- 1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Thamar University, Dhamar, Yemen
- 2Oral and Maxillofacial Surgery, Department of Dentistry, All India Institute of Medical Sciences, Jodhpur, India
- 3Medical Microbiology and Hygiene, Marburg University Hospital, Marburg, Germany
- 4Department of Oral and Maxillofacial Surgery, University Hospital Marburg Universitätsklinikum Giessen und Marburg GmbH, Marburg, Germany
- 5Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden
Various dental, maxillofacial, and orthopedic surgical procedures (DMOSP) have been known to produce bioaerosols, that can lead to the transmission of various infectious diseases. Hence, a systematic review (SR) aimed at generating evidence of aerosols generating DMOSP that can result in the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), further investigating their infectivity and assessing the role of enhanced personal protective equipment (PPE) an essential to preventing the spreading of SARS-CoV-2 during aerosol-generating procedures (AGPs). This SR was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) guidelines based on a well-designed Population, Intervention, Comparison, Outcomes and Study (PICOS) framework, and various databases were searched to retrieve the studies which assessed potential aerosolization during DMOSP. This SR included 80 studies (59 dental and 21 orthopedic) with 7 SR, 47 humans, 5 cadaveric, 16 experimental, and 5 animal studies that confirmed the generation of small-sized < 5 μm particles in DMOSP. One study confirmed that HIV could be transmitted by aerosolized blood generated by an electric saw and bur. There is sufficient evidence that DMOSP generates an ample amount of bioaerosols, but the infectivity of these bioaerosols to transmit diseases like SARS-CoV-2 generates very weak evidence but still, this should be considered. Confirmation through isolation and culture of viable virus in the clinical environment should be pursued. An evidence provided by the current review was gathered by extrapolation from available experimental and empirical evidence not based on SARS-CoV-2. The results of the present review, therefore, should be interpreted with great caution.
Introduction
Pandemics are a never-ending entity, as the viruses end up being part of the ecosystem. In less than two decades, the world has faced a SARS outbreak (2002), a MERS outbreak (2012) and finally, at present, the healthcare systems are struggling with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The oral mucous membrane has been reported to have a high affinity for angiotensin-converting enzyme receptor 2 (ACE2) which is responsible for the entrance of the virus into human cells, then starting its replications [1]. Thus, the saliva may contain more viral load, and oral and maxillofacial surgeons face a substantial risk of exposure to SARS-CoV-2, as their actual field of work is close to both the oral cavity and the nasopharynx/oropharynx [2].
Aerosol-generating procedures (AGPs) are defined as any medical, dental, or patient care procedure that yields in the generation of airborne particles ≤ 5 μm in size [3]. Particles < 5 μm are produced by several dental procedures, posing an increased risk of transmission of respiratory infections such as COVID-19 [4, 5]. Aerosols thus refer to liquid and solid particles ( ≤ 5 μm) which dehydrate and thus retain in the air for hours before falling on the ground in a larger distance (>>2 m or 6 feet, respectively) or entering the respiratory system, whereas, droplets are described as larger entities (>5 μm) that rapidly drop to the ground due to the force of gravity, typically 3–6 feet of the carrier [6]. Droplets and splatter are a mixture of air, water, saliva, and/or solid particles, becoming visible to the naked eye when >50 μm.
All dental surgical procedures performed with a high-speed rotating handpiece, ultrasonic scaler, and water air syringe are AGPs that are mostly contaminated with blood, bacteria, viruses, and fungi [7–12]. Similar bioaerosols are generated by various orthopedic procedures owing to the use of high-speed, power drilling and cutting tools, electrocautery, and pulse lavage [13–15]. It is already a known fact that bone and tooth cutting with high-speed burs in combination with external irrigation produces aerosols, further tossing the particles into space [16–20]. Thus, dental, maxillofacial, and orthopedic procedures (DMOSP) are at the highest risk with increased bacterial and viral load [21–24].
Literature evidence is supported by scattered data that DMOSP generates various amounts of bioaerosols [24–28]. The maxillofacial procedures include simple extractions to complex bone drilling and cutting procedures, and there is a scarcity of data for isolated oral and maxillofacial procedures. Hence, a single review compiling all data was the need of the hour.
Thus, a systematic review was conducted to identify whether there is scientific evidence supporting that DMOSP is AGPs and whether bioaerosols produced at DMOSP can transmit SARS-CoV-2, thus leading to the transmission of COVID-19. Furthermore, there is still a conspicuous lack of scientific evidence substantiating that enhanced PPE is necessary to protect during oral and maxillofacial surgery (OMFS) when dealing with suspected or confirmed patients with COVID-19 in this current pandemic outbreak. The authors hypothesize that enhanced PPE using respirators (N95 or FFP 2/3) would be sufficient and more effective than standard PPE without respirators or equivalents in protecting dentists and surgeons against SARS-CoV-2 during AGPs in suspected and confirmed COVID-19 cases.
Methodology
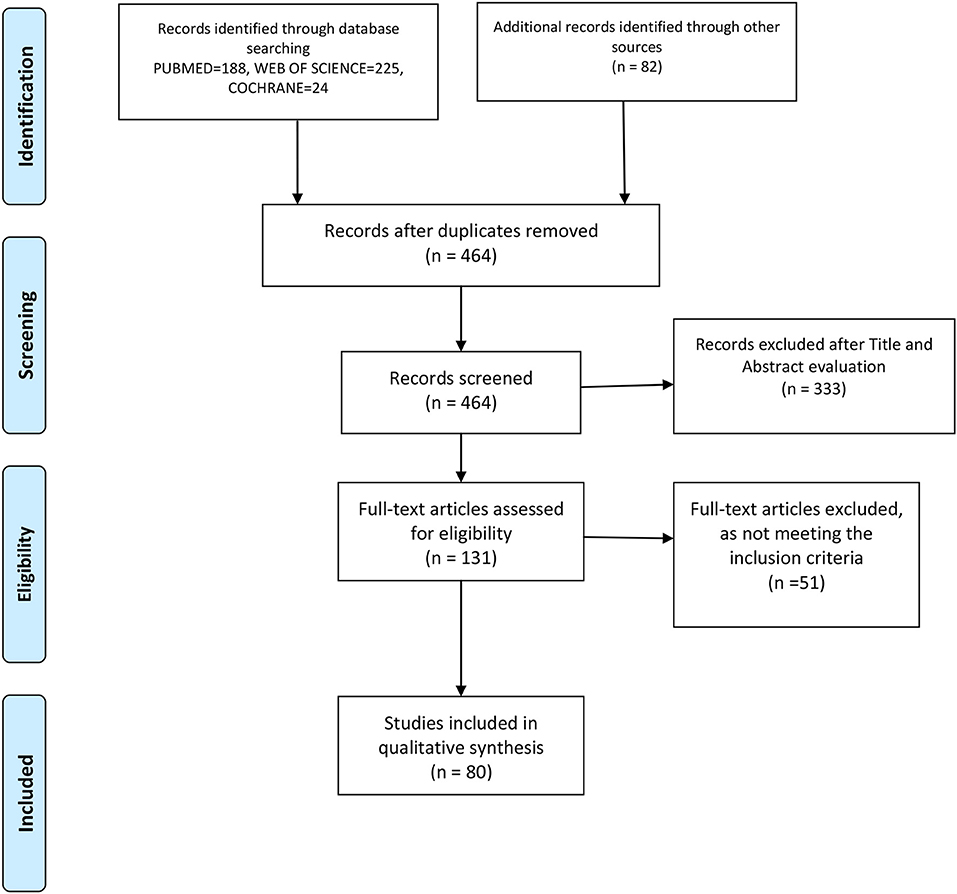
We performed this review following the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) [29], and the flow diagram is shown in Figure 1, in combination with the Network Meta-Analyses of Health Care Interventions, and was registered in PROSPERO with No. CRD42020192912.
Literature search
Two independent reviewers searched various databases, such as PUBMED, Cochrane, and Web of Science using the keywords “aerosols,” “bioaerosols,” “transmission,” “oral,” “microbes,” “maxillofacial,” and “orthopedics.” All the online databases of various oral and maxillofacial surgery journals (IJOMS, BJOMS, JOMS, and JOCMF) and orthopedic journals (Bone and Joint Journal, Spine, JBJS-America, and European Spine Journal) were searched robustly. All the gray literature was searched using references of the tentatively selected articles for further identification of potentially eligible articles, followed by random screening of the first 100 articles on Google Scholar. All English language articles whose full texts were available till 10th March 2022 were included in this study.
Selection criteria
Based on the objective of this rapid systematic review, there were two inclusion criteria based on the research question (PICOS) as shown in Table 1.
No restrictions were placed on the design of the article, publication year, or author's country. However, studies of non-English origin, conference abstracts, protocols, and case reports or lack of full-text availability were excluded from the current study.
Data extraction
The following information was extracted: authors, type of study, how outcomes were measured, type of surgical procedures, type of surgical instruments used, conclusion, evidence of aerosol generation, evidence of transmission risk, and type of microbial species.
Any disagreement regarding the eligibility or data extracted was discussed among themselves to finalize a decision.
Synthesis of results
The results were presented narratively to answer the prior authors' clinical questions as stated in Table 1. Inter-rater reliability was confirmed using the Kappa coefficient.
Results
Figure 1 is a flowchart on the process of article evaluation for inclusion in the present systematic review. Based on the literature search, out of 131 articles assessed for eligibility, only 80 were included in the qualitative analysis.
Characteristics of included studies
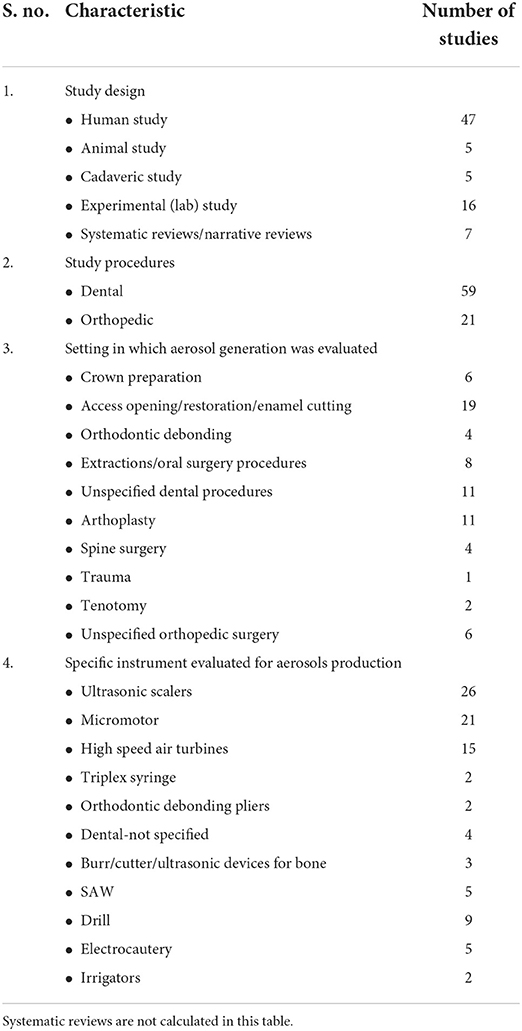
Characteristics of the included studies in this review have been summarized in Supplementary Table 1 with individual characteristics in Table 2.
Characteristics of aerosols generated in DMOSP
Aerosols spread
Aerosol spread was evaluated by the majority of the studies, with most studies measuring spread of bacteria during surgery with Petri dishes placed at regular intervals to identify aerial spread of microbes [16–18, 20, 30–67], The other methods used to confirm aerosol spread were laser beam for illumination with photographic analysis [21, 68], shadowgraphy [19], sodium fluorescein for illumination [4, 69], UV illumnination [70, 71].
Spectrophotometric analysis [72–74], gravimetric impactor [75], Kastle–Meyer test [76], particle sensor [37, 77], splashed area on face by magnification [78], particle counter [79–81], leucomalachite green presumptive test [10, 82], concentrations of hemoglobin in air [59, 67, 83–87], and air sampling [88, 89].
Particle size and composition
All systematic reviews [11, 20, 24, 25, 27, 28, 90] generation of small-sized < 5 μm particles in DMOSP. The particle size generated corresponds to droplet nuclei that could carry viruses [19]. The composition of dental aerosols differ between patients, but it consists of organic and inorganic particles.
Distance between two operator bays
Contamination chances are minimal in open-plan dental clinics at a distance of 5 m or more [73].
Blood in aerosols
Two authors [67, 87] observed that aerosols generated from electrocautery, drills, and saws in orthopedic surgeries contained hemoglobin. Even animal studies [15, 67, 85, 87, 91] confirmed Hb in aerosols. Similar results were found in human studies during oral and maxillofacial surgeries [76, 83, 84]. One of the studies even confirmed blood contamination of the internal part of the visor in 4% of cases after oral surgery procedures [76].
It was estimated that Hb inhaled by a surgeon in an hour range from 0.04 to 0.68 μg [24].
Contamination of personnel
Microorganisms were found on PPEs, such as sleeves, masks, face shields, and chest of the scrubs, justifying the usage of PPE to prevent further spread [26]. Contamination was evaluated with most contamination confined to the patient, operator, and assistant [72], and maximum concentration was found near the mouth of the dentist [31]. Most of the particles are concentrated ~60 cm from the patient's mouth [61].
HIV and other viruses in aerosols
ΦX174 phage was found in the aerosols generated after oral prophylaxis in a laboratory study [31]. Johnson et al. noted that HIV can be cultured from aerosolized particles from the bone saw, but not from te electrocautery [92]. Johnson et al. [85] estimated the inhalational risk of viruses for surgeons to be <1 HIV or 180 hepatitis B viruses per procedure.
Characteristics of aerosols generated by various instruments
Micromotor and conventional air turbines
Low speeds generated the least aerosol particles, and the largest aerosol particles and the high-speed handpiece generated the greatest amount and size of splatter particles [33]. Sergis et al. [93] identified a threshold for rotation speeds for radial atomization between 80,000 and 100,000 rpm. Droplet particle size of < 5 μm was only detectable above baseline levels at revolutions > 80,000 rpm [93]. Whereas, Clarkson et al. [90] confirmed respirable aerosols when the speed is >60,000 rpm. The contamination of face masks is usually seen with high-speed rotary instruments [59].
Scalers
Both piezoelectric scalers and ultrasonic scalers are prone to generate a higher level of aerosols [63]. Droplet size tends to vary from 5 to 300 μm corresponding to droplet nuclei that can contain viruses [94].
Triplex syringe
Triplex syringe generated the largest amount of aerosols (particle size: 1.73 ± 2.23 μm) [4].
Two studies [67, 87] have compared aerosols generated by powered bone cutting tools like saws and drills and electrocautery. The aerosols generated by these procedures have been subcategorized into small particles (0.3–0.5 μm), medium particles (0.5–5 μm), and large particles (>5 μm) as explained by Sharma et al. [24].
Saw
The oscillating saw tends to produce the majority of medium-sized particles (56–68%) along with small-sized particles (28–40%) [24].
Drill
Jewett et al. [87] compared high-speed, air-powered drills, which produce 47% medium, 38% large, and 17% small particles, to high-speed drills with continuous irrigation which produce 31% medium, 59% large, and 9% small-sized particles.
Electrocautery
Electrocautery shows a predominance of small-sized particles both in cutting and coagulation mode. “Cutting” mode produced 90–95% small-sized particles as compared to coagulation mode with 60–78% small-sized particles [24].
Interrater reliability
Interrater reliability was evaluated using Cohen's Kappa coefficient which came out to be 0.81 confirming a substantial level of agreement among the reviewers.
Overall evidence regarding whether DMOSP generating bioaerosols can result in the transmission of SARS-CoV-2
There is sufficient evidence from various studies that the surgical procedures which used high powered instruments that emit or require water for cooling like ultrasonic scalers, air-water syringes, air polishing, piezo surgical handpiece, extractions using motorized handpieces, as well as bone drilling with high-speed rotary instruments (>60,000 rpm), high powered drills, oscillating saws, and electrocoagulation (cutting and coagulation mode) produce respirable aerosols which are <0.5 μm [24–28, 89, 95–97].
The patient, operator, and assistant are at the maximum risk of exposure [72] as the small particles tend to be retained in the respiratory tract. Thus, conventional masks seem insufficient against high-risk AGPs [25].
For these procedures, airborne transmission-based precautions using full PPE, procedural mitigation (high volume suction, rubber dam, preprocedural mouth rinses, and antimicrobial coolants), and 15–30 min as fallow time (the time required to allow larger droplets to settle before environmental cleaning) are required along with N95 [90]. In the operating room, the best way to decrease aerosol load is OR ventilation, with 15 air changes per hour removing 90% aerosols within 15–20 min [98, 99].
For those dental surgical procedures that use powered low-velocity instruments and may produce droplet particles > 5 μm, including 3-in-1 syringe (air-only/water-only), slow speed/electric handpiece (i.e., <60,000 rpm), surgical implant procedure, and surgical handpiece, standard PPE, and procedural mitigation without fallow time are required [90].
Finally, for those dental surgical procedures which do not use powered instruments and may produce splatter but are unlikely to produce aerosol particles < 5 μm such as tooth extraction (using forceps/elevator), manual scaling, inhalation sedation, local anesthetic administration, and standard PPE without procedural mitigation and fallow time will be sufficient [90].
In addition, there is inconclusive evidence to support the creation of infectious aerosols during DMOSP, and their potential to transmit infectious diseases like SARS CoV-2 is questionable.
Discussion
COVID-19 as a pandemic is currently far away from being contained in a majority of countries and represents a serious potential threat to healthcare workers (HCWs), who are disproportionately affected to a higher degree during the current pandemic outbreak [23, 100]. There is uncertainty in the literature regarding the aerosol generation during DMOSP and its associated risk of viral transmission.
In this context, potential airborne transmission of SARS-CoV-2 via aerosols [101] as the fourth way of transmission [102] though controversially discussed is considered a significant risk [103], particularly for all those medical professionals working in close vicinity to the bronchotracheal, nasal and paranasal, oral, and oropharyngeal system [23, 104]. Furthermore, the surgeries in operation theater tend to produce splashes or sprays of body fluids that also cannot be ignored.
Although conclusive data regarding concrete numbers of incidence among dental and OMFS HCWs are lacking, some reports are indicating that dentists and OMFS are among those at elevated risk [105, 106] for transmission by patients including asymptomatic or before onset patients. In addition, considering potential aerosol transmission, due to the specific characteristics of their working environment, oral surgeons may inadvertently contribute to the cross-transmission of SARS-CoV-2 from patient to patient and HCWs by an aerosol transmission during and for some time after performing AGPs [74], especially when there is an exposure to high concentrations of aerosols in a relatively closed environment such as in surgeries [5, 105]. If it is considered highly probable of airborne transmission [5] and SARS CoV-2 is transmitted via aerosols [6], then the medical masks would be inadequate [5] because aerosols can both penetrate and circumnavigate masks, e.g., if compromised by moisture or if worn inadequately [5]. Face shields, too, would provide only partial protection as they leave open gaps between the shield and the HCW, and 6 feet of separation would not protect from aerosols that remain suspended in the air or are carried by air currents [5, 6].
Do dental and maxillofacial surgical procedures generate bioaerosols (and if so, which ones), which can result in the transmission of SARS-CoV-2?
In this context, understanding aerosol transmission and its implications in dentistry is essential, as oral surgery environments with AGPs convey high risk of aerosolized transmission [23, 34], with high-speed drilling, water-air 3-1 syringe, ultrasonic scaling, and piezosurgery generally considered to be high-risk transmitters [105, 106]. In OMFS, especially tracheostomy, tracheostomy care, airway suctioning, abscess drainage, and wound irrigation (e.g., hydro-jet lavage) need to be added to this list according to the WHO recommendations [3, 107] based on prior experiences with SARS-CoV-1 [102]. Although the production of aerosols during these AGPs goes generally accepted, there is overall only weak to moderate evidence that these aerosols will in fact cause aerosol-based transmission.
In this context, it needs to be stressed that in most dental procedures suction serves as a relevant mitigation factor, reducing splatter and aerosol distribution. Chairside high-volume evacuators (HVEs) or more expensive HEPA (high-efficiency particulate arrestor) filters may reduce contamination by the operating site by 67–75% [74] to around 90% [108], or 99.7%, respectively, of particles measuring 0.3 μm in diameter [105]. Next, water or saline cooling procedures, though highly responsible for aerosolization, in turn, reduce immediate local virus load by dilution. In contrast, especially in OMFS procedures such as tracheostomy, which are usually lacking efficient suction and dilution effects, ventilation and airway-related procedures, therefore, may carry a higher risk of transmission as was shown for SARS-CoV-1 [102].
Ishihama et al. [10] assessed high-speed rotary instruments during surgery of impacted third molars and found only indirect evidence supporting the generation of aerosols during oral surgery. Even high-speed rotary instruments, and ultrasonic scalers, showed evidence of blood-contaminated droplets [109]. Comparing extraoral osteotomies in terms of orthopedic osteotomies, Nogler et al. [17, 18] confirmed contamination of OR and personnel. Even Pluim et al. [79] found moderate evidence that sawing of bone when using an oscillating saw can produce aerosols within the respirable range. Therefore, aerosol formation during OMFS bone-cutting procedures needs to be considered as a potential risk factor and the question arises whether there are potential infectious agents present in these aerosols.
Are aerosolized airborne droplets (and to which extent is splatter) in DMOSP infective?
Particles ≤ 10 μm are considered respirable particles which are capable of reaching the lower airways, whereas particles with 10–100 μm are considered inspirable particles, i.e., limited to reaching the upper airways [102]. As viral RNA (though no viable virus) has been detected in the air associated with droplets smaller than 5 μm, the droplets may maintain infectivity [5]. SARS-CoV has been reported to travel more than six feet [110]. There is a high probability termed “beyond a reasonable doubt,” [5] that, e.g., patients' breathing, talking, and less likely coughing [102], e.g., during surgery may cause a mix of potentially infective droplets and aerosols. Microdroplets small enough to remain aloft in the air thus pose a risk of exposure at distances beyond 1–2 m from an infected patient [5], and as aerosols are estimated to travel between up to 4.5 m [111] and 27 feet (around 8 m), or room-scale [102], respectively, and stay viable for hours [112].
In this context, Klompas et al. [6] pointed out that the presence of aerosols will not automatically cause aerosol-based transmission as this depends—besides route of exposure—on factors such as the size of the inoculum, duration of exposure, and host defenses. So far, low reproduction numbers of COVID-19 (rather similar to influenza, i.e., R0 ≈ 2 as opposed to classical airborne viruses such as measles, with R0 ≈ 18) [102] indicate that either a high virus load is required, or aerosols are not the dominant mode of transmission [6].
The diameter of a mature HIV particle is 100 nm [113] and Johnson et al. [92] noted that HIV could be cultured from aerosols of a bone saw. SARS-CoV-2 is an enveloped virus ≈ 0.1 μm in diameter [114]. In an experimental study by Lee BU et al. [115] in which it was assumed that 8.97 × 10−5% of a respiratory fluid particle from a patient with COVID-19 is occupied by SARS-CoV-2, hence the minimum size of a respiratory particle that can contain SARS-CoV-2 is calculated to be ~9.3 μm. If the patient supposedly has a high viral load, then it can decrease the minimum size of respiratory particles containing SARS-CoV-2, thereby increasing the probability of aerosol generation of the viruses [115]. It was found that the virus SARS-CoV-2 was viable even after 3 h, with limited loss of viability [116].
This is of utmost importance, as the presence of SARS-CoV-2 is also reported in particles ranging between 0.25 and 1.0 μm [112]. Thus, theoretically, a bioaerosol carrying viruses might remain within the proximity of the dental chair even after the patient leaves.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been found in infected saliva [117] thus local virus load also explains discussion on preprocedural mouth rinsing (e.g., chlorhexidine CHX), which leads to a mean reduction of 68.4% colony-forming bacterial units in dental aerosols [117]. It has been proven to be efficient against several infectious viruses [105]. However, there is currently no evidence for the use of hydrogen peroxide mouth rinsing [118, 119], even though its use was initially publicized [23, 120, 121].
According to recent Cochrane reviews, there is currently no evidence yet relating to the benefits and risks of healthcare workers using antimicrobial mouthwashes or nasal sprays to protect themselves from contracting COVID-19 [122, 123]. Nevertheless, it should always be kept in mind that airborne transmission via aerosols remains an imponderable threat, especially to oral surgeons even though so far un-proven [102] and still speculative. This uncertainty may be because it is difficult to detect contaminated air, as infectious aerosols are usually extremely dilute, and it is hard to collect and culture fine particles [124]. As aerosol transmission is classified as obligate, preferential, or opportunistic, based on the agent's capacity to be transmitted and to induce the disease through fine-particle aerosols and other routes [124, 125], an opportunistic transmission potential should be assigned to SARS-CoV-2 [5].
Is additional standard personal protective equipment essential to prevent the spreading of SARS-CoV-2 and thus COVID-19 during aerosol-generating DMOSP?
Standard local disinfection and decontamination protocols plus pandemic-adapted distancing procedures should always be ensured as a basic principle. There have been many recommendations from respective governmental or health service institutions of different countries for HCWs, and many recommendations are heterogeneous and epidemiological data relative to their effectiveness against COVID-19 are limited [126].
Therefore, from a clinical point of view, the most contingent question arises as to which is an adequate/appropriate PPE for DMOSP and whether this question can be answered from an evidence-based point of view. All OR personnel should be considered contaminated after each procedure and PPE should be preferred with well-established donning and doffing practices [24]. However, to save resources, PPE can be chosen depending on the planned procedure and the infection status of the patient [23].
So far, according to consensus, power air-purifying respirators (PAPR), which were scarcely available during the outbreak, have not been considered mandatory to safely avoid aerosol-borne transmission in OMFS [23]. At present, N95/FFP2 for AGPs and N99/FFP3 masks with valves [23] for surgery in infected patients, respectively, are most frequently recommended, instead [127–131].
Chu et al. [132] concluded in their systematic review regarding the spread of viruses via aerosols, that respirators would be more protective than medical masks alone. Even another systematic review by Sobti et al. [25] has confirmed that conventional masks do not offer protection against high-risk AGPs. This is in contrast with a study by Bartoszko et al. [126] regarding the use of medical masks vs. N95 respirators in preventing laboratory-confirmed viral infection and respiratory illness specifically in HCWs, analyzed four RCTs including coronavirus and concluded that the use of medical masks did not increase the rate of laboratory-confirmed respiratory infection (OR 1.06) or clinically respiratory illness (OR 1.49).
Nevertheless, at least for AGPs, N95 respirators/FFP-2 masks at present are unanimously recommended by national and international guidelines. The underlying rationale most probably relates to the high level of viral exposure from droplet clouds rather than transmission by the airborne route [102, 133], but is also due to the conspicuous lack of understanding of the detailed mechanisms of SARS-CoV-2 transmission, which may also explain the discrepancy of the recommendation to protect the HCWs with surgical masks vs. respirators.
Accordingly, there is inconsistency in recommendations for routine care and non-AGPs of COVID-19 [5] as the WHO, Public Health England, and the Public Health Agency of Canada recommend the use of medical/surgical masks for non-AGPs [107, 134, 135] in contrast to several societies and national associations recommending N95/FFP2 also for non-AGPs over the less expensive and more readily available medical masks [126].
According to Zimmermann and Nkenke [23], for routine care of low-risk patients (i.e., symptoms-free), the use of medical masks and gloves to protect against droplet transmission is considered sufficient.
As a consequence, at least under pandemic conditions, to save resources [23] and according to available evidence as presented in this article, it may seem reasonable to differentiate between low-risk and high-risk dental and OMFS procedures, with just the latter ones requiring special precautions to prevent droplet and especially aerosolized disease transmission. For low-risk treatments, current empirical data and the absence of clear scientific evidence for aerosol transmission of SARS-CoV-2 provide sufficient rationale for the use of surgical masks, which in analogy should apply in DMOSP.
There are several limitations of this review: (1) The included studies did not directly study an association between potential aerosolization during DMOSP and SARS-CoV-2. (2) Until now, there is no evidence comparing surgical masks vs. respirators regarding SARS-CoV-2 transmission, as all studies so far available dealt with other viruses (influenza and SARS-CoV-1 viruses) rather than SARS CoV-2. Thus, as there was no indirectness and extreme heterogeneity in the included studies, the confidence of evidence for this review must be rated as very low-quality evidence. (3) As a consequence, any evidence provided by the current review was gathered by extrapolation from available experimental and empirical evidence not based on SARS-CoV-2. The results of the present review, therefore, should be interpreted with great caution.
Conclusion
As there is laboratory experimental evidence supporting that dental and OMF aerosol transmission of the viable virus is possible, the risk for SARS-CoV-2 transmission from dental and OMF AGPs, therefore, needs to be confirmed through isolation and culture of viable virus in the clinical environment. At present, according to available very weak/inconclusive evidence, the transmission of SARS-CoV-2 via infective aerosol during AGPs, so far, must remain speculative and controversial. As, however, this is a probable opportunistic way of transmission which cannot be sufficiently excluded and therefore should not be dismissed out of hand prematurely, proper and equally important properly applied protective equipment (i.e., N95 respirators or FFP-2 masks or above regarding mouth and nose protection) should always be used during AGPs. Last but not least, there is an urgent need for studies comparing respirators to surgical masks during dental and OMFS AGPs for protection against SARS-CoV-2 transmission.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors have read and approved the final version of the manuscript.
Conflict of interest
Author AN was employed by University Hospital Marburg UKGM GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/froh.2022.974644/full#supplementary-material
References
1. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
2. Liu L, Wei Q, Alvarez X, Wang H, Du Y, Zhu H, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. (2011) 85:4025–30. doi: 10.1128/JVI.02292-10
3. World Health Organization. Considerations for the Provision of Essential Oral Health Services in the Context of COVID-19. (2020). Available online at: https://www.who.int/publications/i/item/who-2019-nCoV-oral-health-2020.1 (accessed January 20, 2021).
4. Han P, Li H, Walsh LJ, Ivanovski S. Splatters and aerosols contamination in dental aerosol generating procedures. Appl Sci. (2021) 11:1914. doi: 10.3390/app11041914
5. Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis. (2020) 71:2311–3. doi: 10.1093/cid/ciaa939
6. Klompas M, Baker MA, Rhee C. Airborne transmission of SARS-CoV-2 theoretical considerations and available evidence. JAMA. (2020) 324:441–2. doi: 10.1001/jama.2020.12458
7. Bentley CD, Burkhart NW, Crawford JJ. Evaluating spatter and aerosol contamination during dental procedures. J Am Dent Assoc. (1994) 125:579–84. doi: 10.14219/jada.archive.1994.0093
8. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. (2004) 135:429–37. doi: 10.14219/jada.archive.2004.0207
9. Sacchetti R, Baldissarri A, De Luca G, Lucca P, Stampi S, Zanetti F. Microbial contamination in dental unit waterlines: comparison between Er:YAG laser and turbine lines. Ann Agric Environ Med. (2006) 13:275–9.
10. Ishihama K, Iida S, Koizumi H, Wada T, Adachi T, Isomura-Tanaka E, et al. High incidence of blood exposure due to imperceptible contaminated splatters during oral surgery. J Oral Maxillofac Surg. (2008) 66:704–10. doi: 10.1016/j.joms.2007.06.663
11. Szymanska J, Sitkowska J. Bacterial contamination of dental unit waterlines. Environ Monit Assess. (2013) 185:3603–11. doi: 10.1007/s10661-012-2812-9
12. Al-Eid RA, Ramalingam S, Sundar C, Aldawsari M, Nooh N. Detection of visually imperceptible blood contamination in the oral surgical clinic using forensic luminol blood detection agent. J Int Soc Prevent Communit Dent. (2018) 8:327–32. doi: 10.4103/jispcd.JISPCD_10_18
13. Simpson AH, Dall G, Haas JG. COVID-19: potential transmission through aerosols in surgical procedures and blood products. Bone Joint Res. (2020) 9:200–1. doi: 10.1302/2046-3758.94.BJR-2020-0130
14. Hirschmann MT, Hart A, Henckel J, Sadoghi P, Seil R, Mouton C. COVID-19 coronavirus: recommended personal protective equipment for the orthopaedic and trauma surgeon. Knee Surg Sports Traumatol Arthrosc. (2020) 28:1690–8. doi: 10.1007/s00167-020-06022-4
15. Yeh HC, Muggenburg BA, Guilmette RA, Snipes MB, Turner RS, Jones RK, et al. Characterization of aerosols produced during total hip replacement surgery in dogs with 51Cr-labeled blood. J Aerosol Sci. (1995) 26:511–8. doi: 10.1016/0021-8502(94)00119-J
16. Nogler M, Lass-Fl€orl C, Wimmer C, Mayr E, Bach C, Ogon M. Contamination during removal of cement in revision hip arthroplasty. A cadaver study using ultrasound and high-speed cutters. J Bone Joint Surg Br. (2003) 85:436e439. doi: 10.1302/0301-620X.85B3.12451
17. Nogler M, Lass-Fl€orl C, Ogon M, Mayr E, Bach C, Wimmer C. Environmental and body contamination through aerosols produced by high-speed cutters in lumbar spine surgery. Spine. (2001) 26:2156e2159. doi: 10.1097/00007632-200110010-00023
18. Nogler M, Lass-Fl€orl C, Wimmer C, Bach C, Kaufmann C, Ogon M. Aerosols produced by high-speed cutters in cervical spine surgery: extent of environmental contamination. Eur Spine J. (2001) 10:274e277. doi: 10.1007/s005860100310
19. Nogler M, Wimmer C, Lass-Fl€orl C, Mayr E, Trobos S, Gegenhuber C. Contamination risk of the surgical team through ROBODOC's high-speed cutter. Clin Orthop Relat Res. (2001) 387:225e231. doi: 10.1097/00003086-200106000-00030
20. Putzer D, Lechner R, Coraca-Huber D, Mayr A, Nogler M, Thaler M. The extent of environmental and body contamination through aerosols by hydro-surgical debridement in the lumbar spine. Arch Orthop Trauma Surg. (2017) 137:743e747. doi: 10.1007/s00402-017-2668-0
21. Paulo AC, Correia-Neves M, Domingos T, Murta AG, Pedrosa J. Influenza infectious dose may explain the high mortality of the second and third wave of 1918-1919 influenza pandemic. PLoS ONE. (2010) 5:e11655. doi: 10.1371/journal.pone.0011655
22. Burdorf A, Porru F, Rugulies R. The COVID-19 pandemic: consequences for occupational health. Scand J Work Environ Health. (2020) 46:229–230. doi: 10.5271/sjweh.3893
23. Zimmermann M, Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J Craniomaxillofac Surg. (2020) 48:521–6. doi: 10.1016/j.jcms.2020.03.011
24. Sharma S, John R, Patel S, Neradi D, Kishore K, Dhillon MS. Bioaerosols in orthopedic surgical procedures and implications for clinical practice in the times of COVID-19: a systematic review and meta-analysis. J Clin Orthopaed Trauma. (2021) 17:239–53. doi: 10.1016/j.jcot.2021.03.016
25. Sobti A, Fathi M, Mokhtar MA, Mahana K, Rashid MS, Polyzois I, et al. Aerosol generating procedures in trauma and orthopaedics in the era of the Covid-19 pandemic; what do we know? Surgeon. (2021) 19:e42–8. doi: 10.1016/j.surge.2020.08.001
26. Nóbrega MT, Bastos RT, Mecenas P, de Toledo IP, Richardson-Lozano R, Altabtbaei K, et al. Aerosol generated by dental procedures: a scoping review. J Evid Based Med. (2021) 14:303–12. doi: 10.1111/jebm.12461
27. Clementini M, Raspini M, Barbato L, Bernardelli F, Braga G, Di Gioia C, et al. Aerosol transmission for SARS-CoV-2 in the dental practice. A review by SIdP Covid-19 task-force. Oral Dis. (2022) 28:852–7. doi: 10.1111/odi.13649
28. Innes N, Johnson IG, Al-Yaseen W, Harris R, Jones R, Kc S, et al. A systematic review of droplet and aerosol generation in dentistry. J Dentist. (2021) 105:103556. doi: 10.1016/j.jdent.2020.103556
29. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
30. Putzer D, Coraça-Huber D, Huber C, Boschert H, Thaler M, Nogler M. The spatial distribution of aerosols in high-speed bone burring with external irrigation. J Microbiol Methods. (2021) 184:106205. doi: 10.1016/j.mimet.2021.106205
31. Liu Z, Zhang P, Li Y, Yang W, Guo J, Liu J, et al. Assessment of spatial concentration variation and deposition of bioaerosol in a dental clinic during oral cleaning. Build Environ. (2021) 202:108024. doi: 10.1016/j.buildenv.2021.108024
32. Ionescu AC, Cagetti MG, Ferracane JL, Garcia-Godoy F, Brambilla E. Topographic aspects of airborne contamination caused by the use of dental handpieces in the operative environment. J Am Dent Assoc. (2020) 151:660–667. doi: 10.1016/j.adaj.2020.06.002
33. Gund M, Isack J, Hannig M, Thieme-Ruffing S, Gärtner B, Boros G, et al. Contamination of surgical mask during aerosol-producing dental treatments. Clin Oral Investig. (2021) 25:3173–80. doi: 10.1007/s00784-020-03645-2
34. Zemouri C, Volgenant CM, Buijs MJ, Crielaard W, Rosema NA, Brandt BW, et al. Dental aerosols: microbial composition and spatial distribution. J Oral Microbiol. (2020) 12:1762040. doi: 10.1080/20002297.2020.1762040
35. Morris BJ, Kiser CJ, Laughlin MS, Sheth MM, Dunn WR, Elkousy HA, et al. A localized laminar flow device decreases airborne particulates during shoulder arthroplasty: a randomized controlled trial. J Shoulder Elbow Surg. (2021) 30:580–6. doi: 10.1016/j.jse.2020.08.035
36. Kirschbaum S, Hommel H, Strache P, Horn R, Falk R, Perka C. Laminar air flow reduces particle load in TKA—even outside the LAF panel: a prospective, randomized cohort study. Knee Surg Sports Traumatol Arthrosc. (2021) 29:3641–7. doi: 10.1007/s00167-020-06344-3
37. Anis HK, Curtis GL, Klika AK, Piuzzi NS, Otiso J, Richter SS, et al. In-room ultraviolet air filtration units reduce airborne particles during total joint arthroplasty. J Orthopaed Res. (2020) 38:431–7. doi: 10.1002/jor.24453
38. Divya R, Senthilnathan KP, Kumar MP, Murugan PS. Evaluation of aerosol and splatter contamination during minor oral surgical procedures. Drug Invent Today. (2019) 12:1845–8. doi: 10.1101/2020.06.25.154401
39. Watanabe A, Tamaki N, Yokota K, Matsuyama M, Kokeguchi S. Use of ATP bioluminescence to survey the spread of aerosol and splatter during dental treatments. J Hosp Infect. (2018) 99:303–5. doi: 10.1016/j.jhin.2018.03.002
40. Kobza J, Pastuszka JS, Bragoszewska E. Do exposures to aerosols pose a risk to dental professionals? Occup Med. (2018) 68:454–8. doi: 10.1093/occmed/kqy095
41. Nunes LM, Viana TS, da Silva GM, Botelho MP, Capel LM. Identificação da população fúngica em amostras de ar coletadas em clínica de ensino de odontopediatria. Revista da ABENO. (2018) 18:84–92. doi: 10.30979/rev.abeno.v18i3.566
42. Adhikari A, Kurella S, Banerjee P, Mitra A. Aerosolized bacteria and microbial activity in dental clinics during cleaning procedures. J Aerosol Sci. (2017) 114:209–18. doi: 10.1016/j.jaerosci.2017.09.019
43. Singh A, Shiva Manjunath RG, Singla D, Bhattacharya HS, Sarkar A, Chandra N. Aerosol, a health hazard during ultrasonic scaling: a clinico-microbiological study. Indian J Dent Res. (2016) 27:160–2. doi: 10.4103/0970-9290.183131
44. Jimson S, Kannan I, Jimson S, Parthiban J, Jayalakshmi M. Evaluation of airborne bacterial contamination during procedures in oral surgery clinic. Biomed Pharmacol J. (2015) 8SE:669–75. doi: 10.13005/bpj/765
45. Chuang C-Y, Cheng H-C, Yang S, Fang W, Hung PC, Chuang SY. Investigation of the spreading characteristics of bacterial aerosol contamination during dental scaling treatment. J Dent Sci. (2014) 9:294–6. doi: 10.1016/j.jds.2014.06.002
46. Polednik B. Aerosol and bioaerosol particles in a dental office. Environ Res. (2014) 134:405–9. doi: 10.1016/j.envres.2014.06.027
47. Manarte-Monteiro P, Carvalho A, Pina C, Oliveira H, Manso MC. Air quality assessment during dental practice: aerosols bacterial counts in an universitary clinic. Revista Portuguesa de Estomatologia Medicina Dentária e Cirurgia Maxilofacial. (2013) 54:2–7. doi: 10.1016/j.rpemd.2012.10.002
48. Yen-Tseng L. Surveillance of Methicillin-Resistance Staphylococcus aureus in a Periodontal Clinic. (Master's thesis) (2013). Available online at: https://escholarship.org/uc/item/2bp9n086 (accessed May 22, 2022).
49. Pasquarella C, Veronesi L, Napoli C, Castiglia P, Liguori G, Rizzetto R, et al. Microbial environmental contamination in Italian dental clinics: amulticenter study yielding recommendations for standardized sampling methods and threshold values. Sci Total Environ. (2012) 420:289–99. doi: 10.1016/j.scitotenv.2012.01.030
50. Guida M, Gallé F, Di Onofrio V, Nastro RA, Battista M, Liguori G, et al. Environmental microbial contamination in dental setting: a local experience. J Prev Med Hyg. (2012) 53:207–12. doi: 10.15167/2421-4248/jpmh2012.53.4.350
51. Kucukdurmaz F, Imren Y, Akkoyunlu Y, Tuncay I, Sen C. Domestic electric drills in the service of orthopaedic surgery: a potential and preventable source of surgical site infections. Acta Orthop Traumatol Turc. (2012) 46:455e9. doi: 10.3944/AOTT.2012.2794
52. Labaf H, Owlia P, Taherian A, Haghgoo R. Quantitative analysis of changes in bacterial aerosols during endodontic, periodontic and prosthodontic treatments. African J Microbiol Res. (2011) 5:4946–4948. doi: 10.5897/AJMR11.820
53. Dutil S, Meriaux A, de Latremoille MC, Lazure L, Barbeau J, Duchaine C. Measurement of airborne bacteria and endotoxin generated during dental cleaning. J Occup Environ Hyg. (2009) 6:121–30. doi: 10.1080/15459620802633957
54. Pina-Vaz I, Pina-Vaz C, Carvalho MF, Azevedo A. Evaluating spatter and aerosol contamination during opening of access cavities in endodontics. Rev Clín Pesq Odontol. (2008) 4:77–83.
55. Azari MR, Ghajari A, Nejad MRM, Nasiree NF. Airborne microbial contamination of dental units. Tanaffos. (2008) 7:54–7.
56. Greco PM, Lai CH. A new method of assessing aerosolized bacteria generated during orthodontic debonding procedures. Am J Orthod Dentofacial Orthop. (2008) 133:S79–87. doi: 10.1016/j.ajodo.2006.08.021
57. Motta RH, Groppo FC, Bergamaschi CC, Ramacciato JC, Baglie S, de Mattos-Filho TR. Isolation and antimicrobial resistance of Staphylococcus aureus isolates in a dental clinic environment. Infect Control Hosp Epidemiol. (2007) 28:185–90. doi: 10.1086/510867
58. Shivakumar KM, Prashant GM, Madhu Shankari GS, Subba Reddy VV, Chandu GN. Assessment of atmospheric microbial contamination in a mobile dental unit. Indian J Dent Res. (2007) 18:177–80. doi: 10.4103/0970-9290.35828
59. Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH. Bacterial aerosols in dental practice - a potential hospital infection problem? J Hosp Infect. (2006) 64:76–81. doi: 10.1016/j.jhin.2006.04.011
60. Timmerman MF, Menso L, Steinfort J, vanWinkelhoff AJ, van derWeijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. (2004) 31:458–62. doi: 10.1111/j.1600-051X.2004.00511.x
61. Prospero E, Savini S, Annino I. Microbial aerosol contamination of dental healthcare workers' faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. (2003) 24:139–41. doi: 10.1086/502172
62. Toroglu MS, Haytaç MC, Köksal F. Evaluation of aerosol contamination during debonding procedures. Angle Orthodontist. (2001) 71:299–306. doi: 10.1043/0003-3219(2001)071
63. Grenier D. Quantitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. (1995) 61:3165–8. doi: 10.1128/aem.61.8.3165-3168.1995
64. Yeh HC, Jones RK, Muggenburg BA, Turner RS, Lundgren DL, Smith JP. Characterization of aerosols produced during surgical procedures in hospitals. Aerosol Sci Technol. (1995) 22:151e161. doi: 10.1080/02786829408959736
65. Legnani P, Checchi L, Pelliccioni GA, D'Achille C. Atmospheric contamination during dental procedures. Quintessence Int. (1994) 25:435–9.
66. Earnest R, Loesche W. Measuring harmful levels of bacteria in dental aerosols. J Am Dent Assoc. (1991) 122:55–7. doi: 10.14219/jada.archive.1991.0187
67. Heinsohn P, Jewett DL, Balzer L, Bennett CH, Seipel P, Rosen A. Aerosols created by some surgical power tools: particle size distribution and qualitative hemoglobin content. Appl Occup Environ Hyg. (1991) 6:773e6. doi: 10.1080/1047322X.1991.10389727
68. Takanabe Y, Maruoka Y, Kondo J, Yagi S, Chikazu D, Okamoto R, et al. Dispersion of aerosols generated during dental therapy. Int J Environ Res Public Health. (2021) 18:11279. doi: 10.3390/ijerph182111279
69. Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. JADA. (1998) 129:1241–9. doi: 10.14219/jada.archive.1998.0421
70. Wendlandt R, Thomas M, Kienast B, Schulz AP. In-vitro evaluation of surgical helmet systems for protecting surgeons from droplets generated during orthopaedic procedures. J Hosp Infect. (2016) 94:75–9. doi: 10.1016/j.jhin.2016.05.002
71. Veena HR, Mahantesha S, Joseph PA, Patil SR, Patil SH. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infect Public Health. (2015) 8:260–65. doi: 10.1016/j.jiph.2014.11.004
72. Llandro H, Allison JR, Currie CC, Edwards DC, Bowes C, Durham J, et al. Evaluating splatter and settled aerosol during orthodontic debonding: implications for the COVID-19 pandemic. Br Dent J. (2021). doi: 10.1038/s41415-020-2503-9. [Epub ahead of print].
73. Holliday R, Allison JR, Currie CC, Edwards DC, Bowes C, Pickering K, et al. Evaluating contaminated dental aerosol and splatter in an open plan clinic environment: Implications for the COVID-19 pandemic. J Dent. (2021) 105:103565. doi: 10.1016/j.jdent.2020.103565
74. Allison JR, Currie CC, Edwards DC, Bowes C, Coulter J, Pickering K, et al. Evaluating aerosol and splatter following dental procedures: addressing new challenges for oral health care and rehabilitation. J Oral Rehabil. (2020) 48:61–72. doi: 10.1111/joor.13098
75. Graziani F, Izzetti R, Lardani L, Totaro M, Baggiani A. Experimental evaluation of aerosol production after dental ultrasonic instrumentation: an analysis on fine particulate matter perturbation. Int J Environ Res Public Health. (2021) 18:3357. doi: 10.3390/ijerph18073357
76. Aguilar-Duran L, Bara-Casaus JJ, Aguilar-Duran S, Valmaseda- Castellon E, Figueiredo R. Blood spatter in oral surgery: prevalence and risk factors. JADA. (2020) 151:438–43. doi: 10.1016/j.adaj.2020.02.026
77. Matys J, Grzech-Leśniak K. Dental aerosol as a hazard risk for dental workers. Materials. (2020) 13:5109. doi: 10.3390/ma13225109
78. Nejatidanesh F, Khosravi Z, Goroohi H, Badrian H, Savabi O. Risk of contamination of different areas of dentist's face during dental practices. Int J Prevent Med. (2013) 4:611.
79. Pluim JME, Jimenez-Bou L, Gerretsen RRR, Loeve AJ. Aerosol production during autopsies: the risk of sawing in bone. Forensic Sci Int. (2018) 289:260–7. doi: 10.1016/j.forsciint.2018.05.046
80. Pereira ML, Vilain R, Leivas TP, Tribess A. Measurement of the concentration and size of aerosol particles and identification of the sources in orthopedic surgeries. HVAC R Research. (2012) 18:588e601.
81. Sotiriou M, Ferguson SF, Davey M, Wolfson JM, Demokritou P, Lawrence J, et al. Measurement of particle concentrations in a dental office. Environ Monit Assess. (2008) 137:351–61. doi: 10.1007/s10661-007-9770-7
82. Ishihama K, Koizumi H, Wada T, Iida S, Tanaka S, Yamanishi T, et al. Evidence of aerosolised floating blood mist during oral surgery. J Hosp Infect. (2009) 71:359–64. doi: 10.1016/j.jhin.2008.12.005
83. Cristina ML, Spagnolo AM, Sartini M, Dallera M, Ottria G, Lombardi R, Perdelli F. Evaluation of the risk of infection through exposure to aerosols and spatters in dentistry. Am J Infect Control. (2008) 36:304–7. doi: 10.1016/j.ajic.2007.07.019
84. Perdelli F, Spagnolo AM, Cristina ML, Sartini M, Malcontenti R, Dallera M, et al. Evaluation of contamination by blood aerosols produced during various healthcare procedures. J Hosp Infect. (2008) 70:174–9. doi: 10.1016/j.jhin.2008.06.012
85. Johnson MC, Schwarz APD, Sandfort BDR, Buchan RM. Characterization of blood-containing aerosol generated during canine total hip replacement surgery. Appl Occup Environ Hyg. (1997) 12:739e743. doi: 10.1080/1047322X.1997.10387757
86. Heinsohn P, Jewett DL. Exposure to blood-containing aerosols in the operating room: a preliminary study. Am Ind Hyg Assoc J. (1993) 54:446e53. doi: 10.1080/15298669391354946
87. Jewett DL, Heinsohn P, Bennett C, Rosen A, Neuilly C. Bloodcontaining aerosols generated by surgical techniques: a possible infectious hazard. Am Ind Hyg Assoc J. (1992) 53:228e31.23. doi: 10.1080/15298669291359564
88. Kedjarune U, Kukiattrakoon B, Yapong B, Chowanadisai S, Leggat P. Bacterial aerosols in the dental clinic: effect of time, position and type of treatment. Int Dent J. (2000) 50:103–7. doi: 10.1002/j.1875-595X.2000.tb00807.x
89. Bennett AM, Fulford MR, Walker JT, Bradshaw DJ, Martin MV, Marsh PD. Microbial aerosols in general dental practice. Br Dent J. (2000) 189:664–67. doi: 10.1038/sj.bdj.4800859
90. Clarkson JRC, Richards D, Robertson C, Aceves-Martins M, on on behalf of the CoDER Working Group. Aerosol Generating Procedures and Their Mitigation in International Dental Guidance Documents - A Rapid Review. (2020). Available online at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi049n1xKzuAhXUURUIHY5lAuQQFjABegQIAxAC&url=https%3A%2F%2Foralhealth.cochrane.org%2Fsites%2Foralhealth.cochrane.org%2Ffiles%2Fpublic%2Fuploads%2Frapid_review_of_agps_in_international_dental_guidance_documents.pdf&usg=AOvVaw1Zs0oSqZhABGjJgeIfG_rthhttphttps:// (accessed January 20, 2021).
91. Micik RE, Miller RL, Mazzarella MA, Ryge G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J Dent Res. (1969) 48:49–56. doi: 10.1177/00220345690480012401
92. Johnson GK, Robinson WS. Human immunodeficiency virus-1 (HIV-1) in the vapors of surgical power instruments. J Med Virol. (1991) 33:47–50. doi: 10.1002/jmv.1890330110
93. Sergis A, Wade WG, Gallagher JE, Morrell AP, Patel S, Dickinson CM, et al. Mechanisms of atomization from rotary dental instruments and its mitigation. J Dent Res. (2021) 100:261–7. doi: 10.1177/0022034520979644
94. Mirbod P, Haffner EA, Bagheri M, Higham JE. Aerosol formation due to a dental procedure: insights leading to the transmission of diseases to the environment. J R Soc Interface. (2021) 18:20200967. doi: 10.1098/rsif.2020.0967
95. Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles, 2nd ed. New York, NY: Wiley (1999).
96. SBAR NSS,. Assessing the Evidence Base for Medical Procedures Which Create a Higher Risk of Respiratory Infection Transmission From Patient to Healthcare Worker. (2020). Available online at: https://hps.scot.nhs.uk/web-resources-container/sbar-assessing-the-evidence-base-for-medical-procedures-which-create-a-higher-risk-of-respiratory-infection-transmission-from-patient-to-healthcare-worker/ (accessed January 20, 2021).
97. SBAR NSSSLWG,. Ventilation, Water Environmental Cleaning in Dental Surgeries Relating to COVID-19. (2020). Available online at: https://www.scottishdental.org/ventilation-water-and-environmental-cleaning-in-dental-surgeries-relating-to-covid-19/ (accessed January 20, 2021).
98. Wong KC, Leung KS. Transmission and prevention of occupational infections in orthopaedic surgeons. J Bone Joint Surg. (2004) 86:1065e1076. doi: 10.2106/00004623-200405000-00029
99. Spagnolo AM, Ottria G, Amicizia D, Perdelli F, Cristina ML. Operating theatre quality and prevention of surgical site infections. J Prev Med Hyg. (2013) 54:131e137.
100. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. (2020) 105:100–1. doi: 10.1016/j.jhin.2020.03.002
101. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. (2020) 67:568–76. doi: 10.1007/s12630-020-01591-x
102. Sommerstein R, Fux CA, Vuichard-Gysin D, Abbas M, Marschall J, Balmelli C, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. (2020) 9:100. doi: 10.1186/s13756-020-00763-0
103. Schwartz KL, Murti M, Finkelstein M, Leis JA, Fitzgerald-Husek A, Bourns L, et al. Lack of COVID-19 transmission on an international flight. CMAJ. (2020) 192:E410. doi: 10.1503/cmaj.75015
104. Patel ZM, Fernandez-Miranda J, Hwang PH, Nayak JV, Dodd RL, Sajjadi H, et al. In reply: precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurgery. (2020) 87:E162–3. doi: 10.1093/neuros/nyaa156
105. Ge ZY, Yang LM, Xia JJ, Fu XH, Zhang YZ. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. (2020) 21:361–8. doi: 10.1631/jzus.B2010010
106. Warnakulasuriya S. Protecting dental manpower from COVID-19 infection. Oral Dis. (2020) 27 (Suppl. 3):651–4. doi: 10.1111/odi.13410
107. Rational Rational Use of Personal Protective Equipment for Coronavirus Disease (COVID-19) and Considerations During Severe Shortages: Interim Guidance 6 April 2020. Geneva, World Health Organization (2020). Available online at: https://apps.who.int/iris/handle/10665/331695 (accessed January 21, 2021).
108. Narayana TV, Mohanty L, Sreenath G, Vidhyadhari P. Role of preprocedural rinse and high volume evacuator in reducing bacterial contamination in bioaerosols. J Oral Maxillofac Pathol. (2016) 20:59–65. doi: 10.4103/0973-029X.180931
109. Yamada H, Ishihama K, Yasuda K, Hasumi-Nakayama Y, Shimoji S, Furusawa K. Aerial dispersal of blood-contaminated aerosols during dental procedures. Quintessence Int. (2011) 42:399–405.
110. Kutter JS, Spronken MI, Fraaij PL, Fouchier RA, Herfst S. Transmission routes of respiratory viruses among humans. Curr Opin Virol. (2018) 28:142–51. doi: 10.1016/j.coviro.2018.01.001
111. Loh NW, Tan Y, Taculod J, Gorospe B, Teope AS, Somani J, et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. (2020) 67:893–4. doi: 10.1007/s12630-020-01634-3
112. Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. (2020) 582:557–60. doi: 10.1038/s41586-020-2271-3
113. German Advisory Committee Blood (Arbeitskreis Blut) Subgroup Subgroup ‘Assessment of Pathogens Transmissible by Blood'. Human Immunodeficiency Virus (HIV). Transfus Med Hemother. (2016) 43:203–22. doi: 10.1159/000445852
114. Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. (2020) 9:e57309. doi: 10.7554/eLife.57309.sa2
115. Lee BU. Minimum sizes of respiratory particles carrying SARS-CoV-2 and the possibility of aerosol generation. Int J Environ Res Public Health. (2020) 17:6960. doi: 10.3390/ijerph17196960
116. Van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Eng J Med. (2020) 382:1564–7. doi: 10.1056/NEJMc2004973
117. Marui VC, Souto MLS, Rovai ES, Romito GA, Chambrone L, Pannuti CM. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. (2019) 150:1015–26.e1011. doi: 10.1016/j.adaj.2019.06.024
118. Gottsauner MJ, Michaelides I, Schmidt B, Scholz KJ, Buchalla W, Widbiller M, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. (2020) 24:3707–13. doi: 10.1007/s00784-020-03549-1
119. Meister TL, Brüggemann Y, Todt D, Conzelmann C, Müller JA, Groß R, et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. (2020) 222:1289–92. doi: 10.1093/infdis/jiaa471
120. Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. (2020) 99:481–7. doi: 10.1177/0022034520914246
121. Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. (2020) 12:9. doi: 10.1038/s41368-020-0075-9
122. Burton MJ, Clarkson JE, Goulao B, Glenny A-M, McBain AJ, Schilder AG, et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev. (2020) 9:CD013626. doi: 10.1002/14651858.CD013626.pub2
123. Burton MJ, Clarkson JE, Goulao B, Glenny AM, McBain AJ, Schilder AG, et al. Antimicrobial mouthwashes (gargling) and nasal sprays to protect healthcare workers when undertaking aerosol-generating procedures (AGPs) on patients without suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev. (2020) 9:Cd013628. doi: 10.1002/14651858.CD013628.pub2
124. Roy CJ, Milton DK. Airborne transmission of communicable infection–the elusive pathway. N Engl J Med. (2004) 350:1710–1712. doi: 10.1056/NEJMp048051
125. Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. (2019) 32:372–9. doi: 10.1097/QCO.0000000000000563
126. Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses. (2020) 14:365–73. doi: 10.1111/irv.12745
127. Centers for Disease Control Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html#anchor_1607453984225 (accessed January 20, 2021).
128. European Centre for Disease Prevention Control. Guidance for Wearing and Removing Personal Protective Equipment in Healthcare Settings for the Care of Patients With Suspected or Confirmed COVID-19. European Centre for Disease Prevention and Control. Available online at: https://www.ecdc.europa.eu/en/publications-data/guidance-wearing-and-removing-personal-protective-equipment-healthcare-settings (accessed January 20, 2021).
129. French French Society of Stomatology M-FS, Oral S. Practitioners specialized in oral health and coronavirus disease 2019: professional guidelines from the French society of stomatology, maxillofacial surgery and oral surgery, to form a common front against the infectious risk. J Stomatol Oral Maxillofac Surg. (2020) 121:155–8. doi: 10.1016/j.jormas.2020.03.011
130. Occupational Safety Health Administrations. OSHA Guidance for Dentistry Workers and Employers. Available online at: https://www.osha.gov/coronavirus/control-prevention/dentistry (accessed January 20, 2021).
131. The American Society of Dentist Anesthesiologists. Interim Guidance for Dentist Anesthesiologists Practicing in the Office-Based Setting During the COVID-19 Pandemic. (2020). Available online at: https://www.asdahq.org/sites/default/files/Guidance%20ASDA%204.14.20.pdf (accessed January 20, 2021).
132. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schunemann HJ, Authors C-SURGEs. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. (2020) 395:1973–87. doi: 10.1016/S0140-6736(20)31142-9
133. Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, et al. High SARS-CoV-2 attack rate following exposure at a choir practice — Skagit County, Washington. MMWR Morb Mortal Wkly Rep March. (2020) 2020:606–10. doi: 10.15585/mmwr.mm6919e6
134. Public Health Agency of Canada. Coronavirus Disease (COVID-19): For Health Professionals. Public Health Agency of Canada. Available online at: https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals.html (accessed January 20, 2021).
135. Public Health England. When to Use a Surgical Face Mask or FFP3 Respirator. Available online at: https://www.rdash.nhs.uk/wp-content/uploads/2017/08/appendix-47-surgical-face-mask-ffp3.pdf (accessed January 20, 2021).
Keywords: COVID-19, maxillofacial surgery, aerosols, aerosol generating dental procedure, systematic review, orthopedic, coronavirus disease 2019 (COVID-19), SARS-CoV-2
Citation: Al-Moraissi EA, Kaur A, Günther F, Neff A and Christidis N (2022) Can aerosols-generating dental, oral and maxillofacial, and orthopedic surgical procedures lead to disease transmission? An implication on the current COVID-19 pandemic. Front. Oral. Health 3:974644. doi: 10.3389/froh.2022.974644
Received: 21 June 2022; Accepted: 04 July 2022;
Published: 01 August 2022.
Edited by:
Yiu Yan Leung, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Dion Tik Shun Li, The University of Hong Kong, Hong Kong SAR, ChinaLau Sze Lok Alfred, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2022 Al-Moraissi, Kaur, Günther, Neff and Christidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Essam Ahmed Al-Moraissi, ZHJlc3NhbWFsbW9yYWlzc2lAZ21haWwuY29t; ZHJfZXNzYW1hbG1vcmFpc3NpQHlhaG9vLmNvbQ==
 Essam Ahmed Al-Moraissi
Essam Ahmed Al-Moraissi Amanjot Kaur
Amanjot Kaur Frank Günther3
Frank Günther3 Andreas Neff
Andreas Neff Nikolaos Christidis
Nikolaos Christidis