
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oral. Health, 09 October 2023
Sec. Oral Cancers
Volume 4 - 2023 | https://doi.org/10.3389/froh.2023.1239961
This article is part of the Research TopicReviews in the Prevention and Early Detection of Oral CancersView all 5 articles
 Kanokporn Bhalang1*
Kanokporn Bhalang1* Kittipong Danuthai2
Kittipong Danuthai2
The aims of this study were to investigate the sensitivity and specificity of toluidine blue and/or vinegar in oral cancer screening and to examine the correlation between clinical screening using toluidine blue and vinegar and the expression of the tumor marker p53 and proliferation marker Ki67, respectively. The study consisted of 87 patients with suspected oral squamous cell carcinoma lesions. Toluidine blue and/or vinegar were applied to the lesions, followed by biopsies. The tissues were diagnosed histopathologically and underwent immunohistochemical process for p53 or Ki67. The results revealed that the sensitivity and specificity of oral cancer screening using toluidine blue were 93% and 46%, respectively; whereas the sensitivity and specificity using vinegar were 85% and 81%, respectively. A statistically significant correlation between the use of vinegar and the expression of Ki67 (p = 0.019) was observed. Although there was a difference in the expression of p53 between specimens that were positive and negative to toluidine blue, the correlation did not reach a significant level. Based on the results from this study, vinegar has a lower sensitivity than toluidine blue but a higher specificity for oral cancer screening. The results of the clinical screening using vinegar correlated with the expression of Ki67 at the cellular level.
Oral cancer is a global health problem (1). Oral squamous cell carcinoma (OSCC) is the most common cancer occurring in the oral cavity (2). In Thailand, the current five-year survival rate of 20%–30% is quite low (3). Early diagnosis is very important and can lead to improved survival rates. One diagnostic technique that is widely used internationally is the application of toluidine blue to suspected lesions (4, 5). The proposed mechanism of toluidine blue in early cancer detection is that toluidine blue is taken up by dysplastic cells, which have an increased density of nuclear material (6). Another potential early detection method is the use of 5% acetic acid, which has been utilized for cervical and oral cancer screening (7). These developments are more distinct in cancerous epithelium because it has high nuclear and protein concentration (9). A clinical screening technique would be more trustworthy if it correlates with cellular markers used for cancer detection. Several investigators suggest that protein p53, the product of a tumor suppressor gene, is one of the most interesting candidates for oral cancer detection (10, 11). p53 functions as a transcription factor controlling the cell cycle and the process of apoptosis (12), and disruptions in these result in cancer development. Mutation of p53 changes the property of p53 protein resulting in its accumulation in the nucleus (13). A study has reported that toluidine blue positive cells have an allelic loss at chromosome 17p, which is the p53 locus (14). It was revealed that p53 gene mutates late in carcinogenesis and could be associated with the invasive phenotype of oral squamous cell carcinoma (15). Thus correlating the expression of p53 expression and toluidine blue positive lesions might shed some light into the benefit of using the solution. Another marker to consider is Ki67, which functions to control cellular proliferation and is found only in proliferating cells (16). In normal epithelium Ki67 is found in the basal cell layer, but in malignant transforming tissue, Ki67 can be seen in every layer of the epithelium (17). The increase in number of genetic materials and protein in cancer tissues makes them positive to vinegar application can be confirmed by investigating the level of Ki67 protein. As the expression of Ki67 is significantly higher in tissues with poorer differentiated squamous cell carcinoma and more severe dysplasia (18), there is a prognostic value in using vinegar if it is correlated with the expression of Ki67.
Our previous study revealed that there was a difference in the expression of p53 in vinegar positive lesions (8). However, to the authors' knowledge, there has never been other studies that investigate the correlations between clinical screening of oral squamous cell carcinoma and immunohistochemically stained lesions. The aim of this study was to examine the application of toluidine blue and 5% acetic acid in the detection of oral premalignant lesions and to determine the association between clinical screening using toluidine blue and vinegar and the expression of p53 and Ki67, respectively.
Eighty-seven patients with a clinical diagnosis of precancerous lesions or oral squamous cell carcinoma were recruited to participate in our study. They were from Rajvithi Hospital and Faculty of Dentistry, Chulalongkorn University Hospital, Bangkok, Thailand. The study was performed with informed consent and protocols approved by the Committees on Investigations Involving Human Subjects of the institutions.
An oral medicine specialist recorded clinical findings, took pictures of the lesions, and picked the areas to be examined. Patients were then randomized into three interventions. Toluidine blue application was conducted on 33 patients. Five percent acetic acid (vinegar) application was conducted on a second group of 30 patients. Both toluidine blue and vinegar were applied to a third group of 24 patients.
For the application of toluidine blue, a cotton bud soaked in 1% acetic acid was used to clean the lesion prior to the application of toluidine blue with a different cotton tip for 30 s. Subsequently, a third cotton bud soaked with 1% acetic acid was used to remove any excess toluidine blue on the lesion. The patient then rinsed out their mouth. A positive lesion was the one which color changed to blue, while a negative lesion was with no change. The area was then photographed and an incisional biopsy was performed at the blue-stained area.
For acetic acid application, a gauze soaked with vinegar was applied to a clean and dry lesion for one minute. The researcher noted the changes and took picture of the lesion. A positive finding was designated when a lesion changed to opaque white. When a lesion did not change or changed to transparent white, a negative finding was indicated. An incisional biopsy was performed at the area that had turned opaque white.
For the 24 patients to which both substances were applied, application of vinegar was conducted first, followed by the application of toluidine blue. A positive finding was indicated for a lesion with color changed to both opaque white and blue, while a negative finding was a lesion not demonstrating these combined changes. An incisional biopsy was performed at the area that turned opaque white and had stained blue. If the areas that had stained blue and turned opaque white were not coincident, incisional biopsies were performed at both areas. If the lesion did not stain or change color, a biopsy was performed at the central area of the lesion.
The specimen was routinely processed for histology, and sections were cut and then stained with hematoxylin and eosin for histopathological (final) diagnosis. For tissues obtained from patients receiving toluidine blue application, the next tissue section was used for the immunohistochemical study of tumor marker, p53 (19). For tissues obtained from patients receiving vinegar application, the next tissue section was used for the immunohistochemical study of proliferation marker, Ki67. A monoclonal antibody, anti-Ki67 (MIB 1, diluted 1:100; Dako, Denmark) was utilized as the primary antibody. The Envision plus kit (Dako, Denmark) was used for secondary antibody. The reaction was revealed by using 0.03% diaminobenzidine (DAB) solution. The Ki67 positive cells were counted under a light microscope at 400× magnification. The researcher picked three areas on each section for investigation. The cells were quantified by two researchers and then averaged. Positive controls were sections with known Ki67 overexpression. The negative control was performed with no primary antibody. The tissues from the 24 patients who had both substances applied on the lesions were not included in the immunohistochemical analysis.
Sensitivity and specificity of toluidine blue and/or vinegar for oral cancer detection were calculated. The correlation between the results of the vinegar application and the final diagnoses was determined using Fisher's exact test. Differences in the percentage of p53/Ki67 positive cells between toluidine blue/vinegar positive and negative lesions were compared using the Mann–Whitney test. A p-value less than 0.05 was considered statistically significant.
As seen in Table 1, our study comprised 87 subjects (56% male/44% female) ranging in age from 25 to 86 years (average age 61.5 ± 12.38 years). Sample lesions were most commonly found on the lateral tongue (31.0%), buccal mucosa (20.7%), and floor of mouth (18.4%). Sixty-seven lesions received toluidine blue application, of which 58 were positively stained. Of these, 52 of were disease positive by histopathological diagnosis (Table 2). Of the 9 lesions negative to toluidine blue staining, 4 received a positive histopathological diagnosis. Thus, the sensitivity of toluidine blue for oral cancer detection was 92.86%, and the specificity was 45.45%. Vinegar was applied to 83 lesions, with 56 lesions showing positive results (Table 3). Upon histopathological diagnosis, 56 of these were seen to be disease positive. Of the 27 lesions negative to 5% acetic acid treatment, 9 of these were deemed disease positive. These results indicated that the sensitivity of vinegar for oral cancer detection was 85.25%, while the specificity was 81.82%. We applied both toluidine blue and 5% acetic acid to 27 lesions, finding 23 lesions positive to both substances (Table 4). Twenty-two of these were positive for disease by histopathological diagnosis. Negative staining results were observed for 5 lesions, 1 of which was found to be disease positive. The sensitivity and specificity when using both reagents for oral cancer screening were thus 95.65% and 80%, respectively. Using Fisher's exact test to evaluate the relationship between the results of oral cancer screening using toluidine blue or vinegar and the results of the final diagnoses showed significant correlations (p = 0.000 and p = 0.004, respectively).
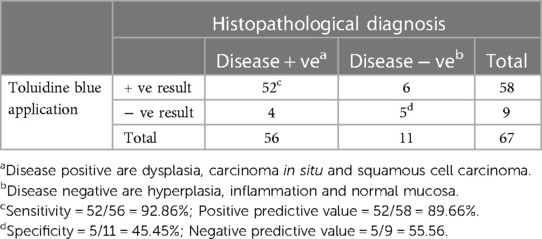
Table 2. Results from toluidine blue (TB) application and the histopathological diagnoses (67 specimens from 54 patients).
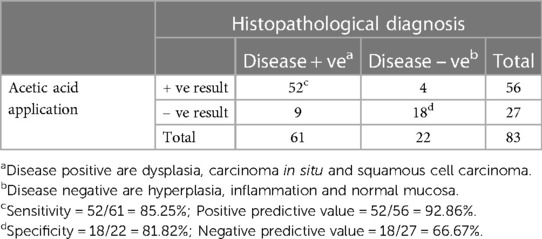
Table 3. Results from 5% acetic acid application and the histopathological diagnoses (83 specimens from 57 patients).
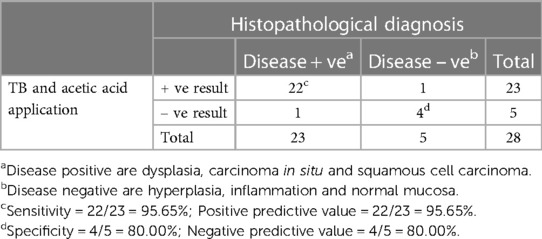
Table 4. Results from both toluidine blue (TB) and 5% acetic acid application and the histopathological diagnoses (28 specimens from 24 patients).
Figure 1 shows the results of the immunohistochemical staining for p53. Normal mucosa demonstrated little to no observable staining (Figure 1A). Scattered cells with positive staining were noted in samples of dysplasia (Figure 1B). Oral squamous cell carcinoma samples displayed many positively stained cells arranged in cord-like structures, suggesting a clonogenic origin (Figure 1C). Although the percentage of cells with p53 staining in all toluidine blue positive specimens at 4.93 ± 1.32% was higher than that of the toluidine blue negative specimens at1.49 ± 0.97%, the difference between these two groups was not significant (p = 0.198) (Table 5). The results of the immunohistochemical staining for Ki67 are seen in Figure 2. Scant, if any, staining was noted in normal oral mucosa (Figure 2A), Dysplastic samples, however, showed robust staining in the suprabasal epithelial layer (Figure 2B). In contrast, in samples of oral squamous cell carcinoma widespread positive staining was seen throughout the epithelial cell layers (Figure 2C). We found increased numbers of specimens positive for Ki67 staining as the histopathological diagnosis rose in severity from normal mucosa (1/33%), to epithelial hyperplasia and chronic inflammation (4/44.4%), through epithelial dysplasia and carcinoma in situ (9/81.8%), and oral squamous cell carcinoma (24/88.9%) (Table 6). The percentage of stained cells based on severity was seen to follow the same trend (Figure 3). The positive cells for Ki67 in all vinegar positive specimens was 3.23 ± 0.58% and that of the vinegar negative specimens was 1.45 ± 0.45%, with this difference being significant (p = 0.018) (Table 7).
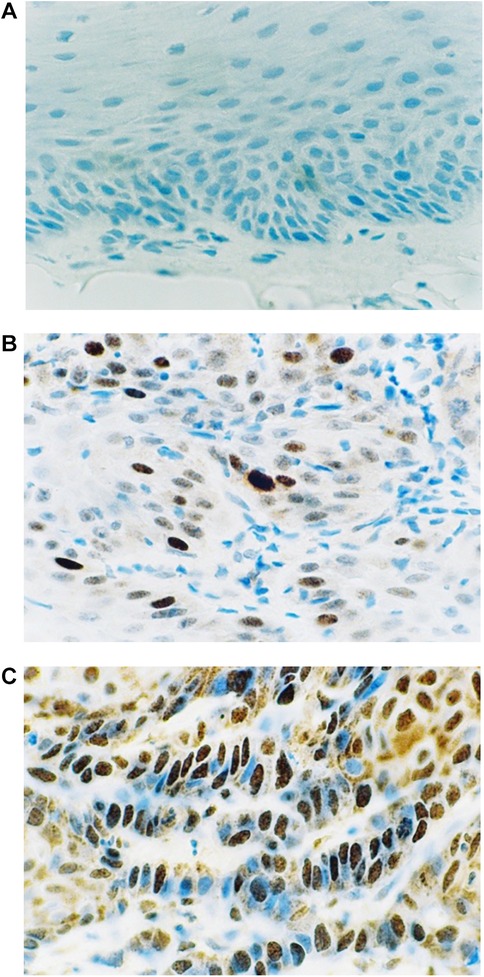
Figure 1. Immunohistochemical staining for p53. (A) Normal oral mucosa (B) epithelial dysplasia (C) oral squamous cell carcinoma.
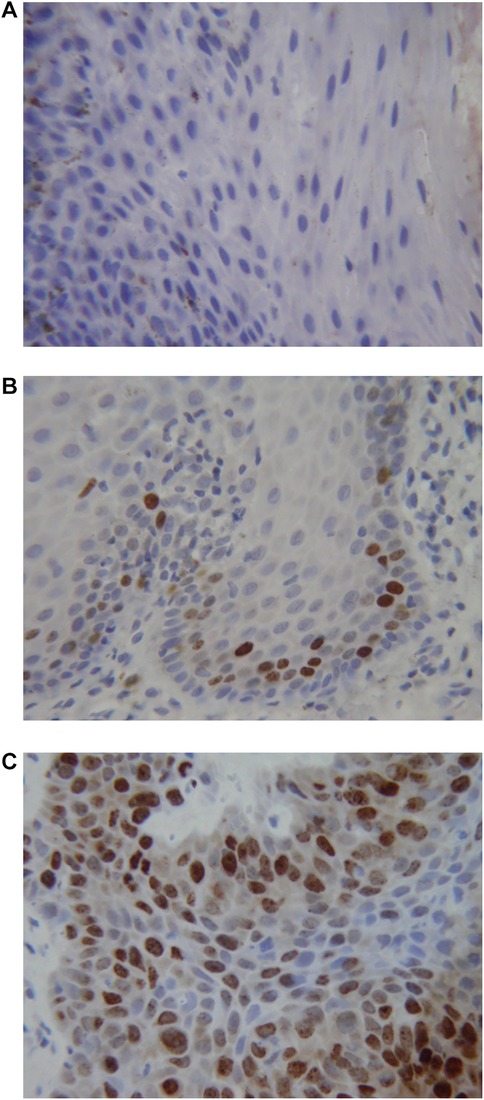
Figure 2. Immunohistochemical staining for Ki67. (A) Normal oral mucosa (B) epithelial dysplasia (C) oral squamous cell carcinoma.
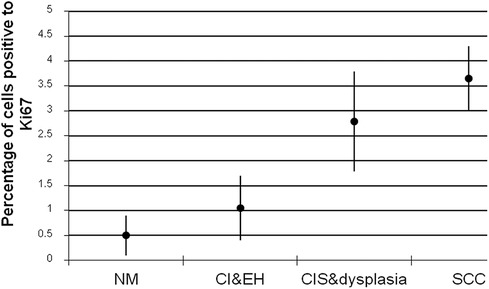
Figure 3. Percentage of cells positive to Ki67 antibody ± standard deviations in each group of specimens (NM, normal mucosa; CI, chronic inflammation; EH, epithelial hyperplasia; CIS, carcinoma in situ; SCC, squamous cell carcinoma).
The patients in our study were 61.8 ± 11.8 years old with a male to female ratio of 6 to 5 is comparable with that of other studies (1, 20). Betel nut chewing is still common in the Thai elderly female population (8). This is consistent with higher number of female patients in our study. The lesion location distribution is also comparable to those found in other studies (1, 20), with the exception of a higher percentage of lesions on the buccal mucosa (23%) in our study.
When we compared the sensitivity and specificity of toluidine blue in the present study to the sensitivity and specificity of toluidine blue used for oral cancer screening from other studies (21–24), our results had a comparable sensitivity, but a much lower specificity. The reported low specificity here could stem from the low number of control lesions and the inclusion of all blue stained lesions. It has been reported that pale royal blue stained lesions are unrelated to any histological feature (25). The sensitivity and specificity of the vinegar used for oral cancer screening were higher when compared with the use for cervical cancer detection (7, 9, 26–28). When both substances were used on the same lesion, the sensitivity reached 96% and the specificity was as high as 80%.
The association between oral cancer screening using toluidine blue or vinegar and final diagnoses indicated that toluidine blue and vinegar reacted better with malignant or dysplastic tissues than with normal tissues, warranting the application of toluidine blue and 5% acetic acid in oral cancer screening. As vinegar has a comparable sensitivity to toluidine blue but a higher specificity in oral cancer screening, future study evaluating the use of vinegar for the detection of oral premalignant lesions in rural communities will be of value.
Our results revealed that the observable transformations due to vinegar use in oral cancer screening and the results of final diagnoses were associated with a significant difference in Ki67 positive cells between tissues that were positive to vinegar screening and those that were not. Although the number of p53 stained cells were higher in specimens that were positive to toluidine blue than those negative to toluidine blue, the difference did not reach a significant level. This could stem from the difficulty in separating lesions that are truly positive to toluidine blue (dark royal blue stain) and false positives (pale blue stain) as stated by a recent publication that pale blue lesions have no histological significance (25). The number of cells positive to Ki67 grouped by the final diagnoses showed an association between the advancement of the lesions and the level of Ki67 antibody. The average percentage of cells positive to Ki67 antibody in specimens of oral squamous cell carcinoma, carcinoma in situ, and epithelial dysplasia was significantly higher than less affected specimens. This revealed that the immunohistopathological diagnosis of oral premalignant and malignant lesions are correlated with the number of cells positive to Ki67 antibody. Our findings further warrant the application of both p53 and Ki67 antibody reaction for oral cancer treatment planning and prognosis determination.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Committee on Investigations Involving Human Subjects of Faculty of Dentistry, Chulalongkorn University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KB conducted the clinical part of the research and wrote the manuscript. KD conducted the laboratory part of the research. All authors contributed to the article and approved the submitted version.
We would also like to extend our heartfelt gratitude to Anocha Suesuwan for her help with data collection and to Kevin Tompkins for his assistance in reviewing this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990s. Int J Cancer. (1999) 80:827–41. doi: 10.1002/(SICI)1097-0215(19990315)80:6%3C827::AID-IJC6%3E3.0.CO;2-P
3. Vatanasapt V, Sriamporn S. Oral cavity. In: Deerasamee S, Martin N, Sontipong S, editors. Cancer in Thailand vol II, 1992-1994, IARC technical report No. 34. Lyon: IARC (1999). p. 26–9.
4. Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol. (1998) 85:444–6. doi: 10.1016/S1079-2104(98)90071-3
5. Rosenberg D, Cretin S. Use of meta-analysis to evaluate tolonium chloride in oral cancer screening. J Oral Surg. (1989) 67:621–7. doi: 10.1016/0030-4220(89)90286-7
6. Rajmohan M. Assessment of oral mucosa in normal, precancer and cancer using chemiluminescent illumination, toluidine blue supravital staining and oral exfoliative cytology [Dissertation]. Chennai: Talmilnadu Dr. M.G. R Medical University (2005).
7. Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, et al. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol’s iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. (2003) 106:404–8. doi: 10.1002/ijc.11245
8. Bhalang K, Suesuwan A, Dhanuthai K, Sannikorn P, Luangjarmekorn L, Swasdison S. The application of acetic acid in the detection of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2008) 106(3):371–6. doi: 10.1016/j.tripleo.2008.01.017
9. Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, et al. Visual inspection of the uterine cervix after the application of acetic aicid in the detection of cervical carcinoma and its precursors. Cancer. (1998) 83:2150–6. doi: 10.1002/(SICI)1097-0142(19981115)83:10%3C2150::AID-CNCR13%3E3.0.CO;2-0
10. Rich AM, Kerdpon D, Reade PC. P53 expression in oral precancer and cancer. Aust Dent J. (1999) 44:103–5. doi: 10.1111/j.1834-7819.1999.tb00209.x
11. Kaur J, Srivastava A, Ralhan R. Overexpression of p53 protien in betel- and tobacco-related human oral dysplasia and squamous cell carcinoma in India. Int J Cancer. (1994) 58:340–5. doi: 10.1002/ijc.2910580305
12. Warnakulasuriya KAAS, Johnson NW. Expression of p53 mutant nuclear phosphoprotien in oral carcinoma and potentially malignant oral lesions. J Oral Pathol Med. (1992) 21:404–8. doi: 10.1111/j.1600-0714.1992.tb01028.x
13. Mielcarek-Kuchta D, Olofsson J, Golusinski W. P53, Ki67 and cyclin D1 as prognosticators of lymph node metastases in laryngeal carcinoma. Eur Arch Otorhinolaryngol. (2003) 260(10):549–54. doi: 10.1007/s00405-003-0651-6
14. Ibrahim SO, Lillehaug JR, Johannessen AC, Liavaag PG, Nilsen R, Vasstrand EN. Expression of biomarkers (p53, transforming growth factor alpha, epidermal growth factor receptor, c-erbB-2/neu and the proliferative cell nuclear antigen) in oropharyngeal squamous cell carcinomas. Oral Oncol. (1999) 35(3):302–13. doi: 10.1016/S1368-8375(98)00120-1
15. Shahnavaz SA, Regezi JA, Bradley G, Dubé ID, Jordan RC. P53 gene mutations in sequential oral epithelial dysplasias and squamous cell carcinomas. J Pathol. (2000) 190(4):417–22. doi: 10.1002/(SICI)1096-9896(200003)190:4%3C417::AID-PATH544%3E3.0.CO;2-G
16. Epstein JB, Zhang L, Poh C, Nakamura H, Berean K, Rosin M. Increased allelic loss in toluidine blue-positive oral premalignant lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2003) 95(1):45–50. doi: 10.1067/moe.2003.97
17. Hong MK, Laskin WB, Herman BE, Johnston MH, Vargo JJ, Steinberg SM, et al. Expansion of the Ki-67 proliferative compartment correlates with degree of dysplasia in Barrett’s esophagus. Cancer. (1995) 75(2):423–9. doi: 10.1002/1097-0142(19950115)75:2%3C423::AID-CNCR2820750202%3E3.0.CO;2-5
18. Takkem A, Barakat C, Zakaraia S, Zaid K, Najmeh J, Ayoub M, et al. Ki-67 prognostic value in different histological grades of oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pac J Cancer Prev. (2018) 19(11):3279–86. doi: 10.31557/APJCP.2018.19.11.3279
19. Kurokawa H, Zhang M, Matsumoto S, Yamashita Y, Tanaka T, Tomoyose T. The relationship of the histologic grade at the deep invasive front and the expression of Ki-67 antigen and p53 protein in oral squamous cell carcinoma. J Oral Pathol Med. (2005) 34(10):602–7. doi: 10.1111/j.1600-0714.2005.00358.x
20. Neville BW, Damm DD, Allen CM, Chi AC. Oral & maxillofacial pathology. 4th ed. Philadephia: W.B. Saunders (2015).
21. Mashberg A. Final evaluation of tolonium chloride rinse for screening of high-risk patients with asymptomatic squamous carcinoma. J Am Dent Assoc. (1983) 106:319–23. doi: 10.14219/jada.archive.1983.0063
22. Onofre MA, Sposto MR, Navarro CM, Scully C. Assessment of the blue toluidine stain in oral lesions with suspicious of malignancy. J Dent Res. (1995) 74:782.
23. Warnakulasuriya KAAS, Johnson NW. Sensitivity and specificity of OraScan toluidine blue mouthrinse in the detetion of oral cancer and precancer. J Oral Pathol Med. (1996) 25:97–103. doi: 10.1111/j.1600-0714.1996.tb00201.x
24. Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2001) 91:535–40. doi: 10.1067/moe.2001.112949
25. Gandolfo S, Pentenero M, Broccoletti R, Pagano M, Carrozzo M, Scully C. Toluidine blue uptake in potentially malignant oral lesions in vivo: clinical and histological assessment. Oral Oncol. (2006) 42:89–95. doi: 10.1016/j.oraloncology.2005.06.016
26. University of Zimbabwe/JHPIEGO Cervical Cancer Project. Visual inspection with acetic acid for cervical cancer screening: test qualities in a primary-care setting. Lancet. (1999) 353:869–73. doi: 10.1016/S0140-6736(98)07033-0
27. Belinson J, Pretorius R, Zhang W, Wu LY, Qiao YL, Elson P. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. (2001) 98:441–4.11530126
Keywords: oral squamous cell carcinoma, oral cancer, toluidine blue, acetic acid, p53, Ki67
Citation: Bhalang K and Danuthai K (2023) Expression of p53 in toluidine blue positive oral squamous cell carcinoma lesions and expression of Ki67 in vinegar positive oral squamous cell carcinoma lesions. Front. Oral. Health 4:1239961. doi: 10.3389/froh.2023.1239961
Received: 14 June 2023; Accepted: 20 September 2023;
Published: 9 October 2023.
Edited by:
Ricardo D. Coletta, Campinas State University, BrazilReviewed by:
Jayanth Kumar Vadivel, Saveetha Dental College and Hospitals, India© 2023 Bhalang and Danuthai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanokporn Bhalang a2Fub2twb3JuLmJoQGNodWxhLmFjLnRo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.