
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oral. Health, 28 January 2022
Sec. Oral Cancers
Volume 2 - 2021 | https://doi.org/10.3389/froh.2021.815606
This article is part of the Research TopicOral Oncology: From Precise Surgery to Precision Medicine and SurgeryView all 7 articles
 K. S. Rathan Shetty
K. S. Rathan Shetty Vinayak Kurle
Vinayak Kurle P. Greeshma
P. Greeshma Veena B. Ganga
Veena B. Ganga Samskruthi P. Murthy*
Samskruthi P. Murthy* Siddappa K. Thammaiah
Siddappa K. Thammaiah P. Krishna Prasad
P. Krishna Prasad Purushottham Chavan
Purushottham Chavan Rajshekar Halkud
Rajshekar Halkud R. Krishnappa
R. KrishnappaMore than half of patients with oral cancer recur even after multimodality treatment and recurrent oral cancers carry a poorer prognosis when compared to other sites of head and neck. The best survival outcome in a recurrent setting is achieved by salvage surgery; however, objective criteria to select an ideal candidate for salvage surgery is difficult to frame, as the outcome depends on various treatment-, tumor-, and patient-related factors. The following is summarizes various tumor- and treatment-related factors that guide our decision-making to optimize oncologic and functional outcomes in surgical salvage for recurrent oral cancers. Short disease-free interval, advanced tumor stage (recurrent and primary), extracapsular spread and positive tumor margins in a recurrent tumor, regional recurrence, and multimodality treatment of primary tumor all portend worse outcomes after surgical salvage. Quality of life after surgical intervention has shown improvement over 1 year with a drastic drop in pain scores. Various trials are underway evaluating the combination of immunotherapy and surgical salvage in recurrent head and neck tumors, including oral cavity, which may widen our indications for salvage surgery with improved survival and preserved organ function.
Oral cavity squamous cell carcinoma (OCSCC) is one of the common cancers globally and their oncologic outcome has remained stable over decades [1]. Tumor relapses occur in 25–30% of cases in early-stage OCSCC and 50–60% of cases in advanced OCSCC [2–5]. Managing relapsed tumors is difficult due to exhausted treatment options, as most of the patients are previously heavily treated with surgery with/without adjuvant therapy. These tumors represent resistant clones of cells that have escaped treatment and, hence, have guarded prognosis [6]. Immunotherapy has shown promising results in recurrent/metastatic tumors of head and neck (R/M HN) and has emerged as the first-line treatment modality for the recurrent disease that is not amenable for curative-intent management [7]. Specific guidelines are framed regarding the choice of systemic therapy in R/M HN setting, which considers previous treatment, performance status of the patient, and combined positive score (CPS) [8].
Tumor resection with adequate margins and adjuvant therapy form the main treatment modality in both the primary and recurrent setting in OCSCC and offer the best survival benefit [6, 9–11]. However, no specific criteria exist to precisely select the patients for surgical salvage (SS). The available literature on recurrent oral cancers (ReOC) is retrospective and is commonly represented along with other head and neck sites. Most patients with OCSCC have field cancerization, making differentiation between second primary and recurrent tumor hard and are often reported together. Objective criteria to accurately select patients for SS are difficult to frame, as the success of SS is based on numerous factors and vary from patient to patient. Apart from the anatomical and functional constraints, poorly vascularized bed, heavily pretreated areas of healing, performance status, comorbidities, previous treatment morbidity, and various recurrent tumor factors also play a crucial role in decision-making.
We comprehensively reviewed literature from Medline and Embase databases to include studies evaluating outcomes on salvage surgery in ReOC. The following review summarizes tumor- and treatment-related factors reported in these studies that guide our decision-making to optimize oncologic and functional outcomes in SS for ReOC.
Two-thirds of patients with head and neck cancer recur with most presenting in <24 months from completion of treatment for primary tumor [6, 11]. The rates of local, regional, and locoregional recurrences in OCSCC are 31.2–62.6%, 24–51.1%, and 4.1–16.3%, respectively [12–18]. Knowledge and understanding of recurrence pattern are of substantial importance, as it aids in early detection, assessment of resectability, and preoperative planning.
Previous surgery and/or radiation therapy (RT) open planes of tumor barrier and bring changes in anatomical orientation, which may render locally recurrent tumor unresectable even when detected early. Tongue cancers migrate longitudinally along muscles fibers and lymphovascular planes and previous resection may result in early involvement of mylohyoid muscle, which portends a worse prognosis [19, 20]. Primary buccal cancers involving masticator space, most often present with local recurrence above supramandibular notch, bringing recurrent tumor close to vital areas such as foramen ovale, pterygoid plates, and cavernous sinus making resection more complicated [21]. Unexpected local recurrences in buccal cancer are seen secondary to retrograde infiltration of the nerve [21, 22]. Local relapse commonly occurs at the anastomotic site of previous reconstructive flap within flap tissue or a remote oral cavity site [23].
Local recurrences carry better prognosis after SS than regional/locoregional recurrences [24]. Sessions et al. demonstrated that SS for recurrent tongue (local) cancer had a better 5-year cumulative survival rate than those with regional recurrence (48.6 vs. 30.5%, respectively) [25]. Although, Mucke et al. aimed to investigate the impact of recurrence interval on survival, authors also found that patients with local recurrence fared better than those with regional recurrence [5-year overall survival (OS) 37.5 vs. 21.5%, respectively] [26]. Positive surgical margin in SS is a predictor of poor prognosis [27, 28]. Use of frozen section to evaluate margins intraoperatively is advisable, especially in a salvage setting, as most of the surrounding tissue is fibrotic and makes margin assessment difficult. The frozen section is assessed from resected specimen rather than tumor bed [29].
Regional recurrences are commonly seen in level II nodal region [30]. Unusual regional recurrences occur in ReOC at intraparotid, prelaryngeal, and retropharyngeal sites [30, 31]. However, patients with unusual regional recurrences have a poorer SS success rate (21.7 vs. 68.8%, p < 0.001) and 5-year disease-specific survival (DSS) (23.8 vs. 60.8%, p < 0.001) than those without unusual regional recurrences [31]. Mizrachi et al. evaluated 1,302 cases of oral cancer for regional recurrence in RT-naïve patients. 15% of patients developed regional recurrence and most (87%) patients underwent salvage treatment [32]. Patients who underwent SS with adjuvant chemoradiation (CTRT) therapy had better DSS than surgery alone or non-SS. Regional recurrence is best managed with surgery with or without adjuvant therapy. Another study demonstrated that regional relapse in a previously treated neck and with short disease-free interval (DFI) was associated with a significant reduction in survival [33].
Oral cavity examination has an easier access when compared to other head and neck sites. In a recurrent setting, tissue changes from previous treatment preclude recurrent tumor identification. The onset of new or persistent symptoms should alert the treating surgeon for active intervention. Surveillance with CT) or MRI scan is considered as standard protocol in most centers. Diffusion-weighted MRI is invaluable in identifying recurrent tumors even in asymptomatic patients. PET/CT surveillance can also be used and a short time to positive PET/CT carries poorer outcomes in ReOC [34].
Disease-free interval is defined as the time interval between completion of primary treatment and occurrence of recurrent tumor. Various studies have predicted that short DFI is associated with poorer survival and is an independent predictor of outcome in ReOC [15, 26, 34]. There is heterogeneity in defining optimal cutoff for early vs. late recurrence. Two studies that considered 18 months as optimal cutoff to define early vs. late recurrence have reported lower OS with recurrences <18 months when compared to recurrences occurring >18 months (20.5 vs. 42.3% and 27.6 vs. 38.2%, respectively) [26, 34]. Liao et al. reported an optimal cutoff for interval to relapse as 10 months and late recurrence was associated with significantly better 5-year DSS and OS (p < 0.0001) [15]. A recent study by Hosni et al. has shown that early recurrence in patients with OCSCC treated with surgical resection and before initiating planned postoperative RT is seen in 15% of cases [35]. They noted that patients with oral tongue primary, microscopic positive margin, pathological tumor (pT) stage III/IV, and pathological nodal (pN) stage II/III were associated with early recurrence on multivariate analysis. The 3-year OS significantly (p = 0.001) dropped from 71% (95% CI, 67–75%) to 41% (95% CI, 30–56%) in patients with no early recurrence to those who recurred before planning adjuvant therapy, respectively. Hence, an early recurrence carries a worse prognosis and must be factored during decision-making and patient counseling.
Various staging and risk stratifications of the patient have been proposed to aid in choosing the right patient for salvage treatment [5, 20, 36–38]. Yueh et al. proposed the first prognostic staging system for 308 persistent, recurrent, and second primary tumors of the oral cavity and oropharynx [36]. They proposed a composite 4-stage system with three variables, namely, tumor nodal metastasis (TNM) staging of recurrent tumor, weight loss (no weight loss, <20%, and ≥20%), and deep muscle invasion (i.e., mylohyoid/constrictor muscle involvement for oral cavity and pharyngeal tumors, respectively). For patients with T2N0M0, no weight loss and for patients with T2N0M0, <20% weight loss, 1-year survival rate was 88.2 and 71.9%, respectively, and 1-year survival rate dropped to 32.6% when weight loss was ≥ 20% and/or with deep muscle invasion. However, for patients with metastasis, the 1-year survival rate was 4.2%, regardless of weight loss. An important finding was that persistent tumors fare worse than recurrent tumors, which, in turn, fare worse than second primary tumors.
Lacy et al. also proposed a new staging system for recurrent oral cavity and oropharyngeal cancers, which included primary tumor (initial) TNM staging and extent of recurrence (local, regional, and distant recurrences) [20]. The 2-year survival rate was 54 and 41% for stage I (initial TNM stage I with local recurrence) and stage II (initial TNM stage I with regional recurrence or stage II with local recurrence), respectively, and 18 and 3% of 2-year survival rate for stage III (initial TNM stages III or IV with local or regional recurrence) and stage IV (any patient with distant metastasis), respectively. The following study highlights the fact that local recurrence has better outcomes than regional recurrent tumors. Sun et al. also proposed a staging system that incorporated both the primary and recurrent tumor staging with OCSCC [10]. Authors reported a significant survival difference between the proposed stages (p = 0.000) and those with locoregional recurrences, multiple nodes at recurrence and incompletely excised recurrent tumor fared worse. Tam et al. investigated survival outcome in ReOC. On recursive partition analysis, they identified the three risk groups: (1) the high-risk group (patients who received adjuvant CTRT/RT after initial therapy), (2) the intermediate-risk group (previous surgery alone and age ≥ 62 years), and (3) the low-risk group (previous surgery alone and age <62 years) with 5-year OS rate of 10, 39, and 74%, respectively [37]. Authors concluded that patients belonging to the high-risk group must be considered for noncurative intent treatment. Although risk stratification aids surgeon in choosing the right patient and counseling them regarding oncologic outcomes, they are not validated on external data and may not be applicable universally.
The indications for adjuvant therapy after SS in ReOC remain the same as the primary setting [39, 40]. In radiation-naïve patients, adjuvant radiation does not pose any specific morbidity. However, reirradiation (ReRT) must be suggested with caution in patients who received RT for primary tumors. Tumors that recur at the site of the previous radiation field after >50 Gy of total radiation dose within 6 months are considered to have radioresistant tumor [41] and the survival benefit with ReRT must be critically balanced against ReRT induced adverse effects. With conformal radiotherapy techniques such as intensity-modulated radiotherapy (IMRT) and volumetric modulated arch therapy, ReRT is now possible. Janot et al. conducted a randomized controlled trial to compare ReRT concurrent with chemotherapy vs. observation after SS in patients with recurrent head and neck carcinoma [42]. The trial reported a significantly improved DSS with adjuvant ReRT [hazards ratio (HR) 1.68, 95% CI, 1.13–2.50; p = 0.01] when compared to no ReRT. However, acute toxicity (≥grade 3) and late toxicity (≥grade 3) were seen in 28 and 39% of patients in ReRT arm, respectively. The RT technique used was three-dimensional (3D) conformal radiotherapy and IMRT was not used in the trial. Pathological risk features in the RT arm of the trial were suspicious/involved margin (22%), vascular emboli/perineural infiltration/diffuse infiltration (54%), and capsular rupture (70%). The trial included 18% of ReOC cases. May et al. suggested postoperative ReRT in recurrent head and neck cancers with positive surgical margin and perineural invasion, based on negative impact on progression-free survival when postoperative ReRT was omitted in cases with positive margin (HR: 8.894, 95% CI: 1.742–45.403) and perineural invasion (HR: 3.391, 95% CI: 1.140–10.089) [43].
Decisions regarding radiation dose and fractionation for adjuvant ReRT may vary from center to center. A recent retrospective multi-institutional study evaluated the efficacy of IMRT ReRT in 505 patients with recurrent/second primary tumor of head and neck (16.6% oral cavity subsite) [44]. This study concluded that doses 50 to 66 Gy were adequate after removing gross disease. The acute and late toxicity rates were 22.1 and 16.7%, respectively. Hyperfractionation and elective neck irradiation were not associated with oncologic benefit. Adjuvant RT must be considered after SS when feasible to achieve best survival.
Radiation-naïve patients with ReOC after SS have a 5-year OS, recurrence-free survival, and locoregional control rates of 59, 60, and 74%, respectively, and seem similar to locally advanced patients with nonrecurrent oral cancer treated with multimodality therapy [3, 45]. A study compared outcomes of ReOC after early OSCC (RT naïve) vs. advanced OSCC who received multimodality treatment with no recurrence. They showed that early OSCC with recurrence fared worse than advanced OSCC that did not recur [46]. However, the study is inherently biased as advanced OSCC with no recurrence represent favorable tumor biology than early tumors with recurrence. Various studies (as per comprehensive literature search) published after 2010, which have evaluated survival outcomes after SS in ReOC, are represented in Table 1.
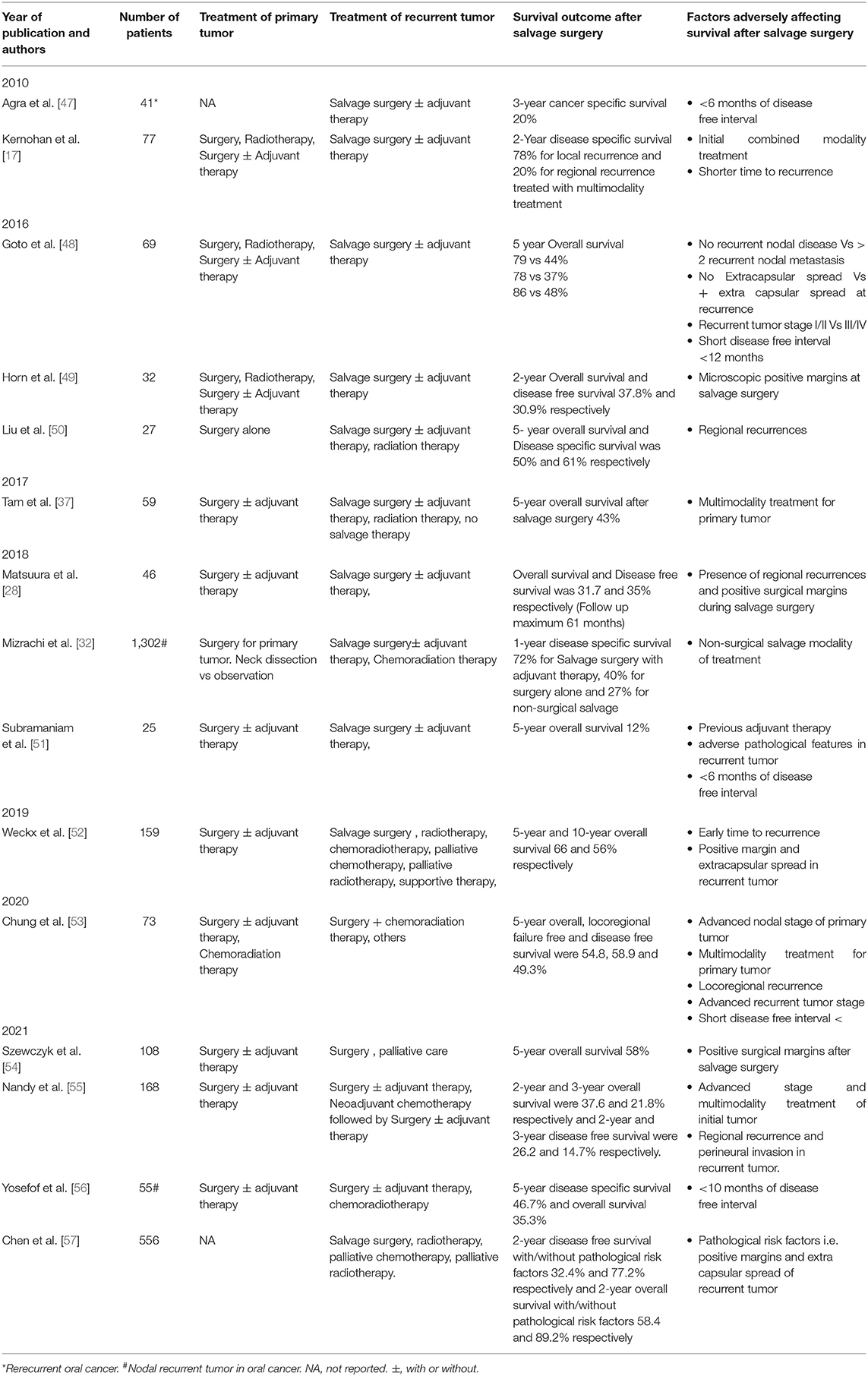
Table 1. Studies evaluating survival outcomes after salvage surgery for recurrent oral cancer in the recent decades.
Extracapsular spread (ECS) is a strong predictor of systemic spread [48]. Presence of ECS in the primary tumor is a known predictor of poor outcomes; however, its presence in primary tumor has also been associated with poor survival even after salvage of ReOC, regardless of TNM staging or DFI [52]. Tumor thickness of >10 mm in a recurrent tumor is associated with shorter OS [15]. Adverse pathological features such as lymphovascular and perineural invasion are associated with a worse prognosis. Primary and recurrent tumor stages have been proven to be poor prognosticators for survival after SS [47, 51, 53, 58].
50–60% of patients with recurrent oral cavity and oropharyngeal tumors after their SS are at a greater risk of rerecurrence and <20% develop distant metastasis on follow-up [47]. The clinical staging had no bearing on the rate of rerecurrence in these patients. DFI between first and second recurrence is vital, as second recurrences occurring in <6 months had a 3-year cancer-specific survival of 0% [50]. Hence, patients presenting with rerecurrence and a short DFI are considered for palliative intent management. Second salvage surgery must be reserved for motivated patients with good performance status and with >6 months of DFI only.
In 1970's, Gilbert and Kagan reported that only 18.3% of recurrent oral and oropharyngeal tumors are surgical salvageable due to limitations related to reconstruction [59]. However, with advances in free flap reconstructions and microvascular techniques, most defects can be reconstructed with success rates of >90% in most series [60].
The overall complication rates are 18–24.1% in the recurrent setting [49, 61, 62]. Previous treatment details such as the extent of resection(especially mandible/maxilla), available vessels for microvascular anastomosis, radiation dose, time since RT completion, comorbidities, smoking, and preexisting wound infection all play a critical role in preoperative planning [63, 64]. Prior radiotherapy has shown to reduce graft bed vascularization continuously with increasing total RT dose and time after RT [65].
Microvascular reconstruction has shown a success rate of 92–96.8% in the ReOC setting [61, 66]. Selecting an appropriate recipient vessel in the neck is crucial for a successful outcome. Recipient vessels are prone to spasm and anastomotic complications due to inadvertent injury resulting from difficult dissection through fibrotic bed. Offodile et al. studied outcomes of sequential microvascular reconstruction for recurrent and second primary oral cancers [67]. The incidence of free flap failure was 1.6 and 8.1% in second and third sequential reconstructions. The duration of hospital stay and re-exploration for venous occlusion were higher with subsequent free flaps. This study showed that anterolateral thigh (ALT) flap was the most common flap used and free fibula flap was commonly used at first recurrence, highlighting that most salvage resection involved bone. In salvage settings, the microvascular anastomosis to contralateral superior thyroid/facial vessels and ipsilateral superficial temporal/transverse cervical vessels were frequent [67].
The lure to perform locoregional flaps in ReOC when survival outcome is limited must be weighed against the higher incidence of complications and wound dehiscence. Pectoralis major myocutaneous flap and extended vertical lower trapezius flaps have been described to reconstruct heavily pretreated defects in ReOC with acceptable outcomes [68, 69]. However, the requisite volume or tissue component required to restore integrity and function can be easily achieved by free flaps.
Many factors create a hostile environment for normal wound healing in a salvage setting; hence, a comprehensive approach, which includes intricate preoperative planning and preparation of the patient, is vital to achieve favorable outcome.
Extensive resection and radiation in ReOC are known to cause significant morbidity. A recent study by Horn et al. is one of the first to study quality of life (QOL) measures after SS in ReOC [70]. The QOL was assessed according to the European Organization for Research and Treatment of Cancer (EORTC) and utilized general questionnaire QLQ-C30 and specific tool QLQ-H&N35 questionnaires. They reported significant drop in global QOL during the first 3 months after SS; however, QOL recovered to baseline over a year. Other domains such as role functioning, emotional functioning, and physical functioning showed decrease in scores for first 3 months, but recovered over a year. Swallowing and speech items were rated the worst, nevertheless mean scores increased after a year. An important finding in this study was significant reduction in pain scores at 3 months after SS and remained way below baseline values even after a year. SS in ReOC is justified, as most domains in QOL improved over a year with significant reduction in pain scores.
Although SS offers the best chance of survival in ReOC, the oncologic outcome is still poorer, especially when compared to other subsites. Various trials are underway to assess the feasibility of immunotherapy in recurrent head and neck tumors (including oral cavity) planned for SS in neoadjuvant and adjuvant settings (Table 2). With the emergence of further trials testing the impact of combining immunotherapy and SS therapy in recurrent tumors of head and neck, it may be possible to widen the indications for SS and optimize oncologic outcome without compromising organ function and preserve QOL.

Table 2. Illustrative listing of clinical trials examining impact of combining immunotherapy in salvage surgery setting in recurrent head and neck cancers.
Surgical salvage remains the standard of treatment in ReOC when resectable, as it offers the best chance of survival. Patients with advanced stage recurrent tumors and short DFI are not ideal candidates for SS. Various risk stratification systems have been proposed, which help in triaging the patient for curative intent modalities of salvage treatment. ECS and positive margins in recurrent settings portend poor outcomes and such patients must be advised adjuvant therapy when feasible. With the rising application of immunotherapy in managing recurrent head and neck tumors planned for SS, we can further make way to expand indications for SS with an improved oncologic outcome.
KS contributed to the conceptualization. SM and KS contributed to the writing original draft. SM, KS, VK, PG, VG, and PP contributed to the review and editing. ST, PC, RH, and RK contributed to the supervision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We extend our sincere thanks to Dr Manish Devendra Mair, Consultant, Head and Neck Surgery, University Hospitals of Leicester NS Trust, UK for help in literature search.
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. (2016) 25:16–27. doi: 10.1158/1055-9965.EPI-15-0578
2. Lydiatt DD, Robbins KT, Byers RM, Wolf PF. Treatment of stage I and II oral tongue cancer. Head Neck. (1993) 15:308–12. doi: 10.1002/hed.2880150407
3. Zittel S, Moratin J, Horn D, Metzger K, Ristow O, Engel M, et al. Clinical outcome and prognostic factors in recurrent oral squamous cell carcinoma after primary surgical treatment: a retrospective study. Clin Oral Investig. (2021). doi: 10.1007/s00784-021-04186-y [Online ahead of print].
4. Grégoire V, Lefebvre JL, Licitra L, Felip E. EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2010) 21 Suppl 5:v184–6. doi: 10.1093/annonc/mdq185
5. Tan HK, Giger R, Auperin A, Bourhis J, Janot F, Temam S. Salvage surgery after concomitant chemoradiation in head and neck squamous cell carcinomas - stratification for postsalvage survival. Head Neck. (2010) 32:139–47. doi: 10.1002/hed.21159
6. D'Cruz AK, Vaish R, Dhar H. Oral cancers: current status. Oral Oncol. (2018) 87:64–9. doi: 10.1016/j.oraloncology.2018.10.013
7. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr et al. KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
8. Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. (2019) 99:104460. doi: 10.1016/j.oraloncology.2019.104460
9. Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. (2014) 36:144–51. doi: 10.1002/hed.23227
10. Sun GW, Tang EY, Yang XD, Hu QG. Salvage treatment for recurrent oral squamous cell carcinoma. J Craniofac Surg. (2009) 20:1093–6. doi: 10.1097/SCS.0b013e3181abb307
11. Goodwin WJ Jr. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. (2000) 110 (3 Pt 2 Suppl 93):1–18. doi: 10.1097/00005537-200003001-00001
12. Agra IM, Carvalho AL, Ulbrich FS, de Campos OD, Martins EP, Magrin J, et al. Prognostic factors in salvage surgery for recurrent oral and oropharyngeal cancer. Head Neck. (2006) 28:107–13. doi: 10.1002/hed.20309
13. Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. (2006) 42:789–94. doi: 10.1016/j.oraloncology.2005.11.016
14. Brown JS, Blackburn TK, Woolgar JA, Lowe D, Errington RD, Vaughan ED, et al. A comparison of outcomes for patients with oral squamous cell carcinoma at intermediate risk of recurrence treated by surgery alone or with post-operative radiotherapy. Oral Oncol. (2007) 43:764–73. doi: 10.1016/j.oraloncology.2006.09.010
15. Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, Lee LY, et al. Salvage therapy in relapsed squamous cell carcinoma of the oral cavity: how and when? Cancer. (2008) 112:94–103. doi: 10.1002/cncr.23142
16. Lim YC, Choi EC. Surgery alone for squamous cell carcinoma of the oral cavity: survival rates, recurrence patterns, and salvage treatment. Acta Otolaryngol. (2008) 128:1132–7. doi: 10.1080/00016480801901691
17. Kernohan MD, Clark JR, Gao K, Ebrahimi A, Milross CG. Predicting the prognosis of oral squamous cell carcinoma after first recurrence. Arch Otolaryngol Head Neck Surg. (2010)136:1235–9. doi: 10.1001/archoto.2010.214
18. Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. (2010) 68:1270–5. doi: 10.1016/j.joms.2009.11.016
19. Calabrese L, Bruschini R, Giugliano G, Ostuni A, Maffini F, Massaro MA et al. Compartmental tongue surgery: long term oncologic results in the treatment of tongue cancer. Oral Oncol. (2011) 47:174–9. doi: 10.1016/j.oraloncology.2010.12.006
20. Lacy PD, Spitznagel EL Jr, Piccirillo JF. Development of a new staging system for recurrent oral cavity and oropharyngeal squamous cell carcinoma. Cancer. (1999) 86:1387–95.
21. Lin YW, Chen YF, Yang CC, Ho CH, Wu TC, Yen CY, et al. Patterns of failure after postoperative intensity-modulated radiotherapy for locally advanced buccal cancer: initial masticator space involvement is the key factor of recurrence. Head Neck. (2018) 40:2621–32. doi: 10.1002/hed.25355
22. Geretschläger A, Bojaxhiu B, Crowe S, Arnold A, Manser P, Hallermann W, et al. Outcome and patterns of failure after postoperative intensity modulated radiotherapy for locally advanced or high-risk oral cavity squamous cell carcinoma. Radiat Oncol. (2012) 7:175. doi: 10.1186/1748-717X-7-175
23. Cho Y, Yoon HI, Lee IJ, Kim JW, Lee CG, Choi EC, et al. Patterns of local recurrence after curative resection and reconstruction for oropharyngeal and oral cancers: Implications for postoperative radiotherapy target volumes. Head Neck. (2019) 41:3916–23. doi: 10.1002/hed.25928
24. Gañán L, López M, García J, Esteller E, Quer M, León X. Management of recurrent head and neck cancer: variables related to salvage surgery. Eur Arch Otorhinolaryngol. (2016) 273:4417–24. doi: 10.1007/s00405-016-4093-3
25. Sessions DG, Spector GJ, Lenox J, Haughey B, Chao C, Marks J. Analysis of treatment results for oral tongue cancer. Laryngoscope. (2002) 112:616–25. doi: 10.1097/00005537-200204000-00005
26. Mücke T, Wagenpfeil S, Kesting MR, Hölzle F, Wolff KD. Recurrence interval affects survival after local relapse of oral cancer. Oral Oncol. (2009) 45:687–91. doi: 10.1016/j.oraloncology.2008.10.011
27. Anderson CR, Sisson K, Moncrieff M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. (2015) 51:464–9. doi: 10.1016/j.oraloncology.2015.01.015
28. Matsuura D, Valim TD, Kulcsar MAV, Pinto FR, Brandão LG, Cernea CR et al. Risk factors for salvage surgery failure in oral cavity squamous cell carcinoma. Laryngoscope. (2018) 128:1113–9. doi: 10.1002/lary.26935
29. Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. (2015) 125:2298–307. doi: 10.1002/lary.25397
30. Chan AK, Huang SH, Le LW, Yu E, Dawson LA, Kim JJ, et al. Postoperative intensity-modulated radiotherapy following surgery for oral cavity squamous cell carcinoma: patterns of failure. Oral Oncol. (2013) 49:255–60. doi: 10.1016/j.oraloncology.2012.09.006
31. Feng Z, Niu LX, Zhang JY, Gao Y, Guo CB. Neck recurrence of oral squamous cell carcinoma in unusual sites: retrospective study of 1658 cases. Head Neck. (2016) 38 (Suppl 1):E680–6. doi: 10.1002/hed.24070
32. Mizrachi A, Migliacci JC, Montero PH, McBride S, Shah JP, Patel SG, et al. Neck recurrence in clinically node-negative oral cancer: 27-year experience at a single institution. Oral Oncol. (2018) 78:94–101. doi: 10.1016/j.oraloncology.2018.01.020
33. Kowalski LP. Results of salvage treatment of the neck in patients with oral cancer. Arch Otolaryngol Head Neck Surg. (2002) 128:58–62. doi: 10.1001/archotol.128.1.58
34. Lin HC, Kang CJ, Huang SF, Wang HM, Lin CY, Lee LY, et al. Clinical impact of PET/CT imaging after adjuvant therapy in patients with oral cavity squamous cell carcinoma. Eur J Nucl Med Mol Imaging. (2017) 44:1702–11. doi: 10.1007/s00259-017-3713-5
35. Hosni A, Huang SH, Chiu K, Xu W, Su J, Bayley A, et al. Predictors of early recurrence prior to planned postoperative radiation therapy for oral cavity squamous cell carcinoma and outcomes following salvage intensified radiation therapy. Int J Radiat Oncol Biol Phys. (2019) 103:363–73. doi: 10.1016/j.ijrobp.2018.09.013
36. Yueh B, Feinstein AR, Weaver EM, Sasaki CT, Concato J. Prognostic staging system for recurrent, persistent, and second primary cancers of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. (1998) 124:975–81. doi: 10.1001/archotol.124.9.975
37. Tam S, Araslanova R, Low TH, Warner A, Yoo J, Fung K, et al. Estimating survival after salvage surgery for recurrent oral cavity cancer. JAMA Otolaryngol Head Neck Surg. (2017) 143:685–90. doi: 10.1001/jamaoto.2017.0001
38. Hamoir M, Holvoet E, Ambroise J, Lengelé B, Schmitz S. Salvage surgery in recurrent head and neck squamous cell carcinoma: oncologic outcome and predictors of disease free survival. Oral Oncol. (2017) 67:1–9. doi: 10.1016/j.oraloncology.2017.01.008
39. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. European Organization for Research and Treatment of Cancer Trial 22931. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. (2004) 350:1945–52. doi: 10.1056/NEJMoa032641
40. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB et al. Radiation Therapy Oncology Group 9501/Intergroup. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. (2004) 350:1937–44. doi: 10.1056/NEJMoa032646
41. Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. (2009) 9:134–42. doi: 10.1038/nrc2587
42. Janot F, de Raucourt D, Benhamou E, Ferron C, Dolivet G, Bensadoun RJ, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. (2008) 26:5518–23. doi: 10.1200/JCO.2007.15.0102
43. May ME Jr, Cash ED, Silverman CL, Redman RA, Perez CA, Wilson LD, et al. Prognostic factors and selection criteria in the retreatment of head and neck cancers. Oral Oncol. (2019) 88:85–90. doi: 10.1016/j.oraloncology.2018.11.024
44. Caudell JJ, Ward MC, Riaz N, Zakem SJ, Awan MJ, Dunlap NE, et al. Multi-Institution Reirradiation (MIRI) Collaborative. Volume, dose, and fractionation considerations for IMRT-based reirradiation in head and neck cancer: a multi-institution analysis. Int J Radiat Oncol Biol Phys. (2018) 100:606–17. doi: 10.1016/j.ijrobp.2017.11.036
45. Quinlan-Davidson SR, Morrison WH, Myers JN, Gunn GB, William WN Jr, et al. Recurrent oral cavity cancer: patterns of failure after salvage multimodality therapy. Head Neck. (2017) 39:633–8. doi: 10.1002/hed.24666
46. Katsoulakis E, Leeman JE, Lok BH, Shi W, Zhang Z, Tsai JC, et al. Long-term outcomes in oral cavity squamous cell carcinoma with adjuvant and salvage radiotherapy after surgery. Laryngoscope. (2018) 128:2539–45. doi: 10.1002/lary.27191
47. Agra IM, Filho JG, Martins EP, Kowalski LP. Second salvage surgery for re-recurrent oral cavity and oropharynx carcinoma. Head Neck. (2010) 32:997–1002. doi: 10.1002/hed.21298
48. Goto M, Hanai N, Ozawa T, Hirakawa H, Suzuki H, Hyodo I, et al. Prognostic factors and outcomes for salvage surgery in patients with recurrent squamous cell carcinoma of the tongue. Asia Pac J Clin Oncol. (2016) 12:e141–8. doi: 10.1111/ajco.12087
49. Horn D, Bodem J, Freudlsperger C, Zittel S, Weichert W, Hoffmann J, et al. Outcome of heavily pretreated recurrent oral squamous cell carcinoma after salvage resection: a monocentric retrospective analysis. J Craniomaxillofac Surg. (2016) 44:1061–6. doi: 10.1016/j.jcms.2016.05.005
50. Liu JC, Sopka DS, Mehra R, Lango MN, Fundakowski C, Ridge JA, et al. Early oral tongue cancer initially managed with surgery alone: treatment of recurrence. World J Otorhinolaryngol Head Neck Surg. (2016) 2:193–7. doi: 10.1016/j.wjorl.2016.03.001
51. Subramaniam N, Balasubramanian D, Low TH, Murthy S, Clark JR, Thankappan K, et al. Factors affecting survival in surgically salvaged locoregional recurrences of squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. (2018) 76:1133.e1. doi: 10.1016/j.joms.2017.12.029
52. Weckx A, Riekert M, Grandoch A, Schick V, Zöller JE, Kreppel M. Time to recurrence and patient survival in recurrent oral squamous cell carcinoma. Oral Oncol. (2019) 94:8–13. doi: 10.1016/j.oraloncology.2019.05.002
53. Chung EJ, Park MW, Kwon KH, Rho YS. Clinical outcomes and prognostic factor analysis after salvage surgery for recurrent squamous cell carcinoma of the oral cavity. Int J Oral Maxillofac Surg. (2020) 49:285–91. doi: 10.1016/j.ijom.2019.03.967
54. Szewczyk M, Golusiński P, Pazdrowski J, Golusiński W. Prognostic factors associated with successful salvage surgery in recurrent oral cancer. Diagnostics (Basel). (2021) 11:1105. doi: 10.3390/diagnostics11061105
55. Nandy K, Rai S, Bhatt S, Puj K, Rathod P, Gangopadhyay A. Salvage surgery for recurrent carcinoma of the oral cavity: assessment of prognostic factors. Int J Oral Maxillofac Surg. (2021) 18:S0901-5027(21)00273-3. doi: 10.1016/j.ijom.2021.07.020
56. Yosefof E, Hilly O, Stern S, Bachar G, Shpitzer T, Mizrachi A. Patterns of regional recurrence and salvage treatment in patients with oral cancer. Laryngoscope. (2021). 121–127. doi: 10.1002/lary.29821
57. Chen TC, Lo TH, Huang HC, Wang CW, Yang TL, Lou PJ, et al. Outcomes of salvage treatment in patients with recurrent oral squamous cell carcinoma. Head Neck. (2021) 43:3764–74. doi: 10.1002/hed.26862
58. Mermod M, Tolstonog G, Simon C, Monnier Y. Extracapsular spread in head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. (2016) 62:60–71. doi: 10.1016/j.oraloncology.2016.10.003
59. Gilbert H, Kagan AR. Recurrence patterns in squamous cell carcinoma of the oral cavity, pharynx, and larynx. J Surg Oncol. (1974) 6:357–80. doi: 10.1002/jso.2930060502
60. Paderno A, Piazza C, Bresciani L, Vella R, Nicolai P. Microvascular head and neck reconstruction after (chemo)radiation: facts and prejudices. Curr Opin Otolaryngol Head Neck Surg. (2016) 24:83–90. doi: 10.1097/MOO.0000000000000243
61. Arce K, Bell RB, Potter JK, Buehler MJ, Potter BE, Dierks EJ. Vascularized free tissue transfer for reconstruction of ablative defects in oral and oropharyngeal cancer patients undergoing salvage surgery following concomitant chemoradiation. Int J Oral Maxillofac Surg. (2012) 41:733–8. doi: 10.1016/j.ijom.2012.03.004
62. Kostrzewa JP, Lancaster WP, Iseli TA, Desmond RA, Carroll WR, Rosenthal EL. Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal cancer. Laryngoscope. (2010) 120:267–72. doi: 10.1002/lary.20743
63. Herle P, Shukla L, Morrison WA, Shayan R. Preoperative radiation and free flap outcomes for head and neck reconstruction: a systematic review and meta-analysis. ANZ J Surg. (2015) 85:121–7. doi: 10.1111/ans.12888
64. Kwon D, Genden EM, de Bree R, Rodrigo JP, Rinaldo A, Sanabria A, et al. Overcoming wound complications in head and neck salvage surgery. Auris Nasus Larynx. (2018) 45:1135–42. doi: 10.1016/j.anl.2018.03.008
65. Schultze-Mosgau S, Grabenbauer GG, Radespiel-Tröger M, Wiltfang J, Ries J, et al. Vascularization in the transition area between free grafted soft tissues and pre-irradiated graft bed tissues following preoperative radiotherapy in the head and neck region. Head Neck. (2002) 24:42–51. doi: 10.1002/hed.10012
66. Jung TY, Sung KW, Park SY, Kim SM, Lee JH. Salvage surgery with second free flap reconstruction for recurrent oral squamous cell carcinoma. Heliyon. (2020) 6:e04014. doi: 10.1016/j.heliyon.2020.e04014
67. Offodile AC 2nd, Chang KP, Chen HH, Loesch E, Hung SY, Kao HK. Feasibility and outcomes of the third or more episodes of sequential microvascular reconstruction for recurrent or second primary.Oral Cancer Ann Surg Oncol. (2016) 23:3765–72. doi: 10.1245/s10434-016-5283-3
68. Chen WL, Wang YY, Zhang DM, Fan S, Lin ZY. Extended vertical lower trapezius island myocutaneous flap versus pectoralis major myocutaneous flap for reconstruction in recurrent oral and oropharyngeal cancer. Head Neck. (2016) 38 (Suppl 1):E159–64. doi: 10.1002/hed.23960
69. Chen WL, Li J, Yang Z, Huang Z, Wang J, Zhang B. Extended vertical lower trapezius island myocutaneous flap in reconstruction of oral and maxillofacial defects after salvage surgery for recurrent oral carcinoma. J Oral Maxillofac Surg. (2007) 65:205–11. doi: 10.1016/j.joms.2005.10.056
Keywords: recurrent, salvage surgery, oral cancer, outcome, decision making
Citation: Shetty KSR, Kurle V, Greeshma P, Ganga VB, Murthy SP, Thammaiah SK, Prasad PK, Chavan P, Halkud R and Krishnappa R (2022) Salvage Surgery in Recurrent Oral Squamous Cell Carcinoma. Front. Oral. Health 2:815606. doi: 10.3389/froh.2021.815606
Received: 15 November 2021; Accepted: 27 December 2021;
Published: 28 January 2022.
Edited by:
Zuzana Saidak, University Hospital Center (CHU) of Amiens, FranceReviewed by:
Martin Kauke, Brigham and Women's Hospital and Harvard Medical School, United StatesCopyright © 2022 Shetty, Kurle, Greeshma, Ganga, Murthy, Thammaiah, Prasad, Chavan, Halkud and Krishnappa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samskruthi P. Murthy, c2Ftc2tydXRoaS5tdXJ0aHlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.