- 1Research Department, Dr. Kurt Wolff GmbH & Co. KG, Bielefeld, Germany
- 2Faculty of Dentistry, University of Toronto, Toronto, ON, Canada
- 3Department of Comprehensive Dentistry, School of Dentistry, University of Texas Health San Antonio, San Antonio, TX, United States
Background: The dental pellicle is a thin layer of up to several hundred nm in thickness, covering the tooth surface. It is known to protect the teeth from acid attacks through its selective permeability and it is involved in the remineralization process of the teeth. It functions also as binding site and source of nutrients for bacteria and conditioning biofilm (foundation) for dental plaque formation.
Methods: For this updated literature review, the PubMed database was searched for the dental pellicle and its composition.
Results: The dental pellicle has been analyzed in the past years with various state-of-the art analytic techniques such as high-resolution microscopic techniques (e.g., scanning electron microscopy, atomic force microscopy), spectrophotometry, mass spectrometry, affinity chromatography, enzyme-linked immunosorbent assays (ELISA), and blotting-techniques (e.g., western blot). It consists of several different amino acids, proteins, and proteolytic protein fragments. Some studies also investigated other compounds of the pellicle, mainly fatty acids, and carbohydrates.
Conclusions: The dental pellicle is composed mainly of different proteins, but also fatty acids, and carbohydrates. Analysis with state-of-the-art analytical techniques have uncovered mainly acidic proline-rich proteins, amylase, cystatin, immunoglobulins, lysozyme, and mucins as main proteins of the dental pellicle. The pellicle has protective properties for the teeth. Further research is necessary to gain more knowledge about the role of the pellicle in the tooth remineralization process.
1. Introduction
The dental pellicle (from the latin word “pellicula” which means membrane or thin film) is a very thin biofilm coating the surfaces of human teeth and oral mucosa. It mainly consists of proteins, amino and fatty acids, glycoproteins, carbohydrates, lipids, and other compounds found in saliva, but also those derived from microorganisms, such as bacteria and fungi can be found here (1–16). The dental pellicle has a zeta-potential of −15 to −30 mV. However, with saliva the net-surface changes to −8 to −14 mV (17). These data indicate that the pellicle is anionic (3–6). Jensen et al. have shown that the composition of the dental pellicle is heterogenous among subjects (18). They isolated all proteins found in saliva using anionic and cationic discontinuous polyacrylamide gel electrophoresis. Intact proteins and their proteolytic fragments can be found here. Mainly amylase, acidic proline-rich proteins, mucins (MUC5B and MUC7), cystatins as well as proteolytically derived peptides, amylase, lysozymes, and glycosyltransferase can be found in the pellicle. Acidic phosphoproteins, neutral and basic histatins selectively adsorb to hydroxyapatite (2, 3, 18, 19). Interestingly, no difference in the pellicle composition could be observed between healthy individuals and those with active caries. Here, different mass spectrometry-based techniques were used to investigate the dental pellicle (20). Vacca-Smith et al. found in their in-situ study similar proteins at different time-points during pellicle formation. The amount of proteins was also comparable between time-points of collection (21). The morphological analysis revealed a diameter of adsorbed proteins of 15 ( ± 3) nm (17). The thickness of the pellicle differed in various studies between 2 ( ± 0.5) nm, 18 nm (22), and 100 nm to 1,000 nm depending on the location of the tooth (3, 17). However, high sugary diet changed the pattern of the dental pellicle composition (21). Adhesins from microorganisms can bind to the salivary proteins found in the pellicle. Interestingly, Streptococci bind to amylase and salivary agglutinin glycoprotein (1, 23). However, bacteria are not part of the dental pellicle. Nevertheless, components from bacteria can be found in this protein-containing layer (2).
The pellicle functions as lubrication layer and has protective properties for the dentition. Lubarsky et al. have reported that the pellicle can help defending the teeth from acidic attacks due to its selective permeability. The pellicle proteins can be found in small cracks in enamel as well. This might influence the mechanical properties of the teeth (24). However, full protection of the teeth by the pellicle is not possible (25). Hara et al. showed that the dental pellicle formed after 2 h can protect teeth from demineralization due to erosive challenge for 10 min, but not a longer period. Dentin could not be protected (26). Amaechi et al. confirmed these results for the 1 h enamel pellicle (16). However, in vivo the dental pellicle will be colonized within seconds to minutes (27). Protective characteristics of the pellicle will differ between the individuals and their respective pelliclecomposition.
The formation of the pellicle is a specific and non-random process (3). The binding of salivary proteins, especially aPRPs, statherin, and histatins, are the first proteins that adsorb to hydroxyapatite of enamel (3, 5). More than 100 different proteins can be found in the pellicle (3, 6). Trautmann et al. found a 10 times higher number of proteins from the pellicle compared to the previously published studies: of the 1,188 identified proteins, 68 proteins were found in all individuals (caries-active and healthy) investigated in the study (9, 10). The same research group investigated the composition of pellicle with saliva of the respective subjects. 498 proteins in the dental pellicle, and 1,032 proteins in the saliva could be identified. Additionally, pellicle formation relies on selective adsorption (10). Here, nano-liquid-chromatography-high resolution-mass-spectrometry/mass-spectrometry (LC-HR-MS/MS) techniques were used for the identification of the pellicle composition. The formation of the pellicle takes about 30 to 90 min (10). It has been shown that acids can remove the outer globular layer of the pellicle, but the basal layer stays intact. Acid resistance is mediated by statherins and mucins (3). When discussing protective properties, about 8% of the pellicle-bound proteins have additional antibacterial properties: cystatins, lysozyme, myeloperoxidase, and histatins (5). Delvar et al. have shown that the use of carboxymethylcellulose helps to improve the protective properties of the pellicle (28). The same could be observed for chitosan, which adsorbs on top of the pellicle. Interestingly, individual variations seem to be important in the protective action of the dental pellicle. This was observed by Bruvo et al. with the help of different methods, such as surface microhardness, sodium dodecyl sulfate—poly crylamide gel electrophoresis (SDS-PAGE), and high-pressure liquid chromatography (HPLC) (29). Another study investigated the protective properties of calcium ions, which can also be incorporated into the pellicle (30). Increased calcium concentration in saliva, and in the pellicle, weakens the electrostatic interaction between salivary proteins and the enamel surface. At the same time, thickness and viscoelasticity of the pellicle are increased (31). The density and mechanical properties increase from the outer to the inner layer (22). This was measured by nanoindentation. Using ellipsometry and transmission electron microscopy (TEM), Güth-Thiel et al. observed that the pellicle formation can be separated into a rapid formation-phase within the first minutes after tooth cleaning, and a slowpellicle formation-phase with only minor changes in composition and thickness between 30 and 120 min (11).
While several studies have looked at different aspects of the enamel pellicle, a conclusive and state-of-the-art overview of the different components of the dental pellicle is missing. Due to the advancement in analytical techniques for studying nanoscale objects and processes, there has been an increasing number of new papers in the field of pellicle research, especially in recent years. Therefore, the aim of this review article is to give an updated overview on the composition of the dental pellicle and to identify future research areas.
2. Materials and methods
For this review, the PubMed-database was searched using the following search-terms: “pellicle AND (teeth OR tooth OR enamel OR dentin) AND composition”. The literature search was completed on March 31, 2023. Relevant studies were selected through independent review by JE and FM. Studies with a general focus on the composition of the dental pellicle were included. Exclusion criteria were studies where the pellicle was investigated on restorative materials, or where the effect of different external ingredients on the pellicle was investigated. Studies published before 1990 were excluded.
2.1. Analytical techniques
Several analytical techniques can be used for the research on the dental pellicle. The techniques are presented in short in Table 1. Techniques, after collecting the samples, are used to 1. separate the fractions, and 2. analyze the composition of the respective fractions.
3. Results
The composition of the dental pellicle can be described as highly diverse. The pellicle contains carbohydrates, fatty acids and proteins (Figure 1). Sources of the same are not only saliva, but also diet and also of microbial origin. The included studies can be separated into studies focusing on the carbohydrates, fatty acids and proteins. Table 2 gives an overview of the compounds that have been previously identified in the pellicle.
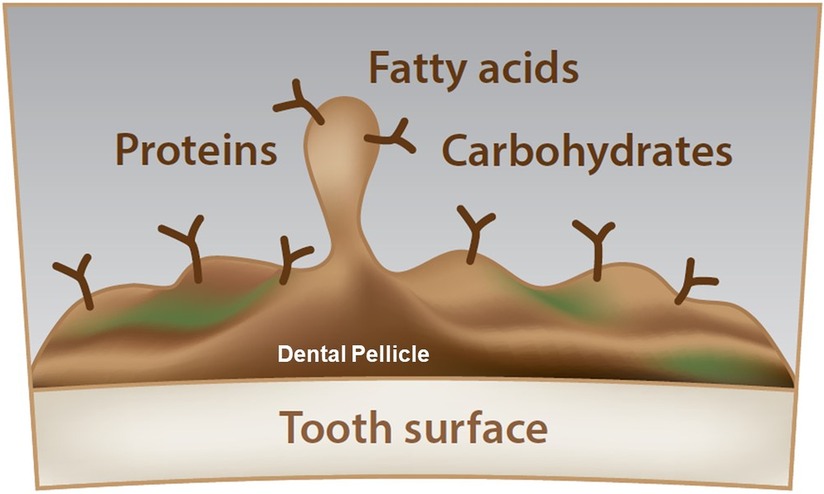
Figure 1. Schematic overview of the dental pellicle. Due to the high complexity, carbohydrates, fatty acids, and proteins cannot be located. The dental pellicle is a thin layer, covering the tooth surface while being the starting point for the bacterial adhesion and biofilm buildup.
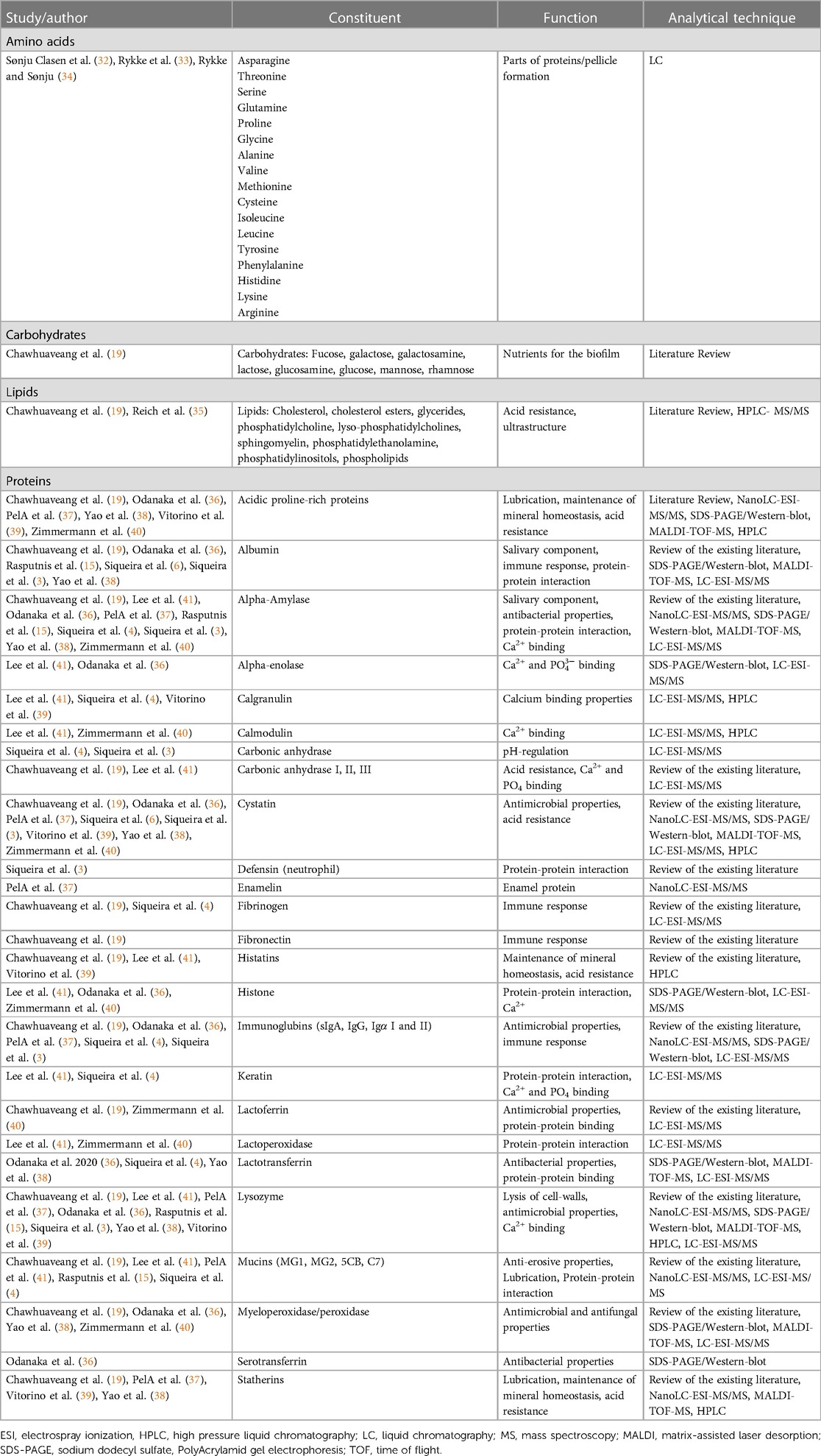
Table 2. Constituents of the dental pellicle and their functions (alphabetical order of proteins and other components).
3.1. Carbohydrates
Carbohydrates of the dental pellicle are mainly derived from diet and microorganisms. However, the composition of carbohydrates is not well-studied. The main function of carbohydrates is as nutritional source for oral bacteria and the biofilm (19).
3.2. Fatty acids
Research on fatty acids in the whole dental pellicle is not well-established in the literature and limited to the characterization of fatty acid profiles of the dental pellicle. Triacylglycerols and phospholipids were found as major compounds. In contrast to this, glycolipids, cholesterol, and cholesterol esters were not found in the pellicle, and saliva (35, 42). Fatty acid profiles (types of fatty acids) of different subjects are comparable. An increase in the total amount of fatty acids can be found over time. However, the total amount of fatty acids is differing between individuals. On the basis of fatty acids, pellicle formation is a selective process, which is not directly correlating with the salivary profile (42).
3.3. Proteins
Characteristics of studies focusing on general composition give an overview of the pellicle composition. Rasputnis et al. found that the ultrastructure of the pellicle on dentin and enamel surfaces is comparable. However, as mostly enamel pellicle has been studied, a final statement on the comparability of the composition and properties of the dentin and enamel pellicle cannot be made (15). Chawhuaveang et al. describe the protective properties of the dental pellicle and gave additional insights into its calcium-binding properties that help protect teeth from demineralization. Another important aspect of the pellicle is serving as the binding site of bacteria to the teeth. With this, the pellicle also contributes to periodontal diseases, as the bacterial biofilm adhering to the teeth is a key-factor for periodontal infections. Insights on pellicle’s selective acid-transportation, which determines its caries-protective properties was given (19). It is important to note that the methodology of the pellicle collection influences the composition (3, 37). While former studies and their respective results are based on a gel-based approach (for protein-composition), more recent studies used gel-based techniques combined with mass spectrometry (MS) or immunologic methods (36, 37, 43–45). Odanaka et al, in addition, have investigated the origin of the proteins found in the pellicle. They are not only derived from salivary glands and saliva, but also from gingival crevicular fluid (36). Another factor influencing pellicle composition seems to be the location of sampling: Upper or lower jaw, anterior or posterior teeth, palatal, lingual or facial (46).
When investigating the dental pellicle, it is not only the location that plays an important role, but also the time point of sampling. The pellicle is fully formed after 120 min but is changing with a high dynamic in composition over time (41). Siqueira et al. found 130 proteins in all samples that were extracted from 3 individuals (4). The number of proteins found in the various studies differs from the respective methods that were used. Statherin, lysozyme, albumin, and amylase were intact proteins found in the study by Yao et al. (38). Interestingly, albumin can be found when sampled in vivo, but when the same saliva was formed in vitro on hydroxyapatite discs, albumin was observed in smaller amounts (47). Histatins, as protective proteins in the oral cavity, can also be found in the pellicle (5). Primary teeth and permanent teeth have a distinct pellicle characteristics: Pellicle from primary teeth forms slower and is thinner, without a globular second layer compared to the pellicle formed on teeth in the permanent dentition (32).
It seems that proteins are also denatured over time. Zimmermann et al. looked at the proteome and peptidome. It can be assumed that the peptidome is derived from the proteome. A clinical trial has shown a unique composition among all subjects (40). Mucins are also known to be part of the pellicle. Levels of mucins are impaired by hyposalivation and reduction of salivary flow (48). The proteolytic activity from different enzymes in the oral cavity seems to influence the formation and composition of the pellicle (39). Proteolysis of the salivary proteins for the formation of the pellicle does not seem to be a random process (49). Proteins differ between sampling sites in the oral cavity. Parotid saliva agglutinin, which is the main binding-site for Streptococcus mutans, was identified in the premolar region of the oral cavity (47). Glycosyltransferase, which is known as virulence factor for streptococci, can be identified in the pellicle (8). Diet, mainly sugar intake (amount and frequency) and possibly also bacterial biofilms have an influence on the pellicle formation and also on the protein degradation of the same (33, 34). In a 2-year in vivo study, Rykke et al. showed different amino acid profiles from saliva and the pellicle indicating that pellicle formation is a selective process (33).
4. Discussion
Several studies have investigated the different components of the dental pellicle (3, 19, 35, 48). However, most studies have been performed several years ago using different molecular technologies. These methods include the most commonly used technique for the identification of proteins, namely western blot (21, 36, 47, 50–52). More sophisticated approaches, such as liquid chromatography (LC) combined with mass spectroscopy (MS), or high-pressure-liquid-chromatography (HPLC) were also used alone or even in combinations (4, 9, 20, 35–41, 53). From those approaches, the composition was found to be more complex compared to that found when using western blot alone. Furthermore, up-to-date technologies for proteomics will probably lead to a deeper insight into the dental pellicle. Another important aspect is the integration of microscopic techniques for the characterization of the dental pellicle. Atomic force microscopy is one of those techniques that might help getting more insights into the dental pellicle-characteristics (54). Additionally, lab-research could provide more information on the function and interaction of the identified proteins. Future research needs also to focus on the variation in pellicle composition with sampling sites, ethnicity, age, gender, known systemic diseases, and others. These differences in composition of pellicle from sampling sites might be attributed to the variation in the composition of the saliva, including the proteins, from different salivary glands (36, 46).
It is established that all known amino acids are present in the dental pellicle (33, 34). The ratio of those varies between the studies indicating individual profiles of the proteins. The most often identified proteins in the pellicle are amylase, lysozyme, statherins, mucins, immunoglobulins, peroxidase, cystatins, albumin, and proline-rich proteins (Table 2). Most proteins have the ability to bind Ca2+, leading to the assumption that the pellicle plays a protective role against tooth demineralization (3, 19, 36). The pellicle can be a reservoir for calcium ions protecting the teeth from attack by acid from bacteria leading to dental caries or from other sources leading dental erosion. Therefore, it is important not to inactivate the Ca2+ but supplement the pellicle. Studies have shown the benefits of adding Ca2+ for oral health, especially protecting teeth from demineralization (55–58).
Compared to the studies investigating proteins, fewer studies have been published on the presence and characterization of lipids or carbohydrates in the dental pellicle (3, 19, 20, 35, 42). There is limited evidence that the pellicle might also be a reservoir for carbohydrates (i.e., sugars). While some of those sugars (e.g., xylitol) might be protective, others might enhance the caries-process, namely glucose or lactose (59, 60). It can be assumed that carbohydrates content of the pellicle are mainly derived from diet and will be metabolized by oral bacteria for their energy supply. Lipids might improve the acid resistance of the pellicle and change the microstructure of the pellicle (19, 35, 42, 61).
This review gives an overview of the pellicle composition. However, the structure and interaction of individual pellicle constituents have not been investigated so far. From a biological point of view, the interaction of components within the pellicle and how these interactions may affect its three-dimensional structure is important, as this might help to understand how the pellicle can be modified to protect the teeth from acids and bacterial adhesion. By reducing bacterial adhesion, risk for dental caries and gum diseases can be reduced (62–64). Interestingly, calcium phosphates lead to a reduction of biofilm formation. Hydroxyapatite particles, which have been shown to prevent dental caries in several clinical trials (65–67), seem to reduce the biofilm formation to a higher extend compared to amorphous calcium phosphates in combination with casein phosphopeptides (62, 68).
From a scientific point of view, the dental pellicle needs to be characterized more thoroughly in a variety of ways. Those include a detailed description of its three-dimensional structure, the interaction of proteins in the pellicle, the interaction of the pellicle with bacteria and fungi during biofilm formation, but also the role of carbohydrates and lipids. While many proteins, carbohydrates and others have been identified as part of the pellicle, their involvement in the remineralization process remains mostly unclear. Casein has been identified as important calcium-binding protein, and others also have calcium-binding abilities, but their specific roles in remineralization need further investigation. Additionally, proteins might penetrate small cracks and demineralized structures, where they could bind calcium and thus enhance remineralization in those non-intact areas.
Future research needs to focus not only on these questions, but also on the modification of the pellicle. Studies have shown that different active agents can be incorporated into the pellicle or alter the biofilm adhesion. Those are hydroxyapatite particles, but also polyphenols (12, 62, 69–72). It is important to keep the oral homeostasis intact and to achieve this, biomimetic approaches are preferred (73–75). This means that biomimetic remineralization using calcium phosphates should be enhanced when modifying the pellicle (57, 58). Additionally, bacterial adhesion needs to be reduced and acidic resistance needs to be enhanced (56, 76). While the pellicle is known to show subtle variations in structure and composition between individuals, there may be beneficial modifications to the pellicle that can be applied uniformly to every single individual with consistent results. This, however, requires further investigation.
5. Conclusions
This review has summarized the composition of dental pellicle. While many proteins have been identified so far, further research needs to be performed especially on the intra-individual composition and the function of the proteins, lipids, and carbohydrates. Furthermore, modification of the pellicle to enhance its beneficial functions is possible, mainly with effective agents to protect teeth from acidic attacks or to inhibit bacterial adhesion. The research focus needs to be based on active biomimetics as they will be positively influencing oral homeostasis. In addition, future research using the most advanced analytical methods needs to focus on the involvement of the dental pellicle in the remineralization process.
Author contributions
JE: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. BG: Conceptualization, Methodology, Writing – review & editing. BA: Conceptualization, Methodology, Writing – review & editing. ES: Methodology, Writing – review & editing. FM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by Dr. Kurt Wolff GmbH & Co. KG, Bielefeld, Germany.
Acknowledgments
The authors would like to kindly thank Markus Morick for preparing the Figure 1.
Conflict of interest
JS, ES, and FM are employees of Dr. Kurt Wolff GmbH & Co KG.
The remaining authors declare that the research was conducted in the absence of any commercial of financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Hannig M, Hannig C. Der initiale orale biofilm – pathogen oder protektiv? Oralprophylaxe. (2007) 29:73–82.
3. Siqueira WL, Custodio W, McDonald EE. New insights into the composition and functions of the acquired enamel pellicle. J Dent Res. (2012) 91(12):1110–8.23018818
4. Siqueira WL, Helmerhorst EJ, Zhang W, Salih E, Oppenheim FG. Acquired enamel pellicle and its potential role in oral diagnostics. Ann N Y Acad Sci. (2007) 1098:504–9. doi: 10.1196/annals.1384.023
5. Siqueira WL, Margolis HC, Helmerhorst EJ, Mendes FM, Oppenheim FG. Evidence of intact histatins in the in vivo acquired enamel pellicle. J Dent Res. (2010) 89(6):626–30. doi: 10.1177/0022034510363384
6. Siqueira WL, Zhang W, Helmerhorst EJ, Gygi SP, Oppenheim FG. Identification of protein components in in vivo human acquired enamel pellicle using LC-ESI-MS/MS. J Proteome Res. (2007) 6(6):2152–60. doi: 10.1021/pr060580k
7. Hannig C, Hannig M, Kensche A, Carpenter G. The mucosal pellicle – an underestimated factor in oral physiology. Arch Oral Biol. (2017) 80:144–52. doi: 10.1016/j.archoralbio.2017.04.001
8. Hannig C, Ruggeri A, Al-Khayer B, Schmitz P, Spitzmüller B, Deimling D, et al. Electron microscopic detection and activity of glucosyltransferase B, C, and D in the in situ formed pellicle. Arch Oral Biol. (2008) 53(11):1003–10. doi: 10.1016/j.archoralbio.2008.04.005
9. Trautmann S, Barghash A, Fecher-Trost C, Schalkowsky P, Hannig C, Kirsch J, et al. Proteomic analysis of the initial oral pellicle in caries-active and caries-free individuals. Proteomics Clin Appl. (2018) 4:e1800143.30548171
10. Trautmann S, Künzel N, Fecher-Trost C, Barghash A, Dudek J, Flockerzi V, et al. Is the proteomic composition of the salivary pellicle dependent on the substrate material? Proteomics Clinical Applications. (2022) 16(3):e2100109. doi: 10.1002/prca.202100109
11. Güth-Thiel S, Kraus-Kuleszka I, Mantz H, Hoth-Hannig W, Hähl H, Dudek J, et al. Comprehensive measurements of salivary pellicle thickness formed at different intraoral sites on si wafers and bovine enamel. Colloids Surf B: Biointerfaces. (2019) 174:246–51. doi: 10.1016/j.colsurfb.2018.11.020
12. Kensche A, Durasch A, Konig B, Henle T, Hannig C, Hannig M. Characterization of the in situ pellicle ultrastructure formed under the influence of bovine milk and milk protein isolates. Arch Oral Biol. (2019) 104:133–40. doi: 10.1016/j.archoralbio.2019.05.021
13. Sterzenbach T, Helbig R, Hannig C, Hannig M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin Oral Investig. (2020) 24(12):4237–60. doi: 10.1007/s00784-020-03646-1
14. Trautmann S, Künzel N, Fecher-Trost C, Barghash A, Schalkowsky P, Dudek J, et al. Deep proteomic insights into the individual short-term pellicle formation on enamel-an in situ pilot study. Proteom Clin Appl. (2020) 14(6):e2070054. doi: 10.1002/prca.202070054
15. Rasputnis W, Schestakow A, Hannig M. The dentin pellicle – a neglected topic in dental research. Arch Oral Biol. (2021) 129:105212. doi: 10.1016/j.archoralbio.2021.105212
16. Amaechi BT, Higham SM, Edgar WM, Milosevic A. Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion. J Dent Res. (1999) 78(12):1821–8. doi: 10.1177/00220345990780120901
17. Young A, Smistad G, Karlsen J, Rolla G, Rykke M. Zeta potentials of human enamel and hydroxyapatite as measured by the coulter DELSA 440. Adv Dent Res. (1997) 11(4):560–5. doi: 10.1177/08959374970110042501
18. Jensen JL, Lamkin MS, Oppenheim FG. Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J Dent Res. (1992) 71:1569–76. doi: 10.1177/00220345920710090501
19. Chawhuaveang DD, Yu OY, Yin IX, Lam WY, Mei ML, Chu CH. Acquired salivary pellicle and oral diseases: a literature review. J Dent Sci. (2021) 16(1):523–9. doi: 10.1016/j.jds.2020.10.007
20. Schulz A, Lang R, Behr J, Hertel S, Reich M, Kümmerer K, et al. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci Rep. (2020) 10(1):697. doi: 10.1038/s41598-020-57531-8
21. Vacca Smith AM, Bowen WH. In situ studies of pellicle formation on hydroxyapatite discs. Arch Oral Biol. (2000) 45(4):277–91. doi: 10.1016/S0003-9969(99)00141-7
22. Zhang YF, Li DY, Yu JX, He HT. On the thickness and nanomechanical properties of salivary pellicle formed on tooth enamel. J Dent. (2016) 55:99–104. doi: 10.1016/j.jdent.2016.10.009
23. Bikker FJ, Cukkemane N, Nazmi K, Veerman EC. Identification of the hydroxyapatite-binding domain of salivary agglutinin. Eur J Oral Sci. (2013) 121(1):7–12. doi: 10.1111/eos.12013
24. Lubarsky GV, D’Sa RA, Deb S, Meenan BJ, Lemoine P. The role of enamel proteins in protecting mature human enamel against acidic environments: a double layer force spectroscopy study. Biointerphases. (2012) 7(1–4):14. doi: 10.1007/s13758-011-0014-6
25. Schestakow A, Bauer C, Hannig M. Ultrastructure of the dentin pellicle and the impact of erosion. Caries Res. (2022) 56:488–95.36310018
26. Hara AT, Ando M, González-Cabezas C, Cury JA, Serra MC, Zero DT. Protective effect of the dental pellicle against erosive challenges in situ. J Dent Res. (2006) 85(7):612–6. doi: 10.1177/154405910608500706
27. Meyer F, Enax J, Epple M, Amaechi BT, Simader B. Cariogenic biofilms: development, properties, and biomimetic preventive agents. Dent J. (2021) 9(8):88. doi: 10.3390/dj9080088
28. Delvar A, Lindh L, Arnebrant T, Sotres J. Interaction of polyelectrolytes with salivary pellicles on hydroxyapatite surfaces under erosive acidic conditions. ACS Appl Mater Interfaces. (2015) 7(38):21610–8. doi: 10.1021/acsami.5b07118
29. Bruvo M, Moe D, Kirkeby S, Vorum H, Bardow A. Individual variations in protective effects of experimentally formed salivary pellicles. Caries Res. (2009) 43(3):163–70. doi: 10.1159/000213887
30. Baumann T, Bereiter R, Lussi A, Carvalho T. The effect of different salivary calcium concentrations on the erosion protection conferred by the salivary pellicle. Sci Rep. (2017) 7:15194.
31. Zeng Q, Zheng J, Yang D, Tang Y, Zhou Z. Effect of calcium ions on the adsorption and lubrication behavior of salivary proteins on human tooth enamel surface. J Mech Behav Biomed Mater. (2019) 98:172–8. doi: 10.1016/j.jmbbm.2019.06.019
32. Sønju Clasen AB, Hannig M, Skjørland K, Sønju T. Analytical and ultrastructural studies of pellicle on primary teeth. Acta Odontol Scand. (1997) 55(6):339–43. doi: 10.1080/00006357.1997.11978411
33. Rykke M, Sönju T, Rölla G. Interindividual and longitudinal studies of amino acid composition of pellicle collected in vivo. Scand J Dent Res. (1990) 98(2):129–34.1971457
34. Rykke M, Sönju T. Amino acid composition of acquired enamel pellicle collected in vivo after 2 h and after 24 h. Scand J Dent Res. (1991) 99(6):463–9.1684876
35. Reich M, Hannig C, Hannig M, Kümmerer K, Kensche A. The lipid composition of the in situ pellicle. Arch Oral Biol. (2022) 142:105493. doi: 10.1016/j.archoralbio.2022.105493
36. Odanaka H, Obama T, Sawada N, Sugano M, Itabe H, Yamamoto M. Comparison of protein profiles of the pellicle, gingival crevicular fluid, and saliva: possible origin of pellicle proteins. Biol Res. (2020) 53(1):3. doi: 10.1186/s40659-020-0271-2
37. PelÁ VT, Ventura TMO, Buzalaf MAR. Optimizing the formation of the acquired enamel pellicle in vitro for proteomic analysis. J Appl Oral Sci. (2020) 28:e20200189. doi: 10.1590/1678-7757-2020-0189
38. Yao Y, Grogan J, Zehnder M, Lendenmann U, Nam B, Wu Z, et al. Compositional analysis of human acquired enamel pellicle by mass spectrometry. Arch Oral Biol. (2001) 46(4):293–303. doi: 10.1016/S0003-9969(00)00134-5
39. Vitorino R, Calheiros-Lobo MJ, Duarte JA, Domingues PM, Amado FM. Peptide profile of human acquired enamel pellicle using MALDI tandem MS. J Sep Sci. (2008) 31(3):523–37. doi: 10.1002/jssc.200700486
40. Zimmerman JN, Custodio W, Hatibovic-Kofman S, Lee YH, Xiao Y, Siqueira WL. Proteome and peptidome of human acquired enamel pellicle on deciduous teeth. Int J Mol Sci. (2013) 14(1):920–34. doi: 10.3390/ijms14010920
41. Lee YH, Zimmerman JN, Custodio W, Xiao Y, Basiri T, Hatibovic-Kofman S, et al. Proteomic evaluation of acquired enamel pellicle during in vivo formation. PloS one. (2013) 8(7):e67919. doi: 10.1371/journal.pone.0067919
42. Reich M, Kümmerer K, Al-Ahmad A, Hannig C. Fatty acid profile of the initial oral biofilm (pellicle): an in-situ study. Lipids. (2013) 48(9):929–37. doi: 10.1007/s11745-013-3822-2
43. Lindh L, Aroonsang W, Sotres J, Arnebrant T. Salivary pellicles. Monogr Oral Sci. (2014) 24:30–9. doi: 10.1159/000358782
44. Li J, Helmerhorst EJ, Corley RB, Luus LE, Troxler RF, Oppenheim FG. Characterization of the immunologic responses to human in vivo acquired enamel pellicle as a novel means to investigate its composition. Oral Microbiol Immunol. (2003) 18(3):183–91. doi: 10.1034/j.1399-302X.2003.00065.x
45. Lie T. Scanning and transmission electron microscope study of pellicle morphogenesis. Scand J Dent Res. (1977) 85(4):217–31.266749
46. Ventura T, Cassiano LPS, Souza ESCM, Taira EA, Leite AL, Rios D, et al. The proteomic profile of the acquired enamel pellicle according to its location in the dental arches. Arch Oral Biol. (2017) 79:20–9. doi: 10.1016/j.archoralbio.2017.03.001
47. Carlén A, Börjesson AC, Nikdel K, Olsson J. Composition of pellicles formed in vivo on tooth surfaces in different parts of the dentition, and in vitro on hydroxyapatite. Caries Res. (1998) 32(6):447–55. doi: 10.1159/000016486
48. Faruque M, Wanschers M, Ligtenberg AJ, Laine ML, Bikker FJ. A review on the role of salivary MUC5B in oral health. J Oral Biosci. (2022) 64(4):392–9. doi: 10.1016/j.job.2022.09.005
49. Lamkin MS, Migliari D, Yao Y, Troxler RF, Oppenheim FG. New in vitro model for the acquired enamel pellicle: pellicles formed from whole saliva show inter-subject consistency in protein composition and proteolytic fragmentation patterns. J Dent Res. (2001) 80(1):385–8. doi: 10.1177/00220345010800011501
50. Gibbins HL, Proctor GB, Yakubov GE, Wilson S, Carpenter GH. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis. (2014) 20(7):707–13. doi: 10.1111/odi.12194
51. Gibbins HL, Yakubov GE, Proctor GB, Wilson S, Carpenter GH. What interactions drive the salivary mucosal pellicle formation? Colloids and surfaces B. Biointerfaces. (2014) 120(100):184–92. doi: 10.1016/j.colsurfb.2014.05.020
52. Mutahar M, Bartlett D, Carpenter G, Moazzez R. Proteins from whole mouth saliva mediate greater protection against severe erosive tooth wear than proteins from parotid saliva using an in vitro model. J Dent. (2020) 95:103319. doi: 10.1016/j.jdent.2020.103319
53. Cabiddu G, Maes P, Hyvrier F, Olianas A, Manconi B, Brignot H, et al. Proteomic characterization of the mucosal pellicle formed in vitro on a cellular model of oral epithelium. J Proteomics. (2020) 222:103797. doi: 10.1016/j.jprot.2020.103797
54. Hannig M, Herzog S, Willigeroth SF, Zimehl R. Atomic force microscopy study of salivary pellicles formed on enamel and glass in vivo. Colloid Polym Sci. (2001) 279(5):479–83. doi: 10.1007/s003960000478
55. Enax J, Amaechi BT, Farah R, Liu JA, Schulze zur Wiesche E, Meyer F. Remineralization strategies for teeth with molar incisor hypomineralization (MIH): a literature review. Dent J. (2023) 11(3):80. doi: 10.3390/dj11030080
56. Enax J, Fabritius HO, Fabritius-Vilpoux K, Amaechi BT, Meyer F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care−state of the art. Open Dent J. (2019) 13:274–87. doi: 10.2174/1874210601913010274
57. Meyer F, Amaechi BT, Fabritius HO, Enax J. Overview of calcium phosphates used in biomimetic oral care. Open Dent J. (2018) 12:406–23. doi: 10.2174/1874210601812010406
58. Cieplik F, Rupp CM, Hirsch S, Muehler D, Enax J, Meyer F, et al. Ca2+ release and buffering effects of synthetic hydroxyapatite following bacterial acid challenge. BMC oral Health. (2020) 20(1):85. doi: 10.1186/s12903-020-01080-z
59. Enax J, Amaechi BT, Schulze zur Wiesche E, Meyer F. Overview on adjunct ingredients used in hydroxyapatite-based oral care products. Biomimetics. (2022) 7(4):250. doi: 10.3390/biomimetics7040250
60. Meyer F, Enax J. Early childhood caries: epidemiology, aetiology, and prevention. Int J Dent. (2018) 2018:7. doi: 10.1155/2018/1415873
61. Kensche A, Reich M, Kümmerer K, Hannig M, Hannig C. Lipids in preventive dentistry. Clin Oral Investig. (2013) 17(3):669–85. doi: 10.1007/s00784-012-0835-9
62. Kensche A, Holder C, Basche S, Tahan N, Hannig C, Hannig M. Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ. Arch Oral Biol. (2017) 80:18–26. doi: 10.1016/j.archoralbio.2017.03.013
63. Schlagenhauf U, Jakob L, Eigenthaler M, Segerer S, Jockel-Schneider Y, Rehn M. Regular consumption of lactobacillus reuteri-containing lozenges reduces pregnancy gingivitis: an RCT. J Clin Periodontol. (2016) 43(11):948–54. doi: 10.1111/jcpe.12606
64. Meyer F, Enax J. Hydroxyapatite in oral biofilm management. Eur J Dent. (2019) 13:287–90. doi: 10.1055/s-0039-1695657
65. Paszynska E, Pawinska M, Enax J, Meyer F, Schulze zur Wiesche E, May T, et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: a 18 months double-blinded randomized clinical trial. Front Public Health. (2023) 11.37533523
66. Limeback H, Enax J, Meyer F. Biomimetic hydroxyapatite and caries prevention: a systematic review and meta-analysis. Can J Dent Hyg. (2021) 55:148–59.34925515
67. Meyer F, Enax J, Amaechi BT, Limeback H, Fabritius H-O, Ganss B, et al. Hydroxyapatite as remineralization agent for children’s dental care. Front Dent Med. (2022) 3:859560. doi: 10.3389/fdmed.2022.859560
68. Grychtol S, Basche S, Hannig M, Hannig C. Effect of CPP/ACP on initial bioadhesion to enamel and dentin in situ. Sci World J. (2014) 2014:512682. doi: 10.1155/2014/512682
69. Kensche A, Pötschke S, Hannig C, Richter G, Hoth-Hannig W, Hannig M. Influence of calcium phosphate and apatite containing products on enamel erosion. Scientific World J. (2016) 2016:1–12. doi: 10.1155/2016/7959273
70. Flemming J, Meyer-Probst CT, Speer K, Kölling-Speer I, Hannig C, Hannig M. Preventive applications of polyphenols in dentistry – a review. Int J Mol Sci. (2021) 22(9):4892. doi: 10.3390/ijms22094892
71. Schestakow A, Nekrashevych Y, Hoth-Hannig W, Hannig M. Influence of periodic polyphenol treatment on the anti-erosive potential of the acquired enamel pellicle—a qualitative exploratory study. J Dent. (2022) 124:104236. doi: 10.1016/j.jdent.2022.104236
72. Schestakow A, Meyer-Probst CT, Hannig C, Hannig M. Prevention of dental biofilm formation with polyphenols: a systematic review. Planta Med. (2022) 89:1026–33.36343637
73. Drago-Serrano ME, Campos-Rodriguez R, Carrero JC, de la Garza M. Lactoferrin: balancing ups and downs of inflammation due to microbial infections. Int J Mol Sci. (2017) 18(3):501.28257033
74. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. (2015) 15(1):30–44. doi: 10.1038/nri3785
75. Meyer F, Enax J. Die mundhöhle als Ökosystem. Biol Unserer Zeit. (2018) 48:62–8. doi: 10.1002/biuz.201810641
Keywords: dental pellicle, proteins, saliva, biofilm, teeth, hydroxyapatite
Citation: Enax J, Ganss B, Amaechi BT, Schulze zur Wiesche E and Meyer F (2023) The composition of the dental pellicle: an updated literature review. Front. Oral. Health 4:1260442. doi: 10.3389/froh.2023.1260442
Received: 26 July 2023; Accepted: 26 September 2023;
Published: 12 October 2023.
Edited by:
Mario Jr Taba, University of São Paulo, BrazilReviewed by:
Marlise Inez Klein, State University of Campinas, BrazilSebastian Aguayo, Pontifical Catholic University of Chile, Chile
© 2023 Enax, Ganss, Amaechi, Schulze zur Wiesche and Meyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frederic Meyer ZnJlZGVyaWMubWV5ZXJAZHJ3b2xmZmdyb3VwLmNvbQ==
 Joachim Enax
Joachim Enax Bernhard Ganss
Bernhard Ganss Bennett T. Amaechi
Bennett T. Amaechi Erik Schulze zur Wiesche1
Erik Schulze zur Wiesche1 Frederic Meyer
Frederic Meyer