
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oral. Health, 11 May 2023
Sec. Oral and Maxillofacial Surgery
Volume 4 - 2023 | https://doi.org/10.3389/froh.2023.1166091
This article is part of the Research TopicReviews in the Neuroscience of Orofacial FunctionsView all 5 articles
Objectives: The present paper aims to systematically review the literature published from 2015 to 2023 on bruxism in children with the aim to compilate the best available evidence.
Materials and Methods: A systematic search in the National Library of Medicine's PubMed, Medline (EBSCO), SCOPUS, and Google Scholar databases was performed to identify all studies on humans assessing genetic, biopsychosocial, and sleep factors assessed with any different approach for sleep bruxism (SB) in children and its interventions. The selected articles were assessed independently by the two authors according to a structured reading of the article's format (PICO). The quality of the articles was evaluated using Quality Assessments Tool for Experimental Bruxism Studies (Qu-ATEBS) and the JBI critical appraisal tools.
Results: A total of 16 articles were included for discussion in the review and grouped into questionnaire/parental-report (n = 7), SB assessment through parental report of SB and clinical examination (n = 4), and instrumental assessment (n = 5) studies. The total quality scores evaluated with STROBE and Qu-ATEBS were high for all included papers. However, in general, there was no control of bias strategies and there was no control group in the intervention studies.
Conclusions: Investigations based on self-report, clinical, and instrumental bruxism assessment showed a positive association with genetics, quality of life aspects (school and emotional functions and overuse of screen-time), mother anxiety and family conformation, diet, alteration in sleep behaviors and architecture, and sleep breathing disorders. Additionally, the literature presents options to increase airway patency and, thus, reduce the occurrence of SB. Tooth wear was not found to be a major sign of SB in children. However, methods of SB assessment are heterogeneous and hamper a reliable comparison of the results.
Sleep bruxism (SB) is a repetitive jaw-muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible during sleep (1). The concept of bruxism shifted from a disorder to a motor activity that may even have potential protective relevance (2, 3).
The etiology of SB is multifactorial (4) and includes biological, psychosocial, and lifestyle factors. According to gene analysis studies (5, 6) and studies among individuals of the same family (7), SB is explained by both environmental and genetic factors (8, 9). Moreover, an imbalance in certain neurotransmitters in the central nervous system (e.g., dopamine and serotonin) play a role in the genesis of masticatory muscle activity (MMA) and SB (10). This imbalance is related to lifestyle factors, like consumption of added sugar and screen-time overuse (11), and psychosocial factors, such as anxiety, depression, and stress, which have also been demonstrated to increase risk for SB (12, 13). Additionally, health issues like breathing disorders (14) and Gastroesophageal Reflux Disease (GERD) (15) have also been suggested as predictors of SB.
Studies that follow cohorts to assess SB are scarce, which hampers the possibility of establishing a cause-consequence relationship. SB could be a transitory activity related to positive consequences in most of the cases (such as reducing cortisol levels during positive stress situations), but in some cases is the symptom of situations that can even compromise the patient's life (obstructive sleep apnea). In this last point, following subjects over time without undergoing intervention for the underlying condition is not viable due to ethical concerns.
Variations in the conception and assessment of SB, population characteristics, and research methodologies among different studies might partially contribute to the inconsistent results. Due to these reasons, clinicians lack enough tools to update and apply an adequate evidence-based dentistry regarding bruxism in children. Therefore, this review aimed to compile the best available evidence about bruxism in children over the last seven years and give evidence-based recommendations for pediatric healthcare givers to treat bruxism in the clinic. The question we aim to answer is, “What is the evidence that supports the assessment and intervention of sleep bruxism in children?”
This systematic review was registered in PROSPERO.
On March 25 2023, a literature review was performed based on a search in PubMed, Medline (EBSCO), SCOPUS, and Google Scholar databases for articles on SB in children and its etiology, assessment, and intervention. The search was filtered to include only papers published from 2015 to 2023 in the English language. The keywords used in the search strategy for each database are included in Table 1. The article screening included two phases: title and abstracts screening, and full-text review. First, all identified titles and abstracts were independently screened by the two authors. The inclusion criteria were: (a) studies on child human subjects (age 0–18 years); (b) studies dealing with SB, evaluated by parental-report [e.g., reporting of sleep tooth-grinding sound (STG) by questionnaire or interview] and/or clinical inspection (e.g., tooth wear, Linea Alba, and/or masticatory muscle pain), and/or instrumental assessment (e.g., scoring of SB episodes based on polysomnography (PSG), polygraphy, or Electromyography (EMG)); and (c) studies having the following designs: observational studies, controlled clinical trials, or randomized controlled clinical trials. The exclusion criteria were: (a) studies on animals; (b) studies on adults; (c) publication types such as editorials, letters, legal cases, interviews, and conference abstracts; (d) studies in which the assessment of SB and/or the outcome measures were not performed with calibrated/validated instruments and/or techniques; (e) studies including children with neurodevelopmental disorders; and (f) retrospective design. Full texts of eligible studies were only obtained when both reviewers were in consensus. Lack of agreement between the reviewers was resolved by discussion between both investigators.
The selected articles were read and assessed independently by the two authors according to a PICO (Population-Exposure-Comparison-Outcome) structured strategy. The population (P) is described in terms of sample size, inclusion criteria, and demographic characteristics. The intervention (I) concerns information on the study design, assessment approach, and measurement of variables. The comparison (C) includes data on the control group depending on the study design (i.e., individuals without SB). The outcome (O) is reported in terms of the possible relationship between different biopsychosocial and lifestyle variables and SB in children. The main conclusion of each study was also included.
The PRISMA checklist for systematic reviews was followed.
Strengthening the Reporting of Observational studies in Epidemiology (STROBE) (16) was used for the quality assessment of case-control and cross-sectional studies. STROBE score, defined as the number of the 22 STROBE items adequately reported divided by the number of applicable items, expressed as a percentage, was determined for the quality assessment of the studies. The 13 STROBE items with several questions (2–15 questions per item, online supplementary) were considered adequately reported when at least 50% of their questions had “yes” answers (after exclusion of the “not applicable” components).
Those studies dealing with intervention of sleep bruxism in children were evaluated with the Quality Assessments Tool for Experimental Bruxism Studies (Qu-ATEBS) (17). A score between 0 and 50 was considered low quality and a score between 51 and 70 was considered high quality. Only studies with a score above 50% in STROBE and above 51 in Qu-ATEBS were included in this systematic review.
Title and abstract reading led to the exclusion of 143 irrelevant articles. The full text was obtained of the remaining 27 articles. Of these, 14 were excluded for not fulfilling the inclusion criteria, so that 13 articles were selected for inclusion in the review. Two more papers were added to the review by searching the reference lists of two previous papers (18, 19). Thus, a total of 16 articles were included in the review. Table 2 includes the list of articles and the reason for exclusion from the review after reading the full text. Based on the type of assessment of SB, the selected articles were then divided into three categories: SB assessment by means of parental report (n = 7), SB assessment through parental report of SB and clinical examination (n = 4), and instrumental assessment (n = 5). The flow diagram for the inclusion of the studies is presented in Figure 1.
The total quality scores evaluated with STROBE for all included papers with cross-sectional, case-control, and epidemiological studies were high. In general, there was no control of bias strategies. The total quality scores evaluated with Qu-ATEBS were high for the two included papers. However, in general, there was no control of bias and there was no control group in the intervention studies.
Seven studies investigated the association between parental-reported SB and lifestyle, habits, psychological and stress aspects, tooth wear, and the effect of two interventions (direct and indirect) for SB in children (Table 3).
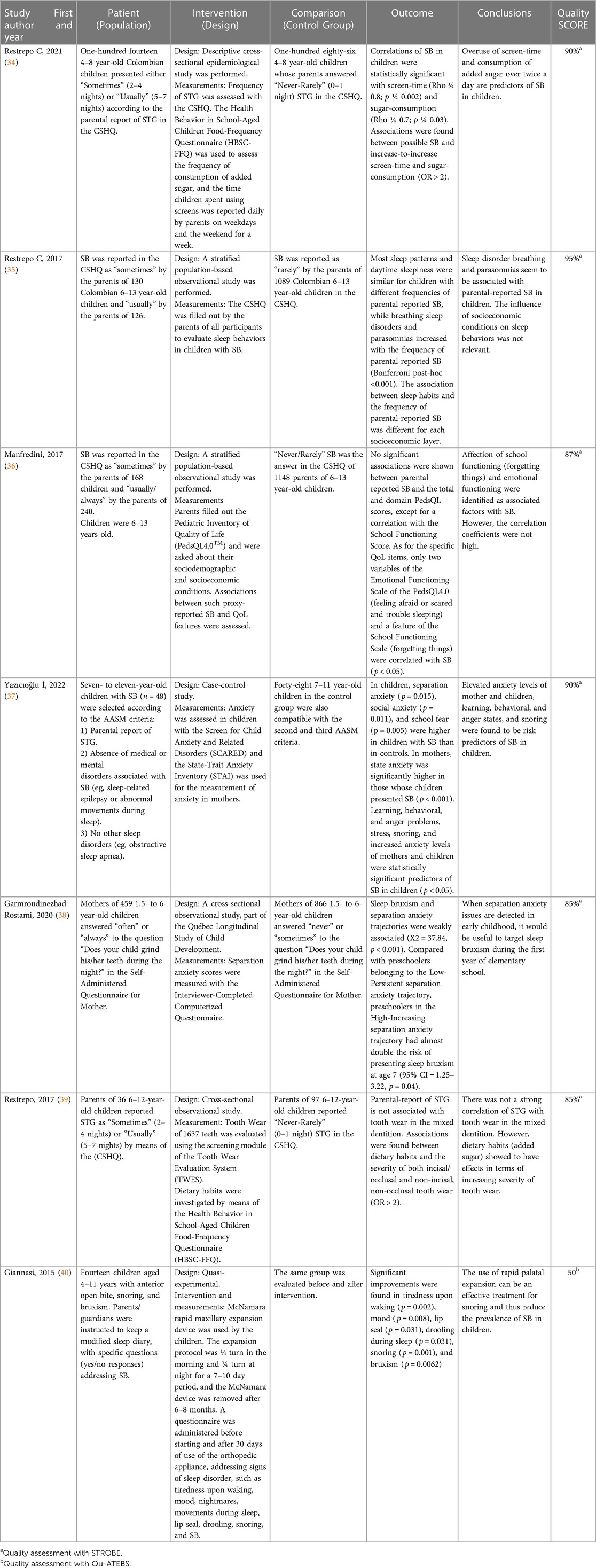
Table 3. PICO-like structured Reading of reviewed articles using parental-reported SB as assessment strategy.
Study designs were strongly heterogeneous, based on the evaluation of SB either in non-patient children (e.g., school students or pediatric patients attending dental clinics of universities for conservative care) or in case-control groups of SB. Methodological differences were also evident from the strategy to assess SB as well as from the outcome measures established in each investigation. All the studies were based on a cross-sectional assessment, except for the two intervention studies. The authors used parental reports based on the Children's Sleep Habits Questionnaire (CSHQ) (34–36, 39) and the Self-Administered Questionnaire for Mother (38) and one used only the report included in the criteria of the American Academy of Sleep Medicine (AASM) (37).
As for the results, six papers found a statistically significant association between SB and breathing disorders and parasomnias (35), forgetting things and feeling afraid or scared (36), and overuse of screen time and added sugar (34). Demographic characteristics were found not to affect the frequency of SB in children (35, 36) and tooth wear was not found to be significantly associated with the frequency of SB in children with mixed dentition (39).
The paper dealing with palatal expansion on SB in children (40) showed reduction of parental-reported SB within the time of the study, but did not present long-term results.
Four investigations calculated the correlation of SB evaluated by means of parental report and clinical examination (tooth wear, Linea Alba, etc.) with genetics and family function (Table 4). All studies except one (44) were based on a case-control design (41–43) with comparison groups of SB vs. non-SB in children. The authors used parental reports of SB and a comprehensive clinical assessment (41, 42) or parental-reported SB and presence of tooth wear (43, 44) to evaluate bruxism.
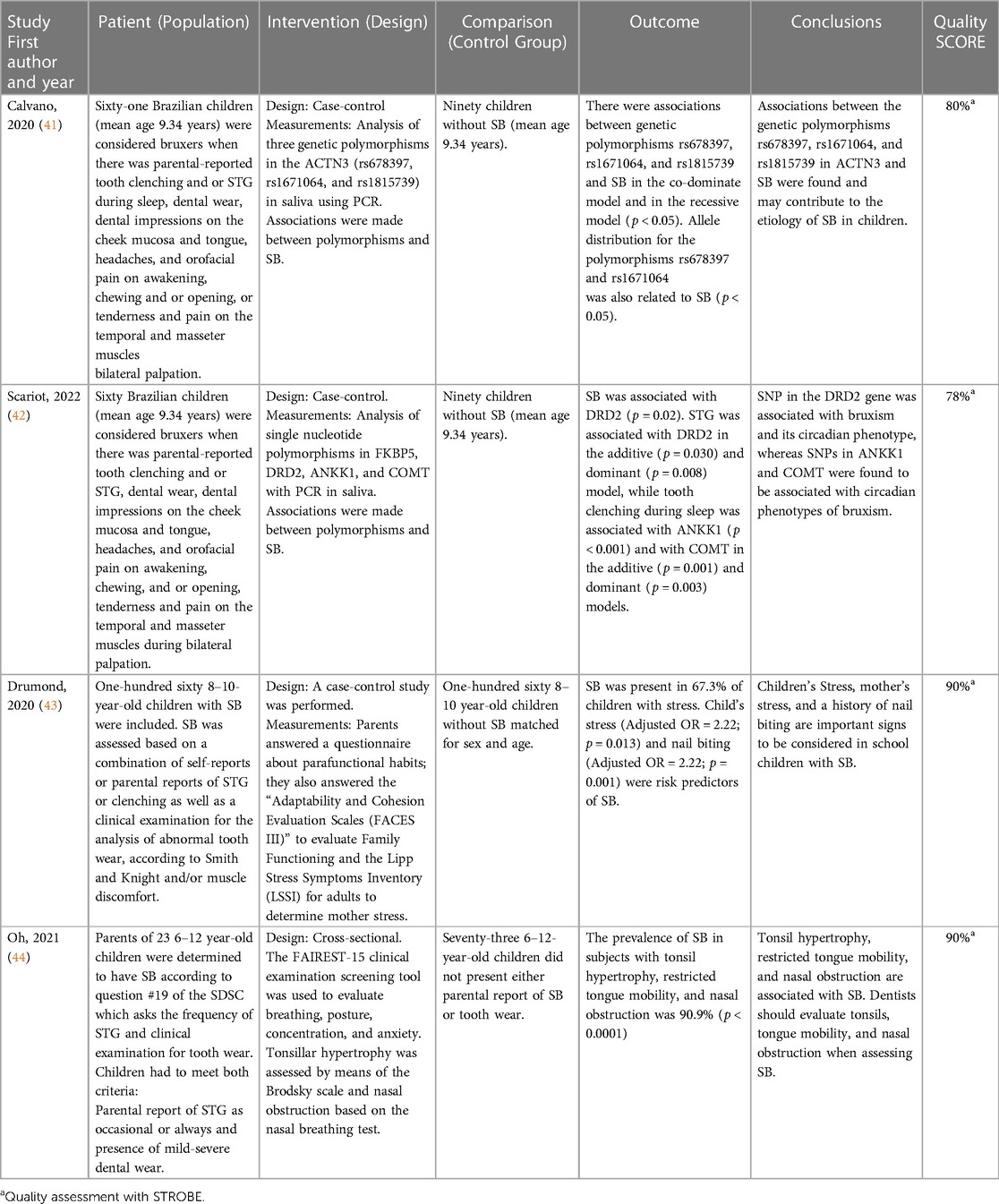
Table 4. PICO-like structured Reading of reviewed articles using parental report and clinical assessment of sleep bruxism as assessment strategy.
The two studies dealing with genetics found certain polymorphisms of Dopamine and Serotonin associated with bruxism (p < 0.05) (41, 42). Another study concluded that nail-biting and mother's and children's stress increase the odds of having SB (OR > 2; p < 0.02) (43). Finally, one study determined that the prevalence of SB among individuals with tonsil hypertrophy, restricted tongue mobility, and nasal obstruction was 90.9% (p < 0.05) (44).
One study evaluated the accuracy of PSG as a tool to assess SB in children (45), another determined the correlation between an EMG device (Grindcare measure) and PSG to evaluate SB/MMA in children (46), and two more evaluated the sleep architecture and physiology in children with SB (47, 48). One last investigation observed the effect of a Mandibular Advancement Device (MAD) to reduce SB by means of the BiteStrip® EMG device and obstructive sleep apnea by means of the ApneaLinkTM Plus in children (49) (Table 5). Subjects were recruited either among patients of a medical hospital (49) or university dental clinics (45–47). The assessment of SB with portable devices used in both studies with EMG for in-home recording were based on single-channel recording (46, 49). In one study, the researchers placed the EMG surface electrode (Grindcare measure) on the temporal muscle for five nights (46) and, in the other one, the BiteStrip® (49) was placed in the masseter muscle for 60 nights.
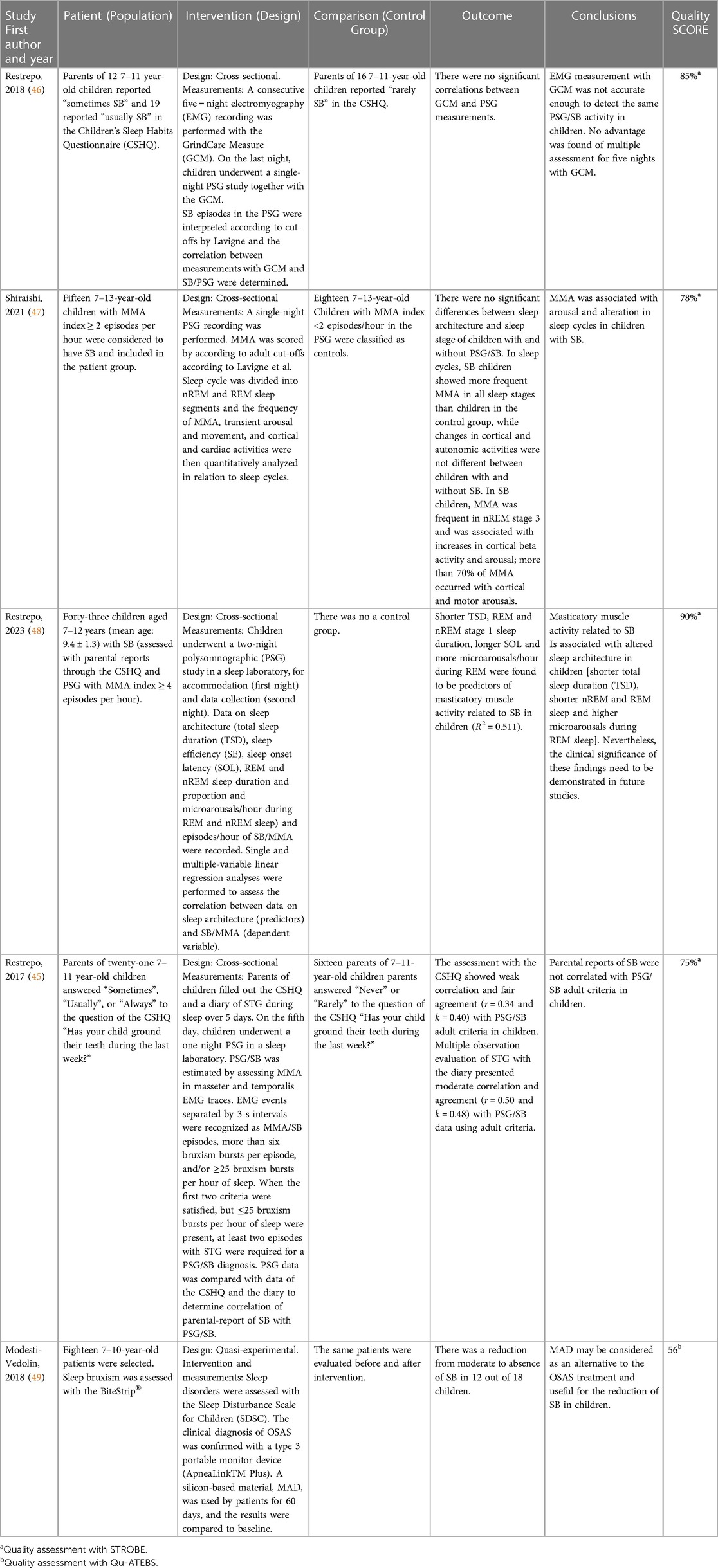
Table 5. PICO-like structured Reading of reviewed articles using EMG and/or PSG either as assessment strategy or outcome measure.
All studies were based on a dichotomous assessment of presence/absence of bruxism, based on a cutoff threshold of two or four EMG episodes per hour of sleep (45–49), even though in one study, the number of EMG events were considered as a continuous variable (48).
As for the findings, two studies did not retrieve any significant associations between SB/MMA and parental-reported SB (45) or between EMG for in-home recording with the Grindcare Measure and SB/MMA in the PSG recordings (46). Regarding the behavior of sleep in children with SB, sleep architecture and sleep MMA in the different stages of sleep significantly differed between children with and without SB. In sleep cycles, SB children showed more frequent MMA in all sleep stages than controls, while cyclic changes in cortical and autonomic activities did not significantly differ between the two groups. In SB children, MMA was the most prevalent in nREM stage 3 and was associated with increases in cortical beta activity and arousal. More than 70% of MMA occurred with cortical and motor arousals (47). Regarding treatment, one study evaluated the effect of a mandibular advancement device (MAD) for the treatment of obstructive sleep apnea syndrome (OSAS) in pediatric patients and one of the outcome measures of that study was the reduction of SB, which went from moderate to absent in 12 of 18 patients (49).
The objective of this review was to compilate the best available evidence about SB in children from the last seven years and to give evidence-based recommendations for pediatric healthcare givers to treat bruxism in the clinic. The results showed that there is available literature using only parental-report of SB as well as parental-report and clinical symptoms of SB (e.g., tooth wear, Linea Alba, impressions in the mucosa and tongue, headaches, and orofacial pain on awakening, chewing and/or opening, and tenderness and pain on the temporal and masseter muscles during bilateral palpation) and SB/PSG. Epidemiological as well as case-control and intervention studies were found. The investigations dealt with genetics, sleep behaviors and architecture, lifestyle and habits, and quality of life, diet, and assessment of reliability of diagnostic tools and parental reports of SB.
The experimental studies addressed the intervention of SB from its origin, evaluating the effect of two types of orthopedic devices (e.g., McNamara and MAD) to reduce the obstruction of the airway aiming to reduce parental-reported SB and SB episodes. Below, each of the topics found in the review will be discussed.
According to the results of this review, there is an association between SB and expression of certain polymorphisms (41) of Dopamine and Serotonin, which are related to coping strategies, the regulation of the wake-sleep cycle and the regulation of hunger and satiety (50). Regarding the Dopaminergic genes, the DRD2, DRD3, and DRD5 receptors (51, 52) represent a predisposing genetic pattern for SB in adults, but DRD2 and catechol-o-methyltransferase (COMT) have been related to SB in children (42), as shown in this review. COMT and DRD2 are associated with disruption of the reward system in the brain (53), stress, authoritarian parenting patterns (54), and deleterious changes in the quality of life (55) of children. These patterns, in turn, have also been recognized in children with SB at the school stage (34, 36).
As will be mentioned later in this review, SB has a close relationship with OSA. In this regard, in adults, the DRD1 rs686 may indicate a predisposition to BS caused by obstructive sleep apnea and or hypopnea (OSA), and the HTR2A rs2770304 polymorphism could contribute to the association between SB and OSA (56). In this review of the literature, these polymorphisms were not related to SB in children. Further studies are necessary to determine the relationship between the genetic factors associated both with respiratory disturbances (e.g., OSA) and SB in children.
Two studies were included in this review that deal with sleep architecture of SB in children (47, 48). One showed that SB was more frequent in nREM 3 (47), while the other indicated that sleep architecture of children with SB was more affected in REM than in nREM sleep (48). This is in agreement with the results of a previous investigation that found that the episodes of SB occur primarily during nREM 2 and during REM sleep (57). In the three studies (47, 48, 57), a relevant percentage of bruxism episodes were associated with arousal. Additionally, within the limitations of the study, Restrepo et al. (48) demonstrated that the odds of high masticatory muscle activity related to SB increased when there was high sleep onset latency, short sleep duration, short REM sleep duration, and high microarousals/hour during REM sleep.
A significant correlation between SB and snoring has been supported by several studies in children (35, 44). Although studies have suggested concomitant occurrence of SB in individuals with OSA, a narrow upper airway, rather than an obstruction of the upper airway, could be a factor contributing to the relationship between snoring and SB in children (58). OSA and its relationship to sleep bruxism is related to an arousal response that is often generated by hypoxemia and respiratory difficulty that triggers a serotoninergic neuronal reaction (56). At the end of an apneic event, frequent snoring, mumbling, gasping, and or STG occur (59). It is imperative to recognize these symptoms early, as children with SB can have a high likelihood of showing problematic daytime behavior that can also be frequently associated with sleep problems (60). Thus, evaluating the upper airway and using other diagnostic tools such as cephalograms and panoramic x-rays to determine airway dimensions and/or obstruction is mandatory in the dental room to search for a transdisciplinary assessment and intervention.
GERD acidifies the gastric tract and causes contraction of the airway, triggering SB/MMA in order to increase the buffer capacity by stimulating the salivary glands (61). Bruxism patients who experience GERD for extensive periods of time are prone to severe tooth wear. Gastric juice has greater erosive effects on both enamel and dentin (62) compared to acids from diet (63). This may be the reason why, when excluding from studies children with GERD, increasing frequency of SB was not correlated with the severity of tooth wear, as demonstrated in one of the articles included in this review (39). According to this evidence, it is mandatory to change the procedures to determine the origin of tooth wear and rethinking tooth wear as a diagnostic criterion to assess SB in children.
Quality of life (QoL) is emerging as an important outcome measure in the current medical literature, but available data on its relationship with SB in children are still scarce. As stated before, polymorphisms in DRD2 related to bruxism phenotypes in children (42) is a genetic variation in the dopamine receptor D2 (DRD2) that may alter dopamine signaling and modify the rewarding effects of food (64) and videogame playing (65) and could explain the relationship between SB, sugar, and screen overuse (34). The consumption of added sugar and excessive screen-time is increasing worldwide (66), increasing the prevalence of sleep problems (67, 68), psychosocial disorders (69, 70), lack of cortisol homeostasis (71), depression and hostility, and Attention Deficit Hyperactivity Disorder-related symptoms (72). All these issues have also been associated with SB in children (35).
On the same hand, alterations in QoL have been associated with SB in children, particularly effects on working memory and emotional regulation (36). Excessive screen-time and added sugar consumption are also risk factors for the same QoL issues (73, 74).
Anxiety in childhood is a frequent occurrence (75). However, it is underdiagnosed because of diverse symptomatology according to the different phases of development. Additionally, the concept of education is changing, and family structure and parenting style are also changing. Parenting requires spending a lot of time and resources on caring for and nurturing children (75). In addition, the unavailability of educational resources, the comparison between peers, and the lack of parenting experience increase parents' and children's tension and anxiety. Mothers have the highest influence on children, as they have the most contact with them (75). This may be the reason why studies have found that there is a correlation between mothers' and children's emotions. As a matter of fact, one of the studies included in this review (43) found that elevated anxiety levels of mothers increase the risk of having SB in children. Additionally, the same study found nail-biting as a predictor of SB and proposed that this could be a consequence of anxiety (43). This is the main reason why it is so important to evaluate the children's relationship with their mothers and the rest of their families when addressing SB in children in the clinic.
The two investigations that were included in this review regarding intervention were focused on improving airway patency (40, 49) in order to reduce the mechanism that triggers SB in children. Other studies evaluating strategies to treat anxiety, psychological issues, and parafunctional habits associated with SB were not included in this review, even though they are extremely necessary.
The study by Giannasi et al. (40) evaluated the parental reports of SB before and after palatal expansion with the McNamara device to increase nasal volume and airflow. The other investigation (49) increased oropharynx dimensions by using MAD in children with respiratory disturbances and the measurement of SB was made with PSG (49). In both cases, the report of SB and SB/MMA decreased.
The quality of studies about bruxism in children varies widely. All studies included in this systematic review were well-conducted, using appropriate study designs and methods. However, there are limitations, such as small sample sizes, lack of appropriate control groups and control of bias, and unclear measures of outcomes. Additionally, there is a lack of consensus about the assessment and treatment of bruxism in children, which leads to variability in study results.
One of the main challenges in conducting studies about SB in children is the difficulty in diagnosing the condition. Bruxism is often identified by parental reports of grinding or clenching teeth during sleep and/or clinical examination, but even the reports and clinical examination are not standardized or accurate in identifying the condition.
Another challenge is the lack of consensus about the etiology of bruxism. Some studies suggest that it is related to psychological factors, such as anxiety or stress, while others point to quality of life, lifestyle and habits, diet, or other medical conditions. This variability in etiology can make it challenging to design studies that adequately control for confounding factors and is difficult to review the studies to synthetize the evidence.
Despite these challenges, there have been several well-conducted studies on SB in children that provide insights into the prevalence, risk factors, outcomes, and intervention of the condition. These studies have highlighted the importance of paying attention to what is generating SB and intervening when necessary.
However, more high-quality studies are needed to better understand the underlying mechanisms, risk factors, and effective interventions for bruxism in children. Future studies should strive to use appropriate study designs and methods, include appropriate controls and measures, and use consistent definitions and diagnostic criteria to improve the quality and comparability of study findings.
Sleep bruxism is a symptom of underlying health, biopsychosocial, lifestyle, and diet conditions of children and their families. According to the findings in this review, methods of SB assessment are heterogeneous and hamper a reliable comparison of the results. However, with the available literature, it is possible to propose a flowchart based on an algorithm to assess and intervene with SB in the clinic (Figure 2). This algorithm contains the main variables that have been studied regarding SB in children and proposes a transdisciplinary approach.
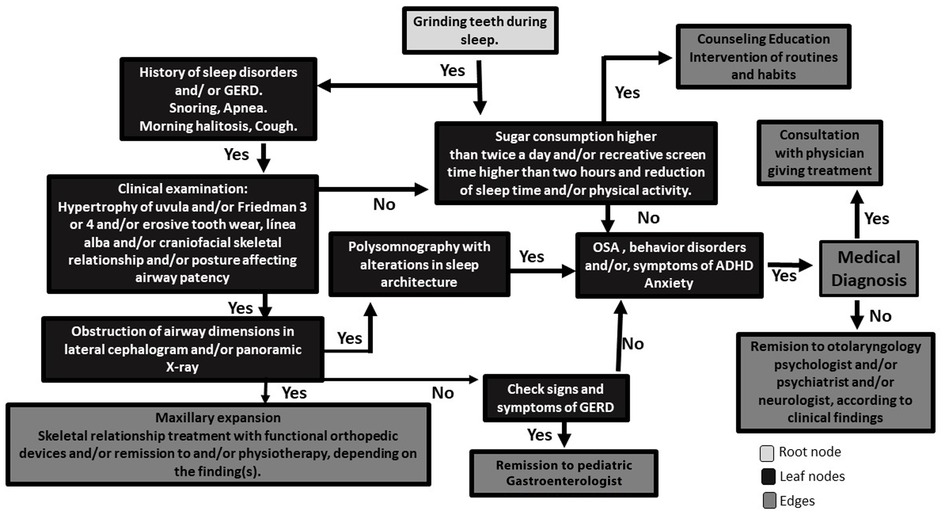
Figure 2. Flowchart based on an algorithm to assess and intervene against SB in the clinic. Starting from the root node (grinding teeth during sleep), go to the next nodes (search for symptoms of underlying diseases and dietary and lifestyle habits). Once you reach the leaf node, the node tells you the predicted outcome. Then the edges are subsets to look at, depending on the Yes/No answers to the steps of the algorithm.
CR-S and EW conceived the review. CR-S and EW read and analyzed the titles and the articles. Both authors wrote the results and discussion. All authors drafted the manuscript and designed the tables. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2023.1166091/full#supplementary-material.
1. Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. (2018) 45:837–44. doi: 10.1111/joor.12663
2. Raphael KG, Santiago V, Lobbezoo F. Is bruxism a disorder or a behavior? Rethinking the international consensus on defining and grading of bruxism. J Oral Rehabil. (2016) 43:791–8. doi: 10.1111/joor.12413
3. Svensson P, Lavigne G. Clinical bruxism semantics beyond academic debates: normo- and patho-bruxism as a new proposal. J Oral Rehabil. (2020) 47:547–8. doi: 10.1111/joor.12967
4. Lobbezoo F, Naeije M. Bruxism is mainly regulated centrally, not peripherally. J Oral Rehabil. (2001) 28:1085–91. doi: 10.1046/j.1365-2842.2001.00839.x
5. Abe Y, Suganuma T, Ishii M, Yamamoto G, Gunji T, Clark GT, et al. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res. (2012) 21:289–96. doi: 10.1111/j.1365-2869.2011.00961.x
6. Oporto GH 5th, Bornhardt T, Iturriaga V, Salazar LA. Single nucleotide polymorphisms in genes of dopaminergic pathways are associated with bruxism. Clin Oral Investig. (2018) 22:331–7. doi: 10.1007/s00784-017-2117-z
7. Rintakoski K, Hublin C, Lobbezoo F, Rose RJ, Kaprio J. Genetic factors account for half of the phenotypic variance in liability to sleep-related bruxism in young adults: a nationwide Finnish twin cohort study. Twin Res Hum Genet. (2012) 15:714–9. doi: 10.1017/thg.2012.54
8. Ahlberg J, Piirtola M, Lobbezoo F, Manfredini D, Korhonen T, Aarab G, et al. Correlates and genetics of self-reported sleep and awake bruxism in a nationwide twin cohort. J Oral Rehabil. (2020) 47:1110–19. doi: 10.1111/joor.13042
9. Lobbezoo F, Visscher CM, Koutris M, Wetselaar P, Aarab G. Bruxism in dentists’ families. J Oral Rehabil. (2018) 45:657–8. doi: 10.1111/joor.12648
10. Smardz J, Martynowicz H, Wojakowska A, Wezgowiec J, Danel D, Mazur G, et al. Lower serotonin levels in severe sleep bruxism and its association with sleep, heart rate, and body mass index. J Oral Rehabil. (2022) 49:422–9. doi: 10.1111/joor.13295
11. Suwa S, Takahara M, Shirakawa S, Komada Y, Sasaguri K, Onozuka M, et al. Sleep bruxism and its relationship to sleep habits and lifestyle of elementary school children in Japan. Sleep Biol. Rhythms. (2009) 7:93–102. doi: 10.1111/j.1479-8425.2009.00394.x
12. Ahlberg J, Lobbezoo F, Ahlberg K, Manfredini D, Hublin C, Sinisalo J, et al. Self-reported bruxism mirrors anxiety and stress in adults. Med Oral Patol Oral Cir Bucal. (2013) 18:7–11. doi: 10.4317/medoral.18232
13. Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. (2009) 23:153–66.19492540
14. Serra-Negra JM, Ribeiro MB, Prado IM, Paiva SM, Pordeus IA. Association between possible sleep bruxism and sleep characteristics in children. Cranio. (2017) 35:315–20. doi: 10.1080/08869634.2016.1239894
15. Sakaguchi K, Yagi T, Maeda A, Nagayama K, Uehara S, Saito-Sakoguchi Y, et al. Association of problem behavior with sleep problems and gastroesophageal reflux symptoms. Pediatr Int. (2014) 56:24–30. doi: 10.1111/ped.12201
16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE)statement: guidelines for reporting observational studies. J Clin Epidemiol. (2008) 61:344–9. doi: 10.1016/j.jclinepi.2007.11.008
17. Dawson A, Raphael KG, Glaros A, Axelsson S, Arima T, Ernberg M, et al. Development of a quality-assessment tool for experimental bruxism studies: reliability and validity. J Orofac Pain. (2013) 27:111–22. doi: 10.11607/jop.1065
18. Guo H, Wang T, Niu X, Wang H, Yang W, Qiu J, et al. The risk factors related to bruxism in children: a systematic review and meta-analysis. Arch Oral Biol. (2018) 86:18–34. doi: 10.1016/j.archoralbio.2017.11.004
19. Ribeiro-Lages MB, Jural LA, Magno MB, Vicente-Gomila J, Ferreira DM, Fonseca-Gonçalves A, et al. A world panorama of bruxism in children and adolescents with emphasis on associated sleep features: a bibliometric analysis. J Oral Rehabil. (2021) 48:1271–82. doi: 10.1111/joor.13249
20. Lin LZ, Xu SL, Wu QZ, Zhou Y, Ma HM, Chen DH, et al. Exposure to second-hand smoke during early life and subsequent sleep problems in children: a population-based cross-sectional study. Environ Health. (2021) 20:127. doi: 10.1186/s12940-021-00793-0
21. Sampaio NM, Oliveira MC, Andrade AC, Santos LB, Sampaio M, Ortega A. Relationship between stress and sleep bruxism in children and their mothers: a case control study. Sleep Sci. (2018) 11:239–44. doi: 10.5935/1984-0063.20180038
22. Kobayashi FY, Castelo PM, Politti F, Rocha MM, Beltramin RZ, Salgueiro MDCC, et al. Immediate evaluation of the effect of infrared LED photobiomodulation on childhood sleep bruxism: a randomized clinical trial. Life (Basel). (2022) 12:964. doi: 10.3390/life12070964
23. Oliveira MT, Bittencourt ST, Marcon K, Destro S, Pereira JR. Sleep bruxism and anxiety level in children. Braz Oral Res. (2015) 29:S1806-83242015000100221. doi: 10.1590/1807-3107BOR-2015.vol29.0024
24. Alfano CA, Bower JL, Meers JM. Polysomnography-detected bruxism in children is associated with somatic complaints but not anxiety. J Clin Sleep Med. (2018) 14:23–9. doi: 10.5664/jcsm.6872
25. de Alencar NA, Leão CS, Leão ATT, Luiz RR, Fonseca-Gonçalves A, Maia LC. Sleep bruxism and anxiety impacts in quality of life related to oral health of Brazilian children and their families. J Clin Pediatr Dent. (2017) 41:179–85. doi: 10.17796/1053-4628-41.3.179
26. Ceyhan E, Hasirci E, Gezgin O, Senirkentli GB, Aygun YC. Are lower urinary tract conditions more common in children with sleep bruxism? J Pediatr Urol. (2022) 1:S1477–5131. doi: 10.1016/j.jpurol.2022.11.024
27. Soares-Silva L, Tavares-Silva C, Fonseca-Gonçalves A, Maia LC. Presence of oral habits and their association with the trait of anxiety in pediatric patients with possible sleep bruxism. J Indian Soc Pedod Prev Dent. (2019) 37:245–50. doi: 10.4103/JISPPD.JISPPD_272_18
28. Huynh NT, Desplats E, Bellerive A. Sleep bruxism in children: sleep studies correlate poorly with parental reports. Sleep Med. (2016) 19:63–8. doi: 10.1016/j.sleep.2015.09.023
29. Bellerive A, Montpetit A, El-Khatib H, Carra MC, Remise C, Desplats E, et al. The effect of rapid palatal expansion on sleep bruxism in children. Sleep Breath. (2015) 19:1265–71. doi: 10.1007/s11325-015-1156-4
30. Martins IM, Alonso LS, Vale MP, Abreu LG, Serra-Negra JM. Association between the severity of possible sleep bruxism and possible awake bruxism and attrition tooth wear facets in children and adolescents. Cranio. (2022) Jul 25:1–7. doi: 10.1080/08869634.2022.2102708
31. Gomes MC, Neves ÉT, Perazzo MF, Souza EGC, Serra-Negra JM, Paiva SM, et al. Evaluation of the association of bruxism, psychosocial and sociodemographic factors in preschoolers. Braz Oral Res. (2018) 32:e009. doi: 10.1590/1807-3107bor-2018.vol32.0009
32. Massignan C, de Alencar NA, Soares JP, Santana CM, Serra-Negra J, Bolan M, et al. Poor sleep quality and prevalence of probable sleep bruxism in primary and mixed dentitions: a cross-sectional study. Sleep Breath. (2019) 23:935–41. doi: 10.1007/s11325-018-1771-y
33. Tavares-Silva C, Holandino C, Homsani F, Luiz RR, Prodestino J, Farah A, et al. Homeopathic medicine of Melissa officinalis combined or not with Phytolacca decandra in the treatment of possible sleep bruxism in children: a crossover randomized triple-blinded controlled clinical trial. Phytomedicine. (2019) 58:152869. doi: 10.1016/j.phymed.2019.152869
34. Restrepo C, Santamaría A, Manrique R. Sleep bruxism in children: relationship with screen-time and sugar consumption. Sleep Med X. (2021) 3:100035. doi: 10.1016/j.sleepx.2021.100035
35. Restrepo C, Manfredini D, Lobbezoo F. Sleep behaviors in children with different frequencies of parental-reported sleep bruxism. J Dent. (2017) 66:83–90. doi: 10.1016/j.jdent.2017.08.005
36. Manfredini D, Lobbezoo F, Giancristofaro RA, Restrepo C. Association between proxy-reported sleep bruxism and quality of life aspects in Colombian children of different social layers. Clin Oral Investig. (2017) 21:1351–8. doi: 10.1007/s00784-016-1901-5
37. Yazıcıoğlu İ, Ray PÇ. Evaluation of anxiety levels in children and their mothers and appearance of sleep bruxism in turkish children and associated risk factors: a cross-sectional study. J Oral Facial Pain Headache. (2022) 36:147–54. doi: 10.11607/ofph.3011
38. Garmroudinezhad Rostami E, Touchette É, Huynh N, Montplaisir J, Tremblay RE, Battaglia M, et al. High separation anxiety trajectory in early childhood is a risk factor for sleep bruxism at age 7. Sleep. (2020) 43:zsz317. doi: 10.1093/sleep/zsz317
39. Restrepo C, Manfredini D, Manrique R, Lobbezoo F. Association of dietary habits and parental-reported sleep tooth grinding with tooth wear in children with mixed dentition. BMC Oral Health. (2017) 17:156. doi: 10.1186/s12903-017-0447-5
40. Giannasi LC, Santos IR, Alfaya TA, Bussadori SK, Leitão-Filho FS, de Oliveira LV. Effect of a rapid maxillary expansion on snoring and sleep in children: a pilot study. Cranio. (2015) 33:169–73. doi: 10.1179/2151090314Y.0000000029
41. Calvano Küchler E, Arid J, Palinkas M, Ayumi Omori M, de Lara RM, Napolitano Gonçalves LM, et al. Genetic polymorphisms in ACTN3 contribute to the etiology of bruxism in children. J Clin Pediatr Dent. (2020) 44:180–4. doi: 10.17796/1053-4625-44.3.8
42. Scariot R, Brunet L, Olsson B, Palinkas M, Regalo SCH, Rebellato NLB, et al. Single nucleotide polymorphisms in dopamine receptor D2 are associated with bruxism and its circadian phenotypes in children. Cranio. (2022) 40:152–9. doi: 10.1080/08869634.2019.1705629
43. Drumond CL, Paiva SM, Vieira-Andrade RG, Ramos-Jorge J, Ramos-Jorge ML, Provini F, et al. Do family functioning and mothers’ and children’s stress increase the odds of probable sleep bruxism among schoolchildren? A case control study. Clin Oral Investig. (2020) 24:1025–33. doi: 10.1007/s00784-019-02997-8
44. Oh JS, Zaghi S, Ghodousi N, Peterson C, Silva D, Lavigne GJ, et al. Determinants of probable sleep bruxism in a pediatric mixed dentition population: a multivariate analysis of mouth vs. Nasal breathing, tongue mobility, and tonsil size. Sleep Med. (2021) 77:7–13. doi: 10.1016/j.sleep.2020.11.007
45. Restrepo C, Manfredini D, Castrillon E, Svensson P, Santamaria A, Alvarez C, et al. Diagnostic accuracy of the use of parental-reported sleep bruxism in a polysomnographic study in children. Int J Paediatr Dent. (2017) 27:318–25. doi: 10.1111/ipd.12262
46. Restrepo C, Lobbezoo F, Castrillon E, Svensson P, Santamaria A, Alvarez C, et al. Agreement between jaw-muscle activity measurement with portable single-channel electromyography and polysomnography in children. Int J Paediatr Dent. (2018) 28:33–42. doi: 10.1111/ipd.12308
47. Shiraishi Y, Tachibana M, Shirota A, Mohri I, Taniike M, Yamashiro T, et al. Relationships between cortical, cardiac, and arousal-motor activities in the genesis of rhythmic masticatory muscle activity across sleep cycles in primary sleep bruxism children. Sleep. (2021) 44:zsab156. doi: 10.1093/sleep/zsab156
48. Restrepo C, Lobbezoo F, Castrillon E, Svensson P, Santamaria A, Manfredini D. Correlations between sleep architecture and sleep-related masseter muscle activity in children with sleep bruxism. J Oral Rehabil. (2023):Feb 15. doi: 10.1111/joor.13430. [Epub ahead of print]
49. Modesti-Vedolin G, Chies C, Chaves-Fagondes S, Piza-Pelizzer E, Lima-Grossi M. Efficacy of a mandibular advancement intraoral appliance (MOA) for the treatment of obstructive sleep apnea syndrome (OSAS) in pediatric patients: a pilot-study. Med Oral Patol Oral Cir Bucal. (2018) 23:e656–63. doi: 10.4317/medoral.22580
50. Lechin F, Pardey-Maldonado B, van der Dijs B, Benaim M, Baez S, Orozco B, et al. Circulating neurotransmitters during the different wake-sleep stages in normal subjects. Psychoneuroendocrinology. (2004) 29:669–85. doi: 10.1016/S0306-4530(03)00095-7
51. Oporto GH, Bornhardt T, Iturriaga V, Salazar LA. Genetic polymorphisms in the serotonergic system are associated with circadian manifestations of bruxism. J Oral Rehabil. (2016) 43:805–12. doi: 10.1111/joor.12436
52. Cruz-Fierro N, Martínez-Fierro M, Cerda-Flores RM, Gómez-Govea MA, Delgado-Enciso I, Martínez-De-Villarreal LE, et al. The phenotype, psychotype and genotype of bruxism. Biomed Rep. (2018) 8:264–8. doi: 10.3892/br.2018.1041
53. Macare C, Ducci F, Zhang Y, Ruggeri B, Jia T, Kaakinen M, et al. A neurobiological pathway to smoking in adolescence: tTC12-ANKK1-DRD2 variants and reward response. Eur Neuropsychopharmacol. (2018) 28:1103–14. doi: 10.1016/j.euroneuro.2018.07.101
54. Si S, Su Y, Zhang S, Zhang J. Genetic susceptibility to parenting style: DRD2 and COMT influence creativity. Neuroimage. (2020) 213:116681. doi: 10.1016/j.neuroimage.2020.116681
55. Senzaki S, Pott U, Shinohara I, Moriguchi Y. Roles of culture and COMT Val58Met gene on neural basis of executive function: a comparison between Japanese and American children. Dev Psychobiol. (2021) 63:1053–60. doi: 10.1002/dev.22087
56. Wieckiewicz M, Bogunia-Kubik K, Mazur G, Danel D, Smardz J, Wojakowska A, et al. Genetic basis of sleep bruxism and sleep apnea-response to a medical puzzle. Sci Rep. (2020) 10:7497. doi: 10.1038/s41598-020-64615-y
57. Herrera M, Valencia I, Grant M, Metroka D, Chialastri A, Kothare SV. Bruxism in children: effect on sleep architecture and daytime cognitive performance and behavior. Sleep. (2006) 29:1143–8. doi: 10.1093/sleep/29.9.1143
58. Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin. (2012) 56:387–413. doi: 10.1016/j.cden.2012.01.003
59. Saito M, Yamaguchi T, Mikami S, Watanabe K, Gotouda A, Okada K, et al. Temporal association between sleep apnea–hypopnea and sleep bruxism events. J Sleep Res. (2014) 23:196–203. doi: 10.1111/jsr.12099
60. Tachibana M, Kato T, Kato_Nishimura K, Matsuzawa S, Mohri I, Taniike M. Associations of sleep bruxism with age, sleep apnea, and daytime problematic behaviors in children. Oral Dis. (2016) 22:557–65. doi: 10.1111/odi.12492
61. Li Y, Yu F, Niu L, Hu W, Long Y, Tay FR, et al. Associations among bruxism, gastroesophageal reflux disease, and tooth wear. J Clin Med. (2018) 7:417. doi: 10.3390/jcm7110417
62. Braga SR, De Faria DL, De Oliveira E, Sobral MA. Morphological and mineral analysis of dental enamel after erosive challenge in gastric juice and orange juice. Microsc. Res. Tech. (2011) 74:1083–7. doi: 10.1002/jemt.20998
63. Bartlett DW, Coward PY. Comparison of the erosive potential of gastric juice and a carbonated drink in vitro. J. Oral Rehabil. (2001) 28:1045–7. doi: 10.1046/j.1365-2842.2001.00780.x
64. Ramos-Lopez O, Panduro A, Rivera-Iñiguez I, Roman S. Dopamine D2 receptor polymorphism (C957T) is associated with sugar consumption and triglyceride levels in West Mexicans. Physiol Behav. (2018 Oct 1) 194:532–7. doi: 10.1016/j.physbeh.2018.07.004
65. Weinstein A, Livny A, Weizman A. New developments in brain research of internet and gaming disorder. Neurosci Biobehav Rev. (2017) 75:314–30. doi: 10.1016/j.neubiorev.2017.01.040
66. Fisberg M, Kovalskys I, Gómez G, Rigotti A, Sanabria LYC, García MCY, et al. Total and added sugar intake: assessment in eight latin American countries. Nutrients. (2018) 10:389–96. doi: 10.3390/nu10040389
67. Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. (2015) 21:50–8. doi: 10.1016/j.smrv.2014.07.007
68. Janssen X, Martin A, Hughes AR, Hill CM, Kotronoulas G, Hesketh KR. Associations of screen time, sedentary time and physical activity with sleep in under 5s: a systematic review and meta-analysis. Sleep Med Rev. (2020) 49:101226. doi: 10.1016/j.smrv.2019.101226
69. Yen JY, Ko CH, Yen CF, Wu HY, Yang MJ. The comorbid psychiatric symptoms of internet addiction: attention deficit and hyperactivity disorder (ADHD), depression, social phobia, and hostility. J Adolesc Health. (2007) 41:93–8. doi: 10.1016/j.jadohealth.2007.02.002
70. Del-Ponte B, Quinte GC, Cruz S, Grellert M, Santos IS. Dietary patterns and attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. J Affect Disord. (2019) 252:160–73. doi: 10.1016/j.jad.2019.04.061
71. Wallenius M, Hirvonen A, Lindholm H, Rimpelä AH, Nygård C, Saarni L, et al. Salivary cortisol in relation to the use of information and communication technology (ICT) in school-aged children. Psychology. (2010) 1:88–95. doi: 10.4236/psych.2010.12012
72. Türkoğlu S, Akça ÖF, Türkoğlu G, Akça M. Psychiatric disorders and symptoms in children and adolescents with sleep bruxism. Sleep Breath. (2014) 18:649–54. doi: 10.1007/s11325-013-0928-y
73. Horowitz-Kraus T, Hutton JS. Brain connectivity in children is increased by the time they spend reading books and decreased by the length of exposure to screen-based media. Acta Paediatr. (2018) 107:685–93. doi: 10.1111/apa.14176
74. Loewen OK, Maximova K, Ekwaru JP, Faught EL, Asbridge M, Ohinmaa A, et al. Lifestyle behavior and mental health in early adolescence. Pediatrics. (2019) 143:e20183307. doi: 10.1542/peds.2018-3307
Keywords: sleep bruxism, children, anxiety, sugar, screen time, obstructive sleep apnea, sleep architecture
Citation: Restrepo-Serna C and Winocur E (2023) Sleep bruxism in children, from evidence to the clinic. A systematic review. Front. Oral. Health 4:1166091. doi: 10.3389/froh.2023.1166091
Received: 14 February 2023; Accepted: 12 April 2023;
Published: 11 May 2023.
Edited by:
Nikolaos Christidis, Karolinska Institutet (KI), SwedenReviewed by:
Malin Ernberg, Karolinska Institutet (KI), Sweden© 2023 Restrepo-Serna and Winocur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudia Restrepo Y3Jlc3RyZXBvc0BjZXMuZWR1LmNv
†The author has first authorship
‡The author has last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.