- School of Dentistry, University of Texas Health Science Center at Houston, Houston, TX, United States
Peripheral Calcifying Odontogenic Cyst (PCOC) is the extraosseous form of calcifying odontogenic cyst that is limited to peripheral soft tissue without bony involvement. This case report presents a case of PCOC manifested as a progressive growth of gingival mass in a young male treated with excisional biopsy. Histological examination confirmed diagnosis of PCOC with presence of characteristic ghost cells and sporadic calcifications. No recurrence of the lesion and no complication were noted at three-year follow-up. Review of available literature on PCOC noted a predilection of occurrence in the mandible (61%) and in the anterior area of the jaws (58%). Mean age of patients was 41.7 ± SD25.43 (7–83) and 95% CI [33.6, 49.8] yrs. Mean size of the lesions was 1.38 ± SD1.1 (0.5–4.3) and 95% CI [0.93, 1.83] cm. Gender distribution was noted to be 51.3% male and 48.7% female.
Introduction
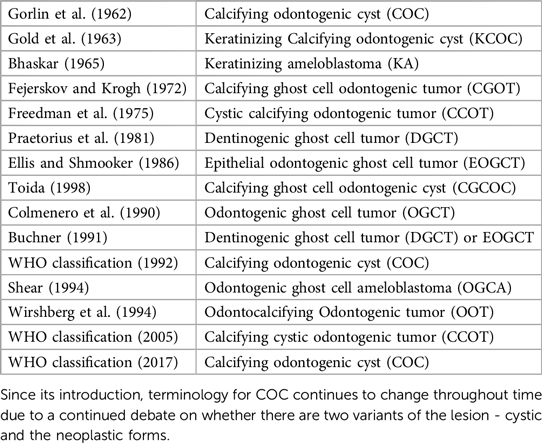
Calcifying odontogenic cyst (COC) is a benign cystic neoplasm of odontogenic origin characterized by an ameloblastoma-like epithelium and ghost cells that have the potential to undergo calcification. COC was first described by Gorlin and colleagues in 1962 (1–3). The incidence of COC is estimated at 1%–3% of all odontogenic cysts and tumors (9, 37–41). Various classification systems and terminologies have been proposed in relation to COC due to ongoing debate as to whether COC is a neoplasm or a developmental cyst (4, 5). The World Health Organization (WHO) first described the lesion as non-neoplastic cystic lesion and used the term COC in 1971. In 1992, the WHO classified it as an odontogenic tumor but continued to use the term COC (5, 29). In 2005, WHO re-designated COC lesion as calcifying cystic odontogenic tumor (CCOT) (5, 29). In 2017, WHO changed the terminology back to COC and reclassified it as a benign odontogenic cyst (5, 29). COCs represent a heterogenous group of lesions that show a variety of clinicopathologic and behavioral features and may coexist with other odontogenic lesions (4, 5). Table 1 shows the various terminologies proposed for COC by different authors.
COC can present as intraosseous (Central Calcifying Odontogenic Cyst) or extraosseous (Peripheral Calcifying Odontogenic Cyst) lesion. The intraosseous central form has unicystic or multicystic radiolucent presentation, well defined borders, and is associated with bony destruction (1–3). The peripheral form (PCOC) represents less than 25% of reported COC cases (6). PCOC is less destructive and typically isolated peripherally in the adjacent soft tissues (6).
The main objective of this article is to discuss PCOC in a clinic case and review available literature to illustrate characteristics of PCOC, progression of changing terminologies, management of PCOC, and differential diagnosis of similar oral lesions.
Case presentation
A 12-year-old Hispanic male patient presented with a chief complaint of a “progressive growth of gingiva” located palatal to the upper right lateral incisor. The patient reported that the lesion slowly increased in size over the span of six months and it was overall asymptomatic unless accidentally pressed then slight tenderness was reported. The patient denied any traumatic event relevant to the appearance of the lesion and denied any interference to eating, speech, or performance of oral hygiene. Due to the location of the lesion, the patient reported that he frequently pressed it with his tongue and would like to have it removed. Review of medical history was non-contributary. The patient was healthy without any medical conditions reported.
Clinical evaluation revealed a 7 mm × 9 mm × 5 mm convex, sessile, firm, depressible, round, pink, exophytic soft tissue lesion located along the palatal gingival margin of the upper right lateral incisor covered with normal keratinized tissue (Figures 1A–C). Periodontal probing noted 2–3 mm probing depth and vitality testing of surrounding teeth were normal. No displacement of teeth and no mobility of teeth were noted. Radiographic assessment noted for normal finding and ruled out endodontic lesions, bony involvement, or bony defects in the area.
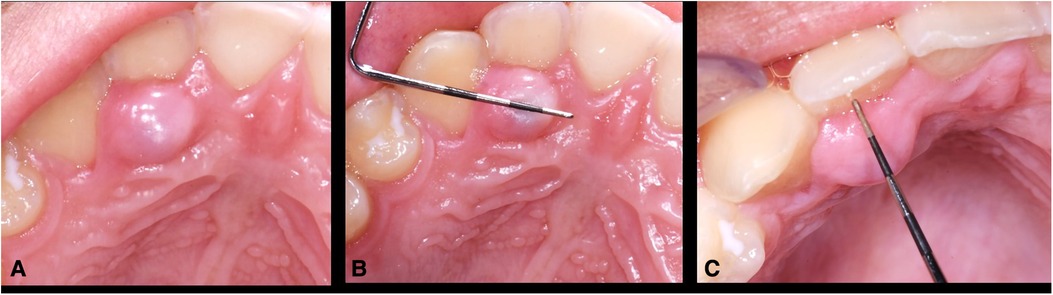
Figure 1. (A–C) Clinical presentation of the lesion. The 7 mm long × 9 mm wide × 5 mm high lesion over palatal gingiva of upper right lateral incisor had grown over the course of 6 months and became tender upon palpation. The lesion was generally firm but compressible pink to red, round, exophytic mass covered with normal keratinized tissue.
On the day of consultation, an excisional biopsy was performed. The lesion was excised in its entirety to bone and sent for histological examination. The patient was prescribed chlorhexidine oral rinse to be used twice a day for one week and over the counter ibuprofen was advised as needed for pain management. The microscopic examination revealed a well-defined cystic area lined with keratinized and non-keratinized epithelium with fibrous connective tissue. The cystic area in the fibrous connective tissue was lined with well-defined layer of palisading ameloblastic-like basal cells, loosely arranged supra-basal epithelial cells resembling stellate reticulum, eosinophilic ghost cells devoid of nuclei, and basophilic calcification. Scattered chronic inflammatory cells were present. A diagnosis of PCOC was confirmed microscopically and photomicrographs are presented in Figures 2A–C. Patient was seen two weeks later for post-operative check with normal healing noted. The patient reported uneventful healing without complications. The patient reported mild discomfort and minimal bleeding of the surgical site for 2–3 days after the procedure and no pain medication was needed post-operatively. At the 3-year follow-up, no recurrence of the lesion was noted and patient continued to report no concern or complication (Figure 3).
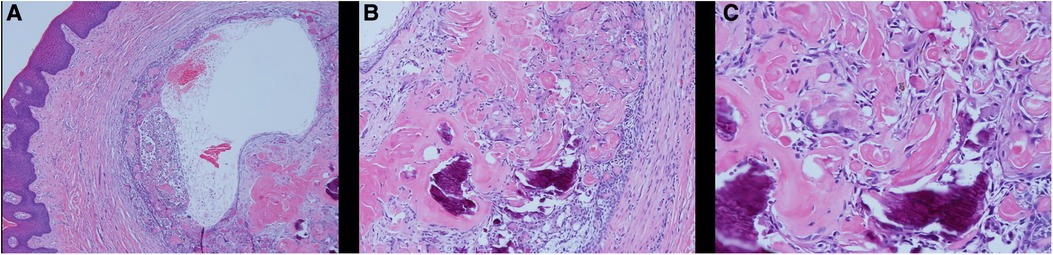
Figure 2. Histological examination of PCOC. Microscopic examination showing an oral soft tissue specimen with keratinized and non-keratinized epithelium with fibrous connective tissue. A cystic area in the fibrous connective tissue lined with well-defined layer of palisading ameloblastic-like basal cells, loosely arranged supra-basal epithelial cells resembling stellate reticulum, eosinophilic ghost cells devoid of nuclei, and basophilic calcification. Scattered chronic inflammatory cells were present. (A) HE × 4; (B) HE × 10; (C) HE × 20).
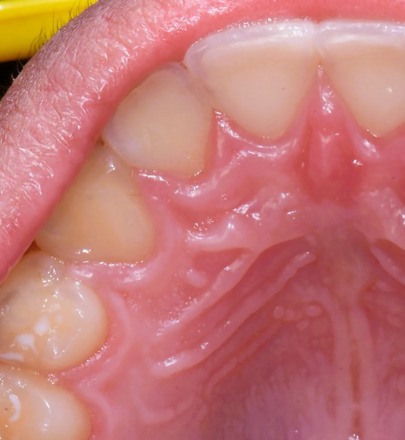
Figure 3. 3-year follow-up of the area after excision of PCOC noted for normal healing and no recurrence of the lesion.
Review of literature
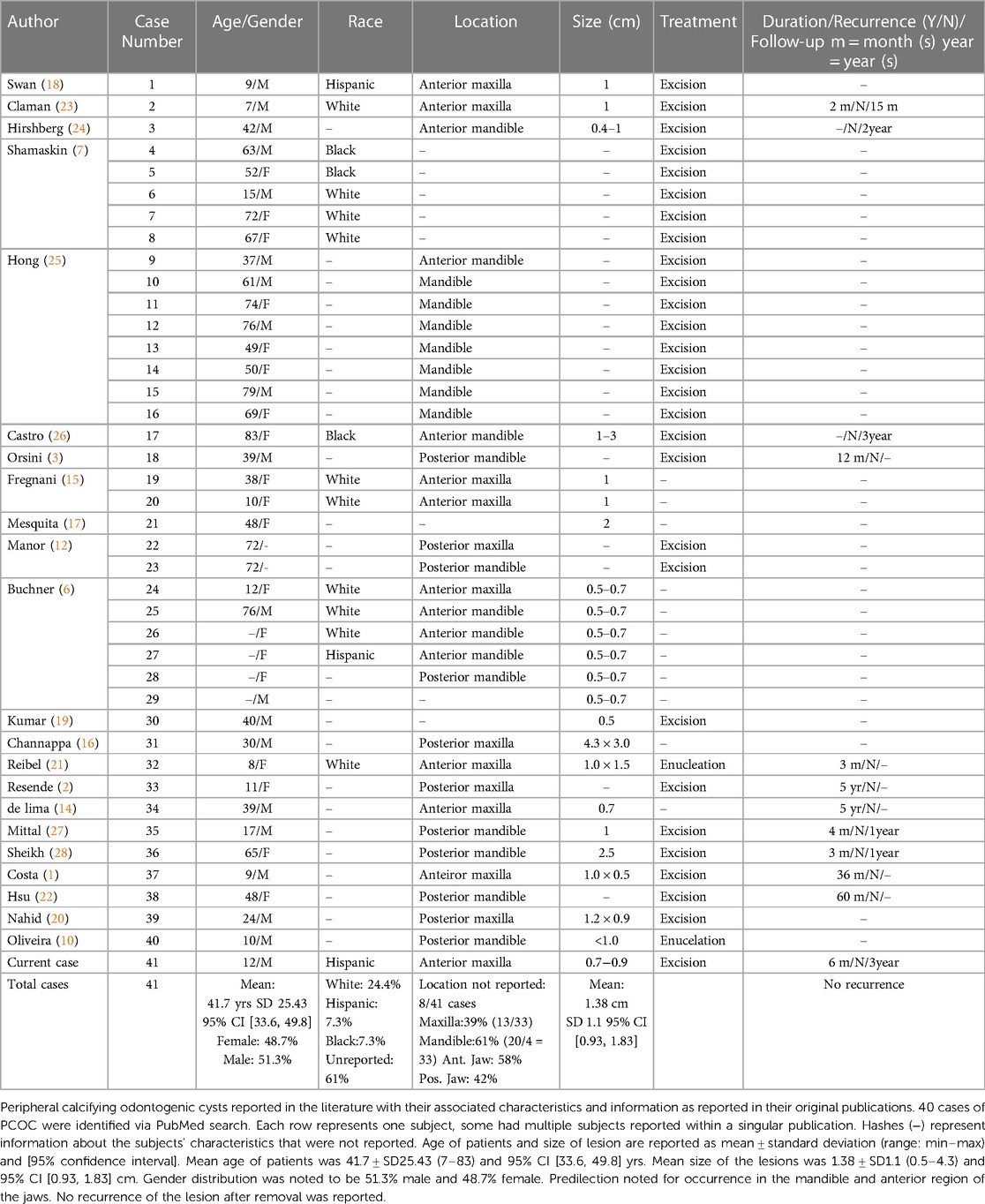
A PUBMED search of case reports on PCOCs published between the years 1962 and 2023 was completed using the keyword: Peripheral Calcifying Odontogenic Cyst. A total of 52 publications were identified. All cases reviewed were published in English. Publications originally reported in a different language were excluded from our review. Review articles and meta-analysis were excluded due to the potential for subjects being accounted for multiple times and over-estimating the incidence of PCOC. Histological analysis and diagnosis of the lesions as PCOC using unique hallmarks of the lesion were required to be included in this study. 22 articles with total of 40 cases of PCOC were reviewed and the descriptive information from these cases and the current case are summarized in Table 2.
Discussion
Review of the 41 cases of PCOC reported noted a mean age of occurrence of 41.7 years old with standard deviation of 25.43 years and a range of 7 to 83 years. Gender distribution was noted to be 48.7% female and 51.3% male. 25 out of 41 cases did not report on the ethnicities of the patients. Of the cases that reported on ethnicities, 62.5% was White, 18.75% was Hispanic and 18.75% was Black. 8 out of 41 cases did not specify location of PCOC occurrence in their reports. Of the 33 cases that reported location of the lesion, a predilection was noted for mandible (61%). A predilection was noted for the anterior area (58%). The size of the PCOC reported had a mean of 1.38 cm (cm) with standard deviation of 1.1 cm and a range of 0.5 cm to 4.3 cm. The duration of the lesion before detection ranged from several months, to multiple years due to many being asymptomatic painless swelling. No recurrence was reported in any of the cases reviewed.
Characteristics of PCOC
PCOC commonly presents as a uniform, painless, firm to soft gingival swelling without unique or distinguishing characteristics that would differentiate itself from other gingival pathologies commonly found in the oral environment. These lesions usually do not involve the underlying bone with very few rare cases reported with absorption of the adjacent alveolar bone, adjacent tooth root resorption, and potential for tooth displacement (1–3, 6, 7). PCOC lesions usually have no distinct radiographical characteristics, except in some case where sporadic calcifications in the lesions may appear on radiographs as radiopaque specks (6, 8). Histological analysis is often required for appropriate classification of these lesions. The most common histopathological findings of PCOC include a cystic lesion lined by epithelium with a well-defined ameloblastic-like basal layer of columnar cells in a palisaded fashion, an overlying layer with cells resembling stellate reticulum, and ghost cells that may be in the epithelial cyst lining or in the fibrous capsule (29). Presence of eosinophilic ghost cells is a characteristic microscopic feature of PCOC, however, may also be seen in other lesions, such as ghost cell odontogenic carcinoma, odontomas, and ameloblastic fibro-odontoma (29). Basophilic calcifications and calcifications of ghost cells can also be noted microscopically in PCOC lesions (29). The function of these ghost cells is currently debated but have been determined to not have a negative impact on the patient or with treatment outcomes (2).
Similar to previous study, our review found the most common site of occurrence for PCOC is the mandibular area, with a predilection for the anterior area of the jaw (2, 6). Previous studies found either similar distribution between genders or a higher percentage of occurrence in females while the present review noted a slightly higher percentage of occurrence in males (51.3%) (2, 6). The size of the PCOC lesions reviewed had a mean of 1.38 cm with standard deviation of 1.1 cm and a range of 0.5 cm to 4.3 cm.
Management of PCOC
Due to their non-aggressive behavior, and low risk of recurrence, PCOC lesions are often treated with enucleation or excisional removal with favorable prognosis. Recurrence of PCOC after treatment has not yet been reported in literature. Excision can be done via conventional surgical blade, laser, or electrosurgery devices. PCOC is often limited in the peripheral oral soft tissue without bony involvement and has an average size of 1.38 cm, therefore complete removal of the lesion if often reported in literature. Alternative management methods of decompression, marsupialization may be considered in lesion of large size. The patient in the present case reported slow growth of the lesion over the span of six month. Due to the limited number of cases available in literature it cannot be concluded if left alone without treatment if PCOC would continue to slowly expand in size or self-arrest at some point. In addition to microscopic examination, it has been suggested that utilization of immunohistochemical investigations on cell proliferation activity may also aid in diagnosis of PCOC and may further contribute to further understanding on debate of its cystic or neoplastic nature (4).
Progression of changing terminologies
In 1962, Gorlin first described the calcifying ghost cell odontogenic cyst (CGCOC) under the term calcifying odontogenic cyst (COC) (11). Since its introduction, its classification and terminology continue to change through time due to a continued debate on whether there are two variants of the lesion—cystic and the neoplastic forms (4, 5). The “monistic” concept believes that all COCs are neoplastic in nature. However, majority of COC are cystic in architecture and appear to be non-neoplastic. Hence, the “dualistic” concept believes that COCs exist as two entities: a cyst or a neoplasm (4, 5). A chorological timeline of the various classification and terminology systems are presented in Table 1 (4, 5).
Differential diagnosis of oral peripheral soft tissue exophytic lesion
Differential diagnosis of exophytic oral lesion with soft tissue enlargement can include various etiologies from reactive, viral, neoplastic, to developmental. Reactive lesions such as peripheral giant cell granuloma (PGCG) or pyogenic granuloma (PG) are inflammatory response to localized irritation or trauma with association to hormonal changes, or certain medications (30). PGCG often presents as a soft tissue purplish-red nodule consisting of multinucleated giant cells in a background of mononuclear stromal cells and extravasated red blood cells (30). PG often presents as smooth or lobulated exophytic lesion that is red-bluish in coloration and histologically noted for mass of angiomatous tissue with proliferation of capillary vessels and infiltrations of plasma cells, lymphocytes and neutrophils (31). Peripheral ossifying fibroma (POF) is a smooth surfaced reactive gingival enlargement to local irritant. POF often has normal squamous surface epithelium with proliferation of fibroblasts, endothelial proliferation, mineralized osteoids or calcifications, and inflammatory cells (34). Mucocele is a mucus retention lesion due to alteration to excretory duct of the salivary gland (35). It has smooth surface with normal coloration to bluish tint (35). Histologically, mucocele usually has hyperplastic parakeratinized stratified squamous epithelium and cystic space containing mucin and mucus-filled cells (35). Presence of salivary gland tissue and sialomucin is a diagnostic histological feature of mucocele (35). Traumatic fibroma usually presents as smooth-surfaced, normal-colored mucosa with sessile or pedunculated base as a reactive hyperplastic lesion to localized trauma (36). Histologically, irritation fibroma usually is noted as non-encapsulated nodular mass composed of fibrous connective tissue with collagen bundles interspersed with fibroblasts, blood vessels and scattered chronic inflammatory cells (36). The overlying squamous epithelium can be hyperkeratotic (36). Viral lesions such as condyloma acuminatum, verruca vulgaris, papilloma are associated with human papilloma virus (HPV) and have rough verrucous surface (32). Histologically theses lesions are noted for acanthosis with overlying hyperkeratosis and distinctive cells termed koilocytes (32). Koilocytes are large keratinocytes with abundant cytoplasm and small pyknotic nuclei often present in the upper layers of the epidermis (32). In addition to microscopic examination, in-situ hybridization, immunohistochemical studies for papillomavirus common antigen, or polymerase chain reaction may be used to confirm presence of HPV or further identify the HPV type (32). Developmental lesions may include PCOC as in this case or adult gingival cyst. The distinctive histological finding of PCOC is presence of ghost cells. Ghost cells are enlarged epithelial cells with eosinophilic cytoplasm and no nucleus which can be found in the lining epithelium and in the cyst lumen (1). Adult gingival cyst is a developmental odontogenic cyst that has mucosal surface lined by stratified squamous epithelium and a fibrous connective tissue capsule containing a cystic lumen lined by flat to cuboidal cells and mild chronic inflammatory cell infiltrates (33). Additional differential diagnosis of peripheral oral soft tissue enlargement may also include benign and malignant neoplasm. Differential diagnoses for oral soft tissue as exophytic lesion are listed in Figure 4 (12, 13, 36). Definitive diagnosis of peripheral oral exophytic lesions can be challenging and histological examination along with clinical correlation often is needed to aid in proper diagnosis.
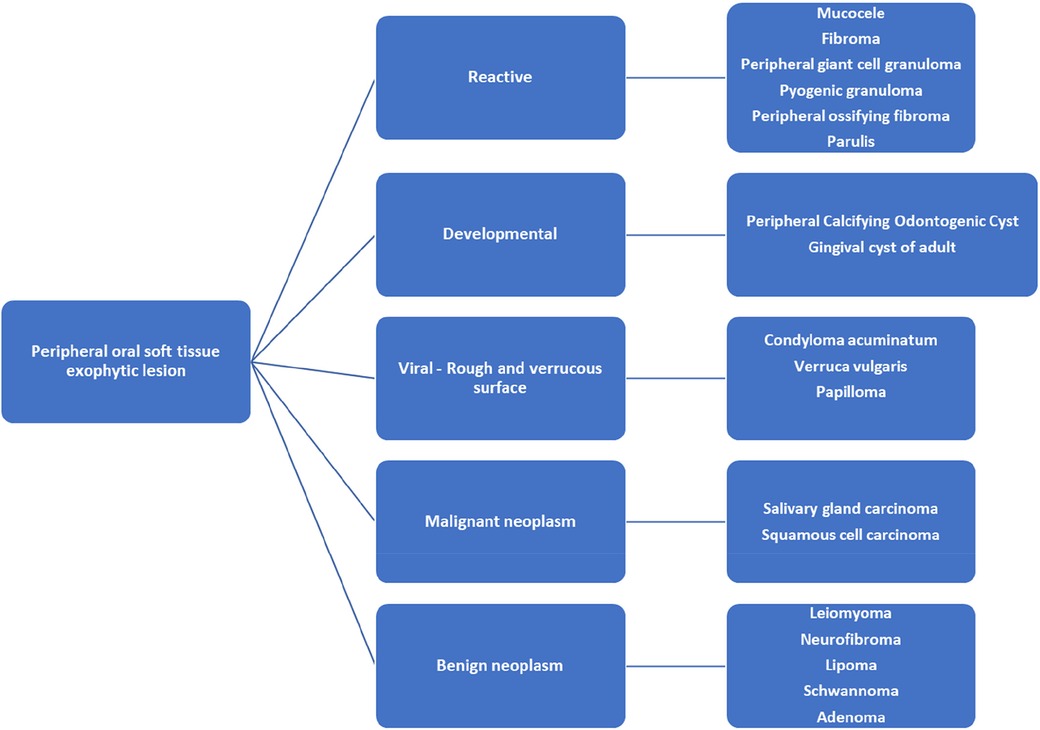
Figure 4. Differential diagnosis of oral lesion as soft tissue enlargement of the oral Mucosa (12, 13, 36).
Limitations of present review
Literature on PCOC is limited to case reports and case series, which is considered lowest level of evidence in evidence-based research. Case reports and series can provide clinicians a useful narrative observational trend of the characteristic, incidence, location, and biological behavior of PCOC and gender, ethnicities, and age of patients. However, meaningful statistical analysis often is difficult with case reports/series due to limited number of case available in the literature and/or missing data. Data reported in case reports may not always be comprehensive and missing data can lead to missed observational trend. Ethnicity, gender, age of the patient, size, and location of the lesion were missing in some of the case reports reviewed. Incidence of PCOC may also be underreported due to the various terminologies and changing classification systems used throughout the years. This case report and review of literature presented a narrative description of the general characteristics on PCOC solely based on 41 case reports available in PubMed utilizing the key term PCOC. Systemic analysis of larger number of cases and inclusion of all available terminologies related to PCOC throughout the years in literature search key terms will provide a higher level of evidence of results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
The authors contributed equally to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors JC and GDT declared that they were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Costa LC, Neto JB, de-Assis EM, Gomes HE, Leitão TJ, Vasconcelos RRC, et al. Peripheral calcifying odontogenic cyst: a rare case report. J Clin Exp Dent. (2018) 10(11):e1140–1144. doi: 10.4317/jced.55137
2. Resende RG, Brito JA, Souza LN, Gomez RS, Mesquita RA. Peripheral calcifying odontogenic cyst: a case report and review of the literature. Head Neck Pathol. (2011) 5(1):76–80. doi: 10.1007/s12105-010-0213-3
3. Orsini G, Fioroni M, Rubini C, Piattelli A. Peripheral calcifying odontogenic cyst. J Clin Periodontol. (2002) 29(1):83–6. doi: 10.1034/j.1600-051x.2002.290113.x
4. Toida M. So-called calcifying odontogenic cyst: review and discussion on the terminology and classification. J Oral Pathol Med. (1998) 27(2):49–52. doi: 10.1111/j.1600-0714.1998.tb02092.x
5. Thinakaran M, Sivakumar P, Ramalingam S, Jeddy N, Balaguhan S. Calcifying ghost cell odontogenic cyst: a review on terminologies and classifications. J Oral Maxillofac Pathol JOMFP. (2012) 16(3):450–3. doi: 10.4103/0973-029X.102519
6. Buchner A, Merrell PW, Carpenter WM. Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med. (2006) 35(7):385–91. doi: 10.1111/j.1600-0714.2006.00437.x
7. Shamaskin RG, Svirsky JA, Kaugars GE. Intraosseous and extraosseous calcifying odontogenic cyst (gorlin cyst). J Oral Maxillofac Surg. (1989) 47(6):562–5. doi: 10.1016/S0278-2391(89)80067-9
8. Buchner A, Merrell PW, Hansen LS, Leider AS. Peripheral (extraosseous) calcifying odontogenic cyst. Oral Surg Oral Med Oral Pathol. (1991) 72(1):65–70. doi: 10.1016/0030-4220(91)90191-E
9. Nalabolu GR, Mohiddin A, Hiremath SK, Manyam R, Bharath TS, Raju PR. Epidemiological study of odontogenic tumours: an institutional experience. J Infect Public Health. (2017) 10(3):324–30. doi: 10.1016/j.jiph.2016.05.014
10. Oliveira EM, da Santana LAM, Silva ER, Souza LN. A calcifying odontogenic cyst associated with compound odontoma mimicking a tooth germ. Case Rep Dent. (2021) 2021:1–4. doi: 10.1155/2021/9991772
11. Gorlin RJ, Pindborg JJ, Clausen FP, Vickers RA. The calcifying odontogenic cyst—a possible analogue of the cutaneous calcifying epithelioma of malherbe: an analysis of fifteen cases. Oral Surg Oral Med Oral Pathol. (1962) 15(10):1235–43. doi: 10.1016/0030-4220(62)90159-7
12. Manor Y, Mardinger O, Katz J, Taicher S, Hirshberg A. Peripheral odontogenic tumours—differential diagnosis in gingival lesions. Int J Oral Maxillofac Surg. (2004) 33(3):268–73. doi: 10.1006/ijom.2003.0508
13. Finkelstein M, Lanzel E, Hellstein J. A guide to clinical differential diagnosis of oral mucosal lesions. (2002). Available at: https://www.dentalcare.com/en-us/ce-courses/ce110
14. de Lima AP, Kitakawa D, Almeida JD, Brandão AAH, Anbinder AL. Peripheral calcifying cystic odontogenic tumour of the maxillary gingiva. BMC Res Notes. (2012) 5(1):455–455. doi: 10.1186/1756-0500-5-455
15. Fregnani ER, Pires FR, Quezada RD, Shih IM, Vargas PA, De Almeida OP. Calcifying odontogenic cyst: clinicopathological features and immunohistochemical profile of 10 cases: calcifying odontogenic cyst. J Oral Pathol Med. (2003) 32(3):163–70. doi: 10.1034/j.1600-0714.2003.00070.x
16. Channappa NK, Krishnapillai R, Rao JBM. Cystic variant of calcifying epithelial odontogenic tumor. J Investig Clin Dent. (2012) 3(2):152–6. doi: 10.1111/j.2041-1626.2011.00092.x
17. Mesquita RA, Lotufo MA, Sugaya NN, de Araújo NS, de Araújo VC. Peripheral clear cell variant of calcifying epithelial odontogenic tumor: report of a case and immunohistochemical investigation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2003) 95(2):198–204. doi: 10.1067/moe.2003.63
18. Swan RH, Houston GD, Moore SP. Peripheral calcifying odontogenic cyst (gorlin cyst). J Periodontol. (1985) 56(6):340–3. doi: 10.1902/jop.1985.56.6.340
19. Kumar U, Vij H, Vij R, Kharbanda J, Aparna I, Radhakrishnan R. Dentinogenic ghost cell tumor of the peripheral variant mimicking epulis. Int J Dent. (2010) 2010:519494–95. doi: 10.1155/2010/519494
20. Nahid R, Bansal M, Gupta K, Pandey S, Tiwari P, Agarwal R. Exophytic gingival growth of the maxillary canine region in a young individual: extremely rare case report of peripheral dentinogenic ghost cell tumor. J Cancer Res Ther. (2020) 16(3):661–4. doi: 10.4103/jcrt.JCRT_180_19
21. Reibel J, Gronbaek AB, Poulsen S. Peripheral ameloblastic fibro-odontoma or peripheral developing complex odontoma: report of a case: peripheral ameloblastic fibro-odontoma. Int J Paediatr Dent. (2011) 21(6):468–70. doi: 10.1111/j.1365-263X.2011.01144.x
22. Hsu HJ, Chen YK, Wang WC, Tseng CH. Peripheral calcifying odontogenic cyst with multinucleated giant cell formation. J Dent Sci. (2019) 14(2):211–2. doi: 10.1016/j.jds.2019.01.003
23. Claman LJ, Rossie KM, Records LE, Colbert G. Peripheral calcifying odontogenic cyst in a child: case report of an unusual lesion. Pediatr Dent. (1987) 9(3):226–8. PMID: 3507639.3507639
24. Hirshberg A, Dayan D, Horowitz I. Dentinogenic ghost cell tumor. Int J Oral Maxillofac Surg. (1987) 16(5):620–5. doi: 10.1016/s0901-5027(87)80117-0
25. Hong SP, Ellis GL, Hartman KS. Calcifying odontogenic cyst. A review of ninety-two cases with reevaluation of their nature as cysts or neoplasms, the nature of ghost cells, and subclassification. Oral Surg Oral Med Oral Pathol. (1991) 72(1):56–64. doi: 10.1016/0030-4220(91)90190-n
26. Castro WH, de Aguiar MC, Gomez RS. Peripheral dentinogenic ghost-cell tumor: a case report. Quintessence Int (Berl). (1997) 28(1):45–7. PMID: 10332354.
27. Mittal N, Sah K, Chandra S, Gupta S, Mittal S, Agarwal M. Extraosseous calcifying cystic odontogenic tumor: an uncommon variant. Natl J Maxillofac Surg. (2013) 4(2):245–8. doi: 10.4103/0975-5950.127662
28. Sheikh J, Cohen MD, Ramer N, Payami A. Ghost cell tumors. J Oral Maxillofac Surg. (2017) 75(4):750–8. doi: 10.1016/j.joms.2016.10.013
29. Bilodeau EA, Hunter KD. Calcifying odontogenic cyst. PathologyOutlines.com website. Available at: https://www.pathologyoutlines.com/topic/mandiblemaxillacalcifyingodontogenic.html (Accessed June 1, 2023).
30. Tandon PN, Gupta SK, Gupta DS, Jurel SK, Saraswat A. Peripheral giant cell granuloma. Contemp Clin Dent. (2012) 3(Suppl 1):S118–21. doi: 10.4103/0976-237X.95121
31. Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: Statpearls. Treasure Island (FL): StatPearls Publishing; (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK556077/ (Updated Octomber 23, 2022).
32. Pennycook KB, McCready TA. Condyloma Acuminata. In: Statpearls. Treasure Island (FL): StatPearls Publishing; (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK547667/ (Updated August 1, 2022).
33. Brod JMM, Passador-Santos F, Soares AB, Sperandio M. Gingival cyst of the adult: report of an inconspicuous lesion associated with multiple agenesis. Case reports in dentistry. (2017) 2017:4346130. doi: 10.1155/2017/4346130
34. Nanda T, Jain S, Nanda S, Gambhir R. Peripheral ossifying fibroma: a clinico-histopathological report. Univers Res J Dent. (2016) 6(2):186. doi: 10.4103/2249-9725.181676
35. More CB, Bhavsar K, Varma S, Tailor M. Oral mucocele: a clinical and histopathological study. J Oral Maxillofac Pathol. (2014) 18(Suppl 1):S72–7. doi: 10.4103/0973-029X.141370
36. Mortazavi H, Safi Y, Baharvand M, Rahmani S, Jafari S. Peripheral exophytic oral lesions: a clinical decision tree. Int J Dent. (2017) 2017:9193831. doi: 10.1155/2017/9193831
37. Buchner A, Merrell PW, Carpenter WM. Relative frequency of central odontogenic tumors: a study of 1,088 cases from northern California and comparison to studies from other parts of the world. J Oral Maxillofac Surg. (2006) 64(9):1343–52. doi: 10.1016/j.joms.2006.05.019
38. de Souza LB, Gordón-Núñez MA, Nonaka CFW, de Medeiros MC, Torres TF, Emiliano GBG. Odontogenic cysts: demographic profile in a Brazilian population over a 38-year period. Med Oral Patol Oral y Cirugía Bucal. (2010) 15(4):e583–590. doi: 10.4317/medoral.15.e583
39. Jones AV, Craig GT, Franklin CD. Range and demographics of odontogenic cysts diagnosed in a UK population over a 30-year period. J Oral Pathol Med. (2006) 35(8):500–7. doi: 10.1111/j.1600-0714.2006.00455.x
40. Grossmann SM, Machado VC, Xavier GM, Moura MD, Gomez RS, Aguiar MCF, et al. Demographic profile of odontogenic and selected nonodontogenic cysts in a Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol and Endod. (2007) 104(6):e35–41. doi: 10.1016/j.tripleo.2007.05.028
Keywords: peripheral calcifying odontogenic cyst, oral pathology and oral medicine, case report, oral lesion, literature review
Citation: Sheng S, Tipton N, Chang J, Meng H-W and Tribble GD (2023) Peripheral calcifying odontogenic cyst: a case report and comprehensive review of 60 years of literature. Front. Oral. Health 4:1223943. doi: 10.3389/froh.2023.1223943
Received: 16 May 2023; Accepted: 11 July 2023;
Published: 4 August 2023.
Edited by:
Romeo Patini, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Santosh R. Patil, New Horizon Dental College and Research Institute, IndiaManabu Yamazaki, Niigata University, Japan
Martinho Campolina Rebello Horta, Pontifical Catholic University of Minas Gerais, Brazil
© 2023 Sheng, Tipton, Chang, Meng and Tribble. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sally Sheng c2FsbHkuc2hlbmdAdXRoLnRtYy5lZHU=
 Sally Sheng
Sally Sheng Nicholas Tipton
Nicholas Tipton Jennifer Chang
Jennifer Chang Hsiu-Wan Meng
Hsiu-Wan Meng Gena D. Tribble
Gena D. Tribble