- 1West China School of Stomatology, Sichuan University, Chengdu, China
- 2State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Smoking seriously affects oral health and causes a variety of oral diseases. Numerous clinical data show that smoking significantly increases the risk of periodontitis, and the duration and amount of smoking are positively correlated with the severity of periodontitis. In fact, smoking creates an environment conducive to the colonization of periodontopathogens, which affects the process of periodontitis. Since subgingival plaque which harbors periodontopathogens is the initiation factor of periodontitis, it is critical to study the impact of smoking on subgingival microbiota for understanding the relationship between smoking and periodontitis. Continuous advances have been made on the understanding of effects of smoking on subgingival plaque and the development of periodontitis. Smoking is observed to enhance the pathogenicity of periodontopathogens, especially the red complex microorganisms, via promoting their colonization and infection, and regulating the expression and function of multiple virulence factors. Furthermore, smoking has a negative impact on periodontal microecological homeostasis, which is reflected in the decrease of commensal bacteria and the increase of periodontopathogens, as well as the changes in the interaction between periodontopathogens and their commensal microbes in subgingival biofilm, thus influencing the pathogenicity of the subgingival plaque. In summary, the mechanism of smoking on subgingival plaque microorganisms represented by the red complex and its effect on the periodontal microecology still need to be further explored. The relevant research results are of great significance for guiding the periodontal clinical treatment of smoking population. This review summarizes the effects and relevant mechanisms of smoking on subgingival plaque and the development of periodontitis.
Introduction
Periodontitis is an inflammatory and destructive disease involving periodontal supporting tissue, including gingiva, periodontal ligament, alveolar bone and cementum [1]. So far, periodontitis is known as the sixth most common human disease because of its high prevalence and great influence [2]. The WHO report shows that the periodontal condition is poor worldwide, with almost 10% of the global population affected severe periodontitis [3]. The results of National Health and Nutrition Examination Surveys (2009–2014) show that 42.2% American adults aged 30–79 suffer from periodontitis, of which 7.8% suffer from severe periodontitis [4]. In 2019, there were 1.1 billion cases of severe periodontitis worldwide. The age-standardized prevalence rate in 2019 was 13109/100000. From 1990 to 2019, the global age-standardized prevalence rate increased by 8.44% [5]. Periodontitis is also related to systemic diseases, such as cardiovascular disease, rheumatoid arthritis, respiratory disease, resulting in more serious effects on general health [2, 6–8]. Therefore, at present, much attention has been paid to the research on pathogenesis, prevention and treatment of periodontitis.
Periodontitis is a multifactorial disease caused by the imbalance among microbes, host and environment. Subgingival plaque and its products are the initiating factors of periodontitis, both of which are closely related to the occurrence and development of periodontitis [9]. When the invasion of microbes and the defense function of the host maintain the dynamic equilibrium of the periodontal microecology, the pathogenic effect of a small number of periodontopathogens can be defended by the immune function of the host [1]. However, some cytokines, prostaglandins and matrix metalloproteinases produced during the disorder of host inflammatory response can mediate the destruction of periodontal tissues and eventually lead to periodontitis [10–12]. There are also some general and local pathogenic factors of periodontitis, such as genetic factors, systemic diseases, dental calculus, anatomical abnormalities, malocclusion, and poor restorations [13, 14]. At the same time, some specific systemic diseases are found to contribute to different types of periodontitis such as aggressive periodontitis [15].
Smoking is a risk factor for a variety of systemic diseases, including lung disease, cardiovascular disease and so on [16–18]. Some studies have shown that smoking can lead to oral dysbacteriosis [19], so it is related to the initiation and development of oral diseases driven by oral microflora, such as periodontitis [20], dental caries [21], periapical periodontitis [22], peri-implantitis [23]. In addition, a link between the occurrence and progression of oral cancer and smoking is also observed [24, 25]. Among the oral diseases related to smoking, periodontitis has been demonstrated to have a strong association with smoking [20, 26, 27]. In fact, smoking is the second highest risk factor for periodontitis [28]. Although the mechanisms that smoking involves in the progress of periodontitis are not fully understood, based on the special relationship between subgingival plaque and periodontitis, one critical mechanism is that smoking influences the balance of subgingival plaque microbiota and periodontal microecology [29, 30]. Here, this review will summarize the effects and relevant mechanisms of smoking on subgingival plaque and the development of periodontitis.
Clinical Correlations Between Smoking and Periodontitis
A number of clinical studies have shown that smoking is closely related to the occurrence, development and severity of periodontitis. By analyzing the general situation of periodontitis in smokers and non-smokers, it was found that smoking habits increased the risk of periodontitis by 90% [31]. At the same time, attribution analysis showed that 74.8% of periodontitis cases in the United States could be attributed to smoking [26]. Studies have found that current smokers are more likely to suffer from periodontitis, while severe periodontitis is more common among current smokers [32].
The gingival condition and probing situation reflect the periodontal status and are often used in the diagnosis of periodontitis. Through oral examination, it is found that there are some significant differences in the periodontal clinical indexes, including plaque index (PI), probing depth (PD) and clinical attachment loss (CAL), between smokers and non-smokers. Some studies have shown that the PI, PD and CAL values in smokers are higher than those in non-smokers [33]. At the same time, the values of PD and CAL are correlated with the concentrations of metabolites of nicotine [33, 34]. On the other hand, the periodontal examination of smokers showed that 93.6% of smokers had one or more CAL > 3 mm [32]. While severe attachment loss, that is, CAL > 7 mm, is also the most popular and appeared in current smokers (27.0%) [32]. At the same time, severe probing depth PD > 7 mm is also the most common among current smokers [32].
The beneficial effects of smoking cessation on periodontal health also suggest the adverse effects of smoking on the prevention and treatment of periodontitis. As far as we know, smoking cessation is an important preventive measure for periodontitis and a necessary adjunct in the treatment of periodontitis. First of all, smoking cessation reduces the incidence of periodontitis. One report showed no significant difference in the prevalence of periodontitis in those who quitted smoking compared to non-smokers, and both were lower than smokers [35]. Smoking cessation can also influence the progressive course of periodontitis. After the assessment of the periodontal status of smokers, non-smokers and quitters, it was found that the periodontal status of quitters was between the other two [36, 37]. Some studies pointed out that certain harmful effects of smoking on periodontium were reversible in the case of smoking cessation [38]. Fiorini et al. [39] found through a systematic review that obvious reversal of periodontitis risk in smokers could be achieved within 10 years after quitting.
In addition to reducing the incidence and risk of progression of periodontitis, smoking cessation is very beneficial to periodontal treatment, especially for non-surgical treatment [40]. In the short term, quitters and non-smokers showed similar responses to periodontal treatment [41]. The degree of changes in PD, CAL and alveolar bone level was similar in quitters and non-smokers under long-term observation and both were lower than smokers [42]. Even after non-surgical treatment, smoking cessation led to reductions in PD and CAL compared to smokers [42–44]. Smoking cessation is also effective in reducing the risk of tooth loss, as Maria Luisa Silveira Souto et al. [45] found through a systematic review and Meta-analysis that the risk of tooth loss in quitters was reduced compared to smokers (RR = 2.60), and was comparable to that of non-smokers (RR = 1.15).
Impact of Smoking on Pathogenicity of Periodontopathogens
The main microorganisms in subgingival plaque can be divided into six different periodontal microbial complexes according to their colonization, distribution in microflora and the relationship with periodontal status [46]. Among them, the red microbial complex is most closely related to periodontitis, including Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola [47]. P. gingivalis is believed to play a key and pioneering role in the progression of periodontitis [21]. One of the main mechanisms of smoking affecting periodontitis is that cigarette smoke and tobacco derivatives affect the pathogenicity of periodontopathogens, especially P. gingivalis.
Growth and Colonization of Periodontopathogens
Multiple studies have revealed that smokers are more susceptible than non-smokers to persistent P. gingivalis infection [48–52]. As a common toxic ingredient in cigarettes, nicotine can inhibit the growth of P. gingivalis in a short time, but P. gingivalis can develop resistance to nicotine in a very short time, and then increase the growth under such a condition [53]. Smoking is thought to create a more anoxic environment, which may favor the growth and colonization of the obligate anaerobes such as P. gingivalis [54, 55]. In addition, under the stimulation of tobacco derivatives such as nicotine and cotinine, the expression of oxidative stress-related proteins in P. gingivalis is up-regulated [56], so that P. gingivalis can survive under oxygen exposure, suggesting the strong adaptability and survival ability of the periodontopathogen to different environmental stress. Biofilm formation of P. gingivalis was also observed to be augmented under the stimulation of cigarette smoke extract (CSE), with a significant increase in biomass, substratum coverage, and maximum and mean thickness apparent [52]. It is notable that CSE-treated P. gingivalis biofilms exhibited a lower pro-inflammatory capacity (TNF-α, IL-6) than control biofilms, which may explain the increased persistence of this pathogen in smokers [57].
The influence of tobacco derivatives on epithelial colonization by periodontopathogens have been reported. Nicotine and cotinine can promote the colonization of the periodontopathogen Aggregatibacter actinomycetemcomitans to epithelial cells at the concentration of 1 mg/ml [58]. However, no positive effect of nicotine on epithelial colonization of P. gingivalis was observed, and the invasiveness of P. gingivalis increases significantly at a concentration of cotinine (100 μg/ml) [59]. Take into consideration that concentration of nicotine and cotinine found in oral cavity of smoking patients is very low [60], whether oral epithelial cells of smokers are more likely to be colonized by periodontopathogens needs to be further studied.
Expression and Function of Virulence Factors of Periodontopathogens
Periodontopathogens can produce various virulence factors, such as extracellular proteases, lipopolysaccharide (LPS) and metabolites, which contact or enter the periodontium to directly destroy cells, or cause local immune and inflammatory response in periodontal tissue to cause tissue damage indirectly. Thus, the role of virulence factors of periodontopathogens is particularly important in the progress of periodontitis, especially for P. gingivalis, which possesses many virulence factors. Using microarrays representative of the P. gingivalis genome, it is revealed that CSE-exposure resulted in differential regulation of 6.8% of P. gingivalis genes, including detoxification and oxidative stress-related genes, DNA repair genes and multiple genes related to P. gingivalis virulence [61]. More studies demonstrated the multiple regulatory effects of smoking on expression or function of virulence factors of P. gingivalis, including the long fimbriae FimA [52, 61], capsular polysaccharides [52], outer membrane proteins RagA and RagB [61], Kgp and Rgp gingipain [62] and LPS [63] (Figure 1).
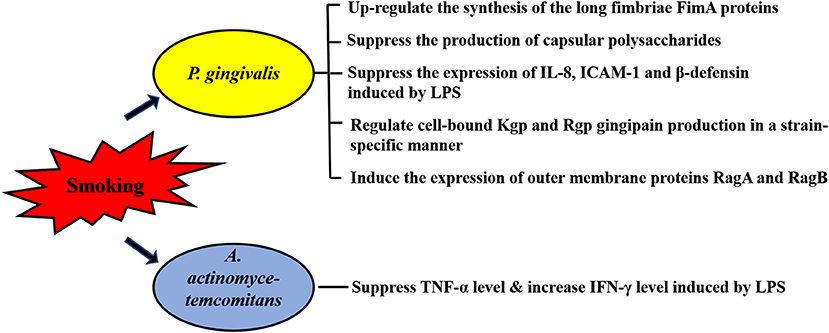
Figure 1. Effects of smoking on virulence factors of periodontopathogens. Expression or function of multiple virulence factors of P. gingivalis are affected by smoking, including the long fimbriae FimA, capsular polysaccharides, outer membrane proteins RagA and RagB, Kgp and Rgp gingipain and lipopolysaccharide (LPS). The immune response caused by LPS of A. actinomycetemcomitans is also influenced by smoking. Intercellular adhesion molecule-1, ICAM-1.
In addition of alterations in gene express profile, a reduced proinflammatory potential is observed in the response of P. gingivalis to CSE. The long fimbriae FimA is one of the main factors responsible for the colonization of the oral cavity by P. gingivalis [64]. Bagaitkar et al. found CSE exposure could up-regulate the synthesis of FimA proteins which induce TLR2 hyposensitivity, thus reducing the host response to P. gingivalis [52]. Given that the production of capsular polysaccharides and the expression of IL-8, intercellular adhesion molecule (ICAM)-1 and β-defensin induced by P. gingivalis LPS are also suppressed by CSE, it is suggested that smoking represents an environmental stress which may promote P. gingivalis colonization and infection via reducing the pro-inflammatory cytokine burden inducing by the virulence factors of the pathogen [52, 62]. CSE also affects the immune response caused by LPS of A. actinomycetemcomitans. An in vivo study reported that both 10 μg and 200 μg nicotine significantly reduced TNF-α levels induced by A. actinomycetemcomitans LPS, but only 200 μg nicotine-treatment resulted in a higher level of IFN-γ induced by A. actinomycetemcomitans LPS [65] (Figure 1).
Impact of Smoking on Periodontal Microecology
Periodontal microecosystem consists of periodontal microbiota, periodontium and various environmental factors including nutrients, temperature, oxygen, pH, etc., as well as interactions between periodontal microbiota and between periodontal microbiota and their hosts. The balance of periodontal microecology can be disturbed by multiple factors from host, microbiota or environment, in which smoking is identified as an important risk factor for homeostasis of periodontal microecology.
A More Anaerobic Environment
Smoking may create a more anaerobic environment in the oral cavity and thus affect the periodontal microecology. Smoking causes gingival vasoconstriction in patients with periodontitis and the pocket oxygen tension of smokers is lower than that of non-smokers [66, 67]. Wu J et al. found that the expression of signal pathways related to aerobic metabolism (the tricarboxylic acid cycle and oxidative phosphorylation of tricarboxylic acid) was reduced in the oral bacteria of smokers, while the expression of non-oxygen metabolic pathways (glycolysis, fructose, galactose and sucrose metabolism and photosynthesis) was enhanced compared to non-smokers [19]. Moreover, decreased local oxygen tension caused by smoking is likely to promote the growth of anaerobic periodontal pathogens such as Fusobacterium, Treponema, P. gingivalis, influencing periodontal microbiota [29, 68–71].
Abundance and Diversity of Subgingival Microbiota
An anaerobic environment caused by smoking has a pronounced impact on the subgingival microbiota, especially on the ratio of anaerobes to aerobes. Compared with non-smokers, the abundance of anaerobic bacteria in subgingival plaque samples of smokers was significant higher, while the abundance of aerobic bacteria was lower [72]. Many studies also showed that smokers were infected with a higher proportion of anaerobic periodontopathogens such as Fusobacterium, Treponema, P. gingivalis, Tannerella forsythia and other periodontal pathogenic bacteria [29, 68–71]. In fact, it is known that subgingival microbial profile is compositionally different in current and never-smokers, with significant differences in the abundance and diversity of subgingival microbiota, although the research results vary from study to study (Table 1). For example, in 2011, Kumar et al. [68] showed that the subgingival biofilm of smokers exhibited a greater diversity in the early stage of subgingival plaque formation (within 7 days) compared with non-smokers, although a decrease in diversity over 7 days was observed. However, in 2015, Camelo-Castillo et al. reported that the bacterial diversity of smoking periodontal patients was higher than that of non-smoking healthy controls but lower than that of non-smoking periodontal patients, after comparing the composition of their subgingival microbiota [55]. In the study of Wu et al., 16S rRNA gene sequencing revealed a lower relative abundance of the phylum Proteobacteria in current smokers (4.6%), compared with never smokers (11.7%), which was also demonstrated at class, genus and operational taxonomic unit (OTU) levels [19]. Moreover, the genera Capnocytophaga, Peptostreptococcus and Leptotrichia were also depleted, while Atopobium and Streptococcus were enriched in smokers [19].
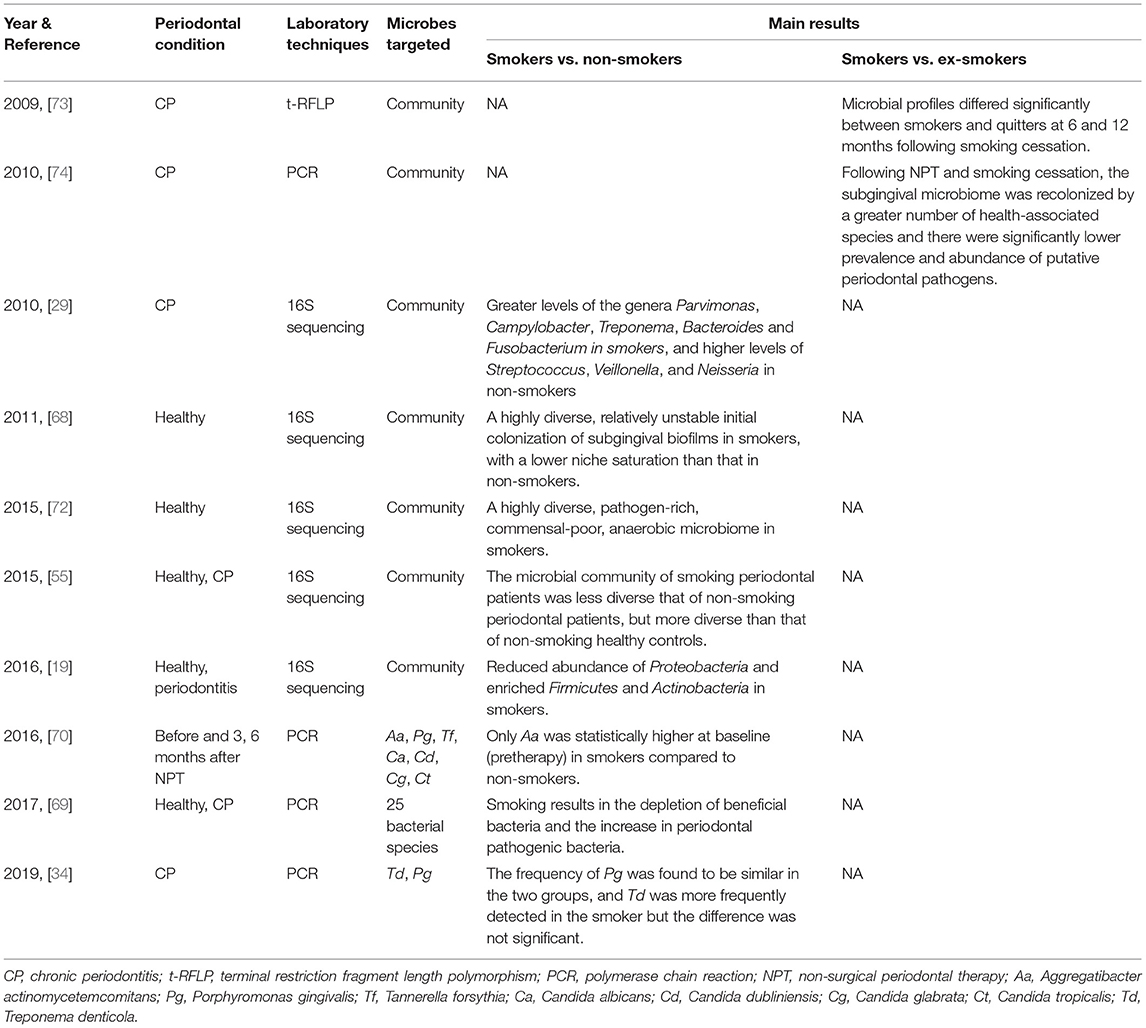
Table 1. Clinical studies on the effects of smoking on abundance and diversity of subgingival microbiota (published between 2006 and 2021).
In the periodontal microecosystem, commensal bacteria exert antagonistic effects on periodontopathogens by secreting antimicrobial substances, competing with pathogens for mucosal surface binding sites, and adjusting environmental pH, so as to maintain the stability of periodontal microecology [75, 76]. However, smoking is found to reduce the abundance of commensal microbes in subgingival plaque and thus change the composition of subgingival microbiota. The numbers of health-related Veillonella, Neisseria and some kinds of Streptococcus such as Streptococcus sanguis decrease significantly in subgingival biofilm of smokers [29].
Besides, alterations in subgingival microbiota could result from smoking cessation (Table 1). The subgingival plaques of smokers and people who had quit smoking for 3, 6, 12 months were compared and significant differences in microbial distribution and species diversity were found between them [73]. A more in-depth study by Delima et al. found that after 12 months of smoking cessation, the prevalence of Porphyromonas endodontalis and Dialister pneumosintes and the levels of Parvimonas micra, Filifactor alocis and Treponema denticola were all reduced in subgingival plaque, but Veillonella parvula levels were increased [74]. However, studies addressing the mechanism of smoking cessation on subgingival biofilm are scarce and the exact mechanism remains unclear.
Interspecies Interaction in Subgingival Plaque
In the process of plaque maturation, streptococci like Streptococcus gordonii that colonize in the early stage provide binding sites for later colonized bacteria such as P. gingivalis, allowing them to attach and form mature biofilms [77]. Huang et al. [78] reported that the stimulation of nicotine enhanced cell growth, biofilm formation and cell aggregation of S. gordonii, as well as up-regulating the expression of 11 genes that encode binding proteins or regulators. These effects of nicotine may enhance the binding and colonization of P. gingivalis in oral cavity, and further promote the development of periodontitis in cigarette smokers. More interestingly, CSE is reported to facilitate P. gingivalis-S. gordonii dual-species biofilm formation in a FimA-dependent manner [57] (Figure 2). It is observed that compared to control biofilms, CSE treatment significantly enhanced the binding of P. gingivalis FimA to glyceraldehyde-3 phosphate dehydrogenase (GAPDH), the cognate FimA ligand on S. gordonii, in a dose-dependent manner [57]. CSE exposure also results in an approximately two-fold increase in the total number of P. gingivalis-S. gordonii microcolonies and a three-fold increase in the microcolony height as compared with the control biofilms without exposure to CSE [57].
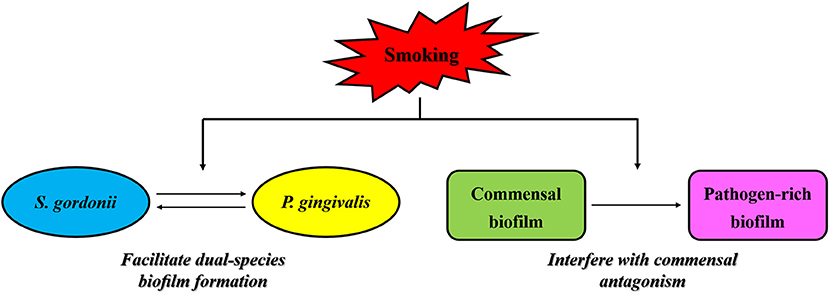
Figure 2. Effects of smoking on interspecies interaction in subgingival plaque. Smoking is reported to facilitate P. gingivalis-S. gordonii dual-species biofilm formation via enhancing the binding of P. gingivalis FimA to glyceraldehyde-3 phosphate dehydrogenase, the cognate FimA ligand on S. gordonii, in a dose-dependent manner. Moreover, smoking is observed to interfere with the antagonistic effects of commensal microbes on periodontal pathogens via a mechanism in which smoke exposure could induce significant transcriptional shifts in commensal biofilms and trigger a florid pro-inflammatory response leading to early commensal death, thus contributing to the formation of pathogen-rich subgingival biofilms and the subsequent development of periodontitis in smokers.
In addition to enhancing the interspecies interaction between P. gingivalis and S. gordonii, smoking is also observed to interfere with the antagonistic effects of commensal microbes on periodontal pathogens, thus contributing to the formation of pathogen-rich subgingival biofilms and the subsequent development of periodontitis in smokers. It is found by Kumar et al. that oral biofilms in clinically healthy smokers are pathogen-rich and commensal-poor, and this pathogen enrichment occurs within 24 h of biofilm formation [68]. To identify the potential mechanism by which smoking creates this altered community structure, they conducted a further study showing that smoke exposure induced significant transcriptional shifts in commensal biofilms, with suppressed essential metabolic functions and increased expression of virulence genes such as LPS, flagella and capsule synthesis, and then triggered a florid pro-inflammatory response, leading to early commensal death, which might preclude niche saturation by commensal microbes and thus, pathogen-rich biofilms in smokers could be formed in the absence of commensal antagonism [79] (Figure 2).
Summary and Outlook
Smoking is a common etiology and risk factor for many diseases throughout the body. For periodontitis, smoking is a critical risk factor, even as important as bacteria for patients with severe periodontitis. Therefore, it is essential to study the mechanisms by which smoking affects the occurrence and development of periodontitis. A brief overview of the effect of smoking on the course of periodontitis can be obtained from the current literature. Firstly, smoking increases the susceptibility of patients to infection of periodontopathogens. Secondly, smoking accelerates the progression of periodontitis via accelerating the destruction of periodontal supporting tissues, which increases the severity of periodontitis. Smoking also hinders the treatment of periodontitis patients and facilitates its recurrence. Overall, the effects of smoking on subgingival bacteria-host interactions are key to these changes, including the effects of smoking on host cells, blood vessels, etc. and immunoinflammatory processes, as well as the effects of smoking on periodontal microecology and periodontal microbial interactions. Although a large amount of literature has been reported, the relevant mechanisms are still less clear, especially with regard to the mechanisms of action of smoking on subgingival microbiota. Even different literature has shown different results in studies on the detection rate of periodontal pathogenic bacteria in smokers and non-smokers and the impact of smoking on the abundance and diversity of subgingival microbiota. Moreover, the differences in the results of studies on the effect of smoking on periodontopathogens caused by different in vivo and in vitro environments are still a challenge to be overcome. In-depth studies on the mechanisms by which smoking affects subgingival plaque and aggravates periodontitis will help to deepen our understanding of the pathogenesis and influence factors of periodontitis, and also help to continue to explore more effective prevention and treatment measures for periodontitis in patients who smoke.
Author Contributions
JZ and QG conceptualized the review. JZ, JY, and JD drafted the manuscript and QG edited the manuscript, with PH providing critical revisions. All authors contributed significantly, read, and approved the final manuscript.
Funding
This work was supported by grants from the Science and Technology Department of Sichuan Province (Grant No. 2021YJ0133) and the Undergraduate Innovation and Training Program of Sichuan University (Grant Nos. C2020112072 and C2021118018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. (2017) 75:7–23. doi: 10.1111/prd.12221
2. Sanz M, Del Castillo AM, Jepsen S, Gonzalez-Juanatey JR, D'Aiuto F, Bouchard P, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. (2020) 47:268–88. doi: 10.1111/jcpe.13189
3. Oral Health. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed March 25, 2020).
4. Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. (2020) 82:257–67. doi: 10.1111/prd.12323
5. Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the global burden of disease study 2019. J Clin Periodontol. (2021). doi: 10.1111/jcpe.13506
6. Ceccarelli F, Saccucci M, Di Carlo G, Lucchetti R, Pilloni A, Pranno N, et al. Periodontitis and rheumatoid arthritis: the same inflammatory mediators? Mediators Inflamm. (2019) 2019:6034546. doi: 10.1155/2019/6034546
7. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
8. Moghadam SA, Shirzaiy M, Risbaf S. The associations between periodontitis and respiratory disease. J Nepal Health Res Counc. (2017) 15:1–6. doi: 10.3126/jnhrc.v15i1.18023
9. Zijnge V, Ammann T, Thurnheer T, Gmur R. Subgingival biofilm structure. Front Oral Biol. (2012) 15:1–16. doi: 10.1159/000329667
10. Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent. (2020) 14:483–95. doi: 10.1055/s-0040-1712718
11. Salminen A, Gursoy UK, Paju S, Hyvarinen K, Mantyla P, Buhlin K, et al. Salivary biomarkers of bacterial burden, inflammatory response, and tissue destruction in periodontitis. J Clin Periodontol. (2014) 41:442–50. doi: 10.1111/jcpe.12234
12. Naruishi K, Nagata T. Biological effects of interleukin-6 on gingival fibroblasts: cytokine regulation in periodontitis. J Cell Physiol. (2018) 233:6393–400. doi: 10.1002/jcp.26521
13. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. (2017) 3:17038. doi: 10.1038/nrdp.2017.38
14. Bui FQ., Almeida-da-Silva CLC, Huynh B, Trinh A, Liu J, Woodward J, et al. Association between periodontal pathogens and systemic disease. Biomed J. (2019) 42:27–35. doi: 10.1016/j.bj.2018.12.001
15. Albandar JM. Aggressive periodontitis: case definition and diagnostic criteria. Periodontol 2000. (2014) 65:13–26. doi: 10.1111/prd.12014
16. Kondo T, Nakano Y, Adachi S, Murohara T. Effects of tobacco smoking on cardiovascular disease. Circ J. (2019) 83:1980–5. doi: 10.1253/circj.CJ-19-0323
17. Kumar A, Cherian SV, Vassallo R, Yi ES, Ryu JH. Current concepts in pathogenesis, diagnosis, and management of smoking-related interstitial lung diseases. Chest. (2018) 154:394–408. doi: 10.1016/j.chest.2017.11.023
18. Wang S, Ungvari GS, Forester BP, Chiu HFK, Wu Y, Kou C, et al. Gender differences in general mental health, smoking, drinking and chronic diseases in older adults in Jilin province, China. Psychiatry Res. (2017) 251:58–62. doi: 10.1016/j.psychres.2017.02.007
19. Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. (2016) 10:2435–46. doi: 10.1038/ismej.2016.37
20. Leite FRM, Nascimento GG, Scheutz F, López R. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med. (2018) 54:831–41. doi: 10.1016/j.amepre.2018.02.014
21. Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol. (2019) 431:2957–69. doi: 10.1016/j.jmb.2019.05.016
22. Segura-Egea JJ, Martin-Gonzalez J, Castellanos-Cosano L. Endodontic medicine: connections between apical periodontitis and systemic diseases. Int Endod J. (2015) 48:933–51. doi: 10.1111/iej.12507
23. Chrcanovic BR, Albrektsson T, Wennerberg A. Smoking and dental implants: a systematic review and meta-analysis. J Dent. (2015) 43:487–98. doi: 10.1016/j.jdent.2015.03.003
24. Bhat MY, Advani J, Rajagopalan P, Patel K, Nanjappa V, Solanki HS, et al. Cigarette smoke and chewing tobacco alter expression of different sets of miRNAs in oral keratinocytes. Sci Rep. (2018) 8:7040. doi: 10.1038/s41598-018-25498-2
25. Rajagopalan P, Patel K, Jain AP, Nanjappa V, Datta KK, Subbannayya T, et al. Molecular alterations associated with chronic exposure to cigarette smoke and chewing tobacco in normal oral keratinocytes. Cancer Biol Ther. (2018) 19:773–85. doi: 10.1080/15384047.2018.1470724
26. Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. J Periodontol. (2000) 71:743–51. doi: 10.1902/jop.2000.71.5.743
27. Holliday RS, Campbell J, Preshaw PM. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. a systematic review of the literature. J Dent. (2019) 86:81–8. doi: 10.1016/j.jdent.2019.05.030
28. Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. (1996) 67:1041–9. doi: 10.1902/jop.1996.67.10.1041
29. Shchipkova AY, Nagaraja HN, Kumar PS. Subgingival microbial profiles of smokers with periodontitis. J Dent Res. (2010) 89:1247–53. doi: 10.1177/0022034510377203
30. Huang C, Shi G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J Transl Med. (2019) 17:225. doi: 10.1186/s12967-019-1971-7
31. de Araujo Nobre M, Malo P. Prevalence of periodontitis, dental caries, and peri-implant pathology and their relation with systemic status and smoking habits: results of an open-cohort study with 22009 patients in a private rehabilitation center. J Dent. (2017) 67:36–42. doi: 10.1016/j.jdent.2017.07.013
32. Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. (2015) 86:611–22. doi: 10.1902/jop.2015.140520
33. BinShabaib M, SS AL, Akram Z, Khan J, Rahman I, Romanos GE, et al. Clinical periodontal status and gingival crevicular fluid cytokine profile among cigarette-smokers, electronic-cigarette users and never-smokers. Arch Oral Biol. (2019) 102:212–7. doi: 10.1016/j.archoralbio.2019.05.001
34. Kanmaz B, Lamont G, Danaci G, Gogeneni H, Buduneli N, Scott DA. Microbiological and biochemical findings in relation to clinical periodontal status in active smokers, non-smokers and passive smokers. Tob Induc Dis. (2019) 17:20. doi: 10.18332/tid/104492
35. Leite FRM, Nascimento GG, Baake S, Pedersen LD, Scheutz F, Lopez R. Impact of smoking cessation on periodontitis: a systematic review and meta-analysis of prospective longitudinal observational and interventional studies. Nicotine Tob Res. (2019) 21:1600–8. doi: 10.1093/ntr/nty147
36. Bergstrom J, Eliasson S, Preber H. Cigarette smoking and periodontal bone loss. J Periodontol. (1991) 62:242–6. doi: 10.1902/jop.1991.62.4.242
37. Haber J, Wattles J, Crowley M, Mandell R, Joshipura K, Kent RL. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol. (1993) 64:16–23. doi: 10.1902/jop.1993.64.1.16
38. Nociti FH, Casati MZ, Duarte PM. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontol 2000. (2015) 67:187–210. doi: 10.1111/prd.12063
39. Fiorini T, Musskopf ML, Oppermann RV, Susin C. Is there a positive effect of smoking cessation on periodontal health? a systematic review. J Periodontol. (2014) 85:83–91. doi: 10.1902/jop.2013.130047
40. Alexandridi F, Tsantila S, Pepelassi E. Smoking cessation and response to periodontal treatment. Aust Dent J. (2018) 63:140–9. doi: 10.1111/adj.12568
41. Preshaw PM, Lauffart B, Zak E, Jeffcoat MK, Barton I, Heasman PA. Progression and treatment of chronic adult periodontitis. J Periodontol. (1999) 70:1209–20. doi: 10.1902/jop.1999.70.10.1209
42. Rosa EF, Corraini P, de Carvalho VF, Inoue G, Gomes EF, Lotufo JP, et al. A prospective 12-month study of the effect of smoking cessation on periodontal clinical parameters. J Clin Periodontol. (2011) 38:562–7. doi: 10.1111/j.1600-051X.2011.01723.x
43. Gugnani N, Gugnani S. Can smoking cessation impact the incidence and progression of periodontitis? Evid Based Dent. (2020) 21:122–3. doi: 10.1038/s41432-020-0136-0
44. Chambrone L, Preshaw PM, Rosa EF, Heasman PA, Romito GA, Pannuti CM, et al. Effects of smoking cessation on the outcomes of non-surgical periodontal therapy: a systematic review and individual patient data meta-analysis. J Clin Periodontol. (2013) 40:607–15. doi: 10.1111/jcpe.12106
45. Souto MLS, Rovai ES, Villar CC, Braga MM, Pannuti CM. Effect of smoking cessation on tooth loss: a systematic review with meta-analysis. BMC Oral Health. (2019) 19:245. doi: 10.1186/s12903-019-0930-2
46. Deng ZL, Szafranski SP, Jarek M, Bhuju S, Wagner-Dobler I. Dysbiosis in chronic periodontitis: key microbial players and interactions with the human host. Sci Rep. (2017) 7:3703. doi: 10.1038/s41598-017-03804-8
47. Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. (2010) 8:481–90. doi: 10.1038/nrmicro2337
48. Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R, Genco RJ. Cigarette smoking increases the risk for subgingival infection with periodontal pathogens. J Periodontol. (1996) 67:1050–4. doi: 10.1902/jop.1996.67.10s.1050
49. Kamma JJ, Nakou M, Baehni PC. Clinical and microbiological characteristics of smokers with early onset periodontitis. J Periodontal Res. (1999) 34:25–33. doi: 10.1111/j.1600-0765.1999.tb02218.x
50. Eggert FM, McLeod MH, Flowerdew G. Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J Periodontol. (2001) 72:1210–20. doi: 10.1902/jop.2000.72.9.1210
51. Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. (2001) 28:377–88. doi: 10.1034/j.1600-051x.2001.028005377.x
52. Bagaitkar J, Demuth DR, Daep CA, Renaud DE, Pierce DL, Scott DA. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS ONE. (2010) 5:e9323. doi: 10.1371/journal.pone.0009323
53. Baek O, Zhu W, Kim HC, Lee SW. Effects of nicotine on the growth and protein expression of Porphyromonas gingivalis. J Microbiol. (2012) 50:143–8. doi: 10.1007/s12275-012-1212-8
54. Al Bataineh MT, Dash NR, Elkhazendar M, Alnusairat DaMH, Darwish IMI, Al-Hajjaj MS, et al. Revealing oral microbiota composition and functionality associated with heavy cigarette smoking. J Transl Med. (2020) 18:421. doi: 10.1186/s12967-020-02579-3
55. Camelo-Castillo AJ, Mira A, Pico A, Nibali L, Henderson B, Donos N, et al. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front Microbiol. (2015) 6:119. doi: 10.3389/fmicb.2015.00119
56. Cogo K, de Andrade A, Labate CA, Bergamaschi CC, Berto LA, Franco GC, et al. Proteomic analysis of Porphyromonas gingivalis exposed to nicotine and cotinine. J Periodontal Res. (2012) 47:766–75. doi: 10.1111/j.1600-0765.2012.01494.x
57. Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments porphyromonas gingivalis-streptococcus gordonii biofilm formation. PLoS ONE. (2011) 6:e27386. doi: 10.1371/journal.pone.0027386
58. Teughels W, Van Eldere J, van Steenberghe D, Cassiman JJ, Fives-Taylor P, Quirynen M. Influence of nicotine and cotinine on epithelial colonization by periodontopathogens. J Periodontol. (2005) 76:1315–22. doi: 10.1902/jop.2005.76.8.1315
59. Cogo K, Calvi BM, Mariano FS, Franco GC, Goncalves RB, Groppo FC. The effects of nicotine and cotinine on porphyromonas gingivalis colonisation of epithelial cells. Arch Oral Biol. (2009) 54:1061–7. doi: 10.1016/j.archoralbio.2009.08.001
60. McGuire JR, McQuade MJ, Rossmann JA, Garnick JJ, Sutherland DE, Scheidt MJ, et al. Cotinine in saliva and gingival crevicular fluid of smokers with periodontal disease. J Periodontol. (1989) 60:176–81. doi: 10.1902/jop.1989.60.4.176
61. Bagaitkar J, Williams LR, Renaud DE, Bemakanakere MR, Martin M, Scott DA, et al. Tobacco-induced alterations to porphyromonas gingivalis-host interactions. Environ Microbiol. (2009) 11:1242–53. doi: 10.1111/j.1462-2920.2008.01852.x
62. Bondy-Carey JL, Galicia J, Bagaitkar J, Potempa JS, Potempa B, Kinane DF, et al. Neutrophils alter epithelial response to porphyromonas gingivalis in a gingival crevice model. Mol Oral Microbiol. (2013) 28:102–13. doi: 10.1111/omi.12008
63. An N, Holl J, Wang X, Rausch MA, Andrukhov O, Rausch-Fan X. Potential suppressive effect of nicotine on the inflammatory response in oral epithelial cells: an in vitro study. Int J Environ Res Public Health. (2021) 18:483. doi: 10.3390/ijerph18020483
64. Missailidis CG, Umeda JE, Ota-Tsuzuki C, Anzai D, Mayer MP. Distribution of fimA genotypes of porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol Immunol. (2004) 19:224–9. doi: 10.1111/j.1399-302X.2004.00140.x
65. Makino A, Yamada S, Okuda K, Kato T. Nicotine involved in periodontal disease through influence on cytokine levels. FEMS Immunol Med Microbiol. (2008) 52:282–6. doi: 10.1111/j.1574-695X.2007.00373.x
66. Scardina GA, Messina P. Morphologic changes in the microcirculation induced by chronic smoking habit: a videocapillaroscopic study on the human gingival mucosa. Am J Dent. (2005) 18:301–4. doi: 10.1016/j.alcohol.2005.10.002
67. Hanioka T, Tanaka M, Takaya K, Matsumori Y, Shizukuishi S. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J Periodontol. (2000) 71:550–4. doi: 10.1902/jop.2000.71.4.550
68. Kumar PS, Matthews CR, Joshi V, de Jager M, Aspiras M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. (2011) 79:4730–8. doi: 10.1128/IAI.05371-11
69. Karasneh JA, Al Habashneh RA, Marzouka NA, Thornhill MH. Effect of cigarette smoking on subgingival bacteria in healthy subjects and patients with chronic periodontitis. BMC Oral Health. (2017) 17:64. doi: 10.1186/s12903-017-0359-4
70. Camargo GA, Abreu MG, Rdos SC, Wenderoscky Lde F, Duque C. Prevalence of periodontopathogens and Candida spp. in smokers after nonsurgical periodontal therapy - a pilot study. Braz Oral Res. (2016) 30:e92. doi: 10.1590/1807-3107BOR-2016.vol30.0092
71. Brook I. The impact of smoking on oral and nasopharyngeal bacterial flora. J Dent Res. (2011) 90:704–10. doi: 10.1177/0022034510391794
72. Mason MR, Preshaw PM, Nagaraja HN, Dabdoub SM, Rahman A, Kumar PS. The subgingival microbiome of clinically healthy current and never smokers. ISME J. (2015) 9:268–72. doi: 10.1038/ismej.2014.114
73. Fullmer SC, Preshaw PM, Heasman PA, Kumar PS. Smoking cessation alters subgingival microbial recolonization. J Dent Res. (2009) 88:524–8. doi: 10.1177/0022034509338676
74. Delima SL, McBride RK, Preshaw PM, Heasman PA, Kumar PS. Response of subgingival bacteria to smoking cessation. J Clin Microbiol. (2010) 48:2344–9. doi: 10.1128/JCM.01821-09
75. Li L, Feng F. Research progress of probiotics and oral health. Oral care industry. (2017) 27:33–7. doi: 10.3969/j.issn.2095-3607.2017.05.011
76. Li M, Zheng L. Advance in streptococcus sanguis and diseases. Journal of Microbiology. (2012) 32:76–8. doi: 10.3969/j.issn.1005-7021.2012.04.015
77. Zhang Y, He J, He B, Huang R, Li M. Effect of tobacco on periodontal disease and oral cancer. Tob Induc Dis. (2019) 17:40. doi: 10.18332/tid/106187
78. Huang R, Li M, Ye M, Yang K, Xu X, Gregory RL. Effects of Nicotine on streptococcus gordonii growth, biofilm formation, and cell aggregation. Appl Environ Microbiol. (2014) 80:7212–8. doi: 10.1128/AEM.02395-14
Keywords: smoking, periodontitis, periodontal microecology, subgingival plaque, subgingival microbiota, periodontopathogen, virulence factor
Citation: Zhang J, Yu J, Dou J, Hu P and Guo Q (2021) The Impact of Smoking on Subgingival Plaque and the Development of Periodontitis: A Literature Review. Front. Oral. Health 2:751099. doi: 10.3389/froh.2021.751099
Received: 31 July 2021; Accepted: 28 September 2021;
Published: 27 October 2021.
Edited by:
Keke Zhang, Wenzhou Medical University, ChinaReviewed by:
Wei Qiu, Southern Medical University, ChinaYaping Gou, Lanzhou University, China
Nadia Rostami, Newcastle University, United Kingdom
Copyright © 2021 Zhang, Yu, Dou, Hu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Guo, Z3VvcWlhbmcyMDE0QHNjdS5lZHUuY24=
 Jiaxin Zhang
Jiaxin Zhang Jialu Yu
Jialu Yu Jinge Dou
Jinge Dou Pingyue Hu
Pingyue Hu Qiang Guo
Qiang Guo