (18), lower gingival inflammation, probing depths (30) and a reduced number of sites with clinical attachment loss ≥3 mm (19). Three studies also investigated the role of dietary practices and found that the combination of a healthy diet and regular exercise habit reduced the odds of severe periodontitis by 10 times (18). Rather than investigate physical activity in general, Han et al. used a modified version of IPAQ to specifically look at the impact of regular walking practices. The study revealed that regular walking significantly lowered the risk of periodontal disease, suggesting that even low-intensity physical activity could be of benefit in reducing periodontitis risk. Moreover, physical activity attenuated the relationship between periodontitis and low socioeconomic status, as periodontitis was significantly associated with low socioeconomic status in non-regular walkers, but not in the regular walking group. This finding could be important in tackling social inequalities in oral health (29).
Two studies used the GPAQ to assess levels of physical activity in different domains, namely transportation, occupation and leisure time (17, 28). The latter refers to bodily movements “performed during free time”, “at the subject's discretion”, and “not required as part of the essential activities of daily living”, while occupational physical activity is differentiated by referring to bodily movements contributing towards the subject's professional duties and includes “carrying/lifting heavy loads, digging or construction work, household chores, etc.” Interestingly, while higher leisure time physical activity was a protective indicator for periodontitis, there was a divergent relationship when occupational physical activity was considered, with greater levels of physical activity at work leading to an increased periodontitis risk (28).
In a large-scale population study of US adults, a significant association was found between increased physical activity and lower periodontitis prevalence in never and former smokers (31). However, while former smokers who met the recommended levels of physical activity were 74% less likely to have periodontitis compared to those who were inactive, this relationship was not significant for current smokers, suggesting that the effect of smoking may override any potential periodontal health benefit from exercise.
4. Mechanisms for the relationship between physical activity and periodontitis
Several underlying mechanisms have been proposed to explain the association between periodontitis and physical activity, which are summarized in Figure 1. Physical activity may directly lower the risk of periodontitis by reducing the level of circulatory proinflammatory mediators associated with periodontal connective tissue attachment loss and alveolar bone resorption (32). A case control study of 751 Australian adults found that those engaging in the recommended amount of leisure time physical activity had lower odds of elevated interleukin-1ß and C-reactive protein (CRP) in gingival crevicular fluid compared to those who were insufficiently active (33). In addition, a dose-response relation was found among periodontitis cases, with increasing levels of physical activity resulting in decreased probability of detectable CRP. Other studies have found that physical activity lowered serum TNF-α, IL-6 and IL-8 levels (25, 32, 34), which could promote periodontal health. Apart from its anti-inflammatory effect, physical activity could suppress periodontal destruction by improving peripheral blood flow and endothelial function (25, 35).
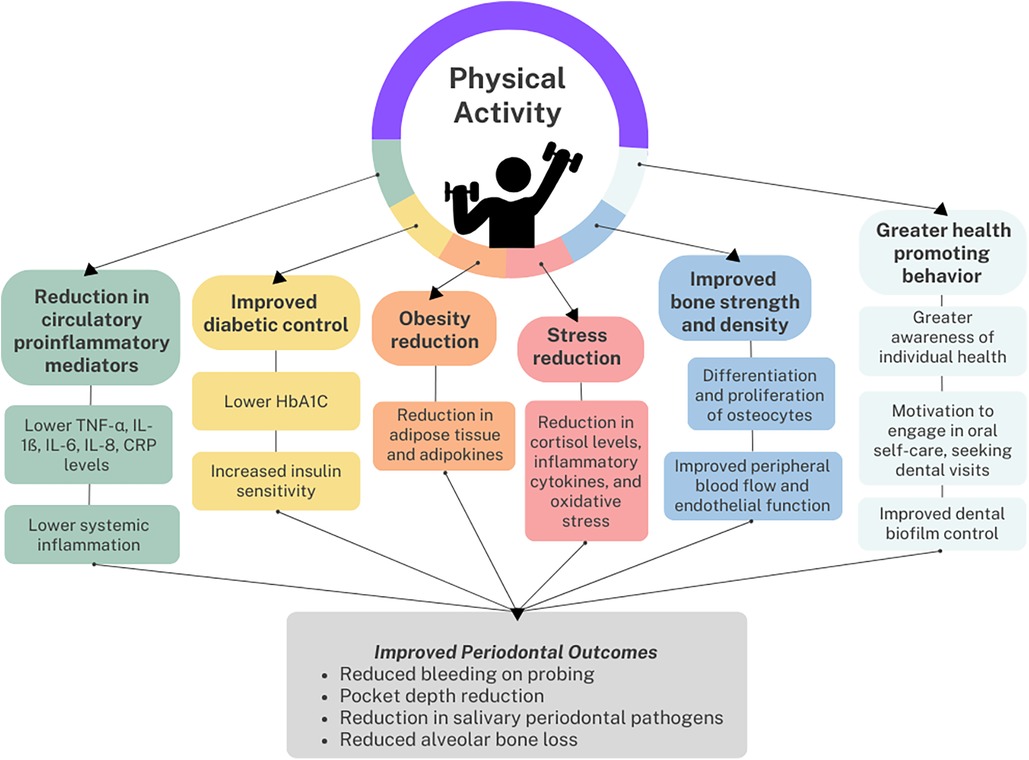
Figure 1. Reported association and the respective potential links between physical activity and periodontal health.
Physical activity could also indirectly protect against periodontitis by reducing known periodontal risk factors, such as diabetes mellitus (36). Physical activity lowers glycated hemoglobin (HbA1C) levels and increases insulin sensitivity, resulting in improved diabetic control (37, 38). While most cross-sectional studies showing an association between physical activity and periodontal disease have excluded diabetes mellitus as a potential confounder, a recent randomized controlled trial was conducted to assess the effect of a 6-month physical activity intervention on the periodontal health of type 2 diabetes mellitus patients. Compared to the control group, those who received the intervention were found to have a significant reduction in bleeding on probing, periodontitis severity, and HbA1C levels (39).
In addition, obesity (40, 41) and intra-abdominal fat (42) have been associated with periodontitis through shared pathways of chronic low-grade inflammation and immune cell dysregulation. Cross-sectional epidemiological studies show that periodontitis is inversely associated with BMI (35) and waist circumference (40). An intervention study among obese men in Japan found a significant decrease in the number of pockets ≥4 mm, the number of teeth with bleeding on probing, and salivary counts of periodontal pathogens including Tannerella forsythia and Treponema denticola (43) after a 12-week exercise program compared to baseline measurements. The study also determined a positive correlation between levels of periodontal pathogens, body weight and fasting insulin, providing further grounds for a mechanism of a shared inflammatory pathway between physical activity, periodontitis and metabolic disorders (32). Physical activity combats obesity by increasing energy expenditure and reducing adipose tissue mass (32), lowering the level of adipokines, macrophages and systemic inflammation (44) which may also benefit periodontal health. While obesity disrupts bone homeostasis by favouring bone resorption which could lead to greater alveolar bone loss (41), the repeated mechanical load and muscular contraction of physical activity promote the differentiation and proliferation of osteocytes. This leads to increased bone strength and density (45), factors which have been associated with less alveolar bone loss (46).
Another potential mechanism is through the stress-reducing effect of physical activity. Randomized controlled trials have demonstrated the positive effects of physical activity on mental health outcomes and a study of over a million American adults found that those who regularly engaged in physical activity reported greater emotional wellbeing than those who were inactive (47). This may be clinically significant for periodontitis patients, as community studies have found psychosocial stress to be associated with greater clinical attachment loss even after adjusting for sociodemographic factors, smoking and diabetes mellitus (48, 49). In a 12-week clinical trial, participants in the intervention group participated in a yoga-based program on top of standard periodontal treatment, while those in the control group only received the periodontal treatment. The intervention group reported reduced stress and had significantly lower plaque scores, bleeding on probing, probing depths and clinical attachment loss upon clinical examination (50). The role of stress is also highlighted in the study by Marruganti et al. (28), who differentiated between leisure-time and occupational physical activity. High levels of occupational physical activity were significantly associated with a greater risk of severe periodontitis, which further increased when combined with low leisure-time physical activity. This was explained through the increased stress experienced when heavy physical labor forms part of one's work, which may result in the activation of the hypothalamic-pituitary-adrenal axis and higher levels of cortisol, pro-inflammatory cytokines and oxidative stress. Conversely, in leisure-time physical activity, psychosocial stress and its associated biological effects are reduced, hence the reverse association is seen with periodontitis.
Finally, it has been proposed that the association could be due to greater awareness and prioritization of health promoting behaviors (31). Individuals who engage in more regular physical activity may have greater motivation to engage in consistent oral hygiene practices or seek regular dental care. This may result in improved periodontal condition given the importance of effective biofilm control in the prevention of a large proportion of periodontal diseases (12). However, behavioral change is unlikely to be the sole explanation as some of the studies demonstrating an association between physical activity and periodontal disease had already excluded oral hygiene habits such as toothbrushing frequency and plaque control in the analysis (18, 19).
5. Periodontal conditions of elite athletes
Given the association between periodontal disease and physical activity, it might be expected that elite athletes, with their active lifestyle and strong emphasis on optimal physical condition, would have high levels of periodontal health. However, cross-sectional studies of this population have nonetheless found evidence of periodontal disease (21–23, 51–54). Gingival bleeding on probing or calculus was found in over three-quarters of UK elite athletes (22), gingivitis in 64% of Dutch athletes (53), and periodontitis in 41% of professional football players (51). Periodontal pocket depths of ≥4 mm were found in 27% of elite athletes from Pakistan (21), 15% of athletes presenting at the dental clinic at the London 2012 Olympic Games (55) and 34% of athletes at the Lima 2019 Pan American Games (52). Furthermore, athletes may have a higher incidence of gingival hypertrophy resulting from anabolic steroid use and “swimmers’ calculus”, a build-up of calculus in frequent swimmers, may arise from prolonged exposure to chlorinated water (56).
These studies are limited by their small sample size and the heterogeneity in both the periodontal measurements and nature of sports represented by the athletes. Additionally, most studies did not provide data to compare the prevalence of periodontal diseases observed in the athletes to a control group or population of a similar age and background, limiting the significance of the findings (51, 53). Gallagher et al. found that 22% of UK elite athletes had pocket depths of ≥4 mm which is only slightly higher than the 19% obtained from England's national oral health survey data of a similar age range (22). Gingivitis has been found to affect almost all adults (57) but data specific to the mid-twenties age range of the athletes of the included studies is scarce. Nonetheless, these results are worrying given the relatively young age of the athletes and the tendency for periodontal disease to become more severe with age due to cumulative damage over time. Moreover, the athletes' oral health problems were discovered to negatively impact their daily activities and training performance (21, 22, 55). Further research could potentially reveal whether underlying biological mechanisms due to the physiology of physical activity or stress place them at greater risk of periodontal disease. With sport encompassing the chosen profession of many professional athletes, this may be all the more likely in light of the data showing the association between occupational physical activity and more severe periodontal disease (28).
Based on the inflammatory nature of periodontitis, it has been proposed that athletes could be prone to periodontal problems due to the immunomodulatory effects of strenuous exercise regimes. While individuals engaging in regular moderate physical activity have improved immune function compared to those with a sedentary lifestyle, prolonged intense physical activity has been associated with a short-term depression of immune function up to 24 h after exercise (58), resulting in an “open window” of increased susceptibility to infection. A systematic review of inflammatory biomarkers in high and moderate intensity physical activity revealed that the former could lead to significantly higher levels of inflammatory mediators, especially IL-6, due to muscle damage during the physical activity (59). The review also suggested that intense physical activity could impair immune response if not accompanied by adequate rest (60). Changes in saliva have also been attributed to poorer periodontal conditions in athletes. During exercise, mouth breathing and fluid loss can result in reduced salivary flow and dry mouth (23, 61), which could create a more favorable environment for periodontal pathogens. Salivary cortisol levels, which have been associated with more severe periodontitis (62), were significantly increased after physical activity of high intensity but not low intensity (63). From a behavioral perspective, athletes may place greater focus on improving physical fitness and ignore other aspects of health including oral care. Periodontal disease could arise due to poor compliance with dental appointments and oral hygiene instruction (64), as well as frequent consumption of carbohydrate-rich sports drinks which have been associated with higher BPE scores (21). Finally, as several of the studies on elite athletes' periodontal conditions collected data in the lead up to or during high profile athletic events such as the Olympics (53, 55) and regional championships (52), the impact of psychological stress in the athletes surveyed should be considered when interpreting these results.
6. Summary
Emerging evidence of the association between physical activity and periodontal disease may hold promise that exercise interventions, which are already being used to promote general health and wellbeing, could also provide clinical benefit in the prevention and management of periodontal diseases.
Studies have demonstrated an association between increased physical activity and reduced periodontal disease severity through clinical measures such as clinical attachment loss, probing depths, and bleeding on probing. The mechanisms underlying this association have been explained through the effect of physical activity in reducing systemic inflammation and shared risk factors such as diabetes mellitus, obesity and stress. There is evidence that even low intensity physical activity such as walking could reduce periodontal risk (29). On the other hand, prolonged high intensity physical activity could result in greater periodontal risk due to higher levels of inflammatory mediators associated with muscle damage and repair. While studies have highlighted the prevalence of periodontal diseases in elite athletes, the small sample size and lack of population data and a control group create uncertainty as to the clinical significance of such findings.
This review is subject to several limitations, including selection bias arising from the broad literature search conducted. Since the current evidence is limited and largely cross-sectional, causation cannot be inferred. As was previously conducted for smoking cessation and diabetes mellitus management, randomized controlled trials are needed to assess the impact of exercise-based interventions on periodontal treatment outcomes. Moreover, the existing available research focuses largely on data from population surveys. There is a need for laboratory and animal-based studies focusing on the processes underlying the observed associations to derive mechanistic insights into how the host-microbiome interaction might be affected by physical activity.
Another challenge of the present topic is the heterogeneity in the measurement of physical activity and periodontal disease. Physical activity is a broad construct complicated by its ubiquity in daily life activities including leisure, transport and work. The nature of exercise can also vary significantly, having been described with differing and ill-defined terminology such as aerobic, endurance, strength, flexibility or resistance-based activities. While the impact of variations in the definition of physical activity is minimized as the majority of the included studies used standardized measurement tools such as the IPAQ or GPAQ, these measures rely on self-report measures of physical activity, which may be subject to bias and inaccuracy. Future intervention studies should include standardized methodological variables to quantify and determine the intensity, types, volume and frequency of physical activity, especially given the findings that show divergent outcomes when physical activity is separately analysed under the domains of occupation and leisure-time activities. More recent studies have defined periodontitis using the updated 2017 World Workshop periodontitis case definition, but earlier studies relied on simple epidemiological measures such as BPE which may underestimate periodontal disease.
Finally, while the studies included in this review have attempted to control for variables that could affect the relationship between exercise and periodontal disease, it is acknowledged that there may be additional unaccounted confounders. Longitudinal cohort studies may shed light on these confounders and establish temporality. The interrelationship of confounding factors such as stress further complicates the investigation of the mechanisms underlying how physical activity affects periodontal disease.
With greater awareness of the socioeconomic cost of non-communicable diseases as well as the role of lifestyle habits in their prevention, recent consensus reports in dentistry have emphasised behavioral change and risk factor control as first steps in the management of oral diseases (12). Knowledge on the role, if any, that exercise plays in periodontal therapy would enable the development of evidence-based clinical practice guidelines and provide dental practitioners with key information to motivate patients to improve both their oral and systemic health.
Author contributions
CC: Conceptualization, Writing – original draft. AC: Writing – review & editing. CC: Writing – review & editing. YT: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Better Health—NHS: National Health Service (NHS); Available at: https://www.nhs.uk/better-health/
2. Healthy Exercise for All Campaign: Leisure and Cultural Services Department, Government of HKSAR; (2023). Available at: https://www.lcsd.gov.hk/en/healthy/
3. Move Your Way: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services; (2023). Available at: https://health.gov/moveyourway
4. Burini RC, Anderson E, Durstine JL, Carson JA. Inflammation, physical activity, and chronic disease: an evolutionary perspective. Sports Med Health Sci. (2020) 2(1):1–6. doi: 10.1016/j.smhs.2020.03.004
5. Saqib ZA, Dai J, Menhas R, Mahmood S, Karim M, Sang X, et al. Physical activity is a medicine for non-communicable diseases: a survey study regarding the perception of physical activity impact on health wellbeing. Risk Manag Healthc Policy. (2020) 13:2949–62. doi: 10.2147/RMHP.S280339
6. WHO. Noncommunicable diseases: World Health Organization; (2022). Available at: https://www.who.int/health-topics/noncommunicable-diseases#tab=tab_1
7. WHO. Global status report on physical activity 2022. Geneva: World Health Organization (2022). Available at: https://www.who.int/teams/health-promotion/physical-activity/global-status-report-on-physical-activity-2022#:∼:text=Let’s%20get%20moving!&text=Regular%20physical%20activity%20promotes%20both,recommended%20levels%20of%20physical%20activity
8. WHO. Global recommendations on physical activity for health. Geneva: World Health Organization (2010). Available at: https://www.who.int/publications/i/item/9789241599979
9. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. (2012) 380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9
10. Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the global burden of disease study 2019. J Clin Periodontol. (2021) 48(9):1165–88. doi: 10.1111/jcpe.13506
11. Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. (2017) 44(5):456–62. doi: 10.1111/jcpe.12732
12. Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. (2020) 47(Suppl 22):4–60. doi: 10.1111/jcpe.13290
13. Ramseier CA, Woelber JP, Kitzmann J, Detzen L, Carra MC, Bouchard P. Impact of risk factor control interventions for smoking cessation and promotion of healthy lifestyles in patients with periodontitis: a systematic review. J Clin Periodontol. (2020) 47(Suppl 22):90–106. doi: 10.1111/jcpe.13240
14. Tonetti MS, Eickholz P, Loos BG, Papapanou P, van der Velden U, Armitage G, et al. Principles in prevention of periodontal diseases: consensus report of group 1 of the 11th European workshop on periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol. (2015) 42(Suppl 16):S5–11. doi: 10.1111/jcpe.12368
15. Carra MC, Detzen L, Kitzmann J, Woelber JP, Ramseier CA, Bouchard P. Promoting behavioural changes to improve oral hygiene in patients with periodontal diseases: a systematic review. J Clin Periodontol. (2020) 47(Suppl 22):72–89. doi: 10.1111/jcpe.13234
16. Javid A Z, Seal CJ, Heasman P, Moynihan PJ. Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J Hum Nutr Diet. (2014) 27(6):523–32. doi: 10.1111/jhn.12184
17. Almohamad M, Krall Kaye E, Mofleh D, Spartano NL. The association of sedentary behaviour and physical activity with periodontal disease in NHANES 2011–2012. J Clin Periodontol. (2022) 49(8):758–67. doi: 10.1111/jcpe.13669
18. Marruganti C, Traversi J, Gaeta C, Ferrari Cagidiaco E, Parrini S, Discepoli N, et al. Adherence to Mediterranean diet, physical activity level, and severity of periodontitis: results from a university-based cross-sectional study. J Periodontol. (2022) 93(8):1218–32. doi: 10.1002/JPER.21-0643
19. Bawadi HA, Khader YS, Haroun TF, Al-Omari M, Tayyem RF. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. J Periodontal Res. (2011) 46(1):74–81. doi: 10.1111/j.1600-0765.2010.01314.x
20. SDCEP. Prevention and treatment of periodontal diseases in primary care Scottish dental clinical effectiveness programme. Dundee, Scotland: Scottish Dental Clinical Effectiveness Programme (2014).
21. Khan K, Qadir A, Trakman G, Aziz T, Khattak MI, Nabi G, et al. Sports and energy drink consumption, oral health problems and performance impact among elite athletes. Nutrients. (2022) 14(23):5089. doi: 10.3390/nu14235089
22. Gallagher J, Ashley P, Petrie A, Needleman I. Oral health and performance impacts in elite and professional athletes. Community Dent Oral Epidemiol. (2018) 46(6):563–8. doi: 10.1111/cdoe.12392
23. Ashley P, Di Iorio A, Cole E, Tanday A, Needleman I. Oral health of elite athletes and association with performance: a systematic review. Br J Sports Med. (2015) 49(1):14–9. doi: 10.1136/bjsports-2014-093617
24. Ahn Y-B, Shin M-S, Byun J-S, Kim H-D. The association of hypertension with periodontitis is highlighted in female adults: results from the fourth Korea national health and nutrition examination survey. J Clin Periodontol. (2015) 42(11):998–1005. doi: 10.1111/jcpe.12471
25. Iwasaki M, Yoshihara A, Suwama K, Zaitsu T, Suzuki S, Ihira H, et al. A cross-sectional study of the association between periodontitis and physical activity in the Japanese population. J Periodontal Res. (2023) 58(2):350–9. doi: 10.1111/jre.13095
26. Rodríguez-Archilla AP-C D. Influence of physical exercise on periodontal disease: a meta-analysis. Int J Dent Sci. (2022) 4(1):21–6.
27. WHO. Physical activity (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/physical-activity
28. Marruganti C, Baima G, Grandini S, Graziani F, Aimetti M, Sanz M, et al. Leisure-time and occupational physical activity demonstrate divergent associations with periodontitis: a population-based study. J Clin Periodontol. (2023) 50(5):559–70. doi: 10.1111/jcpe.13766
29. Han SJ, Bae KH, Lee HJ, Kim SJ, Cho HJ. Association between regular walking and periodontitis according to socioeconomic status: a cross-sectional study. Sci Rep. (2019) 9(1):12969. doi: 10.1038/s41598-019-49505-2
30. Hwang SY, Jang JH, Park JE. Association between healthy lifestyle (diet quality, physical activity, normal body weight) and periodontal diseases in Korean adults. Int J Environ Res Public Health. (2022) 19(7). doi: 10.3390/ijerph19073871
31. Al-Zahrani MS, Borawski EA, Bissada NF. Increased physical activity reduces prevalence of periodontitis. J Dent. (2005) 33(9):703–10. doi: 10.1016/j.jdent.2005.01.004
32. Codella R, Della Guardia L, Terruzzi I, Solini A, Folli F, Varoni EM, et al. Physical activity as a proxy to ameliorate inflammation in patients with type 2 diabetes and periodontal disease at high cardiovascular risk. Nutr Metab Cardiovasc Dis. (2021) 31(8):2199–209. doi: 10.1016/j.numecd.2021.04.022
33. Sanders AE, Slade GD, Fitzsimmons TR, Bartold PM. Physical activity, inflammatory biomarkers in gingival crevicular fluid and periodontitis. J Clin Periodontol. (2009) 36(5):388–95. doi: 10.1111/j.1600-051X.2009.01394.x
34. Ferreira RO, Corrêa MG, Magno MB, Almeida A, Fagundes NCF, Rosing CK, et al. Physical activity reduces the prevalence of periodontal disease: systematic review and meta-analysis. Front Physiol. (2019) 10:234. doi: 10.3389/fphys.2019.00234
35. Shimazaki Y, Egami Y, Matsubara T, Koike G, Akifusa S, Jingu S, et al. Relationship between obesity and physical fitness and periodontitis. J Periodontol. (2010) 81(8):1124–31. doi: 10.1902/jop.2010.100017
36. Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European federation of periodontology. J Clin Periodontol. (2018) 45(2):138–49. doi: 10.1111/jcpe.12808
37. Asfaw MS, Dagne WK. Physical activity can improve diabetes patients’ glucose control; A systematic review and meta-analysis. Heliyon. (2022) 8(12):e12267. doi: 10.1016/j.heliyon.2022.e12267
38. Yun I, Joo HJ, Park YS, Park EC. Association between physical exercise and glycated hemoglobin levels in Korean patients diagnosed with diabetes. Int J Environ Res Public Health. (2022) 19(6). doi: 10.3390/ijerph19063280
39. Wernicke K, Grischke J, Stiesch M, Zeissler S, Kruger K, Bauer P, et al. Influence of physical activity on periodontal health in patients with type 2 diabetes mellitus. A blinded, randomized, controlled trial. Clin Oral Investig. (2021) 25(11):6101–7. doi: 10.1007/s00784-021-03908-6
40. Al-Zahrani MS, Bissada NF, Borawskit EA. Obesity and periodontal disease in young, middle-aged, and older adults. J Periodontol. (2003) 74(5):610–5. doi: 10.1902/jop.2003.74.5.610
41. Zhao P, Xu A, Leung WK. Obesity, bone loss, and periodontitis: the interlink. Biomolecules. (2022) 12(7):865. doi: 10.3390/biom12070865
42. Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. J Dent Res. (2001) 80(7):1631–6. doi: 10.1177/00220345010800070701
43. Omori S, Uchida F, Oh S, So R, Tsujimoto T, Yanagawa T, et al. Exercise habituation is effective for improvement of periodontal disease status: a prospective intervention study. Ther Clin Risk Manag. (2018) 14:565–74. doi: 10.2147/TCRM.S153397
44. Khan MS, Alasqah M, Alammar LM, Alkhaibari Y. Obesity and periodontal disease: a review. J Family Med Prim Care. (2020) 9(6):2650–3. doi: 10.4103/jfmpc.jfmpc_283_20
45. Sun W, Zhang XA, Wang Z. The role and regulation mechanism of Chinese traditional fitness exercises on the bone and cartilage tissue in patients with osteoporosis: a narrative review. Front Physiol. (2023) 14:1071005. doi: 10.3389/fphys.2023.1071005
46. Yu B, Wang CY. Osteoporosis and periodontal diseases—an update on their association and mechanistic links. Periodontol. (2022) 89(1):99–113. doi: 10.1111/prd.12422
47. Chekroud SR, Gueorguieva R, Zheutlin AB, Paulus MP, Krumholz HM, Krystal JH, et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: a cross-sectional study 2018.
48. Ng SK, Keung Leung W. A community study on the relationship between stress, coping, affective dispositions and periodontal attachment loss. Community Dent Oral Epidemiol. (2006) 34(4):252–66. doi: 10.1111/j.1600-0528.2006.00282.x
49. Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress, and inadequate coping behaviors to periodontal disease. J Periodontol. (1999) 70(7):711–23. doi: 10.1902/jop.1999.70.7.711
50. Sudhanshu A, Sharma U, Vadiraja HS, Rana RK, Singhal R. Impact of yoga on periodontal disease and stress management. Int J Yoga. (2017) 10(3):121–7. doi: 10.4103/0973-6131.213468
51. Botelho J, Vicente F, Dias L, Júdice A, Pereira P, Proença L, et al. Periodontal health, nutrition and anthropometry in professional footballers: a preliminary study. Nutrients. (2021) 13(6):1792. doi: 10.3390/nu13061792
52. Opazo-García C, Moya-Salazar J, Chicoma-Flores K, Contreras-Pulache H. Oral health problems in high-performance athletes at 2019 pan American games in Lima: a descriptive study. BDJ Open. (2021) 7:21. doi: 10.1038/s41405-021-00078-1
53. Kragt L, Moen MH, Van Den Hoogenband C-R, Wolvius EB. Oral health among Dutch elite athletes prior to rio 2016. Phys Sportsmed. (2019) 47(2):182–8. doi: 10.1080/00913847.2018.1546105
54. Needleman I, Ashley P, Meehan L, Petrie A, Weiler R, McNally S, et al. Poor oral health including active caries in 187 UK professional male football players: clinical dental examination performed by dentists. Br J Sports Med. (2016) 50(1):41–4. doi: 10.1136/bjsports-2015-094953
55. Needleman I, Ashley P, Petrie A, Fortune F, Turner W, Jones J, et al. Oral health and impact on performance of athletes participating in the London 2012 Olympic games: a cross-sectional study. Br J Sports Med. (2013) 47(16):1054–8. doi: 10.1136/bjsports-2013-092891
56. Budd SC, Egea J-C. Sport, periodontal consequences and athletic patients. In: Sport and oral health: A concise guide. Cham: Springer International Publishing (2017). p. 71–4.
57. Seong J, Bartlett D, Newcombe RG, Claydon NCA, Hellin N, West NX. Prevalence of gingival recession and study of associated related factors in young UK adults. J Dent. (2018) 76:58–67. doi: 10.1016/j.jdent.2018.06.005
58. Gleeson M. Immune function in sport and exercise. J Appl Physiol. (2007) 103(2):693–9. doi: 10.1152/japplphysiol.00008.2007
59. Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine response to strenuous exercise. Can J Physiol Pharmacol. (1998) 76(5):505–11. doi: 10.1139/y98-055
60. Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise-A systematic review. Front Physiol. (2019) 10:1550. doi: 10.3389/fphys.2019.01550
61. Mulic A, Tveit AB, Songe D, Sivertsen H, Skaare AB. Dental erosive wear and salivary flow rate in physically active young adults. BMC Oral Health. (2012) 12:8. doi: 10.1186/1472-6831-12-8
62. Botelho J, Machado V, Mascarenhas P, Rua J, Alves R, Cavacas MA, et al. Stress, salivary cortisol and periodontitis: a systematic review and meta-analysis of observational studies. Arch Oral Biol. (2018) 96:58–65. doi: 10.1016/j.archoralbio.2018.08.016
63. McGuigan MR, Egan AD, Foster C. Salivary cortisol responses and perceived exertion during high intensity and low intensity bouts of resistance exercise. J Sports Sci Med. (2004) 3(1):8–15.24497815
Keywords: physical activity, periodontitis, modifiable risk factor, periodontal disease, elite athlete, general public
Citation: Chan CCK, Chan AKY, Chu CH and Tsang YC (2023) Physical activity as a modifiable risk factor for periodontal disease. Front. Oral. Health 4:1266462. doi: 10.3389/froh.2023.1266462
Received: 25 July 2023; Accepted: 31 October 2023;
Published: 13 November 2023.
Edited by:
Rafael Aiello Bomfim, Federal University of Mato Grosso do Sul, BrazilReviewed by:
Francisco Wilker Mustafa Gomes Muniz, Federal University of Pelotas, BrazilTaciane Menezes Da Silveira, Federal University of Pelotas, Brazil
© 2023 Chan, Chan, Chu and Tsang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiu Cheung Tsang ZWx2aXN0QGhrdS5oaw==
 Charlotte Cheuk Kwan Chan
Charlotte Cheuk Kwan Chan Alice Kit Ying Chan
Alice Kit Ying Chan Chun Hung Chu
Chun Hung Chu Yiu Cheung Tsang
Yiu Cheung Tsang