- 1Department of Oral and Maxillofacial Surgery, Harvard School of Dental Medicine, Boston, MA, United States
- 2Pathology, Lino Rossi Research Center, Department of Biomedical, Surgical and Dental Sciences, Università degli Studi di Milano, Milan, Italy
- 3Division of Oral Medicine and Dentistry, Brigham and Women's Hospital, Boston, MA, United States
- 4Department of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, MA, United States
- 5Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
- 6Medicine, Harvard Medical School, Boston, MA, United States
As the incidence of cancer continues to increase, so too will the use of various forms of cancer therapeutics and their associated oral and dental complications. Although many of the acute and chronic oral toxicities of cancer therapy are largely unavoidable, appropriate and timely management of these complications has the potential to alleviate morbidity and improve outcomes. Undoubtedly, the substantial short- and long-term impacts of cancer therapy on the health of the oral cavity requires increased awareness, prevention, and treatment by multidisciplinary healthcare teams consisting of medical oncologists, dentists, and other oral healthcare specialists. This mini review provides a brief purview of the current state of clinical oncology and its impact on oral health. The topics introduced here will be further investigated throughout the remainder of the “Oral Complications in Cancer Patients” mini-review series.
Introduction
Cancer accounted for roughly 10 million deaths in 2020, serving as a leading cause of mortality globally [1]. Cancer incidence is continuing to grow [2], reflecting population growth and aging, as well as the increasing prevalence of cancer risk factors associated with socioeconomic development. In the United States (US), half of men and one-third of women will develop cancer throughout their lifetime [3–5].
Many cancer treatment modalities, such as surgery, radiotherapy, chemotherapy (neoadjuvant, adjuvant, and/or concurrent), and hematopoietic stem cell transplantation, as well as supportive care measures (e.g., antiresorptive therapies) have the potential to cause various oral complications (Figure 1) [6]. More novel cancer therapeutics, such as targeted therapies [e.g., epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKI)] and emerging immunotherapies, have also demonstrated oral side effects [7–9]. As the incidence of cancer continues to increase, so too will the use of various forms of cancer therapeutics and their associated oral and dental complications.
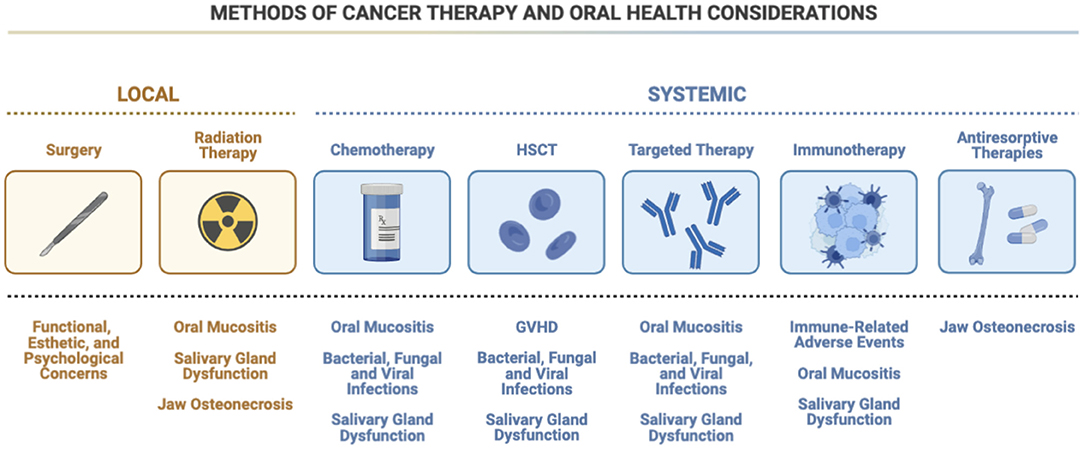
Figure 1. Methods of cancer therapy and oral health considerations. There are many types of possible therapies used to treat cancer, each with their own oral health side effects. For example, chemotherapy and targeted therapy designed to treat both local and systemic cancers carry with them similar concerns for oral mucositis, infection, and salivary gland dysfunction. Antiresorptive therapies may raise the possibility of osteonecrosis, which is also a concern when radiation therapy is used, although the possibility of emergent oral mucositis and salivary gland dysfunction are less prevalent with antiresorptive therapies. HSCT, hematopoietic stem cell transplantation; GVHD, graft-vs.-host disease; HSV, herpes simplex virus.
This mini review provides a brief purview of the current state of clinical oncology and its impact on oral health. Clinical oncology consists of three primary disciplines: surgical oncology, radiation oncology, and medical oncology. Basic principles of clinical oncology, recent advancements in cancer therapeutics, and various oral health complications associated with cancer treatment will be discussed. Finally, the authors will consider various approaches to promoting oral health before, during, and after cancer treatment. The topics introduced here will be further investigated throughout the remainder of the “Oral Complications in Cancer Patients” mini-review series.
Cancer Epidemiologic Trends
According to the Global Burden of Disease (GBD) study, cancer imposes the largest burden of any disease in the world, exceeding that of ischemic heart disease and stroke [10]. In 2018, over 18 million new cases of cancer were diagnosed; the most prevalent cancers among men were lung (1.37 million cases), prostate (1.28 million cases), and stomach (0.68 million), whereas women were most likely to be diagnosed with cancers of the breast (2.09 million cases), lung (0.72 million cases), and cervix/uterus (0.57 million cases) [11]. After ischemic heart disease, cancer remains the second leading cause of death worldwide, followed by stroke, and chronic obstructive pulmonary disease [12]. Over the last 15 years, the incidence of cancer has increased by 28%, which is 3-fold higher than the increase in mortality over the same period (~9%) [12]. Overall, individuals between the ages of 0–74 have a 10.6% risk of dying from cancer; men are most likely to die from lung, liver, and stomach cancer, whereas women are most likely to die from breast, lung, and cervix/uterus cancer [12]. By 2030, cancer is projected to be the leading cause of global mortality, surpassing that of ischemic heart disease [13].
Cancers of the head and neck (HNC) are a heterogeneous group of malignancies that comprise the ninth and seventh most common cancer in the US and world, respectively [2, 4]. Each year, head and neck squamous cell carcinoma (HNSCC) is diagnosed in over half a million patients and is responsible for over 380,000 deaths globally [14]. Oral squamous cell carcinoma (OSCC), a major concern among dentists, oral medicine providers, and other oral healthcare specialists, accounted for approximately 145,000 deaths worldwide in 2012 [15]. In the US, OSCC is responsible for roughly 3% of cancers in men and 2% of cancers in women, most of which are diagnosed after the age of 50 [16]. Five-year survival rates for OSCC are ~70%, although this number fluctuates substantially depending on anatomical/histologic subtype and grade/stage at the time of diagnosis [17].
Cancer Therapy and Associated Oral Health Complications
Surgical Management
Surgical management remains a mainstay of modern cancer treatment, including for HNSCC. While removal of simple, early stage tumors may result in minimal side effects, surgical treatment of more advanced stage lesions can produce numerous esthetic, functional, and psychological sequelae. Potential impacts of surgery on oral function include difficulty tasting, speaking, chewing, and swallowing, whereas the excision of mucosal surfaces, loss in soft tissue volume, and removal of bone may result in substantial esthetic deformities [18]. Maxillofacial prosthodontics is an essential component of oral rehabilitation in patients with oral cancer undergoing surgical management.
The primary goals of maxillofacial prosthodontics are to restore oral function, improve facial esthetics, and enhance quality of life [19]. For example, maxillofacial prosthodontists fabricate various appliances, such as obturators, partial bridges, etc., to support ongoing cancer treatment and for patients following surgical procedures [20]. By utilizing computed tomography imaging, medical modeling technology, and virtual surgical planning (VSP), maxillofacial prosthodontists are able to accurately pre-plan the prosthetic and dental rehabilitation of patients undergoing complex surgical reconstruction [21, 22]. Undoubtedly, the maxillofacial prosthodontist is an essential member of the cancer team and plays a vital role in achieving optimal treatment outcomes for cancer patients.
Radiotherapy
Radiotherapy remains one of the primary treatment modalities for both localized and late-stage cancers. Further, various randomized control trials have demonstrated a superior tumor response to radiotherapy and concurrent chemotherapy, rather than radiotherapy alone, for numerous advanced tumors including HNCs [23–25]. The side effects and toxicities related to radiotherapy, however, have the potential to impart a substantial amount of morbidity and worsen quality of life for many patients. Despite recent advances in radiotherapy technique and delivery, many of the dental and oral complications related to head and neck radiation, such as oral mucositis, salivary gland dysfunction, radiation caries, and osteoradionecrosis, are still largely prevalent among this cancer population.
Oral Mucositis
Oral mucositis (OM) is fairly ubiquitous among patients receiving radiation therapy to the head and neck [26]. Symptoms of OM include irritation, discomfort, and pain that often precedes an erythematous mucosal lesion, which may ultimately progress to frank ulceration [27]. OM frequently occurs 2–3 weeks following high-dose radiation therapy to the head and neck (e.g., 60–70 Gy), and symptoms typically worsen with increasing radiation dose. Although any mucosal surface can potentially develop OM, non-keratinized tissues (e.g., buccal mucosa, lateral tongue, soft palate, and floor of mouth) are at a greater risk than keratinized tissues (e.g., attached gingiva, hard palate, dorsal tongue) [28]. The addition of concurrent chemotherapy or targeted therapies with radiation therapy has been shown to increase the severity, duration, and extent of OM [29]. The morbidity associated with OM includes pain, nutritional compromise often necessitating a feeding tube, reduction in quality of life, interruptions in cancer therapy, possible concomitant infections, and increased treatment costs [28]. Photobiomodulation, which uses red or near-infrared light to beneficially influence cellular metabolism to repair tissue damage caused by injury or disease, has been shown to be effective in preventing OM [30, 31].
Salivary Gland Dysfunction and Radiation Caries
Radiotherapy to the head and neck region is a common cause of salivary gland dysfunction, with doses of 50 Gy or higher imparting the highest risk for this complication [32, 33]. Patients may experience xerostomia in as little as 1 week after initiating radiation therapy, with the potential for permanent salivary gland dysfunction with continued exposure [34]. Disruption in the normal salivary flow may result in numerous oral complications, such as dysgeusia, dysphagia, problems with speech, oral candidiasis, and dental caries, while severely diminishing quality of life [35]. The enhanced risk of tooth decay, known as radiation caries, is thought to be a direct result of radiotherapy-induced salivary gland acinar degeneration and interstitial fibrosis [36, 37]. Teeth exposed to radiation may also be more prone to decay due to changes in the composition of dental hard tissue, such as loss of enamel prism structure, degeneration of odontoblast processes, and obliteration of dentinal tubules [38].
While various pharmacologic agents have been suggested as possible interventions for preventing radiation-induced salivary gland dysfunction, such as parasympathomimetic drugs, parasympatholytic drugs, and cytoprotective agents, the evidence to support their efficacy is of admittedly poor quality [34, 39–42]. Some clinicians and patients may opt for non-pharmacologic products such as toothpastes, mouthrinses, mouth sprays, and gels, as well as sugar-free gums and lozenges to reduce symptoms of xerostomia [43]. Fluoride supplementation, varnish, and regular oral hygiene check-ups are also essential to reduce the risk of developing radiation caries. In patients treated with radiotherapy, the daily application of 1% sodium fluoride gel has the potential to significantly reduce the incidence of caries [44].
Osteoradionecrosis
Osteoradionecrosis (ORN) of the jaw is a potentially severe iatrogenic disease of devitalized bone caused by radiation therapy of the head and neck that fails to heal or remodel [45–48]. Early proposed pathophysiologic mechanisms of ORN focused on hypoxic, hypovascular, and hypocellular tissue resulting in tissue breakdown and a non-healing wound [47, 49, 50]. More recent research, however, has favored the radiation-induced fibrosis theory whereby abnormal fibroblast activity leads to inflammation, local tissue injury, and eventually tissue necrosis [46, 51]. Common signs and symptoms of ORN include oral dysesthesia, paresthesia, pain, trismus, ulceration and necrosis of oral mucosa, malodor, pathologic fractures, draining fistulas, and deterioration in dental hygiene practices. Despite conflicting evidence, hyperbaric oxygen therapy (HBO) may be utilized in an attempt to prevent ORN of the jaws in adults receiving radiotherapy to the head and neck [52].
Chemotherapy and Hematopoietic Stem Cell Transplantation
Chemotherapeutic agents comprise a vast group of chemicals designed to halt the growth of cancer cells, either through inducing apoptosis or preventing their replication. These agents produce their toxic effects by targeting rapidly proliferating cells, such as the basal cells of the mucosal layer as well as the acinar and ductal cells of the salivary glands [53]. The oral side effects of chemotherapy are relatively common and may include OM, candidiasis and other oral infections (including bacterial, viral, and fungal infections), xerostomia, oral bleeding, and potentially periodontal disease [53, 54]. Although concomitant chemotherapy often produces OM, the concomitant use of targeted agents may further alter mucositis risk, severity, and course [55, 56].
Hematopoietic stem cell transplantation (HSCT), on the other hand, involves the transplantation of healthy hematopoietic stem cells to patients with dysfunctional or depleted bone marrow for the treatment of various cancers, immune-deficiency syndromes, and hemoglobinopathies [57]. HSCT carries the risk of numerous acute and chronic complications that may impact the oral cavity, such as OM, oral candidiasis, herpes simplex virus (HSV) recrudescence, and graft-vs.-host disease (GVHD).
Graft-vs.-Host Disease
Graft-vs.-host disease (GVHD) is a major cause of morbidity and non-relapse mortality in patients undergoing allogeneic HSCT, with over 50% of patients developing chronic GVHD [58, 59]. Chronic GVHD is an alloimmune condition caused by donor T-cells recognizing and attacking antigens expressed on normal host tissues [60]. Oral chronic GVHD is characterized by mucosal, lichen planus-like changes presenting as erythematous and/or ulcerative lesions. Patients may experience oral pain, sensitivity to spicy/acidic foods, alcohol, and certain mouthwashes, xerostomia, difficulty speaking/swallowing, and taste changes that may predispose patients to decreased oral intake, nutritional deficiencies, and oral infections [61, 62]. Dentists and oral medicine specialists are important identifiers of this potentially debilitating disease. The most commonly used topical therapies for oral chronic GVHD include high-potency corticosteroids and calcineurin inhibitors, whereas systemic therapy includes corticosteroids, calcineurin inhibitors, and many other immunomodulatory agents [63].
Antiresorptive and Antiangiogenic Therapy
Medication-related osteonecrosis of the jaw (MRONJ) is a potentially debilitating condition characterized by non-healing exposed bone in patients who have used either antiresorptive or antiangiogenic agents [64, 65]. High-dose regimens of antiresorptive medications, like bisphosphonates and receptor activator of nuclear factor kappa B ligand (RANKL) inhibitors (e.g., denosumab), are frequently used to prevent skeletal-related adverse events in adults with malignancies involving bone [66]. Although the pathophysiology of MRONJ has been greatly debated, most hypotheses suggest the role of altered bone remodeling, oversuppression of bone resorption, and angiogenesis inhibition as key mechanisms resulting in this disease process [67].
Dentists and other oral healthcare specialists play a vital role in preventing or minimizing a patient's risk of developing MRONJ. Dental assessments and the provision of prophylactic dental care prior to initiation of antiangiogenic or antiresorptive therapy have been shown to decrease a patient's chances of developing MRONJ [68]. In patients with MRONJ, treatment is divided into either conservative (such as maintaining optimal oral hygiene, eliminating soft and hard tissue disease, antibiotic therapy, and the use of antibacterial mouthwashes) or surgical management [68]. Although the success rate of surgical treatment for MRONJ has proven to be high [69], the side effects of these invasive resections have the potential to impart a substantial amount of morbidity.
Targeted Therapies and Immunotherapies
Targeted therapies, which target specific genes and proteins involved in the growth and survival of cancer cells, and immunotherapies, which stimulate a patient's own immune system to combat cancer, are quickly becoming central pillars of cancer treatment. Although the introduction of targeted therapies and immunotherapies have revolutionized treatment for numerous types of malignancies, they have also produced novel side effects known as immune-related adverse events (irAE). While these adverse events have been observed in nearly all parts of the body, their impact on the oral cavity is notable [70].
Immune-Related Adverse Events
Although immunotherapy has made an indelible mark on the field of cancer therapeutics, irAEs associated with their use are unfortunately commonplace [71]. These adverse events are thought to arise from a loss of tolerance to self-antigens, which results in organ toxicity [72]. irAEs are found in nearly every organ, including the oral cavity where they primarily affect the oral mucosa, salivary glands, and sense of taste [73]. Numerous authors have reported an association between immunotherapy use and numerous oral complications, such as lichenoid reactions, sicca syndrome, vesiculobullous disorders, erythema multiforme (EM), and Steven-Johnson syndrome (SJS) [74–78]. Despite these observations, little is known regarding the etiology, underlying pathophysiology, and appropriate management for these conditions. Nevertheless, appropriate referrals to multidisciplinary teams composed of oncologists, rheumatologists, and oral healthcare specialists should be made to ensure proper case management for this complex patient population.
Promoting Oral Health With Cancer Treatment
Before Cancer Treatment
Obtaining dental clearance is an essential step prior to the initiation of cancer treatment, particularly for patients to be exposed to radiation to the head and neck. The rationale for dental screening prior to cancer therapy derives from numerous studies linking an increased incidence of intra-therapy complications, such as acute dental infections, with poor oral health [79–81].
A complete oral evaluation prior to the initiation of cancer treatment should elucidate the following information: presence of a dental home, date of last dental visit, recognition of past and current dental problems, an oral/dental evidence-based risk assessment such as CAMBRA (caries management by risk assessment), and an evaluation of past and current medication use.
Additionally, the patient should receive oral prophylaxis including professional hygiene therapy as well as eliminate any existing low-grade infections and possible sources of trauma (e.g., trauma from denture or fixed orthodontic appliances) [82]. The National Institute for Dental and Craniofacial Research recommends that elective surgical procedures be postponed until the cessation of cancer therapy, while invasive procedures be completed at least 14 days prior to the initiation of head and/or neck radiation and 7–10 days prior to myelosuppressive chemotherapy [83]. Finally, patients should be educated in proper oral hygiene techniques for the prevention of future dental caries and oral disease that may impact cancer treatment.
During Cancer Treatment
The increased risk of acute oral complications resulting from radiation and/or chemotherapy highlights the importance of continued proper oral hygiene practices and maintenance. Patients should continue to use the modified Bass brushing method with fluoride toothpaste at least two times per day [84], floss one time per day, avoid the use of alcoholic mouthwashes, and remain on a regular hygiene recall schedule with their dentist. The use of removable appliances and prostheses, especially those with the potential to cause hard and soft tissue damage, should be limited. Stimulation of salivary production in those patients demonstrating hyposalivation can be achieved either through the use of sugar-free lozenges or gums (e.g., xylitol) [85, 86].
It is crucial that dentists and other oral healthcare specialists maintain communication with the oncologist throughout the duration of cancer therapy and obtain proper consultation with the oncologist prior to any dental procedures, including prophylaxis. A blood sample should be obtained from patients undergoing chemotherapy roughly 24 h prior to any invasive oral surgical procedures and postponed when the following are present: platelet counts <75,000/mm3; abnormal clotting factors present; and/or absolute neutrophil count of <1,000/mm3 [83].
After Cancer Treatment
Following the completion of cancer therapy, patients should remain on a regular recall schedule as recommended by their dentist and continue practicing proper oral hygiene including the use of fluoride toothpaste and varnish. In patients at higher risk for dental caries (especially post-allogeneic HSCT/GVHD) as well as those with a history of oral malignancy (such as OSCC), a more intensive and frequent recall schedule may be necessary. Further, a patient's hematologic status, such as the resolution of immunosuppression and/or thrombocytopenia following the cessation of cancer therapy, should be assessed prior to dental treatment. Furthermore, the dentist should update the patient's medication list and assess for any anti-resorptive and/or anti-angiogenic medications used throughout the duration of cancer treatment.
Finally, childhood cancer therapy has the potential to result in numerous dental, craniofacial, and soft tissue complications. Children are particularly susceptible to the long-term effects of cancer therapy as treatment typically occurs during the most active stage of growth and organ development [87]. For example, these patients are at an increased risk for dental caries, abnormalities in tooth morphology and composition, hyposalivation, maxillary and mandibular growth disturbances, and temporomandibular dysfunction (TMD) [88, 89]. As such, pediatric and general dentists should be aware of these potential complications and monitor for any abnormal deviations in craniofacial and/or dental growth and development [83].
Conclusion
Innovations and improvements in cancer therapy have substantially increased survivorship in recent years. As a result, there is a growing need for continuing management of the oral health needs of this population. Although many of the acute and chronic oral toxicities of cancer therapy are largely unavoidable, appropriate and timely management of these complications has the potential to alleviate a considerable amount of morbidity. Further, with advances in computational modeling and “Deep Learning” protocols, individuals at risk for developing drug toxicities may be identified and providers may be better equipped to predict which patients and drugs are most likely to induce oral side effects. Finally, the successful management of this complex patient population requires interprofessional collaboration and the utilization of a comprehensive, patient-centered approach with an emphasis on oral health.
Author Contributions
JH and GO wrote the first draft of the manuscript. GH guided and revised the manuscript. NT conceptualized and guided the project. All authors contributed to the article and approved the submitted version.
Funding
GO was supported in part by the Piano di Sostegno alla Ricerca (PSR) 2020, Linea 2: Dotazione Annuale per attività istituzionali, Department of Biomedical, Surgical and Dental Sciences, Università degli Studi di Milano, Milan, Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cancer. World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed January 13, 2022).
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2018. Bethesda, MD: National Cancer Institute (2016).
5. American Cancer Society. Cancer Facts & Figures. (2021). Available online at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed January 13, 2022).
6. Peterson DE, D'Ambrosio JA. Diagnosis and management of acute and chronic oral complications of nonsurgical cancer therapies. Dent Clin North Am. (1992) 36:945–66.
7. Thariat J, Vignot S, Lapierre A, Falk AT, Guigay J, Van Obberghen-Schilling E, et al. Integrating genomics in head and neck cancer treatment: promises and pitfalls. Crit Rev Oncol Hematol. (2015) 95:397–406. doi: 10.1016/j.critrevonc.2015.03.005
8. Jackson LK, Johnson DB, Sosman JA, Murphy BA, Epstein JB. Oral health in oncology: impact of immunotherapy. Support Care Cancer. (2015) 23:1–3. doi: 10.1007/s00520-014-2434-6
9. Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J, Delord JP. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. (2016) 28:254–63. doi: 10.1097/CCO.0000000000000290
10. Burden G, Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. J Am Med Assoc Oncology. (2018) 4:1553–68. doi: 10.1001/jamaoncol.2018.2706
11. International Agency for Research on Cancer. Cancer Fact Sheets. (2022). Available online at: https://gco.iarc.fr/today/fact-sheets-cancers (accessed January 31, 2022).
12. World Health Organization. Global Health Estimates. Available online at https://www.who.int/data/global-health-estimates (accessed January 31, 2022).
13. Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. (2019) 9:217–22. doi: 10.2991/jegh.k.191008.001
14. McDermott JD, Bowles DW. Epidemiology of head and neck squamous cell carcinomas: impact on staging and prevention strategies. Curr Treat Opt Oncol. 20:43. doi: 10.1007/s11864-019-0650-5
15. Suresh GM, Koppad R, Prakash BV, Sabitha KS, Dhara PS. Prognostic indicators of oral squamous cell carcinoma. Ann Maxillofac Surg. (2019) 9:364–70. doi: 10.4103/ams.ams_253_18
16. Schiff BA. Oral Squamous Cell Carcinoma. Merck Manual. (2022). Available online at: https://wwwmerckmanualscom/professional/ear,-nose,-and-throat-disorders/tumors-of-the-head-and-neck/oral-squamous-cell-carcinoma (accessed January 23, 2022).
17. Harris JA, Hunter WP, Hanna GJ, Treister NS, Menon RS. Rural patients with oral squamous cell carcinoma experience better prognosis and long-term survival. Oral Oncol. (2020) 111:105037. doi: 10.1016/j.oraloncology.2020.105037
18. Rogers SN. Quality of life perspectives in patients with oral cancer. Oral Oncol. (2010) 46:445–7. doi: 10.1016/j.oraloncology.2010.02.021
19. Petrovic I, Rosen EB, Matros E, Huryn JM, Shah JP. Oral rehabilitation of the cancer patient: a formidable challenge. J Surg Oncol. (2018) 117:1729–35. doi: 10.1002/jso.25075
20. Phasuk K, Haug SP. Maxillofacial prosthetics. Oral Maxillofac Surg Clin North Am. (2018) 30:487–97. doi: 10.1016/j.coms.2018.06.009
21. Beumer III J, Maruknick MT, Esposito SJ. Maxillofacial Rehabilitation: Prosthodontic and Surgical Management of Cancer-Related, Acquired, and Congenital Defects of the Head and Neck. 3rd ed. Hanover Park, IL: Quintessence Publications (2011).
22. Jaquiéry C, Rohner D, Kunz C, Bucher P, Peters F, Schenk RK, et al. Reconstruction of maxillary and mandibular defects using prefabricated microvascular fibular grafts and osseointegrated dental implants – a prospective study. Clin Oral Implants Res. (2004) 15:598–606. doi: 10.1111/j.1600-0501.2004.01065.x
23. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). J Am Med Assoc. (1999) 281:1623–7. doi: 10.1001/jama.281.17.1623
24. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
25. Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. (1999) 1999:2081–6. doi: 10.1093/jnci/91.24.2081
26. Duncan GG, Epstein JB, Tu D, El Sayed S, Bezjak A, Ottaway J, et al. Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: a report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head Neck. (2005) 27:421–8. doi: 10.1002/hed.20162
27. Sroussi HY, Epstein JB, Bensadoun R-J, Saunders DP, Lalla RV, Migliorati CA, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. (2017) 6:2918–31. doi: 10.1002/cam4.1221
28. Lalla RV, Saunders DP, Peterson DE. Chemotherapy or radiation-induced oral mucositis. Dent Clin North Am. (2014) 58:341–9. doi: 10.1016/j.cden.2013.12.005
29. Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. (2014) 32:2940–50. doi: 10.1200/JCO.2013.53.5633
30. Weissheimer C, Curra M, Gregianin LJ, Daudt LE, Wagner VP, Martins MAT, et al. New photobiomodulation protocol prevents oral mucositis in hematopoietic stem cell transplantation recipients—a retrospective study. Lasers Med Sci. (2017) 32:2013–21. doi: 10.1007/s10103-017-2314-7
31. Cronshaw M, Parker S, Anagnostaki E, Mylona V, Lynch E, Grootveld M. Photobiomodulation and oral mucositis: a systematic review. Dent J. (2020) 8:dj803008. doi: 10.3390/dj8030087
32. Shiboski CH, Hodgson TA, Ship JA, Schiødt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2007) 103(Suppl.):S66.e1–19. doi: 10.1016/j.tripleo.2006.11.013
33. Guchelaar HJ, Vermes A, Meerwaldt JH. Radiation-induced xerostomia: pathophysiology, clinical course and supportive treatment. Support Care Cancer. (1997) 5:281–8. doi: 10.1007/s005200050075
34. Riley P, Glenny A-M, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. (2017) 7:CD012744. doi: 10.1002/14651858.CD012744
35. Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2004) 97:28–46. doi: 10.1016/j.tripleo.2003.07.010
36. Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. (2003) 14:199–212. doi: 10.1177/154411130301400305
37. Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. (2009) 88:894–903. doi: 10.1177/0022034509343143
38. Lieshout HFJ, Bots CP. The effect of radiotherapy on dental hard tissue—a systematic review. Clin Oral Investig. (2013) 18:17–24. doi: 10.1007/s00784-013-1034-z
39. Wiseman LR, Faulds D. Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia. Drugs. (1995) 49:143–55. doi: 10.2165/00003495-199549010-00010
40. Epstein JB, Burchell JL, Emerton S, Le ND, Silverman S Jr. A clinical trial of bethanechol in patients with xerostomia after radiation therapy. A pilot study. Oral Surg Oral Med Oral Pathol. (1994) 77:610–4. doi: 10.1016/0030-4220(94)90320-4
41. Rode M, Šmid L, Budihna M, Gašsperšsič D, Rode M, Šoba E. The influence of pilocarpine and biperiden on pH value and calcium, phosphate, and bicarbonate concentrations in saliva during and after radiotherapy for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. (2001) 2001:509–14. doi: 10.1067/moe.2001.115984
42. Brizel DM, Wasserman T. The influence of intravenous amifostine on xerostomia and survival during radiotherapy for head and neck cancer: two year follow-up of a prospective randomized trial. J Clin Orthod. (2004) 22(14_suppl.):5536. doi: 10.1200/jco.2004.22.90140.5536
43. Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. J Oral Rehabil. (2007) 34:724–32. doi: 10.1111/j.1365-2842.2006.01718.x
44. Dreizen S, Brown LR, Daly TE, Drane JB. Prevention of xerostomia-related dental caries in irradiated cancer patients. J Dent Res. (1977) 56:99–104. doi: 10.1177/00220345770560022101
45. Zehr L, Cooper JS. Osteoradionecrosis, Mandible. StatPearls Treasure Island, FL: StatPearls Publishing (2017).
46. Rivero JA, Shamji O, Kolokythas A. Osteoradionecrosis: a review of pathophysiology, prevention and pharmacologic management using pentoxifylline, α-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol. (2017) 124:464–71. doi: 10.1016/j.oooo.2017.08.004
47. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. (1983) 41:283–8. doi: 10.1016/0278-2391(83)90294-X
48. Harris M. The conservative management of osteoradionecrosis of the mandible with ultrasound therapy. Br J Oral Maxillofac Surg. (1992) 30:313–8. doi: 10.1016/0266-4356(92)90181-H
49. Jacobson AS, Buchbinder D, Hu K, Urken ML. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. (2010) 46:795–801. doi: 10.1016/j.oraloncology.2010.08.007
50. Beumer J, Harrison R, Sanders B, Kurrasch M. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck Surg. (1984) 6:819–27. doi: 10.1002/hed.2890060404
51. Delanian S, Lefaix J-L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. (2004) 73:119–31. doi: 10.1016/j.radonc.2004.08.021
52. El-Rabbany M, Duchnay M, Raziee HR, Zych M, Tenenbaum H, Shah PS, et al. Interventions for preventing osteoradionecrosis of the jaws in adults receiving head and neck radiotherapy. Cochr Database Systemat Rev. (2019). 2019:CD011559. doi: 10.1002/14651858.CD011559.pub2
53. Poulopoulos A, Papadopoulos P, Andreadis D. Chemotherapy: oral side effects and dental interventions-a review of the literature. Stomatol Dis Sci. (2017) 1:35–49. doi: 10.20517/2573-0002.2017.03
54. National Cancer Institute. Oral Complications of Chemotherapy and Head/Neck Radiation (PDQ®)–Health Professional Version. Available online at: https://www.cancer.gov/about-cancer/treatment/side-effects/mouth-throat/oral-complications-hp-pdq#_417 (accessed January 23, 2022).
55. Sonis ST. Oral mucositis in head and neck cancer: risk, biology, and management. Am Soc Clin Oncol Educ Book. (2013) 2013:e236–40. doi: 10.14694/EdBook_AM.2013.33.e236
56. Keil F, Selzer E, Berghold A, Reinisch S, Kapp KS, De Vries A, et al. Induction chemotherapy with docetaxel, cisplatin and 5-fluorouracil followed by radiotherapy with cetuximab for locally advanced squamous cell carcinoma of the head and neck. Eur J Cancer. (2013) 49:352–9. doi: 10.1016/j.ejca.2012.08.004
57. Khaddour K, Hana CK, Mewawalla P. Hematopoietic Stem Cell Transplantation. StatPearls Treasure Island, FL: StatPearls Publishing (2021).
58. Vogelsang GB, Pavletic SZ. Chronic Graft versus Host Disease: Interdisciplinary Management. Cambridge: Cambridge University Press (2009). doi: 10.1017/CBO9780511576751
59. Mays JW, Fassil H, Edwards DA, Pavletic SZ, Bassim CW. Oral chronic graft-versus-host disease: current pathogenesis, therapy, and research. Oral Dis. (2013) 19:327–46. doi: 10.1111/odi.12028
60. Berger AM, Shuster JL, Von Roenn JH. Principles and Practice of Palliative Care and Supportive Oncology. Philadelphia, PA: Lippincott Williams & Wilkins (2007).
61. Treister NS, Cook EF Jr, Antin J, Lee SJ, Soiffer R, Woo S-B. Clinical evaluation of oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2008) 14:110–5. doi: 10.1016/j.bbmt.2007.06.017
62. Schubert MM, Sullivan KM, Morton TH, Izutsu KT, Peterson DE, Flournoy N, et al. Oral manifestations of chronic graft-v-host disease. Arch Intern Med. (1984) 144:1591–5. doi: 10.1001/archinte.1984.00350200087014
63. Devita VT. Cancer: Principles & Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins Publishers (2001).
64. Di Fede O, Panzarella V, Mauceri R, Fusco V, Bedogni A, Lo Muzio L, et al. The dental management of patients at risk of medication-related osteonecrosis of the jaw: new paradigm of primary prevention. Biomed Res Int. (2018) 2018:2684924. doi: 10.1155/2018/2684924
65. Akashi M, Kusumoto J, Takeda D, Shigeta T, Hasegawa T, Komori T, et al. Literature review of perioperative antibiotic administration in surgery for medication-related osteonecrosis of the jaw. Oral Maxillofac Surg. (2018) 22:369–78. doi: 10.1007/s10006-018-0732-8
66. Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiødt M. Medication-related osteonecrosis of the jaw: prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev. (2018) 69:177–87. doi: 10.1016/j.ctrv.2018.06.007
67. Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American association of oral and maxillofacial surgeons' position paper on medication-related osteonecrosis of the jaw−2022 update. J Oral Maxillofac Surg. (2022) 2022:8. doi: 10.1016/j.joms.2022.02.008
68. Hellstein JW, Adler RA, Edwards B, Jacobsen PL, Kalmar JR, Koka S, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dental Assoc. (2011) 142:1243–51. doi: 10.14219/jada.archive.2011.0108
69. Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. (2009) 67(5Suppl.):85–95. doi: 10.1016/j.joms.2009.01.006
70. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
71. Shazib MA, Woo S-B, Sroussi H, Carvo I, Treister N, Farag A, et al. Oral immune-related adverse events associated with PD-1 inhibitor therapy: a case series. Oral Dis. (2020) 26:325–33. doi: 10.1111/odi.13218
72. Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. (2015) 125:3384–91. doi: 10.1172/JCI80011
73. Xu Y, Wen N, Sonis ST, Villa A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): an analysis of 4,683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women's Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer. (2021) 127:1796–804. doi: 10.1002/cncr.33436
74. Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res. (2016) 22:4023–9. doi: 10.1158/1078-0432.CCR-15-2872
75. Nayar N, Briscoe K, Penas PF. Toxic epidermal necrolysis–like reaction with severe satellite cell necrosis associated with nivolumab in a patient with ipilimumab refractory metastatic melanoma. J Immunother. (2016) 2016:149–52. doi: 10.1097/CJI.0000000000000112
76. Salati M, Pifferi M, Baldessari C, Bertolini F, Tomasello C, Cascinu S, et al. Stevens–Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol. (2018) 29:283–4. doi: 10.1093/annonc/mdx640
77. Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens–Johnson syndrome in non-melanoma patients. Eur J Cancer. (2017) 81:237–9. doi: 10.1016/j.ejca.2017.03.026
78. Harris JA, Huang K, Miloslavsky E, Hanna GJ. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oral Dis. (2021) 2021:14000. doi: 10.1111/odi.14000
79. Graber CJ, de Almeida KN, Atkinson JC, Javaheri D, Fukuda CD, Gill VJ, et al. Dental health and viridans streptococcal bacteremia in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. (2001) 27:537–42. doi: 10.1038/sj.bmt.1702818
80. Kennedy HF, Morrison D, Tomlinson D, Gibson BES, Bagg J, Gemmell CG. Gingivitis and toothbrushes: potential roles in viridans streptococcal bacteraemia. J Infect. (2003) 46:67–70. doi: 10.1053/jinf.2002.1084
81. Peterson DE, Overholser CD. Increased morbidity associated with oral infection in patients with acute nonlymphocytic leukemia. Oral Surg Oral Med Oral Pathol. (1981) 51:390–3. doi: 10.1016/0030-4220(81)90148-1
82. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis. Circulation. (2007) 116:1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095
83. National Institute of Dental and Craniofacial Research. Dental Provider's Oncology Pocket Guide. Washington, DC: US Department of Health and Human Services (2009).
84. Poyato-Ferrera M, Segura-Egea JJ, Bullón-Fernández P. Comparison of modified Bass technique with normal toothbrushing practices for efficacy in supragingival plaque removal. Int J Dent Hyg. (2003) 1:110–4. doi: 10.1034/j.1601-5037.2003.00018.x
85. Meurman JH, Grönroos L. Oral and dental health care of oral cancer patients: hyposalivation, caries and infections. Oral Oncol. (2010) 46:464–7. doi: 10.1016/j.oraloncology.2010.02.025
86. Lajer C, Buchwald C, Nauntofte B, Specht L, Bardow A, Jensdottir T. Erosive potential of saliva stimulating tablets with and without fluoride in irradiated head and neck cancer patients. Radiother Oncol. (2009) 93:534–8. doi: 10.1016/j.radonc.2009.06.028
87. Proc P, Szczepańska J, Herud A, Zubowska M, Fendler W, Młynarski W. Dental caries among childhood cancer survivors. Medicine. (2019) 98:e14279. doi: 10.1097/MD.0000000000014279
88. Hartnett E. Integrating oral health throughout cancer care. Clin J Oncol Nurs. (2015) 19:615–9. doi: 10.1188/15.CJON.615-619
Keywords: cancer, clinical oncology, oral health, oral complications, cancer therapy
Citation: Harris JA, Ottaviani G, Treister NS and Hanna GJ (2022) An Overview of Clinical Oncology and Impact on Oral Health. Front. Oral. Health 3:874332. doi: 10.3389/froh.2022.874332
Received: 12 February 2022; Accepted: 24 March 2022;
Published: 25 April 2022.
Edited by:
Ricardo Santiago Gomez, Federal University of Minas Gerais, BrazilReviewed by:
Mushfiq Hassan Shaikh, Western University, CanadaSibel Elif Gultekin, Gazi University, Turkey
Elin Hadler-Olsen, UiT the Arctic University of Norway, Norway
Copyright © 2022 Harris, Ottaviani, Treister and Hanna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Glenn J. Hanna, Z2xlbm5faGFubmFAZGZjaS5oYXJ2YXJkLmVkdQ==
†These authors have contributed equally to this work
 Jack A. Harris
Jack A. Harris Giulia Ottaviani
Giulia Ottaviani Nathaniel S. Treister
Nathaniel S. Treister Glenn J. Hanna
Glenn J. Hanna