
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oral. Health, 06 September 2021
Sec. Oral Infections and Microbes
Volume 2 - 2021 | https://doi.org/10.3389/froh.2021.735634
This article is part of the Research TopicWomen in Oral Infections and Microbes: 2021View all 5 articles
 Anjali Y. Bhagirath1,2,3†
Anjali Y. Bhagirath1,2,3† Manoj Reddy Medapati2,3†
Manoj Reddy Medapati2,3† Vivianne Cruz de Jesus2,3
Vivianne Cruz de Jesus2,3 Sneha Yadav4
Sneha Yadav4 Martha Hinton1,2
Martha Hinton1,2 Shyamala Dakshinamurti1,2
Shyamala Dakshinamurti1,2 Devi Atukorallaya2,3*
Devi Atukorallaya2,3*Pregnancy is a tightly regulated immunological state. Mild environmental perturbations can affect the developing fetus significantly. Infections can elicit severe immunological cascades in the mother's body as well as the developing fetus. Maternal infections and resulting inflammatory responses can mediate epigenetic changes in the fetal genome, depending on the developmental stage. The craniofacial development begins at the early stages of embryogenesis. In this review, we will discuss the immunology of pregnancy and its responsive mechanisms on maternal infections. Further, we will also discuss the epigenetic effects of pathogens, their metabolites and resulting inflammatory responses on the fetus with a special focus on craniofacial development. Understanding the pathophysiological mechanisms of infections and dysregulated inflammatory responses during prenatal development could provide better insights into the origins of craniofacial birth defects.
Pregnancy has been described quite aptly as an immunological balancing act consisting of both activation and regulation. Sir Peter Medawar described the fetus as a semi-allograft that is somehow not rejected by the maternal immune system [1, 2]. Medawar proposed three possible mechanisms for the tolerance of the fetal semi-allograft: an energy or tolerance by the maternal immune system, a physical barrier such as the placenta, and, suppression of the fetal allo-antigens. Studies that followed also showed that while the embryo indeed exhibits paternal major histocompatibility complex (MHC) antigens [3, 4]. These could incite a response if recognized by the maternal immune system, a cooperative mechanism has somehow evolved which results in a successful pregnancy. It is in fact a combination of signals and responses to and from the placenta that modulates the maternal immune system.
The paternal seminal fluid contains several antigens and immunomodulatory molecules such as class Ia, Ib, and class II MHCs [5]. Murine uterine dendritic cells have been shown to cross-present the paternal antigens to activate the maternal T-cells and facilitate immune tolerance toward them [6]. Post-fertilization, the blastocyst “hatches” from the zona pellucida that surrounds it just prior to implantation. Studies on mouse embryos have shown that the embryos express MHC class I proteins [3, 4]. If the zona pellucida is removed prematurely, the maternal cytotoxic T-cells can recognize and kill these embryos [7]. This suggests a protective role for the zona pellucida toward the embryo. It has now been shown that a response by the uterine innate immune system is in fact important for a successful implantation of the embryo [8, 9]. Studies on implantation failures have found that pre-existing microbial invasion or colonization of the endometrium (as seen in sexually transmitted diseases or vaginitis) can result in failed implantations after in vitro-fertilization as well as spontaneous abortions [10].
Post-implantation, the primary site for materno-fetal contact is at the interface between the maternal decidua and the extravillous trophoblasts of the blastocyst. Despite being genetically distinct, somehow there is a lack of maternal antigenic stimulation toward the invading blastocyst [11, 12]. The major component of the maternal decidua is the decidual stromal cells which influence several immunologic activities. Tolerance is mediated by cytokines and the MHCs from the circulating leucocytes. This helps the mother “understand” the fetal signals and not perceive them as a threat. The interface between these two entities (the maternal decidua and the extravillous trophoblasts), shapes the immunologic relations through the course of the pregnancy.
In the first trimester, the fetal extravillous trophoblasts and the maternal decidual cells exhibit unique human leukocyte antigen (HLA) and chemokine receptor profiles [13–15]. The HLAs expressed by the fetal membranes have been found to be tolerogenic rather than immunogenic [16, 17]. Fetal trophoblasts express one class Ia MHC and three class Ib molecules. However, they do not express the class Ia antigens, HLA-A and HLA-B which are responsible for allograft rejection in humans [18, 19]. Thus, most immunomodulation is proposed to occur via regulation of innate immune responses and natural killer (NK) cells. The maternal decidua was believed to contains regulatory T-cells, macrophages and the NK cells but no B lymphocytes [20]. Benner et al. recently reported that decidual B-cells not only exist but also secrete anti-inflammatory cytokines such as IL-10 [21]. The fetal extravillous trophoblasts also express chemokine receptors such as CX3CL1, CCL14, and CCL4 [22]. The expression of chemokine receptors is suggested to facilitate the migration of the maternal leucocytes and encourages further invasion of the trophoblast [22].
The first trimester, specifically the weeks 3 through 10, is the most crucial period in craniofacial development. The term craniofacial structure is collectively used to describe the structures in the head and neck region. While craniofacial development is initiated in the first trimester of pregnancy, it continues well unto puberty. Any perturbations in the first trimester can have a deterministic effect on the normal development of these structures.
Craniofacial structures are derived from branchial arches and most of the mesenchymal structures in the head and neck are derived from the neural crest cells (NCCs). Neural crest cells after induction, undergo an epithelium-mesenchymal transition before migrating to specific locations and differentiating into several cell types. These transient NCCs are highly sensitive to environmental signals and their migration is guided by molecular signals from their non-neural crest tissue environments. Syndromic disorders arising because of defective NCC development and regulation are termed neurocristopathies. NCCs are characterized by environmentally responsive surface markers including integrin-α4, CD-49d, LPAM-1, Notch 1 and 2 and intracellular markers such as Sonic hedgehog (Shh), Sox, Snail, Slug, Vimentin etc. A discussion on specific signaling pathways and craniofacial development is outside the scope of this review as we have chosen to focus on environmental influences. NCCs are very sensitive to extrinsic factors such as ethanol exposure [23], excessive environmental glucose [24], temperature fluctuations [25], nutritional deficiencies [26] and infections [27]. Immunological investigations into NCCs identified that NCCs exhibit lower expression of HLA class I but no expression of HLA class II suggesting poor immunogenicity [28]. iPSC derived NCCs are shown to exhibit immunosuppressive properties by inhibiting T-cell proliferation and decrease in inflammatory cytokines [28].
Using live cell imaging in zebrafish, Zhu et al., demonstrated that NCCs are capable of phagocytic functions in early embryonic development [29] suggesting diverse functional roles.
The first trimester (Figure 1) is characterized by regulated immune activation within the uterine environment, an idea that was established in a mouse model in 1992 [30]. The second trimester, however, is marked by immune quiescence while parturition is again an event marked by activated inflammation. Over the course of a human pregnancy, the concentration of hormones such as estrogen (estradiol and estriol) and progesterone increase with the highest levels observed during the third trimester. Hormonal changes during pregnancy can alter immune responses to increase susceptibility to infections [31, 32]. The endometrium also gets enriched with macrophages and regulatory T cells (Treg cells). Tregs comprise of both and the cells. Studies have shown that the concentration of Tregs increases in the first trimester, peaks in the second, and decreases in the third [33–35]. Reduced number of Tregs are associated with recurrent pregnancy loss as well as pre-eclampsia [34, 36, 37]. In a healthy pregnancy, while the levels of circulating helper T-cells type 1 and 2 (Th1 and Th2) cells remains unchanged until the third trimester, the levels of NK cells and natural killer T-cells (NKT cells) increases [38–40]. The NK and the NKT cells respond to cytokines secreted by the dendritic cells upon activation by external stressors such as pathogens or their molecules. The cytotoxic effects of the NK cells are associated with recurrent pregnancy loss. This effect is postulated to be associated with an alteration in subset of NK cells rather than overall change in abundance [41, 42].
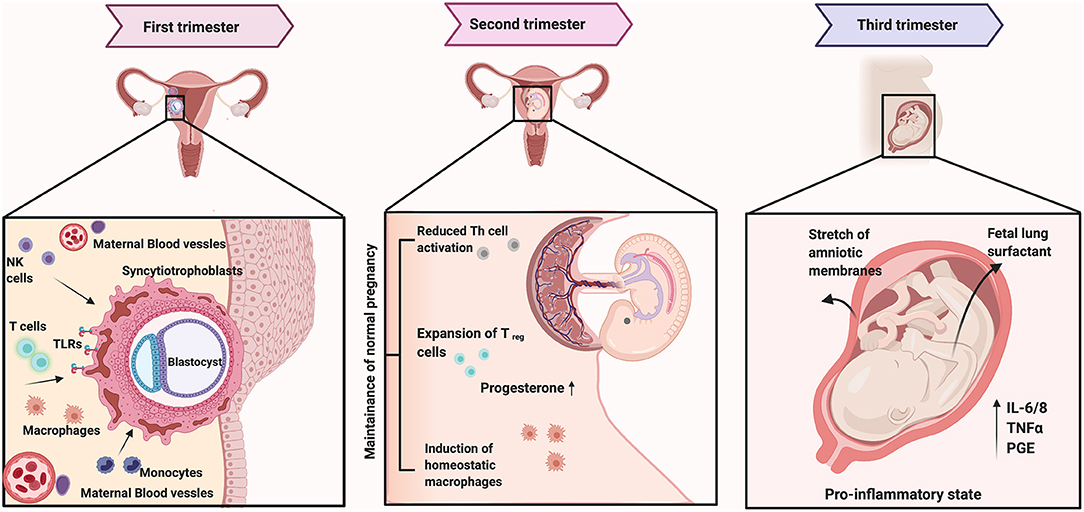
Figure 1. The first trimester in pregnancy is marked by activated inflammation beginning with the adherence of the blastocyst to the uterine wall. The invading trophoblasts from the blastocyst secrete chemokines to recruit maternal innate (monocytes, macrophages, and natural killer cells) and adaptive immune cells [including a restricted subset of CD4+ and CD8+ T cells and regulatory T cells (Treg)]. Simultaneously, there is the proliferation of resident tissue leukocytes, particularly decidual natural killer (dNK) cells and decidual dendritic cells (dDCs). Trophoblasts impart an immature phenotype to local dDCs that encourages differentiation of Tregs and a tolerogenic Th2-polarized environment with high levels of classically anti-inflammatory cytokines, such as IL-10. The second trimester or mid pregnancy is marked with immune senescence with increased progesterone and Treg dominant response. As the pregnancy approaches term, the local indicators of fetal maturity trigger the maternal immune system to undergo a shift toward a pro-inflammatory state again with its peak at term. Further, the stretch of amniotic membranes as well as the fetal lung surfactant have been shown to activate inflammatory responses to facilitate parturition. Figure created using the BioRender software.
Elevated progesterone has a direct effect on the levels of the progesterone induced binding factor (PIBF) by lymphocytes [43]. PIBF levels increase through pregnancy and drops post parturition. High PIBF levels promotes the differentiation of CD4+ T cells into Th2 [44, 45]. The Th2 cells secrete high levels of anti-inflammatory cytokines including IL-4, IL-5, and IL-10 which are crucial for maintaining a homeostatic environment in pregnancy [46, 47]. Aberrations in PIBF can result in altered immune-regulatory functions. The increase in pro-inflammatory cytokines such as IFN-γ and TNF-α can damage the placenta as well as the developing embryo [48].
While most craniofacial developmental anomalies (syndromic or non-syndromic) originate during the first trimester, they become diagnostically apparent only during the second trimester as tissues undergo maturation e.g., craniosynostosis. Endogenous estrogen is important for maintaining bone and cartilage. Mesenchymal stem cells exhibit estrogen receptors during chondrogenesis and facilitate their proliferation [49]. Exogenous estrogen and estrogen-like chemicals can disrupt normal craniofacial development [50]. Exogenous estrogen (Estradiol) has been shown to enhance the cytotoxicity of NK cells and increase the production of IFN-γ [51]. Similarly, estradiol has also been shown to enhance expression of pattern recognition receptors and toll like receptor-4 as well as stimulate low grade production proinflammatory cytokines such as TNF-α in peritoneal macrophages [52]. Zebrafish larvae exposed to exogenous estrogen showed craniofacial malformations [53]. Estradiol E2 has been shown to impair NCC migration [54], and affect several signaling pathways such as Bmp2a and Wnt [55].
During the second trimester, the corticotropin releasing hormone (CRH) also rises as the syncytiotrophoblasts increase in the placenta. At the same time, the hypothalamic pituitary axis (HPA) is upregulated. The rise in CRH levels results in an increase in the maternal cortisol levels [56]. Cortisol inhibits the progesterone's control of the prostaglandin-inactivating enzymes [57]. In a healthy pregnancy, a balance between these two hormones is important to control the inflammatory responses until the complete term. The rise in CRH through pregnancy is described as paradoxical; It exerts a protective anti-inflammatory effect on the HPA but also leads to an activation of the pro-inflammatory cytokines and keeps the maternal immune system primed by releasing debris from apoptosed extravillous trophoblasts into the maternal system [58].
The process of parturition, is an event marked by activated inflammation. Sacks (1998) compared the inflammatory changes during human parturition akin to sepsis [59]. The uterine membranes and the amniotic fluid become enriched with proinflammatory cytokines and prostaglandins, mediated by the innate immune system in preparation for labor [60, 61]. Signals of fetal maturity, such as fetal surfactant lung protein (SP) and stretch of the amniotic membranes, triggers parturition and birth [62]. It is hypothesized that preterm births due to infections and inflammation could be mediated via macrophages and the SP, however, the exact mechanism is yet unclear. Readers are referred to excellent reviews by Abu-Raya et al. [63] and Peterson et al. [64], which discuss the physiologically relevant inflammatory changes in each of the trimesters in greater details.
While several innate and adaptive responses tightly regulate the inflammatory states in pregnancy, such a state is also very delicate and sensitive to intrinsic and extrinsic environmental factors. Infections can affect all stages of pregnancy but with regards to craniofacial development, the first trimester is most critical. It is important to note that a physiologically regulated inflammation in pregnancy can prove a bane in presence of infections. In a non-pregnant state, minor infections are easily cleared by the body's response mechanism; In pregnancy however, due to the physiological “immunosuppression” the severity of responses to infections is increased and can become detrimental to both the mother and the developing fetus. In the following sections we will review such processes in greater details.
David Barker et al. hypothesized that low birth weight infants presented a higher risk of developing Schizophrenia and attention deficit hyperactivity disorder (ADHD) later in life [65]. This was one of earliest studies linking maternal inflammation to prenatal development as well as an exploration of developmental origins of psychiatric disorders. The hypothesis has evolved over the years and a role of the maternal inflammatory state in several other diseases has been recognized [66, 67]. Maternal immune regulation can be perturbed by several factors including nutritional deficiencies, infections, and risk factors such as smoking and substance abuse. However, the degree of effects observed, depends on whether the inflammatory trigger is acute or chronic. In this section, we will discuss both states, and focus mainly on the effect of dysregulated inflammation induced as a result of infections or dysbiosis in the maternal microbiome.
There are two main regulators of inflammatory responses in a pregnant state: hormones and immune cells. Acute inflammation as observed in infections, is typically short but marked by the infiltration of neutrophils, increase in monocytes, macrophages and pro-inflammatory cytokines [68]. Acute inflammatory reactions mediated by infections present with exaggerated responses and often, result in serious consequences [69]. Studies in pregnant Rhesus monkeys showed that transient chorio-decidual infection with Group B Streptococci induced a cytokine surge in the amniotic fluid resulting in a lung inflammation in the fetus at birth [70]. Cadarlet et al. showed that pregnant ewes injected with LPS, resulted in offspring with lung inflammation, reduced β-cell function, and impaired glucose metabolism [71]. While epigenetic changes will be discussed in greater details later, it is interesting to note that maternal infection has been shown to result in histone modification in fetal immune cells [72] thus priming them for future responses. Monocytes from purified cord blood cells of pre-term infants with exposure to chorioamnionitis were found to exhibit a hypo-responsive transcriptional phenotype to Staphylococcus epidermidis in a subset of genes involved in antigen presentation and activation [73].
Cellular receptors such as TLRs, Nod 1 and 2, and acute phase proteins such as C-reactive proteins (CRPs) are key to eliminating infections with the help of the complement system and the phagocytes. The binding of a pathogen activates the pathogen recognition receptor (PRR). PRRs activate an inflammatory cascade resulting in the release of cytokines, matrix metalloproteinases and other growth factors. Proinflammatory cytokines such as interleukin-1β, 6 and 8 as well as TNF-α stimulate both the release of matrix metalloproteinases as well as prostaglandins, activating the damaging inflammatory response [74, 75].
A balance in the levels of estrogen and progesterone is very important to the maintenance of pregnancy. Progesterone plays a key role in the inhibition of proinflammatory cytokines and prostaglandins. Unfortunately, this “immunosuppressive” effect is disadvantageous to the mother as the severity of response to infections is often exaggerated. While the exact mechanism is unclear it is hypothesized that this effect might be due to two reasons: one, increase in the levels of progesterone increases susceptibility to infections in pregnancy [76]; and second, while inflammatory responses are necessary to clear pathogens, due to the immunological landscape of pregnancy, the severity of infections are increased. Pregnant mice infected with influenza were found to have greater viral replication in their lungs compared to the non-pregnant females with higher levels of TNF-α, CCL2, and 3 as well as CXCL1 [77]. Pregnant mice infected with Toxoplasma gondii showed improved outcomes for those treated with recombinant IFN-γ [78].
Chatterjee et al. used inbred guinea pigs infected with guinea pig CMV (GPCMV), to study the effect of immunization with anti–glycoprotein B (gB) antibodies during pregnancy. The study showed that immunization with hyperimmune anti-gB antibody in early pregnancy reduced both the incidence and the severity of newborn GPCMV infection and prevented growth retardation [79].
Chronic inflammation in pregnancy is often a result of long-persisting, low-grade infections or long-term reaction to an inflammatory stimulus. In pregnancy, chronic inflammation is most observed with systemic diseases in the mother such as obesity, diabetes, hypertension, chronic stress, or low-grade viral infections. Such an inflammatory reaction is marked by the recruitment of monocytes and lymphocytes with tissue damage and may have long-term effects in the developing fetus. Studies have shown that chronic inflammation in the mother is associated with long-term neurodevelopmental disorders in the offsprings [80, 81].
Though a direct role has not been established, it may be hypothesized based on the current scientific evidence that, maternal inflammation (induced by infections) can prime the fetal immune system as well as adversely affect the hypothalamic-pituitary-stress axis (HPA) resulting in long-term effects on fetal development (Figure 2). In Rhesus monkeys, blood cells from juvenile offspring of stress induced mothers exhibited reduced levels of TNF-α and IL-6 when treated with LPS in vitro [82]. Rats exposed to prenatal stress exhibited an altered thymic function coupled with a decrease in total lymphocytes as well as in and lymphocytes [83, 84]. Glucocorticoids are released when the mother is exposed to stressors. Glucocorticoids are lipophilic and thus theoretically, can cross the placenta. While the levels of glucocorticoids reaching the fetus may be small, it may have significant effects on overall development of the fetus. Fetuses and preterm newborns exposed to chronic intrauterine infections have been shown to exhibit elevated amniotic fluid cortisol levels [85] and increased cord blood cortisol levels [86]. Such an increase can persist through the first 3 weeks of neonatal life [87].
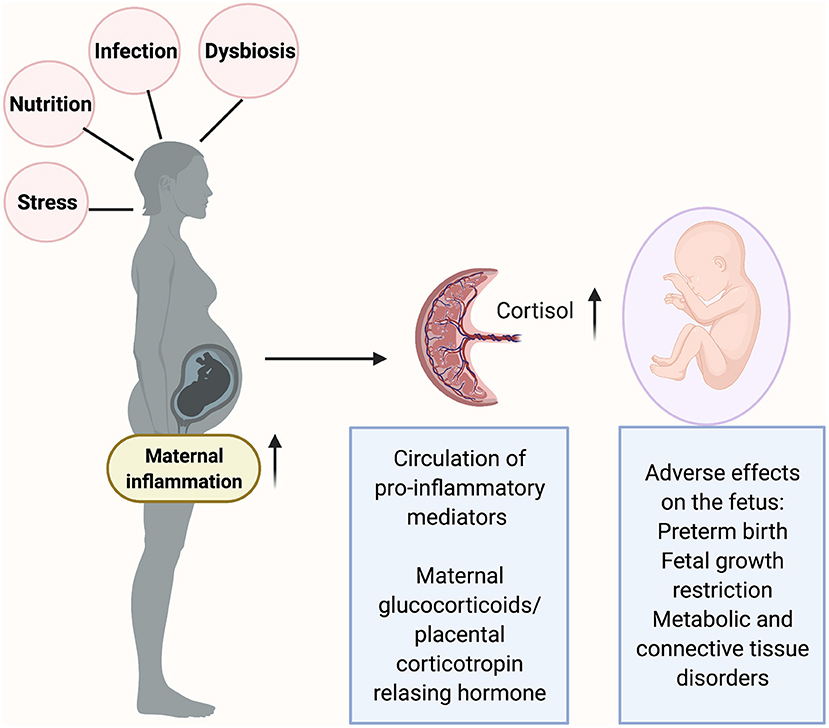
Figure 2. Inflammatory triggers in pregnancy such as maternal infection, stress and malnutrition can cause a release of pro-inflammatory mediators at the maternal-fetal interface (placenta). The placenta produces corticotropin releasing hormone (CRH) in response to stress. Excessive inflammation can result in fetal exposure to the glucocorticoids which in turn can reprogram the fetal hypothalamic-pituitary-adrenal (HPA) axis and alter the fetal developmental processes as well the immune system development. Figure created using the BioRender software.
Exposure of the fetus to glucocorticoids has also been shown to impair muscle growth and skeletal muscle mass [88]. Skeletal growth and development are significantly affected by both infection and chronic inflammation [89, 90]. Pregnant Sprague-Dawley rats injected with bacterial endotoxins resulted in impaired fetal myoblast function, increased protein catabolism, and reduced skeletal muscle growth near term [71]. Embryoid bodies exposed to bacterial LPS resulted in the inhibition of germ layer differentiation [91]. Similarly, Rubella virus infected human induced pluripotent stem cells demonstrated several epigenetic modifications as well as impaired germ layer differentiation [92]. Maternal stress during gestation resulted in fetal growth restriction likely through increased cortisol levels. This was found to be linked to altered muscle growth and skeletal development in both human and animal models [93, 94]. Exposure to corticosteroids during pregnancy has been associated with the risk of developing orofacial defects in animal models however, their effects in humans remain unclear [95, 96].
Along with immunological factors, several other materno-fetal communication “factors” have been identified. Circulating material derived from the fetoplacental unit such as the extracellular vesicles (EVs) [97, 98], microparticles [99], cell-free fetal DNA (cffDNA) [100] as well as fetal microchimeric cells [101] have been shown to play an important role in both health and maternal inflammation state. Extracellular vesicles have been shown to mediate communication between cells by serving as vehicles for proteins, lipids, and microRNAs (miRNAs) and are postulated to play a role in embryo implantation [102], placentation [103], and maintenance of pregnancy [104]. ECVs have also been implicated in the pathogenesis of preecclampsia [105] and preterm births [106], and are touted as prognostic biomarkers [107]. While important, the role of ECVs in pregnancy is yet not fully understood [103, 108, 109].
Microparticles are detected in context of oxidative stress and are hypothesized to be mildly pro-inflammatory in function, though their function remains yet unclear [110]. cffDNAs are derived from apoptotic trophoblasts and their concentration increases with pregnancy. They have been shown to be hypomethylated, thus making them amenable to agonism by TLR 9 [111]. It is hypothesized that cffDNA may serve to prime the TLR-dependent maternal immune responses. However, further studies are needed to establish this. Fetal microchimeric cells (cells transmitted from fetus to mother) have been shown to persist in the maternal system long after gestation [112]. They have been identified at tumor sites including breast cancer [113], thyroid cancers [114] as well as melanomas [115]. While the role of fetal cells is not fully understood, these findings show that while the maternal immune system programs the fetal systems, a reverse mechanism is also simultaneously at play i.e., the fetal molecules also reprogram the maternal immune system.
Studies on maternal inflammation have provided significant insights on the etiology of developmental defects in the fetus but are limited in scope due to significant technical and ethical concerns. Most of the available data has been obtained in animal models with the introduction of systemic infections or sepsis, which are rare occurrences in human pregnancies. Other studies have examined the cause and effect using medical records which includes the risk of exclusion of contributing factors such as diet, gut microbiota, and metabolism. Further research is needed in large population groups of mothers experiencing activated inflammation in pregnancy and adverse developmental outcomes in offspring to examine the association more robustly.
Developmental pathways are complex and depend on both internal and the external signals. Microbial colonization begins in the early stages of life and plays a role in several of these developmental pathways [116]. Most evidence for a direct role of infection in pregnancy comes from bacteria that have been shown to traverse the intact materno-fetal membranes and are hypothesized to have originated from the lower genital tract [117]. However, a study on the ecological succession of the vaginal microbiota through the course of gestation by Rasmussen et al., observed that the vaginal microbiota represents only a small portion of the microbiota in the newborn [118]. Thus, suggesting that the neonate must receive microbes from other sources [118]. Pathogens and their molecules have now been shown to traverse the placenta. Despite these, the sterility of the environment surrounding the fetus has been highly debated [119]. Studies have shown that pregnant women exhibit microbiome profiles distinct from the non-pregnant control groups [120–124]. In this section, we will discuss the existing evidence regarding the possible role of microorganisms from three key sources: the placenta, the vagina, and the oral cavity.
In 1964, Billingham proposed a bi-directional transfer of cellular elements from the placenta which was a paradigm shift for understanding maternal-fetal interactions [1]. While the placenta indeed allows for a bi-directional transfer of molecules, it also serves as a robust barrier against fetal infection by most pathogens [125]. A study by Aagard et al. reported that a normal placenta may harbor its own microbiome [126]. This study also reported the similarity of the isolated phyla to those found on other locations in the body such as the oral cavity, vagina, and the gut [126]. However, the studies that followed, argued both for and against this finding [127–130]. Largely, in the absence of inflammation, the placental microbiome has been described as having a low-abundance and low-biomass. The argument against a placental microbiome centers around possible contamination, systemic infections, and the detection of dead bacteria as whole genome sequencing does not differentiate live from dead [131]. The most recent and compelling evidence in favor of the placental microbiome was a study by Younge et al. [132], where the authors used a mouse model to demonstrate cultivable bacteria from the fetal gut. These bacteria were only isolated in the second trimester and could not be isolated in the third, suggesting that the bacteria could have somehow traversed the placenta to colonize the fetal gut. Further, several studies have reported the association of placental dysbiosis with adverse pregnancy outcomes such as preterm births or premature rupture of membranes [128, 133]. Villitis, an inflammation of the chorionic villi of the placenta caused by infections, can result in miscarriages [134] or adverse fetal outcomes [135, 136]. While the exact mechanism for placenta mediated adverse effects is unclear, several theories have been proposed. One proposed mechanism is that trophoblasts in the first trimester express TLRs (such as TLR-2), which can bind to bacterial endotoxins and peptidoglycans. This can shift the Th-1 balance to a pro-inflammatory state [137]. In dizygotic twins with dichorionic placentas and chronic villitis, the twins exhibited growth differences corelating to the severity of villitis for each placenta [138]. The placenta with higher villitis also showed extensive T-cell infiltration as compared to the non-affected placenta [138].
Viral infection in pregnant women is associated with adverse pregnancy outcomes, including stillbirths and congenital anomalies in the fetus [139–141]. Interestingly, the viral entry mediators such as heparan sulfate, herpesvirus entry mediator A (HveA), HveB, and HveC are not expressed on the syncytiotrophoblasts but only on the extravillous trophoblasts [125, 142]. Despite this, CMV and HSV have been detected in the maternal decidua in both symptomatic and asymptomatic mothers [143–146]. Using mouse models of viral infections, it was shown that the Zika virus in pregnant mice resulted in damage to the placenta, microcephaly, and fetal demise. The authors also showed that the Zika virus exhibited placental tropism [147]. Recently, studies on SARS-CoV-2 infection in pregnant women has shown that the virus causes placental infection, inflammation, and eventually, neurological complications in newborns [148]. This study was one of the many other studies supporting the role of SARS-COV-2 infection in mediating maternal inflammation in pregnancy [149, 150]. While the data on the presence and effect of SARS-CoV-2 in vaginal secretions, amniotic fluid, and breastmilk in infected women is still accruing, studies have reported a concerning role for the virus in pregnancy [151–155]. Further studies are needed to confirm vertical transmission as well as examine its long-term effects in neonates. Thus, while the exact mechanism is yet unclear, it is likely that the viral-mediated maternal immune activation and epigenetic modifications may cause the adverse effects observed in the embryo.
While there are geographical and inter-individual variations in the vaginal microbiota of pregnant women, the consensus remains that, healthy pregnancies are associated with a greater load of Lactobacilli [121, 156, 157]. Lactobacilli were described as colonizers of the vaginal microbiota in 1892 by Albert Döderlein and were hypothesized to play a role in maintaining pH and preventing colonization of other pathologic species [158]. A study in pregnant women in rural Malawi showed that a Lactobacillus deficient vaginal microbiota was associated with shorter term pregnancies. A subset of the cohort in the same study also exhibited an abundance of Peptostreptococcus anaerobius and associated shorter term pregnancies and newborns with lower length for age Z-score [159]. A study using pregnant rats showed that the weights of placenta and the offspring from rats infected with Escherichia coli (observed as a co-colonizer with Lactobacilli) were significantly lower as compared to the non-infected controls [160]. Bacterial dysbiosis in the lower genital tract of pregnant females commonly results in bacterial vaginosis. The pathogens causing vaginosis may vary depending on the cause from Gardnerella vaginalis, Bacteroides, Peptostreptococcus, and Prevotella [161]. Bacterial vaginosis is associated with an increased concentration of endotoxins in cervical mucus or the vaginal fluids [162]. Endotoxins are components of the Gram-negative bacterial cell wall. A study by Kamiyama et al. [163] reported that among women undergoing in vitro fertilization (IVF), a successful pregnancy did not occur if the levels of endotoxin concentration in the menstrual fluids was >200 pg/ml [163]. Bacterial vaginosis has also been shown to result in preterm births, low birth weight of newborn and/or restricted growth of the fetus and sometimes, miscarriages [164–167].
Sexually transmitted diseases (STDs) can result in complications during pregnancy. Maternal gonorrhea is associated with low preterm birth weight, premature rupture of membranes and chorioamnionitis [168–170]. Pregnant rats infected intraperitoneally with Neisseria gonorrhoeae showed a materno-fetal transmission resulting in fetal mortality [171]. Furthermore, studies investigating birth defects and maternal genitourinary infections through the first trimester observed that STDs in pregnancy were associated with significant developmental defects in the newborns [172, 173]. These studies support the theory of vertical transmission via the maternal genitourinary tract for eliciting adverse effects on the fetus.
Studies on the vaginal mycobiome have identified Candida and Saccharomyces as the predominant genera in pregnancy [174]. Fungal infections can result in chorioamnionitis, an inflammation of fetal membranes, an important risk factor for low birth weight, preterm births, and neurodevelopmental defects in the newborn [175–177]. The vaginal virome has been poorly identified due to the difficulties in isolation owing to small viral genomic material and ongoing mutations. However, Herpesviridae, Papillomaviridae, Polomaviridae, and Parvoviridae have been isolated routinely [30]. Though studies of the vaginal virome have not been able to identify specific viruses associated with adverse pregnancy outcomes such as preterm birth, quantitatively, the vaginal viral loads are higher in those who experienced such adverse outcomes [178–180].
A healthy pregnant oral microbiota has been identified to primarily belong to Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Firmicutes, Fusobacteria, Gracilibacteria (GN02), Proteobacteria, Spirochaetes, SR1, Synergistetes and Saccharibacteria (TM7) [181]. Systemic changes in the human body has been shown to exert significant influence on the diversity and the richness of the oral microbiota [182]. Studies have reported a difference in oral microbiome between pregnant and non-pregnant states. Balan et al. reported a difference in bacterial abundance in pregnant patients between the second and the third trimester. There were also significant differences in taxa between the individual patients [183]. Prevotella negrescens was observed to increase in abundance in periodontal plaque samples during the second trimester [184]. Recently, a study in Japanese women observed an increased abundance of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans during early and mid-pregnancy, compared to non-pregnant groups [185]. Candida species were found to be more abundant during mid and late pregnancy [185]. In contrast, DiGiulio et al. observed no significant difference between change in bacterial abundance across and post-pregnancy [157].
Periodontitis is the inflammation of tissues surrounding the teeth mediated by a dysbiosis of dental microbial biofilms. Pregnant women are predisposed to developing periodontitis by the virtue of hormonal changes. It is suggested that women with periodontitis during pregnancy may be at a heightened risk for adverse pregnancy outcomes though the exact mechanism is unknown [186]. Previous studies have identified P. gingivalis and Fusobacterium nucleatum in the amniotic fluid of pregnant females at risk for premature delivery [187, 188] as well as in the placentas of patients with preeclampsia [189, 190]. An abundance of Campylobacter rectus, F. nucleatum, and P. gingivalis, have been associated with adverse outcomes for pregnancy specially if associated with contributing systemic diseases such as diabetes or hypertension [191]. These periodontal pathogens are suggested to mediate their systemic effects by hematogenous dissemination. Increased levels of estrogen and progesterone enhances the permeability of the dental junctional epithelium facilitating the dissemination of periodontal pathogens [192]. P. gingivalis and T. denticola have also been shown to internalize in several types of host cells [193, 194] resulting in altered immune responses as well as mediating epigenetic changes.
Bergeyella, a genus of oral bacteria, isolated from amniotic fluid and the vaginal flora of pregnant women was genetically similar to the clones isolated from the mother's oral subgingival flora, suggesting multiple sources of origin [195]. F. nucleatum has been detected in a wide variety of placental and fetal compartments including amniotic fluids, fetal membranes, cord blood, and neonatal gastric aspirates [179, 196–198].
Syphilis is caused by Treponema palladium and its effect in pregnancy has been investigated from the early 1900's. The route of transmission for T. palladium is highly debated. Spirochetes are known to colonize both from the lower genitourinary tract and the oral cavity. They have also been shown to cross the placentas as early as 9–10 weeks [199–201]. Maternal syphilis has been known to result in preterm births, non-immune hydrops fetalis, spontaneous abortion, and stillbirths [157]. Several consequences for the surviving fetus include growth restriction, orofacial defects, anemia, thrombocytopenia, hepatomegaly, and hydrops fetalis [157, 202–207].
While a direct mechanism for pathogen transmission and adverse outcomes in pregnancy yet remain to be established, studies have definitely highlighted an association. Further, studies are also lacking in several areas of the pregnancy microbiome such as the virome, fungal diversity as well as their metabolites. While reported independently, a cross-species talk and commensalism between different pathogens cannot be ignored. Often such mutualistic associations between pathogens in a dynamic landscape such as pregnancy shapes disease outcomes and thus, must be examined in-depth. Bifidobacteria are amongst the first colonizers of neonatal gut microbiome and play a key role in shaping their immunity [208, 209]. Bifidobacteria strains isolated from maternal breast milk, vaginal, and fecal samples of mother and child combinations have been found to be identical, indicative of multiple transmission routes from the mother to infants [210–212]. Bifidobacteria also serves as carrier of (pro)phages which is important for establishing neonatal virome. The SARS-COV-2 virus has been shown to be associated with enhanced bacterial co-infections [213]. Additionally, SARS-COV-2 binds to the highly expressed oral Angiotensin-Converting Enzyme-2 (ACE-2) receptors [214]. ACE-2 is also highly expressed in the placenta, uterus, and the materno-fetal interface [215, 216]. Fetal ACE-2 plays a key role in the development of the heart, lungs, and brain [217]. The role that this interaction plays in pregnancy and fetal development is yet to be determined.
Figure 3 outlines the most commonly known routes of fetal infection based on the discussed evidence. Overall, recent studies collectively lean toward a role for microbes in fetal development. However, the evidence for the adverse effect of microbes on fetal development point more toward a role of inflammation in the same and further studies are needed to understand and clarify this argument.
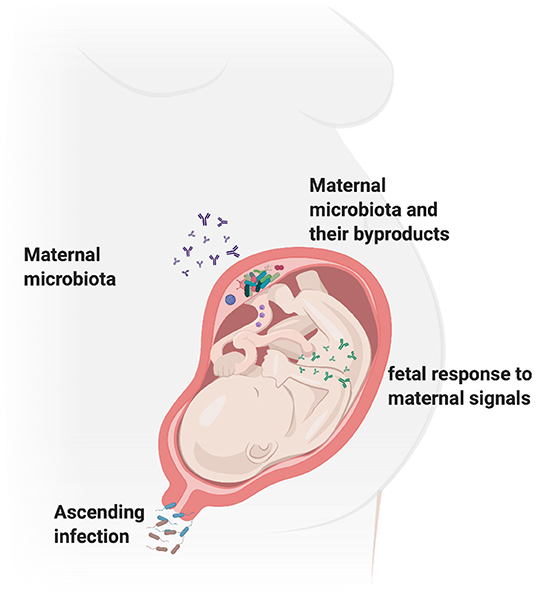
Figure 3. Gestational environment shapes fetal programming. The pregnant vaginal microbiome is known to consist of more than 170 species and studies suggest that the vagina might be a source of microbes for the fetus via vertical transmission in utero or parturition. Maternal microbiome at sites such as the gut and the oral cavity have also been shown to contribute to the development of normal fetal microbiome and mucosal immunity. Bacteria, their metabolites and other by-products have been isolated in the placenta and are also shown to shape fetal immune development post-birth. Thus, the maternal microbiome can impact the fetal development via several mechanisms including altering the maternal inflammation, as well as mediating direct effects on fetal genetics. Figure created using the BioRender software.
Epigenetics is the study of changes or modification in a gene function that are transmitted to the daughter cells without altering the sequence of the respective gene. The epigenetic modification of a specific chromosome in the gametes or zygotes results in the differential gene expression in the somatic cells also known as genomic imprinting [218, 219]. Epigenetic mechanisms are complex and include processes such as methylation, acetylation, phosphorylation, ubiquitylation, and sumoylation of DNA or the post-translational modification of histones. Of these processes, methylation of DNA is the most well-studied mechanism in which a cytosine base in position of C5 in CpG dinucleotides (followed by guanine), undergoes methylation (addition of CH3) [220, 221]. Methylation of DNA is heritable, and relatively stable as compared to histone modifications. Recent studies suggest that pathogen mediated DNA methylation occurs fast and regulates the expression of host genes involved in immune responses [222, 223]. This is carried out by a group of enzymes known as DNA methyltransferases that transfer a methyl group from S-adenyl methionine (SAM) to the fifth carbon in the cytosine base to modify as 5-methyl cytosine. This results in chromatin condensations and disrupted interactions between DNA and the transcription factors. DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B) are expressed and involved in the development of the embryo. The expression of these enzymes is reduced in terminally differentiated cells except for the post-mitotic neurons in the human brain [224, 225]. The diseases caused by fetal epigenetic reprogramming are uniquely regulated and the resulting phenotypes are often delayed (i.e., the effect during fetal exposure is long lasting). While the reprograming of fetal genetic material begins during prenatal development, it continues through the lifetime of an individual [226]. Though not fully understood and limited, there is growing evidence to suggest that microorganisms and their byproducts can mediate epigenetic changes during prenatal development which can contribute to future diseases [227, 228].
While the amniotic fluid is generally believed to be sterile, studies have reported that at least 1% of the women present with subclinical microbial invasion of the amniotic cavity (MIAC) [229]. The majority of sub-clinical MIAC is endogenous in origin. Few of the isolated species are composed of Mycoplasmas (Ureaplasma spp.) and oral bacteria such as Fusobacterium and Streptococcus [230]. While a direct role of these microorganisms on the fetus remains to be explored, studies have shown that they are capable of mediating epigenetic changes.
Internalization is an important mechanism used by several pathogens for dissemination and immune evasion. Internalization of oral pathogens such as P. gingivalis and T. denticola in dental follicle stem cells has shown to reduce the secretion of IL-10 and decreased chemotaxis of PMNs [231]. Though not shown in pregnancy, studies have reported that P. gingivalis [232] as well as their lipopolysaccharides, can downregulate the expression of DNA methylases DNMT3A and DNMT1 in epithelial cells [233, 234]. Infection of periodontal ligament cells with T. denticola increased MMP-2 expression and altered genes encoding for chromatin modification [235]. DNA methylation in periodontitis is not restricted to the site of infection. Increased methylation of the promoter regions of TNF [236] and IL-6 [237] genes have been reported in blood cells, in patients with periodontitis. Further, F. nucleatum, was found to translocate to the placenta in pregnant mouse models and cause adverse outcomes in pregnancy [238]. Independent studies have also associated F. nucleatum with DNA methylation in colorectal cancer and modulate autophagy [239–241] highlighting its role in modulating epigenetics.
C. rectus, another key periodontal pathogen was found to transverse the fetoplacental unit in pregnant mouse models and cause growth restriction in the fetus [242].
Using mice infected with C. rectus, Bobetsis et al. reported DNA hyper-methylations in several regulatory genes in the fetus [243]. This study identified a significant downregulation of insulin-like growth factor 2 (IGF2) as a result of increased methylation of the IGF promoter upon placental infection with C. rectus. Eleven of the six CpG methylation sites in IGF2 exhibited hypermethylation upon infection with C. rectus leading to intra-uterine growth restricted placentas. Studies using fetal cord blood cells in pre-term infants revealed significantly increased levels of methylation of pleomorphic adenoma gene 1 (PLAG1) as well as the Paternally expressed gene 3 (PEG3) [244, 245]. PLAG1 encodes a developmentally regulated, SUMOylated and phosphorylated zinc-finger transcription factor while the PEG3 genes are important in muscle and neuronal lineage development. In African populations, maternal HIV infection was found to be associated with increased methylation of PEG3 gene in the neonates [246]. Metabolites by pathogens have also been shown to mediate epigenetic effects. Folate produced by Bifidobacterium and Lactobacilli generate SAM, resulting in DNA methylations in the host cells [247]. Short chain fatty acids (SCFAs) are a part of free fatty acids generated by bacteria as their metabolic by-products. Patients with severe periodontitis exhibit high SCFA levels in dental biofilms. P. gingivalis derived SCFAs have been shown to induce reactivation of the Epstein-Barr virus and Kaposis's sarcoma associated herpesvirus [248]. This was associated with an increased host histone acetylations and transactivation of viral chromatin [248]. Similarly, Aflatoxins, produced by fungal species commonly found on foods, are also known to be associated with inducing DNA methylations at 71 CpG sites in infant white blood cells [249] and growth faltering in the 1st year [250].
Chorioamnionitis (CA) is marked by an infiltration of the amniotic sac by the maternal neutrophils as a result of infection, leading to a fetal inflammatory response syndrome [251]. DNA methylation patterns in chorionic villi, amnion, and chorion of CA and non-CA patients [244, 252] showed DNA methylation sites close to the promoter regions of immune-response genes such as HLA-E, CXCL4, RAB27A, IRX2, and HSD11B2. In neonatal monocytes, the promoters of innate-immunity genes IL1B, IL6, IL12B, TNF, and CCR2 exhibit significantly higher histone-methylation modifications [253, 254]. These monocytes under CA conditions express significantly lower levels of pro-inflammatory cytokines IL-1β, IL-6, IL-8 that might predispose the fetus to sepsis upon secondary infections [72].
The infection of bladder epithelial cells with E. coli in pregnant women can cause hypermethylation of tumor suppressor gene cyclin dependent kinase 2A (CDK2NA) and result in epithelial dysplasia [245, 255]. CDK2NA is also methylated in HPV-16 induced epithelial dysplasia. Though exceedingly rare but a significant risk factor in pregnancy, the food born pathogen Listeria monocytogenes is known to cause histone modifications. Such modifications can lead to transcriptional activation of MAPK pathways and affect histone modifications in chemokine genes (C-x-C motif) Cxcl2 [256, 257] predisposing the fetus to future immune associated diseases.
It is interesting to note that pathogen mediated DNA methylation is also an effective mechanism for evading immune responses. Addition of methyl donor S-adenosylmethionine (SAM) reduced LPS-mediated inflammatory response in RAW 264.7 macrophages [258]. Similarly, bovine dermal fibroblasts treated with demethylating, and hyper-acetylating agents demonstrated altered responses to LPS stimulation [259]. These studies support the possibility that subclinical chronic infection with pathogens can mediate subtle epigenetic changes, though studies are needed to confirm such an effect in pregnancy.
Gestation is a period of genetic reprogramming in the life of an individual and understanding the role of healthy maternal microbiome vs. a dysbiotic one in fetal development is important. Infection with different types of pathogens mediates different epigenetic effects both transient and stable. Current understanding of the role of microbes in epigenetic modification is limited but ever evolving. Given the differences in human and animal models of infections, further studies are needed to understand infection and inflammation by specific pathogens in the context of epigenetics and pregnancy outcomes. Table 1 lists some key epigenetic modifications by maternal infections and their associated fetal outcomes.
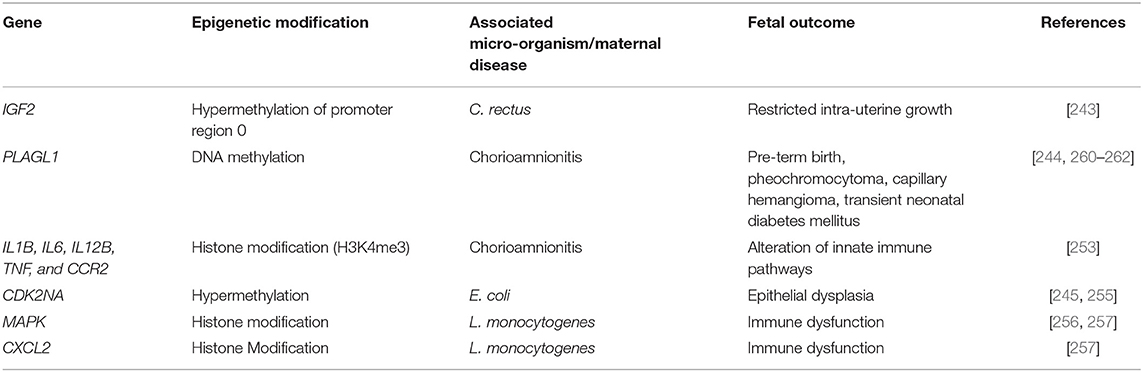
Table 1. Epigenetic modifications on human genes induced by maternal infections and associated fetal adverse outcomes.
Craniofacial development involves complex series of events mediated by cells derived from all three germ layers and the NCCs. The cranial structures include the brain, eyes, ears, nasal, and gustatory apparatuses. The facial structures involve the development of the jaws, teeth, and their associated structures. While genetics is the primary determinant, multivariant signaling and temporal organization are required for the normal development of these structures. Any subtle perturbation in the events above can cause defects in the development of craniofacial structures.
Craniofacial birth defects are the commonest birth defects next only to congenital cardiac defects.
Craniofacial anomalies are typically termed to include cleft lip and/or palate, defects of the central nervous system, eye, jaw, and dental defects. In this section we aim to briefly discuss the studies associating maternal infections and structural development of key craniofacial defects such as neural tube defects, cleft lip/palate, and dental anomalies. Table 2 lists key organisms and the studies associating them with congenital craniofacial defects in newborns.
The research on maternal inflammatory states and infection on the development of neural tube structures and brain development are still ongoing. A strong correlation between the maternal nutritional status, specifically Iron, Magnesium, vitamin B6 and B12 deficiency and the development of neural tube defects in newborns has been reported [274, 275]. Helicobacter pylori, an oral and gastrointestinal pathogen [276], was recently identified in the maternal vaginal microbiome with yeast as a carrier [277]. Maternal H. pylori infection is correlated to the risk of developing pre-eclampsia, spontaneous preterm birth, and intrauterine growth restriction (IUGR) [278]. H. pylori infection is associated with decreased folate, vitamin B12, and ferritin bioavailability to the fetus, and thus, may play a role in the development of NTDs [274, 279]. Maternal syphilis is associated with the development of hydrocephalus in the newborns [280] along with other defects. Viral infections such as maternal varicella-zoster virus infections have been shown to be associated with fetal hydrocephalus, porencephaly, hydranencephaly, calcifications, polymicrogyria, and focal lissencephaly secondary to necrotizing encephalitis [281, 282]. Similarly, maternal hepatitis B and C are also associated with adverse neurological development in the fetus, although more mechanistic studies are needed [283].
Orofacial clefts (OFC) are one of the most widely known and common craniofacial anomalies in newborns [284]. These are characterized by the failure of fusion of facial processes seen overtly as a space or a gap in the upper lip, alveolus, or palate. OFC can be divided into three categories: cleft lip (CL) only, cleft palate (CP) only, and cleft lip associated with cleft palate (CLP). Further, clefting can occur either as isolated (non-syndromic) or as a part of a syndrome with other symptoms. OFC is caused by a combination of environmental factors (e.g., maternal illness, smoking/alcohol consumption, and malnutrition), and genetic predisposition [285, 286]. Studies have reported an association between maternal hyperpyrexia, illnesses, and infections with the occurrence of CLP in the newborns with no gender differences [287], though the mechanism is poorly understood.
Norman Gregg reported a causal association between maternal rubella infection and the occurrence of defects such as OFC in newborns in 1948 [288]. It is now hypothesized that maternal rubella results in an altered hepatic metabolism of vitamin A resulting in the manifestations of the congenital rubella syndrome [289]. A study in Latin-America showed that maternal exposure to acute (influenza) and chronic (syphilis) infections were significantly associated with the incidences of cleft lip cases [263]. The authors hypothesized that the observed association between influenza and cleft lips could be due to the vascular disruption during embryonic period caused by hyperthermia and/or the use of salicylates and may not be directly an effect of the viral infection [263]. Maternal exposure to genitourinary infections has also been associated with cleft lip in the offspring. This association was stronger if the mother reported an exposure to Chlamydia [173]. While the mechanisms involved in the association between chlamydial infection and OFC are not well-characterized, it has been suggested that the chlamydial 60 kD heat shock protein (hsp60), a potent inducer of inflammation, could affect pregnancy outcomes because serum anti-hsp60 antibodies may interfere with the development of the embryo [290]. Maternal toxoplasmosis has also been reported as a causative factor for the development of anophthalmia with oro-orbital and parasagittal clefts in the newborn [272].
Several genes have been identified to be associated with OFCs, however, in terms of gene-environment interaction, the most well-studied genes are the interferon regulatory factor-6 (IRF6) and the poliovirus receptor related-1 (PVRL1). These two gene families are known to modulate immune responses to infections. IRF6 belongs to the IRF gene family which are known to regulate expression of interferons after a viral infection. IRF6 has been shown to be expressed in the maternal endometrial cells as well as the trophectoderm of the fetus [291]. Mutations in the IRF6 gene have been shown to result in Van der Woude's syndrome characterized by cleft lip/palate [292]. The PVRL1 gene also known as the Nectin 1 [293] gene is known to serve as one of the three primary receptors for the alpha herpes virus binding and entry or the herpes virus entry mediator C (HvecC) [294]. Mutations in the PVRL1 gene have been associated with sporadic non-syndromic cleft lip/palate in newborns [295, 296]. A recent study has shown the association of DNA methylation for palate forming genes and non-syndromic cleft lip/palate in both a Brazilian cohort as well as a UK cohort suggesting epigenetic modifiers as contributors to OFC [297]. While an abundance of mechanistic insights exists in term of the role these genes play in palate development, further studies are needed to understand the effect of maternal infections as epigenetic modifiers for these genes.
Dental development begins around 6 weeks post-fertilization with the migration of neural crest cells. As described before, NCCs are highly sensitive to environmental changes and their migration and differentiation is mediated mainly by signals from the surrounding tissue [27, 298]. The dentin and functional ameloblasts for the deciduous and permanent teeth begin activity around 18 and 32 weeks, respectively. Dental anomalies can occur as structural (morphogenetic) defects or a complete absence of a few (oligodontia, hypodontia) or all teeth (anodontia). The occurrence of dental defects has been linked to several causative agents including maternal stress, smoking, alcohol use, hyperpyrexia, and use of antibiotics or other teratogenic causes [299, 300]. Occurrence of dental defects, similar to OFCs, can exist both in isolated forms and as a part of a syndrome. Several studies have reported a correlation between preterm births and lower birth-weight to be associated with delayed tooth eruption [301]. Further, studies have also found that very low birth weight infants are at a higher risk for dental caries and developmental defects of teeth as compared to full-term (term) babies [302–304]. No direct associations between pathogens, epigenetic effects and dental defects have been fully established so far. Thus, we have included a discussion of reports regarding maternal infections and associated dental defects.
Maternal rubella can result in tooth agenesis as well as other morphologic dental abnormalities [305]. Congenital syphilis results in a syndromic dental morphogenic abnormality known as Hutchinson's teeth [268] which is characterized by enamel hypoplasia described as “screw-driver” shaped incisors as well as mulberry or moon's molars [207, 306, 307]. Syndromic dental anomalies are dental defects associated with the development of the jaws such as those in OFCs. Van der Woude's syndrome as described before, is also associated with hypodontia [308]. Viral infection with measles, mumps, chickenpox, rubella, and cytomegalovirus in utero have been shown to result in enamel hypoplasia and hypocalcification of the teeth in newborns [309]. These defects are mostly observed in the primary dentition. If the infection persists postnatally, enamel defects are observed in the permanent dentition as well [310].
While both viral and bacterial infections have been reported in association with craniofacial defects, few subtle differences do exist in their mechanisms and outcomes. In terms of mechanism, bacterial infections activate the complement pathway and mediate acute inflammatory response. When located intracellularly, they are eliminated by T-cells through the activation of pattern recognition receptors eliciting strong but transient responses. Viral infections often remain subclinical and mediate subtle changes via activation of chronic low-grade inflammation and epigenetic modifications. Viruses can remain latent in host cells and in the carrier commensal pathogens for a long time. Indeed, viral receptors have been found on the fetoplacental unit. Viral infection of the placenta can result in the production of soluble immune factors that could mediate long-term adverse effects in the fetus including developmental defects.
While maternal infections do result in craniofacial defects in the fetus as described above, fortunately, the incidence is rare due to continuous patient monitoring and education. Further studies are needed to understand the effect the pathogens and how they mediate fetal development while maintaining infection at subclinical levels.
Materno-fetal health is a global health concern. Studies in animal models of pregnancy such as the zebrafish, rodents, and large primates, have significantly advanced our understanding of the fetal reprogramming in health and disease, however these are not without limitations. As discussed by Leslie Roberts [311], animal models of pregnancy must be viewed with some skepticism. Soncin et al. reported that a mouse model of pregnancy can only mimic the gene expression patterns of human placenta up to the first 16 weeks [260]. Structurally, the rodent placenta is very different from the human placenta. The exchange of maternal nutrients occurs at the intervillous space in humans, in contrast, in rodents, it happens at the capillary interface. Further, the degree of placentation varies significantly between humans and different animal models [172]. Overall, the choice of animal model is often limited due to ethical and experimental considerations but understanding the outcomes when comparing them to human pregnancy is key. Readers are referred to excellent reviews on the subject of human and non-human primates as study models [312–314].
The role of inflammation in adverse outcomes in pregnancy has been well-documented in the literature. While we have discussed inflammation and infection as separate sections in this review, they are deeply interconnected. Though limited, studies have shown isolation of parasites such as plasmodium in the placenta as well as provided evidence for their transplacental transfer [273, 315]. Recently, Humann et al. showed that peptidoglycans from the bacterial cell-wall can cross murine placenta and result in injury to the fetal brain [316]. Reports also highlight the role of microbe derived metabolites in the development of neurodevelopmental disorders [317]. Using germ-free mouse models of pregnancy, Kimura et al. have shown that maternal gut microbiota-derived SCFA can cross the placenta to the developing embryos [318]. Such studies provide experimental support for previous reports linking microbial exposure and fetal gastrointestinal dysfunction as well as the development of neuropsychiatric disorders [317, 319–321]. While the level of sterility of the environment surrounding the fetus is debatable, neither the uterine environment nor the placenta can be considered absolutely sterile. In-fact a distinct microbiome for both have been demonstrated in several studies. As observed with the evolutionary studies in animals, studies in human placenta have suggested mutually learned responses from subclinical infections such as that seen with the insertion of env-like syncytin gene [322, 323]. As discussed before, several bacteria have been shown to internalize to evade immune responses. Thus, the impact of microbiome and dysbiosis in shaping fetal development is as important as the mother's overall health requires in-depth examination (Figure 4).
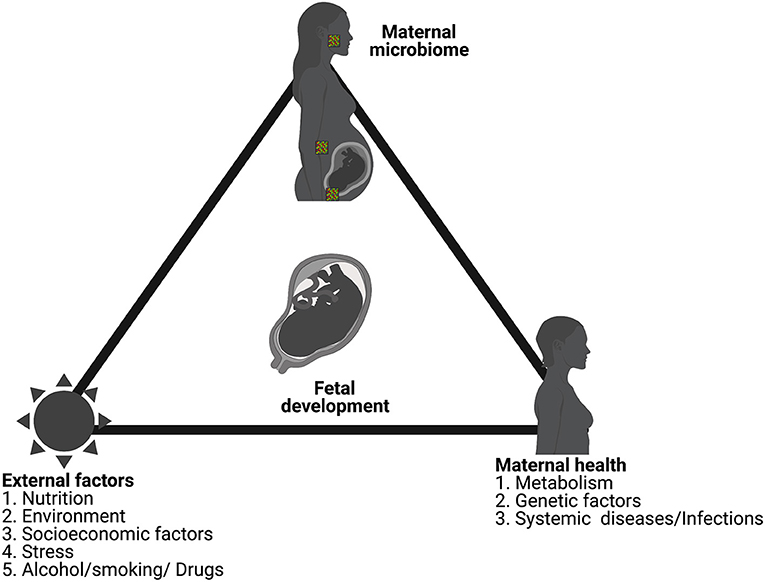
Figure 4. Normal fetal development is a consequence of the overall health of the mother, surrounding environmental factors and the maternal microbiome. Figure created using the BioRender software.
Alterations in maternal inflammatory states, microbiome and infections can have long-lasting effects depending on the phase of fetal development. While the evidence regarding the direct transmission of pathogens from mother to fetus remains debated, the passage of pathogen-derived and immune modulator molecules to the fetus is not far-fetched. Pathogen-derived molecules and toxins are small enough to bypass the placental barrier. These remain undetected by the immune system and can induce subclinical responses in both mother and the fetus. If such molecules are capable of inducing epigenetic modifications under laboratory conditions, would it be possible that such modifications could occur in the fetus or result in adverse effects? Maternal immune responses are known to shape fetal development. With the emergence of new pathogens, we will need a deeper understanding of microbiological immune response mechanisms in pregnancy. The influence of the microbiota and their genes (or the microbiome) upon developmental pathways is an emerging field and the available pool of knowledge is limited, providing a convincing argument to support it. Future studies are needed to understand the physiological effects of both symptomatic and subclinical infections in pregnancy and its outcomes and design appropriate biomarkers for their detection.
AB, MM, and DA conceptualized the work. AB, MM, VJ, SY, MH, SD, and DA participated in collecting materials and writing sections of the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Billingham RE. Transplantation immunity and the maternal-fetal relationship. Trans Stud Coll Physicians Phila. (1964) 31:187–203.
2. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. (1948) 29:58–69.
3. Arcellana-Panlilio MY, Schultz GA. Temporal and spatial expression of major histocompatibility complex class I H-2K in the early mouse embryo. Biol Reprod. (1994) 51:169–83. doi: 10.1095/biolreprod51.2.169
4. Warner CM, Gollnick SO. Expression of H-2K major histocompatibility antigens on preimplantation mouse embryos. Biol Reprod. (1993) 48:1082–7.
5. Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA. Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol. (2009) 182:8080–93. doi: 10.4049/jimmunol.0804018
6. Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. (2007) 117:1399–411. doi: 10.1172/JCI28214
7. Ewoldsen MA, Ostlie NS, Warner CM. Killing of mouse blastocyst stage embryos by cytotoxic T lymphocytes directed to major histocompatibility complex antigens. J Immunol. (1987) 138:2764–70.
8. Raziel A, Schachter M, Strassburger D, Bern O, Ron-El R, Friedler S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil Steril. (2007) 87:198–201. doi: 10.1016/j.fertnstert.2006.05.062
9. Zhou L, Li R, Wang R, Huang HX, Zhong K. Local injury to the endometrium in controlled ovarian hyperstimulation cycles improves implantation rates. Fertil Steril. (2008) 89:1166–76. doi: 10.1016/j.fertnstert.2007.05.064
10. Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. (2016) 215:684–703. doi: 10.1016/j.ajog.2016.09.075
11. Elliot MG, Crespi BJ. Placental invasiveness mediates the evolution of hybrid inviability in mammals. Am Nat. (2006) 168:114–20. doi: 10.1086/505162
12. Ander SE, Diamond MS, Coyne CB. Immune responses at the maternal-fetal interface. Sci Immunol. (2019) 4:eaat6114. doi: 10.1126/sciimmunol.aat6114
13. Ren L, Liu Y-Q, Zhou W-H, Zhang Y-Z. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. (2012) 27:366–74. doi: 10.1093/humrep/der395
14. Papuchova H, Kshirsagar S, Xu L, Bougleux Gomes HA, Li Q, Iyer V, et al. Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc Natl Acad Sci USA. (2020) 117:15772–7. doi: 10.1073/pnas.2000484117
15. Zhou W-H, Du M-R, Dong L, Yu J, Li D-J. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. (2008) 23:2669–79. doi: 10.1093/humrep/den308
16. Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16–human natural killer cells. Blood. (2003) 102:1569–77. doi: 10.1182/blood-2003-02-0517
17. Tilburgs T, Evans JH, Crespo AC, Strominger JL. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proc Natl Acad Sci USA. (2015) 112:13312–7. doi: 10.1073/pnas.1517724112
18. Blaschitz A, Hutter H, Dohr G. HLA Class I protein expression in the human placenta. Early Pregnancy. (2001) 5:67–9.
19. Desoye G, Dohr GA, Motter W, Winter R, Urdl W, Pusch H, et al. Lack of HLA class I and class II antigens on human preimplantation embryos. J Immunol. (1988) 140:4157–9.
20. Solders M, Gorchs L, Gidlof S, Tiblad E, Lundell AC, Kaipe H. Maternal adaptive immune cells in decidua parietalis display a more activated and coinhibitory phenotype compared to decidua basalis. Stem Cells Int. (2017) 2017:8010961. doi: 10.1155/2017/8010961
21. Benner M, Feyaerts D, Garcia CC, Inci N, Lopez SC, Fasse E, et al. Clusters of tolerogenic B cells feature in the dynamic immunological landscape of the pregnant uterus. Cell Rep. (2020) 32:108204. doi: 10.1016/j.celrep.2020.108204
22. Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. (2006) 74:896–904. doi: 10.1095/biolreprod.105.045518
23. Zhang P, Wang G, Lin Z, Wu Y, Zhang J, Liu M, et al. Alcohol exposure induces chick craniofacial bone defects by negatively affecting cranial neural crest development. Toxicol Lett. (2017) 281:53–64. doi: 10.1016/j.toxlet.2017.09.010
24. Wang XY, Li S, Wang G, Ma ZL, Chuai M, Cao L, et al. High glucose environment inhibits cranial neural crest survival by activating excessive autophagy in the chick embryo. Sci Rep. (2015) 5:18321. doi: 10.1038/srep18321
25. Hutson MR, Keyte AL, Hernandez-Morales M, Gibbs E, Kupchinsky ZA, Argyridis I, et al. Temperature-activated ion channels in neural crest cells confer maternal fever-associated birth defects. Sci Signal. (2017) 10:500. doi: 10.1126/scisignal.aal4055
26. Head B, La Du J, Tanguay RL, Kioussi C, Traber MG. Vitamin E is necessary for zebrafish nervous system development. Sci Rep. (2020) 10:15028. doi: 10.1038/s41598-020-71760-x
27. Bayless NL, Greenberg RS, Swigut T, Wysocka J, Blish CA. Zika virus infection induces cranial neural crest cells to produce cytokines at levels detrimental for neurogenesis. Cell Host Microbe. (2016) 20:423–8. doi: 10.1016/j.chom.2016.09.006
28. Fujii S, Yoshida S, Inagaki E, Hatou S, Tsubota K, Takahashi M, et al. Immunological properties of neural crest cells derived from human induced pluripotent stem cells. Stem Cells Dev. (2019) 28:28–43. doi: 10.1089/scd.2018.0058
29. Zhu Y, Crowley SC, Latimer AJ, Lewis GM, Nash R, Kucenas S. Migratory neural crest cells phagocytose dead cells in the developing nervous system. Cell. (2019) 179:74–89.e10. doi: 10.1016/j.cell.2019.08.001
30. McMaster MT, Newton RC, Dey SK, Andrews GK. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol. (1992) 148:1699–705.
31. Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. (2005) 366:1182–8. doi: 10.1016/S0140-6736(05)67481-8
32. Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. (2011) 7:e1002149. doi: 10.1371/journal.ppat.1002149
33. Kallikourdis M, Betz AG. Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS ONE. (2007) 2:e382. doi: 10.1371/journal.pone.0000382
34. Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. (2007) 149:139–45. doi: 10.1111/j.1365-2249.2007.03397.x
35. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. (2004) 112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x
36. Abdolmohammadi Vahid S, Ghaebi M, Ahmadi M, Nouri M, Danaei S, Aghebati-Maleki L, et al. Altered T-cell subpopulations in recurrent pregnancy loss patients with cellular immune abnormalities. J Cell Physiol. (2019) 234:4924–33. doi: 10.1002/jcp.27290
37. Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. (2009) 183:7023–30. doi: 10.4049/jimmunol.0901154
38. Chaouat G, Cayol V, Mairovitz V, Dubanchet S. Localization of the Th2 cytokines IL-3, IL-4, IL-10 at the fetomaternal interface during human and murine pregnancy and lack of requirement for Fas/Fas ligand interaction for a successful allogeneic pregnancy. Am J Reprod Immunol. (1999) 42:1–13. doi: 10.1007/978-94-011-4197-0_6
39. Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol. (2005) 35:3054–63. doi: 10.1002/eji.200425929
40. Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. (2007) 5:405–17. doi: 10.1038/nrmicro1657
41. Yamada H, Morikawa M, Kato EH, Shimada S, Kobashi G, Minakami H. Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. Am J Reprod Immunol. (2003) 50:351–4. doi: 10.1034/j.1600-0897.2003.00095.x
42. Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. (1996) 156:4027–34.
43. Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-related immune modulation of pregnancy and labor. Front Endocrinol. (2019) 10:198. doi: 10.3389/fendo.2019.00198
44. Raghupathy R, Al Mutawa E, Makhseed M, Azizieh F, Szekeres-Bartho J. Modulation of cytokine production by dydrogesterone in lymphocytes from women with recurrent miscarriage. BJOG. (2005) 112:1096–101. doi: 10.1111/j.1471-0528.2005.00633.x
45. Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. (1996) 31:81–95. doi: 10.1016/0165-0378(96)00964-3
46. Szekeres-Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. Am J Reprod Immunol. (2010) 64:77–86. doi: 10.1111/j.1600-0897.2010.00833.x
47. Lin H, Mosmann TR, Guilbert L, TuntiPopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. (1993) 151:4562–73.
48. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. (2018) 49:397–412. doi: 10.1016/j.immuni.2018.07.017
49. Gao Y, Huang E, Zhang H, Wang J, Wu N, Chen X, et al. Crosstalk between Wnt/beta-catenin and estrogen receptor signaling synergistically promotes osteogenic differentiation of mesenchymal progenitor cells. PLoS ONE. (2013) 8:e82436. doi: 10.1371/journal.pone.0082436
50. Cohen SP, LaChappelle AR, Walker BS, Lassiter CS. Modulation of estrogen causes disruption of craniofacial chondrogenesis in Danio rerio. Aquat Toxicol. (2014) 152:113–20. doi: 10.1016/j.aquatox.2014.03.028
51. Nakaya M, Tachibana H, Yamada K. Effect of estrogens on the interferon-gamma producing cell population of mouse splenocytes. Biosci Biotechnol Biochem. (2006) 70:47–53. doi: 10.1271/bbb.70.47
52. Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. (2009) 150:3877–84. doi: 10.1210/en.2009-0098
53. Fushimi S, Wada N, Nohno T, Tomita M, Saijoh K, Sunami S, et al. 17beta-Estradiol inhibits chondrogenesis in the skull development of zebrafish embryos. Aquat Toxicol. (2009) 95:292–8. doi: 10.1016/j.aquatox.2009.03.004
54. Dougherty M, Kamel G, Shubinets V, Hickey G, Grimaldi M, Liao EC. Embryonic fate map of first pharyngeal arch structures in the sox10: kaede zebrafish transgenic model. J Craniofac Surg. (2012) 23:1333–7. doi: 10.1097/SCS.0b013e318260f20b
55. Pashay Ahi E, Walker BS, Lassiter CS, Jonsson ZO. Investigation of the effects of estrogen on skeletal gene expression during zebrafish larval head development. PeerJ. (2016) 4:e1878. doi: 10.7717/peerj.1878
56. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. (2003) 997:136–49. doi: 10.1196/annals.1290.016
57. Patel FA, Challis JR. Cortisol/progesterone antagonism in regulation of 15-hydroxysteroid dehydrogenase activity and mRNA levels in human chorion and placental trophoblast cells at term. J Clin Endocrinol Metab. (2002) 87:700–8. doi: 10.1210/jcem.87.2.8245
58. Petsas G, Jeschke U, Richter DU, Minas V, Hammer A, Kalantaridou S, et al. Aberrant expression of corticotropin-releasing hormone in pre-eclampsia induces expression of fasL in maternal macrophages and extravillous trophoblast apoptosis. Mol Hum Reprod. (2012) 18:535–45. doi: 10.1093/molehr/gas027
59. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. (1998) 179:80–6. doi: 10.1016/S0002-9378(98)70254-6
60. Hadley EE, Richardson LS, Torloni MR, Menon R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am J Reprod Immunol. (2018) 79:2. doi: 10.1111/aji.12776
61. Lappas M. Visfatin regulates the terminal processes of human labour and delivery via activation of the nuclear factor-kappaB pathway. Mol Cell Endocrinol. (2012) 348:128–34. doi: 10.1016/j.mce.2011.07.048
62. Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, et al. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest. (2015) 125:2808–24. doi: 10.1172/JCI78544
63. Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal immunological adaptation during normal pregnancy. Front Immunol. (2020) 11:575197. doi: 10.3389/fimmu.2020.575197
64. Peterson LS, Stelzer IA, Tsai AS, Ghaemi MS, Han X, Ando K, et al. Multiomic immune clockworks of pregnancy. Semin Immunopathol. (2020) 42:397–412. doi: 10.1007/s00281-019-00772-1
65. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and Ischaemic heart disease in England and Wales. Lancet. (1986) 327:1077–81. doi: 10.1016/S0140-6736(86)91340-1
66. Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease cause and prevention. Curr Opin Pediatr. (2015) 27:248–53. doi: 10.1097/MOP.0000000000000191
67. Barker DJ. The origins of the developmental origins theory. J Intern Med. (2007) 261:412–7. doi: 10.1111/j.1365-2796.2007.01809.x
68. Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. (2017) 45:523–38. doi: 10.1515/jpm-2016-0225
69. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. (2014) 5:514. doi: 10.3389/fimmu.2014.00514
70. Adams Waldorf KM, Gravett MG, McAdams RM, Paolella LJ, Gough GM, Carl DJ, et al. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PLoS ONE. (2011) 6:e28972. doi: 10.1371/journal.pone.0028972
71. Cadaret CN, Merrick EM, Barnes TL, Beede KA, Posont RJ, Petersen JL, et al. Sustained maternal inflammation during the early third trimester yields fetal adaptations that impair subsequent skeletal muscle growth and glucose metabolism in sheep. Transl Anim Sci. (2018) 2(Suppl. 1):S14–S8. doi: 10.1093/tas/txy047
72. Bermick J, Gallagher K, denDekker A, Kunkel S, Lukacs N, Schaller M. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS j. (2019) 286:82–109. doi: 10.1111/febs.14728
73. de Jong E, Hancock DG, Wells C, Richmond P, Simmer K, Burgner D, et al. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylococcus epidermidis. Immunol Cell Biol. (2018) 96:792–804. doi: 10.1111/imcb.12037
74. Li W, Mata KM, Mazzuca MQ, Khalil RA. Altered matrix metalloproteinase-2 and−9 expression/activity links placental ischemia and anti-angiogenic sFlt-1 to uteroplacental and vascular remodeling and collagen deposition in hypertensive pregnancy. Biochem Pharmacol. (2014) 89:370–85. doi: 10.1016/j.bcp.2014.03.017
75. Singh N, Prasad P, Das B, Rastogi S. Involvement of matrix metalloproteinases and their inhibitors in endometrial extracellular matrix turnover in Chlamydia trachomatis-infected recurrent spontaneous aborters. Pathog Dis. (2017) 75:7. doi: 10.1093/femspd/ftx007
76. Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, et al. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. (2008) 125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x
77. Chan JF, To KK, Tse H, Lau CC, Li IW, Hung IF, et al. The lower serum immunoglobulin G2 level in severe cases than in mild cases of pandemic H1N1 2009 influenza is associated with cytokine dysregulation. Clin Vaccine Immunol. (2011) 18:305–10. doi: 10.1128/CVI.00363-10
78. Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, et al. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun. (2004) 72:1397–401. doi: 10.1128/IAI.72.3.1397-1401.2004
79. Chatterjee A, Harrison CJ, Britt WJ, Bewtra C. Modification of maternal and congenital cytomegalovirus infection by anti-glycoprotein b antibody transfer in guinea pigs. J Infect Dis. (2001) 183:1547–53. doi: 10.1086/320714
80. Girchenko P, Lahti-Pulkkinen M, Heinonen K, Reynolds RM, Laivuori H, Lipsanen J, et al. Persistently high levels of maternal antenatal inflammation are associated with and mediate the effect of prenatal environmental adversities on neurodevelopmental delay in the offspring. Biol Psychiatry. (2020) 87:898–907. doi: 10.1016/j.biopsych.2019.12.004
81. Lahti-Pulkkinen M, Girchenko P, Tuovinen S, Sammallahti S, Reynolds RM, Lahti J, et al. Maternal hypertensive pregnancy disorders and mental disorders in children. Hypertension. (2020) 75:1429–38. doi: 10.1161/HYPERTENSIONAHA.119.14140
82. Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. (2002) 87:675–81. doi: 10.1210/jcem.87.2.8233
83. Llorente E, Brito ML, Machado P, Gonzalez MC. Effect of prenatal stress on the hormonal response to acute and chronic stress and on immune parameters in the offspring. J Physiol Biochem. (2002) 58:143–9. doi: 10.1007/BF03179851
84. Gotz AA, Stefanski V. Psychosocial maternal stress during pregnancy affects serum corticosterone, blood immune parameters and anxiety behaviour in adult male rat offspring. Physiol Behav. (2007) 90:108–15. doi: 10.1016/j.physbeh.2006.09.014
85. Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Develop. (1988) 28:1599–613. doi: 10.1051/rnd:19881006
86. Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol. (2000) 182:1404–13. doi: 10.1067/mob.2000.106180
87. Miralles R, Hodge R, Kotecha S. Fetal cortisol response to intrauterine microbial colonisation identified by the polymerase chain reaction and fetal inflammation. Arch Dis Child Fetal Neonatal Ed. (2008) 93:F51–4. doi: 10.1136/adc.2006.110130
88. Gokulakrishnan G, Estrada IJ, Sosa HA, Fiorotto ML. In utero glucocorticoid exposure reduces fetal skeletal muscle mass in rats independent of effects on maternal nutrition. Am J Physiol Regul Integr Comp Physiol. (2012) 302:R1143–52. doi: 10.1152/ajpregu.00466.2011
89. Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB j. (2001) 15:1169–80. doi: 10.1096/fj.00-0463
90. Posont RJ, Beede KA, Limesand SW, Yates DT. Changes in myoblast responsiveness to TNFalpha and IL-6 contribute to decreased skeletal muscle mass in intrauterine growth restricted fetal sheep. Transl Anim Sci. (2018) 2(Suppl. 1):S44–S7. doi: 10.1093/tas/txy038
91. Sivasubramaniyan K, Atluri RR, Sarda K, Arvind M, Balaji V, Deb KD. Endotoxin-induced silencing of mesoderm induction and functional differentiation: role of HMGB1 in pluripotency and infection. Regen Med. (2008) 3:23–31. doi: 10.2217/17460751.3.1.23
92. Bilz NC, Willscher E, Binder H, Bohnke J, Stanifer ML, Hubner D, et al. Teratogenic rubella virus alters the endodermal differentiation capacity of human induced pluripotent stem cells. Cells. (2019) 8:870. doi: 10.3390/cells8080870
93. Yates DT, Cadaret CN, Beede KA, Riley HE, Macko AR, Anderson MJ, et al. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive Type I fibers near term. Am J Physiol Regul Integr Comp Physiol. (2016) 310:R1020–9. doi: 10.1152/ajpregu.00528.2015
94. Yates DT, Petersen JL, Schmidt TB, Cadaret CN, Barnes TL, Posont RJ, et al. ASAS-SSR triennnial reproduction symposium: looking back and moving forward-how reproductive physiology has evolved: fetal origins of impaired muscle growth and metabolic dysfunction: lessons from the heat-stressed pregnant ewe. J Anim Sci. (2018) 96:2987–3002. doi: 10.1093/jas/sky164
95. Peterka M, Heringova LH, Sukop A, Peterkova R. Anti-asthma drugs Formoterol and Budesonide (Symbicort) induce orofacial clefts, gastroschisis and heart septum defects in an in vivo model. In Vivo. (2021) 35:1451–60. doi: 10.21873/invivo.12397
96. Kaplan JL, Gunther-Harrington CT, Sutton JS, Stern JA. Multiple midline defects identified in a litter of golden retrievers following gestational administration of prednisone and doxycycline: a case series. BMC Vet Res. (2018) 14:86. doi: 10.1186/s12917-018-1419-y
97. Chang X, Yao J, He Q, Liu M, Duan T, Wang K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (soluble Fms-like tyrosine kinase)-1 and sEng (soluble endoglin) to endothelial cells. Hypertension. (2018) 72:1381–90. doi: 10.1161/HYPERTENSIONAHA.118.11706
98. Cooke WR, Cribbs A, Zhang W, Kandzija N, Motta-Mejia C, Dombi E, et al. Maternal circulating syncytiotrophoblast-derived extracellular vesicles contain biologically active 5'-tRNA halves. Biochem Biophys Res Commun. (2019) 518:107–13. doi: 10.1016/j.bbrc.2019.08.015
99. Aharon A, Brenner B. Microparticles and pregnancy complications. Thromb Res. (2011) 127(Suppl. 3):S67–71. doi: 10.1016/S0049-3848(11)70019-6
100. Alberry M, Maddocks D, Jones M, Abdel Hadi M, Abdel-Fattah S, Avent N, et al. Free fetal DNA in maternal plasma in anembryonic pregnancies: confirmation that the origin is the trophoblast. Prenat Diagn. (2007) 27:415–8. doi: 10.1002/pd.1700
101. Hahn S, Hasler P, Vokalova L, van Breda SV, Than NG, Hoesli IM, et al. Feto-maternal microchimerism: the pre-eclampsia conundrum. Front mmunol. (2019) 10:659. doi: 10.3389/fimmu.2019.00659
102. Kurian NK, Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet. (2019) 36:189–98. doi: 10.1007/s10815-018-1343-x
103. Yang C, Song G, Lim W. Effects of extracellular vesicles on placentation and pregnancy disorders. Reproduction. (2019) 158:R189–96. doi: 10.1530/REP-19-0147
104. Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. (2006) 176:1534–42. doi: 10.4049/jimmunol.176.3.1534
105. Sammar M, Dragovic R, Meiri H, Vatish M, Sharabi-Nov A, Sargent I, et al. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia - A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. (2018) 66:17–25. doi: 10.1016/j.placenta.2018.04.013
106. Menon R, Shahin H. Extracellular vesicles in spontaneous preterm birth. Am J Reprod Immunol. (2021) 85:e13353. doi: 10.1111/aji.13353
107. Zhang W, Lu S, Pu D, Zhang H, Yang L, Zeng P, et al. Detection of fetal trisomy and single gene disease by massively parallel sequencing of extracellular vesicle DNA in maternal plasma: a proof-of-concept validation. BMC Med Genomics. (2019) 12:151. doi: 10.1186/s12920-019-0590-8
108. Buca D, Bologna G, D'Amico A, Cugini S, Musca F, Febbo M, et al. Extracellular vesicles in feto-maternal crosstalk and pregnancy disorders. Int J Mol Sci. (2020) 21:2120. doi: 10.3390/ijms21062120
109. Qamar AY, Mahiddine FY, Bang S, Fang X, Shin ST, Kim MJ, et al. Extracellular vesicle mediated crosstalk between the gametes, conceptus, and female reproductive tract. Front Vet Sci. (2020) 7:850. doi: 10.3389/fvets.2020.589117
110. Patil R, Ghosh K, Satoskar P, Shetty S. Elevated procoagulant endothelial and tissue factor expressing microparticles in women with recurrent pregnancy loss. PLoS ONE. (2013) 8:e81407. doi: 10.1371/journal.pone.0081407
111. Goldfarb IT, Adeli S, Berk T, Phillippe M. Fetal and placental DNA stimulation of TLR9: A mechanism possibly contributing to the pro-inflammatory events during parturition. Reprod Sci. (2018) 25:788–96. doi: 10.1177/1933719117728798
112. Bayes-Genis A, Bellosillo B, de La Calle O, Salido M, Roura S, Ristol FS, et al. Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J Heart Lung Transplant. (2005) 24:2179–83. doi: 10.1016/j.healun.2005.06.003
113. Dubernard G, Aractingi S, Oster M, Rouzier R, Mathieu M-C, Uzan S, et al. Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res. (2008) 10:1–8. doi: 10.1186/bcr1860
114. Cirello V, Recalcati MP, Muzza M, Rossi S, Perrino M, Vicentini L, et al. Fetal cell microchimerism in papillary thyroid cancer: a possible role in tumor damage and tissue repair. Cancer Res. (2008) 68:8482–8. doi: 10.1158/0008-5472.CAN-08-0672
115. Nguyen Huu S, Oster M, Avril MF, Boitier F, Mortier L, Richard MA, et al. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol. (2009) 174:630–7. doi: 10.2353/ajpath.2009.080566
116. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. (2016) 22:713–22. doi: 10.1038/nm.4142
117. Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. (2005) 57:404–11. doi: 10.1203/01.PDR.0000153869.96337.90
118. Rasmussen MA, Thorsen J, Dominguez-Bello MG, Blaser MJ, Mortensen MS, Brejnrod AD, et al. Ecological succession in the vaginal microbiota during pregnancy and birth. ISME J. (2020) 14:2325–35. doi: 10.1038/s41396-020-0686-3
119. Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. (2017) 5:48. doi: 10.1186/s40168-017-0268-4
120. Bisanz JE, Enos MK, PrayGod G, Seney S, Macklaim JM, Chilton S, et al. Microbiota at multiple body sites during pregnancy in a rural tanzanian population and effects of moringa-supplemented probiotic yogurt. Appl Environ Microbiol. (2015) 81:4965–75. doi: 10.1128/AEM.00780-15
121. Freitas AC, Chaban B, Bocking A, Rocco M, Yang S, Hill JE, et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci Rep. (2017) 7:9212. doi: 10.1038/s41598-017-07790-9
122. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. (2012) 150:470–80. doi: 10.1016/j.cell.2012.07.008
123. Goltsman DSA, Sun CL, Proctor DM, DiGiulio DB, Robaczewska A, Thomas BC, et al. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome Res. (2018) 28:1467–80. doi: 10.1101/gr.236000.118
124. Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. (2014) 2:4. doi: 10.1186/2049-2618-2-4
125. Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Strauss JF, et al. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. (2002) 67:1572–9. doi: 10.1095/biolreprod.102.004325
126. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. (2014) 6:237ra65. doi: 10.1126/scitranslmed.3008599
127. Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol. (2019) 220:267.e1–39. doi: 10.1016/j.ajog.2018.10.018
128. Seferovic MD, Pace RM, Carroll M, Belfort B, Major AM, Chu DM, et al. Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. Am J Obstet Gynecol. (2019) 221:146.e1–23. doi: 10.1016/j.ajog.2019.04.036
129. Leon LJ, Doyle R, Diez-Benavente E, Clark TG, Klein N, Stanier P, et al. Enrichment of clinically relevant organisms in spontaneous preterm-delivered placentas and reagent contamination across all clinical groups in a large pregnancy cohort in the United Kingdom. Appl Environ Microbiol. (2018) 84:e00483. doi: 10.1128/AEM.00483-18
130. Gschwind R, Fournier T, Kennedy S, Tsatsaris V, Cordier AG, Barbut F, et al. Evidence for contamination as the origin for bacteria found in human placenta rather than a microbiota. PLoS ONE. (2020) 15:e0237232. doi: 10.1371/journal.pone.0237232
131. Kliman HJ. Comment on “the placenta harbors a unique microbiome”. Sci Transl Med. (2014) 6:254le4.
132. Younge N, McCann JR, Ballard J, Plunkett C, Akhtar S, Araújo-Pérez F, et al. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight. (2019) 4:19. doi: 10.1172/jci.insight.127806
133. Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, et al. The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol. (2016) 214:627.e1–16. doi: 10.1016/j.ajog.2016.01.193
134. Bae GE, Yoon N, Choi M, Hwang S, Hwang H, Kim JS. Acute placental villitis as evidence of fetal sepsis: an autopsy case report. Pediatr Dev Pathol. (2016) 19:165–8. doi: 10.2350/15-06-1656-CR.1
135. Nowak C, Joubert M, Jossic F, Masseau A, Hamidou M, Philippe HJ, et al. Perinatal prognosis of pregnancies complicated by placental chronic villitis or intervillositis of unknown etiology and combined lesions: About a series of 178 cases. Placenta. (2016) 44:104–8. doi: 10.1016/j.placenta.2016.04.017
136. Doyle RM, Harris K, Kamiza S, Harjunmaa U, Ashorn U, Nkhoma M, et al. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS ONE. (2017) 12:e0180167. doi: 10.1371/journal.pone.0180167
137. Koga K, Izumi G, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. (2014) 63:587–600. doi: 10.1111/aji.12258
138. Yusuf K, Kliman HJ. The fetus, not the mother, elicits maternal immunologic rejection: lessons from discordant dizygotic twin placentas. J Perinat Med. (2008) 36:291–6. doi: 10.1515/JPM.2008.054
139. Brasil P, Pereira JP Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. (2016) 375:2321–34. doi: 10.1056/NEJMoa1602412
140. Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G, et al. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLOS Negl Trop Dis. (2016) 10:e0004517. doi: 10.1371/journal.pntd.0004517
141. Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. (2016) 387:228. doi: 10.1016/S0140-6736(16)00006-4
142. Parry S, Holder J, Halterman MW, Weitzman MD, Davis AR, Federoff H, et al. Transduction of human trophoblastic cells by replication-deficient recombinant viral vectors. Promoting cellular differentiation affects virus entry. Am J Pathol. (1998) 152:1521–9.
143. Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol. (2003) 77:13301–14. doi: 10.1128/JVI.77.24.13301-13314.2003
144. McDonagh S, Maidji E, Ma W, Chang HT, Fisher S, Pereira L. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis. (2004) 190:826–34. doi: 10.1086/422330
145. Kapranos NC, Kotronias DC. Detection of herpes simplex virus in first trimester pregnancy loss using molecular techniques. In Vivo. (2009) 23:839–42.
146. Finger-Jardim F, Teixeira LO, de Oliveira GR, Barral MF, da Hora VP, Goncalves CV, et al. Herpes simplex virus: prevalence in placental tissue and incidence in neonatal cord blood samples. J Med Virol. (2014) 86:519–24. doi: 10.1002/jmv.23817
147. Miner Jonathan J, Cao B, Govero J, Smith Amber M, Fernandez E, Cabrera Omar H, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. (2016) 165:1081–91. doi: 10.1016/j.cell.2016.05.008
148. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11:3572. doi: 10.1038/s41467-020-17436-6
149. Yan J, Guo J, Fan C, Juan J, Yu X, Li J, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. (2020) 223:111.e1–14. doi: 10.1016/j.ajog.2020.04.014
150. Zhang L, Dong L, Ming L, Wei M, Li J, Hu R, et al. Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) infection during late pregnancy: a report of 18 patients from Wuhan, China. BMC Pregnancy Childbirth. (2020) 20:394. doi: 10.1186/s12884-020-03026-3
151. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. (2020) 25:39. doi: 10.1186/s40001-020-00439-w
152. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. (2020) 395:760–2. doi: 10.1016/S0140-6736(20)30365-2
153. Lee JK, Oh SJ, Park H, Shin OS. Recent updates on research models and tools to study virus-host interactions at the placenta. Viruses. (2019) 12:5. doi: 10.3390/v12010005
154. Gross R, Conzelmann C, Muller JA, Stenger S, Steinhart K, Kirchhoff F, et al. Detection of SARS-CoV-2 in human breastmilk. Lancet. (2020) 395:1757–8. doi: 10.1016/S0140-6736(20)31181-8
155. Algarroba GN, Hanna NN, Rekawek P, Vahanian SA, Khullar P, Palaia T, et al. Confirmatory evidence of the visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. (2020) 223:953–4. doi: 10.1016/j.ajog.2020.08.106
156. Callahan BJ, DiGiulio DB, Goltsman DSA, Sun CL, Costello EK, Jeganathan P, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc NatI Acad Sci USA. (2017) 114:9966–71. doi: 10.1073/pnas.1705899114
157. DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. (2015) 112:11060–5.
158. Antony KM, Ma J, Aagaard KM. The microbiome and development: a mother's perspective. Semin Reprod Med. (2014) 32:14–22. doi: 10.1055/s-0033-1361818
159. Doyle R, Gondwe A, Fan Y-M, Maleta K, Ashorn P, Klein N, et al. A Lactobacillus-deficient vaginal microbiota dominates postpartum women in rural Malawi. Appl Environ Microbiol. (2018) 84:e02150–17. doi: 10.1128/AEM.02150-17
160. Wroblewska-Seniuk K, Nowicki S, Le Bouguénec C, Nowicki B, Yallampalli C. Maternal/fetal mortality and fetal growth restriction: role of nitric oxide and virulence factors in intrauterine infection in rats. Am J Obstet Gynecol. (2011) 205:83.e1–7. doi: 10.1016/j.ajog.2011.02.049
161. Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol. (2011) 110:1105–28. doi: 10.1111/j.1365-2672.2011.04977.x
162. Platz-Christensen JJ, Mattsby-Baltzer I, Thomsen P, Wiqvist N. Endotoxin and interleukin-1 alpha in the cervical mucus and vaginal fluid of pregnant women with bacterial vaginosis. Am J Obstet Gynecol. (1993) 169:1161–6. doi: 10.1016/0002-9378(93)90274-M
163. Kamiyama S, Teruya Y, Nohara M, Kanazawa K. Impact of detection of bacterial endotoxin in menstrual effluent on the pregnancy rate in in vitro fertilization and embryo transfer. Fertil Steril. (2004) 82:788–92. doi: 10.1016/j.fertnstert.2004.01.054
164. Dingens AS, Fairfortune TS, Reed S, Mitchell C. Bacterial vaginosis and adverse outcomes among full-term infants: a cohort study. BMC Pregnancy Childbirth. (2016) 16:278. doi: 10.1186/s12884-016-1073-y
165. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Eng J Med. (2000) 342:1500–7. doi: 10.1056/NEJM200005183422007
166. Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Dev Disabil Res Rev. (2002) 8:3–13. doi: 10.1002/mrdd.10008
167. Isik G, Demirezen S, Donmez HG, Beksac MS. Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. J Cytol. (2016) 33:135–40. doi: 10.4103/0970-9371.188050
168. Johnson HL, Ghanem KG, Zenilman JM, Erbelding EJ. Sexually transmitted infections and adverse pregnancy outcomes among women attending inner city public sexually transmitted diseases clinics. Sex Transm Dis. (2011) 38:167–71. doi: 10.1097/OLQ.0b013e3181f2e85f
169. Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect. (2013) 89:672–8. doi: 10.1136/sextrans-2013-051118
170. Heumann CL, Quilter LAS, Eastment MC, Heffron R, Hawes SE. Adverse birth outcomes and maternal Neisseria gonorrhoeae Infection: a population-based cohort study in Washington state. Sex Transm Dis. (2017) 44:266–71. doi: 10.1097/OLQ.0000000000000592
171. Nowicki S, Selvarangan R, Anderson G. Experimental transmission of Neisseria gonorrhoeae from pregnant rat to fetus. Infect Immun. (1999) 67:4974–6. doi: 10.1128/IAI.67.9.4974-4976.1999
172. Carter AM. Comparative studies of placentation and immunology in non-human primates suggest a scenario for the evolution of deep trophoblast invasion and an explanation for human pregnancy disorders. Reproduction. (2011) 141:391–6. doi: 10.1530/REP-10-0530
173. Howley MM, Feldkamp ML, Papadopoulos EA, Fisher SC, Arnold KE, Browne ML, et al. Maternal genitourinary infections and risk of birth defects in the national birth defects prevention study. Birth Defects Res. (2018) 110:1443–54. doi: 10.1002/bdr2.1409
174. Zheng N-N, Guo X-C, Lv W, Chen X-X, Feng G-F. Characterization of the vaginal fungal flora in pregnant diabetic women by 18S rRNA sequencing. Eur J Clin Microbiol Infect Dis. (2013) 32:1031–40. doi: 10.1007/s10096-013-1847-3
175. Barton M, Shen A, O'Brien K, Robinson JL, Davies H, Simpson K, et al. Early-onset invasive candidiasis in extremely low birth weight infants: perinatal acquisition predicts poor outcome. Clin Infect Dis. (2017) 64:921–7. doi: 10.1093/cid/cix001
176. Benirschke K, Raphael SI. Candida albicans infection of the amniotic sac. Am J Obstet Gynecol. (1958) 75:200–2. doi: 10.1016/0002-9378(58)90572-6
177. Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida chorioamnionitis leads to preterm birth and adverse fetal-neonatal outcome. Infect Dis Obstet Gynecol. (2017) 2017:9060138. doi: 10.1155/2017/9060138
178. Stout MJ, Tuuli MG, Macones GA, Wylie TN, Wylie KM. 30: Diversity of the vaginal virome is associated with preterm birth. Am J Obstet Gynecol. (2018) 218:S23. doi: 10.1016/j.ajog.2017.10.441
179. Gonzales-Marin C, Spratt DA, Allaker RP. Maternal oral origin of Fusobacterium nucleatum in adverse pregnancy outcomes as determined using the 16S-23S rRNA gene intergenic transcribed spacer region. J Med Microbiol. (2013) 62:133–44. doi: 10.1099/jmm.0.049452-0
180. Wylie KM, Wylie TN, Cahill AG, Macones GA, Tuuli MG, Stout MJ. The vaginal eukaryotic DNA virome and preterm birth. Am J Obstet Gynecol. (2018) 219:189. e1–12. doi: 10.1016/j.ajog.2018.04.048
181. Perera M, Al-hebshi NN, Speicher DJ, Perera I, Johnson NW. Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J Oral Microbiol. (2016) 8:32762. doi: 10.3402/jom.v8.32762
182. Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. (2009) 28:405–11. doi: 10.1089/dna.2009.0874
183. Balan P, Chong YS, Umashankar S, Swarup S, Loke WM, Lopez V, et al. Keystone species in pregnancy gingivitis: a snapshot of oral microbiome during pregnancy and postpartum period. Front Microbiol. (2018) 9:2360. doi: 10.3389/fmicb.2018.02360
184. Gursoy M, Haraldsson G, Hyvonen M, Sorsa T, Pajukanta R, Kononen E. Does the frequency of Prevotella intermedia increase during pregnancy? Oral Microbiol Immunol. (2009) 24:299–303. doi: 10.1111/j.1399-302X.2009.00509.x
185. Fujiwara N, Tsuruda K, Iwamoto Y, Kato F, Odaki T, Yamane N, et al. Significant increase of oral bacteria in the early pregnancy period in Japanese women. J Investig Clin Dent. (2017) 8:12189. doi: 10.1111/jicd.12189
186. Bobetsis YA, Graziani F, Gursoy M, Madianos PN. Periodontal disease and adverse pregnancy outcomes. Periodontol. (2000) 83:154-74. doi: 10.1111/j.1471-0528.2005.00827.x
187. Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. (2007) 78:1249–55. doi: 10.1902/jop.2007.060368
188. Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. (2004) 72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004
189. Vanterpool SF, Been JV, Houben ML, Nikkels PG, De Krijger RR, Zimmermann LJ, et al. Porphyromonas gingivalis within placental villous mesenchyme and umbilical cord stroma is associated with adverse pregnancy outcome. PLoS ONE. (2016) 11:e0146157. doi: 10.1371/journal.pone.0146157
190. Barak S, Oettinger-Barak O, Machtei EE, Sprecher H, Ohel G. Evidence of periopathogenic microorganisms in placentas of women with preeclampsia. J Periodontol. (2007) 78:670–6. doi: 10.1902/jop.2007.060362
191. Cobb CM, Kelly PJ, Williams KB, Babbar S, Angolkar M, Derman RJ. The oral microbiome and adverse pregnancy outcomes. Int J Womens Health. (2017) 9:551–9. doi: 10.2147/IJWH.S142730
192. Carrillo-de-Albornoz A, Figuero E, Herrera D, Bascones-Martinez A. Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. J Clin Periodontol. (2010) 37:230–40. doi: 10.1111/j.1600-051X.2009.01514.x
193. Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. (1995) 63:3878–85. doi: 10.1128/iai.63.10.3878-3885.1995
194. Inagaki S, Kimizuka R, Kokubu E, Saito A, Ishihara K. Treponema denticola invasion into human gingival epithelial cells. Microb Pathog. (2016) 94:104–11. doi: 10.1016/j.micpath.2016.01.010
195. Han YW, Ikegami A, Bissada NF, Herbst M, Redline RW, Ashmead GG. Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. J Clin Microbiol. (2006) 44:1475–83. doi: 10.1128/JCM.44.4.1475-1483.2006
196. Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. (2009) 47:38–47. doi: 10.1128/JCM.01206-08
197. Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS ONE. (2013) 8:e56131. doi: 10.1371/journal.pone.0056131
198. Cahill RJ, Tan S, Dougan G, O'Gaora P, Pickard D, Kennea N, et al. Universal DNA primers amplify bacterial DNA from human fetal membranes and link Fusobacterium nucleatum with prolonged preterm membrane rupture. Mol Hum Reprod. (2005) 11:761–6. doi: 10.1093/molehr/gah234
199. Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. (2003) 189:861–73. doi: 10.1067/S0002-9378(03)00470-8
200. Harter CA, Benirschke K. Fetal syphilis in the first trimester. Am J Obstet Gynecol. (1976) 124:705–11. doi: 10.1016/S0002-9378(16)33340-3
201. Nathan L, Bohman VR, Sanchez PJ, Leos NK, Twickler DM, D WJG. In utero infection with Treponema pallidum in early pregnancy. Prenat Diagn. (1997) 17:119–23. doi: 10.1002/(SICI)1097-0223(199702)17:2<119::AID-PD39>3.0.CO;2-T
202. Ingall D, Sanchez P. Infectious Diseases of the Fetus and Newborn Infant. Amsterdam: W.B Saunders (2001).
203. Gameiro VS, Labronici PJ, Rosa IM, Silva JA. Congenital syphilis with bone lesion: case report. Rev Bras Ortop. (2017) 52:740–2. doi: 10.1016/j.rboe.2017.10.002
204. Brion LP, Manuli M, Rai B, Kresch MJ, Pavlov H, Glaser J. Long-bone radiographic abnormalities as a sign of active congenital syphilis in asymptomatic newborns. Pediatrics. (1991) 88:1037–40.
205. Nissanka-Jayasuriya EH, Odell EW, Phillips C. Dental stigmata of congenital syphilis: a historic review with present day relevance. Head Neck Pathol. (2016) 10:327–31. doi: 10.1007/s12105-016-0703-z
206. Bauer WH. Tooth buds and jaws in patients with congenital syphilis: correlation between distribution of treponema pallidum and tissue reaction. Am J Pathol. (1944) 20:297–319.
207. Hillson S, Grigson C, Bond S. Dental defects of congenital syphilis. Am J Phys Anthropol. (1998) 107:25–40. doi: 10.1002/(SICI)1096-8644(199809)107:1<25::AID-AJPA3>3.0.CO;2-C
208. Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. (2017) 81:e00036–17. doi: 10.1128/MMBR.00036-17
209. Turroni F, Milani C, Duranti S, Ferrario C, Lugli GA, Mancabelli L, et al. Bifidobacteria and the infant gut: an example of co-evolution and natural selection. Cell Mol Life Sci. (2018) 75:103–18. doi: 10.1007/s00018-017-2672-0
210. Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol. (2009) 75:965–9. doi: 10.1128/AEM.02063-08
211. Milani C, Mancabelli L, Lugli GA, Duranti S, Turroni F, Ferrario C, et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl Environ Microbiol. (2015) 81:7078–87. doi: 10.1128/AEM.02037-15
212. Mikami K, Takahashi H, Kimura M, Isozaki M, Izuchi K, Shibata R, et al. Influence of maternal bifidobacteria on the establishment of bifidobacteria colonizing the gut in infants. Pediatr Res. (2009) 65:669–74. doi: 10.1203/PDR.0b013e31819ed7a8
213. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. (2020) 81:266–75. doi: 10.1016/j.jinf.2020.05.046
214. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80. 8. doi: 10.1016/j.cell.2020.02.052
215. Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. (2008) 295:R1953–61. doi: 10.1152/ajpregu.90592.2008
216. Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE. (2020) 15:e0230295. doi: 10.1371/journal.pone.0230295
217. Song R, Preston G, Yosypiv IV. Ontogeny of angiotensin-converting enzyme 2. Pediatr Res. (2012) 71:13–9. doi: 10.1038/pr.2011.7
218. Robertson KD. DNA methylation and human disease. Nat Rev Genet. (2005) 6:597–610. doi: 10.1038/nrg1655
219. Isles AR, Holland AJ. Imprinted genes and mother-offspring interactions. Early Human Dev. (2005) 81:73–7. doi: 10.1016/j.earlhumdev.2004.10.006
220. Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. (1944) 79:137–58. doi: 10.1084/jem.79.2.137
221. Hotchkiss RD. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem. (1948) 175:315–32.
222. Pacis A, Tailleux L, Morin AM, Lambourne J, MacIsaac JL, Yotova V, et al. Bacterial infection remodels the DNA methylation landscape of human dendritic cells. Genome Res. (2015) 25:1801–11. doi: 10.1101/gr.192005.115
223. Pacis A, Mailhot-Leonard F, Tailleux L, Randolph HE, Yotova V, Dumaine A, et al. Gene activation precedes DNA demethylation in response to infection in human dendritic cells. Proc Natl Acad Sci USA. (2019) 116:6938–43. doi: 10.1073/pnas.1814700116
224. Goto K, Numata M, Komura JI, Ono T, Bestor TH, Kondo H. Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Different Res Biol Diversity. (1994) 56:39–44. doi: 10.1046/j.1432-0436.1994.56120039.x
225. Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, et al. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. (2010) 13:423–30. doi: 10.1038/nn.2514
226. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. (2014) 94:1027–76. doi: 10.1152/physrev.00029.2013
227. Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. (2007) 8:253–62. doi: 10.1038/nrg2045
228. Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. (2001) 293:1089–93. doi: 10.1126/science.1063443
229. Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. (2008) 199:375 e1–5. doi: 10.1016/j.ajog.2008.06.040
230. DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. (2010) 64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x
231. Hieke C, Kriebel K, Engelmann R, Muller-Hilke B, Lang H, Kreikemeyer B. Human dental stem cells suppress PMN activity after infection with the periodontopathogens Prevotella intermedia and Tannerella forsythia. Sci Rep. (2016) 6:39096. doi: 10.1038/srep39096
232. Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. (2011) 4:409–19. doi: 10.1038/mi.2010.83
233. de Camargo Pereira G, Guimaraes GN, Planello AC, Santamaria MP, de Souza AP, Line SR, et al. Porphyromonas gingivalis LPS stimulation downregulates DNMT1, DNMT3a, and JMJD3 gene expression levels in human HaCaT keratinocytes. Clin Oral Investig. (2013) 17:1279–85. doi: 10.1007/s00784-012-0816-z
234. Martins MD, Jiao Y, Larsson L, Almeida LO, Garaicoa-Pazmino C, Le JM, et al. Epigenetic modifications of histones in periodontal disease. J Dent Res. (2016) 95:215–22. doi: 10.1177/0022034515611876
235. Ateia IM, Sutthiboonyapan P, Kamarajan P, Jin T, Godovikova V, Kapila YL, et al. Treponema denticola increases MMP-2 expression and activation in the periodontium via reversible DNA and histone modifications. Cell Microbiol. (2018) 20:4. doi: 10.1111/cmi.12815
236. Kojima A, Kobayashi T, Ito S, Murasawa A, Nakazono K, Yoshie H. Tumor necrosis factor-alpha gene promoter methylation in Japanese adults with chronic periodontitis and rheumatoid arthritis. J Periodontal Res. (2016) 51:350–8. doi: 10.1111/jre.12314
237. Kobayashi T, Ishida K, Yoshie H. Increased expression of interleukin-6 (IL-6) gene transcript in relation to IL-6 promoter hypomethylation in gingival tissue from patients with chronic periodontitis. Arch Oral Biol. (2016) 69:89–94. doi: 10.1016/j.archoralbio.2016.05.018
238. Stockham S, Stamford JE, Roberts CT, Fitzsimmons TR, Marchant C, Bartold PM, et al. Abnormal pregnancy outcomes in mice using an induced periodontitis model and the haematogenous migration of Fusobacterium nucleatum sub-species to the murine placenta. PLoS ONE. (2015) 10:e0120050. doi: 10.1371/journal.pone.0120050
239. Tahara T, Hirata I, Nakano N, Tahara S, Horiguchi N, Kawamura T, et al. Potential link between Fusobacterium enrichment and DNA methylation accumulation in the inflammatory colonic mucosa in ulcerative colitis. Oncotarget. (2017) 8:61917–26. doi: 10.18632/oncotarget.18716
240. Tahara T, Yamamoto E, Suzuki H, Maruyama R, Chung W, Garriga J, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. (2014) 74:1311–8. doi: 10.1158/0008-5472.CAN-13-1865
241. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. (2017) 170:548–63.e16. doi: 10.1016/j.cell.2017.07.008
242. Offenbacher S, Riche EL, Barros SP, Bobetsis YA, Lin D, Beck JD. Effects of maternal Campylobacter rectus infection on murine placenta, fetal and neonatal survival, and brain development. J Periodontol. (2005) 76(Suppl. 11):2133–43. doi: 10.1902/jop.2005.76.11-S.2133
243. Bobetsis YA, Barros SP, Lin DM, Weidman JR, Dolinoy DC, Jirtle RL, et al. Bacterial infection promotes DNA hypermethylation. J Dent Res. (2007) 86:169–74. doi: 10.1177/154405910708600212
244. Liu Y, Hoyo C, Murphy S, Huang Z, Overcash F, Thompson J, et al. DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol. (2013) 208:395.e1-7. doi: 10.1016/j.ajog.2013.02.006
245. Fonseca-Silva T, Farias LC, Cardoso CM, Souza LR, Carvalho Fraga CA, Oliveira MV, et al. Analysis of p16(CDKN2A) methylation and HPV-16 infection in oral mucosal dysplasia. Pathobiology. (2012) 79:94–100. doi: 10.1159/000334926
246. Goodrich JM, Reddy P, Naidoo RN, Asharam K, Batterman S, Dolinoy DC. Prenatal exposures and DNA methylation in newborns: a pilot study in Durban, South Africa. Environ Sci Process Impacts. (2016) 18:908–17. doi: 10.1039/C6EM00074F
247. Cortese R, Lu L, Yu Y, Ruden D, Claud EC. Epigenome-Microbiome crosstalk: A potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. (2016) 11:205–15. doi: 10.1080/15592294.2016.1155011
248. Yu X, Shahir AM, Sha J, Feng Z, Eapen B, Nithianantham S, et al. Short-chain fatty acids from periodontal pathogens suppress histone deacetylases, EZH2, and SUV39H1 to promote Kaposi's sarcoma-associated herpesvirus replication. J Virol. (2014) 88:4466–79. doi: 10.1128/JVI.03326-13
249. Hernandez-Vargas H, Castelino J, Silver MJ, Dominguez-Salas P, Cros MP, Durand G, et al. Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in the Gambia. Int J Epidemiol. (2015) 44:1238–48. doi: 10.1093/ije/dyv027
250. Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM, et al. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. (2007) 36:1119–25. doi: 10.1093/ije/dym122
251. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. (2015) 213(Suppl. 4):S29–52. doi: 10.1016/j.ajog.2015.08.040
252. Konwar C, Price EM, Wang LQ, Wilson SL, Terry J, Robinson WP. DNA methylation profiling of acute chorioamnionitis-associated placentas and fetal membranes: insights into epigenetic variation in spontaneous preterm births. Epigenetics Chromatin. (2018) 11:63. doi: 10.1186/s13072-018-0234-9
253. Bermick JR, Lambrecht NJ, denDekker AD, Kunkel SL, Lukacs NW, Hogaboam CM, et al. Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics. (2016) 8:99. doi: 10.1186/s13148-016-0265-7
254. Fong G, Gayen Nee' Betal S, Murthy S, Favara M, Chan JSY, Addya S, et al. DNA methylation profile in human cord blood mononuclear leukocytes from term neonates: effects of histological chorioamnionitis. Front Pediatr. (2020) 8:437. doi: 10.3389/fped.2020.00437
255. Tolg C, Bägli DJ. Uropathogenic Escherichia coli infection: potential importance of epigenetics. Epigenomics. (2012) 4:229–35. doi: 10.2217/epi.12.5
256. Hamon MA, Batsché E, Régnault B, Tham TN, Seveau S, Muchardt C, et al. Histone modifications induced by a family of bacterial toxins. Proc Natl Acad Sci USA. (2007) 104:13467–72. doi: 10.1073/pnas.0702729104
257. Schmeck B, Beermann W, van Laak V, Zahlten J, Opitz B, Witzenrath M, et al. Intracellular bacteria differentially regulated endothelial cytokine release by MAPK-dependent histone modification. J Immunol. (2005) 175:2843–50. doi: 10.4049/jimmunol.175.5.2843
258. Ji J, Xu Y, Zheng M, Luo C, Lei H, Qu H, et al. Methionine attenuates lipopolysaccharide-induced inflammatory responses via DNA methylation in macrophages. ACS Omega. (2019) 4:2331–6. doi: 10.1021/acsomega.8b03571
259. Green BB, Kerr DE. Epigenetic contribution to individual variation in response to lipopolysaccharide in bovine dermal fibroblasts. Vet Immunol Immunopathol. (2014) 157:49–58. doi: 10.1016/j.vetimm.2013.10.015
260. Soncin F, Khater M, To C, Pizzo D, Farah O, Wakeland A, et al. Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development. (2018) 145:dev156273. doi: 10.1242/dev.156273
261. Jarmalaite S, Laurinaviciene A, Tverkuviene J, Kalinauskaite N, Petroska D, Bohling T, et al. Tumor suppressor gene ZAC/PLAGL1: altered expression and loss of the nonimprinted allele in pheochromocytomas. Cancer Genet. (2011) 204:398–404. doi: 10.1016/j.cancergen.2011.07.002
262. Lemeta S, Jarmalaite S, Pylkkanen L, Bohling T, Husgafvel-Pursiainen K. Preferential loss of the nonimprinted allele for the ZAC1 tumor suppressor gene in human capillary hemangioblastoma. J Neuropathol Exp Neurol. (2007) 66:860–7. doi: 10.1097/nen.0b013e318149ee64
263. Molnárová A, Palenčár D, Fekiačová D, Bieliková E, Tichá E, Ujházy E. Orofacial clefts and infections during pregnancy. Biologia. (2018) 73:629–35. doi: 10.2478/s11756-018-0065-y
264. Avgil M, Ornoy A. Herpes simplex virus and Epstein-Barr virus infections in pregnancy: consequences of neonatal or intrauterine infection. Reprod Toxicol. (2006) 21:436–45. doi: 10.1016/j.reprotox.2004.11.014
265. Ornoy A, Tenenbaum A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol. (2006) 21:446–57. doi: 10.1016/j.reprotox.2005.12.007
266. Mercado M, Ailes EC, Daza M, Tong VT, Osorio J, Valencia D, et al. Zika virus detection in amniotic fluid and Zika-associated birth defects. Am J Obstet Gynecol. (2020) 222:610.e1-13. doi: 10.1016/j.ajog.2020.01.009
267. Carvalho IF, Alencar PNB, Carvalho de Andrade MD, Silva PGB, Carvalho EDF, Araújo LS, et al. Clinical and x-ray oral evaluation in patients with congenital Zika Virus. J Appl Oral Sci. (2019) 27:e20180276. doi: 10.1590/1678-7757-2018-0276
268. Hutchinson J. Clinical lecture on heredito-syphilitic struma: and on the teeth as a means of diagnosis. Br Med J. (1861) 1:515–7. doi: 10.1136/bmj.1.20.515
269. Carter TC, Olney RS, Mitchell AA, Romitti PA, Bell EM, Druschel CM, et al. Maternal self-reported genital tract infections during pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. (2011) 91:108–16. doi: 10.1002/bdra.20749
270. Chaim W, Mazor M, Wiznitzer A. The prevalence and clinical significance of intraamniotic infection with Candida species in women with preterm labor. Arch Gynecol Obstet. (1992) 251:9–15. doi: 10.1007/BF02718273
271. Obermair HM, Bhagwanani G, Caldas R, Doyle H, Smoleniec J, Adno A. Candida chorioamnionitis associated with late stillbirth: A case report. Case Rep Womens Health. (2020) 27:e00239. doi: 10.1016/j.crwh.2020.e00239
272. Arce-Estrada GE, Gomez-Toscano V, Cedillo-Pelaez C, Sesman-Bernal AL, Bosch-Canto V, Mayorga-Butron JL, et al. Report of an unsual case of anophthalmia and craniofacial cleft in a newborn with Toxoplasma gondii congenital infection. BMC Infect Dis. (2017) 17:459. doi: 10.1186/s12879-017-2565-8
273. Rashidi A, Roullet M. Malaria during pregnancy with parasite sequestration in the villous chamber. Blood. (2013) 121:2173. doi: 10.1182/blood-2012-10-465096
274. Felkner M, Suarez L, Liszka B, Brender JD, Canfield M. Neural tube defects, micronutrient deficiencies, and Helicobacter pylori: a new hypothesis. Birth Defects Res A Clin Mol Teratol. (2007) 79:617–21. doi: 10.1002/bdra.20382
275. Nashaat EH, Mansour GM. Arch Gynecol Obstet. (2014) 289:1197–202. doi: 10.1007/s00404-013-3138-8
276. Brawner KM, Kumar R, Serrano CA, Ptacek T, Lefkowitz E, Morrow CD, et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. (2017) 10:1169–77. doi: 10.1038/mi.2016.131
277. Sanchez-Alonzo K, Matamala-Valdes L, Parra-Sepulveda C, Bernasconi H, Campos VL, Smith CT, et al. Intracellular presence of Helicobacter pylori and Its virulence-associated genotypes within the vaginal yeast of term pregnant women. Microorganisms. (2021) 9:131. doi: 10.3390/microorganisms9010131
278. den Hollander WJ, Schalekamp-Timmermans S, Holster IL, Jaddoe VW, Hofman A, Moll HA, et al. Helicobacter pylori colonization and pregnancies complicated by preeclampsia, spontaneous prematurity, and small for gestational age birth. Helicobacter. (2017) 22:12364. doi: 10.1111/hel.12364
279. Golalipour MJ, Sedehi M, Qorbani M. Does maternal Helicobacter pylori infection increase the risk of occurrence of neural tube defects in newborns in Northern Iran? Neurosciences. (2012) 17:219–25.
280. Arnold SR, Ford-Jones EL. Congenital syphilis: A guide to diagnosis and management. Paediatr Child Health. (2000) 5:463–9. doi: 10.1093/pch/5.8.463
281. Al-Katawee YA, Al-Hasoun YA, Taha MN, Al-Moslem K. Congenital varicella-zoster virus infection. A rare case of severe brain and ocular malformations without limb or cutaneous involvement in a newborn after maternal subclinical infection. Saudi Med J. (2005) 26:869–71.
282. Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG. (2011) 118:1155–62. doi: 10.1111/j.1471-0528.2011.02983.x
283. Salemi JL, Whiteman VE, August EM, Chandler K, Mbah AK, Salihu HM. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J Viral Hepat. (2014) 21:e144–53. doi: 10.1111/jvh.12250
284. Mossey P, Catilla E, editors. Global registry and database on craniofacial anomalies: report of a WHO Registry Meeting on Craniofacial Anomalies/Main. Geneva: World Health Organization (2003).
285. Funato N, Nakamura M. Identification of shared and unique gene families associated with oral clefts. Int J Oral Sci. (2017) 9:104–9. doi: 10.1038/ijos.2016.56
286. Malic CC, Lam M, Donelle J, Richard L, Vigod SN, Benchimol EI. Incidence, risk factors, and mortality associated with orofacial cleft among children in Ontario, Canada. JAMA Netw Open. (2020) 3:e1921036. doi: 10.1001/jamanetworkopen.2019.21036
287. Peterka M, Tvrdek M, Likovsky Z, Peterkova R, Fara M. Maternal hyperthermia and infection as one of possible causes of orofacial clefts. Acta Chir Plast. (1994) 36:114–8.
288. Beswick RC, Warner R, Warkany J. Congenital anomalies following maternal rubella. Am J Dis Child. (1949) 78:334–48. doi: 10.1001/archpedi.1949.02030050347007
289. Mawson AR, Croft AM. Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int J Environ Res Public Health. (2019) 16:3543. doi: 10.3390/ijerph16193543
290. Witkin SS, Neuer A, Giraldo P, Jeremias J, Tolbert V, Korneeva IL, et al. Chlamydia trachomatis infection, immunity, and pregnancy outcome. Infect Dis Obstet Gynecol. (1997) 5:128–32. doi: 10.1002/(SICI)1098-0997(1997)5:2<128::AID-IDOG7>3.0.CO;2-W
291. Fleming JA, Song G, Choi Y, Spencer TE, Bazer FW. Interferon regulatory factor 6 (IRF6) is expressed in the ovine uterus and functions as a transcriptional activator. Mol Cell Endocrinol. (2009) 299:252–60. doi: 10.1016/j.mce.2008.10.025
292. Blanton SH, Cortez A, Stal S, Mulliken JB, Finnell RH, Hecht JT. Variation in IRF6 contributes to nonsyndromic cleft lip and palate. Am J Med Genet A. (2005) 137A:259–62. doi: 10.1002/ajmg.a.30887
293. Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. (1999) 145:539–49. doi: 10.1083/jcb.145.3.539
294. Krummenacher C, Baribaud I, Eisenberg RJ, Cohen GH. Cellular localization of nectin-1 and glycoprotein D during herpes simplex virus infection. J Virol. (2003) 77:8985–99. doi: 10.1128/JVI.77.16.8985-8999.2003
295. Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, et al. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. (2000) 25:427–30. doi: 10.1038/78119
296. Oner DA, Tastan H. Identification of novel variants in the PVRL1 gene in patients with nonsyndromic cleft lip with or without cleft palate. Genet Test Mol Biomarkers. (2016) 20:269–72. doi: 10.1089/gtmb.2015.0276
297. Alvizi L, Ke X, Brito LA, Seselgyte R, Moore GE, Stanier P, et al. Differential methylation is associated with non-syndromic cleft lip and palate and contributes to penetrance effects. Sci Rep. (2017) 7:2441. doi: 10.1038/s41598-017-02721-0
298. Yan Y, Zhang XT, Wang G, Cheng X, Yan Y, Fu YJ, et al. Zika virus induces abnormal cranial osteogenesis by negatively affecting cranial neural crest development. Infect Genet Evol. (2019) 69:176–89. doi: 10.1016/j.meegid.2019.01.023
299. Jacobsen PE, Henriksen TB, Haubek D, Østergaard JR. Prenatal exposure to antiepileptic drugs and dental agenesis. PLoS ONE. (2014) 9:e84420. doi: 10.1371/journal.pone.0084420
300. Al-Ani AH, Antoun JS, Thomson WM, Merriman TR, Farella M. Maternal smoking during pregnancy is associated with offspring hypodontia. J Dent Res. (2017) 96:1014–9. doi: 10.1177/0022034517711156
301. Paulsson L, Arvini S, Bergström N, Klingberg G, Lindh C. The impact of premature birth on dental maturation in the permanent dentition. Clin Oral Invest. (2019) 23:855–61. doi: 10.1007/s00784-018-2501-3
302. Aine L, Backström MC, Mäki R, Kuusela AL, Koivisto AM, Ikonen RS, et al. Enamel defects in primary and permanent teeth of children born prematurely. J Oral Pathol Med. (2000) 29:403–9. doi: 10.1034/j.1600-0714.2000.290806.x
303. Cruvinel VR, Gravina DB, Azevedo TD, Rezende CS, Bezerra AC, Toledo OA. Prevalence of enamel defects and associated risk factors in both dentitions in preterm and full term born children. J Appl Oral Sci. (2012) 20:310–7. doi: 10.1590/S1678-77572012000300003
304. Gravina DB, Cruvinel VR, Azevedo TD, Toledo OA, Bezerra AC. Enamel defects in the primary dentition of preterm and full-term children. J Clin Pediatr Dent. (2013) 37:391–5. doi: 10.17796/jcpd.37.4.8q77717841781527
305. De Coster PJ, Marks LA, Martens LC, Huysseune A. Dental agenesis: genetic and clinical perspectives. J Oral Pathol Med. (2009) 38:1–17. doi: 10.1111/j.1600-0714.2008.00699.x
308. Oberoi S, Vargervik K. Hypoplasia and hypodontia in Van der Woude syndrome. Cleft Palate Craniofac J. (2005) 42:459–66. doi: 10.1597/04-028.1
309. Stagno S, Pass RF, Dworsky ME, Alford CA. Congenital and perinatal cytomegalovirus infections. Semin Perinatol. (1983) 7:31–42.
310. Jaskoll T, Abichaker G, Htet K, Bringas JP, Morita S, Sedghizadeh PP, et al. Cytomegalovirus induces stage-dependent enamel defects and misexpression of amelogenin, enamelin and dentin sialophosphoprotein in developing mouse molars. Cells Tissues Organs. (2010) 192:221–39. doi: 10.1159/000314909
311. Roberts L. Small, furry and powerful: are mouse lemurs the next big thing in genetics? Nature. (2019) 570:151–4. doi: 10.1038/d41586-019-01789-0
312. Weber H. Factors affecting the choice of species. In: Wolfe-Coote S, editor. The laboratory Primate Cape Town (2005). p. 259–72
313. Su RW, Fazleabas AT. Implantation and establishment of pregnancy in human and nonhuman primates. Adv Anat Embryol Cell Biol. (2015) 216:189–213. doi: 10.1007/978-3-319-15856-3_10
314. Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol. (2018) 315:R1123–53. doi: 10.1152/ajpregu.00391.2017
315. Ouédraogo A, Tiono AB, Diarra A, Bougouma ECC, Nébié I, Konaté AT, et al. Transplacental transmission of Plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J Trop Med. (2012) 2012:109705. doi: 10.1155/2012/109705
316. Humann J, Mann B, Gao G, Moresco P, Ramahi J, Loh LN, et al. Bacterial peptidoglycan traverses the placenta to induce fetal neuroproliferation and aberrant postnatal behavior. Cell Host Microbe. (2016) 19:901. doi: 10.1016/j.chom.2016.05.017
317. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. (2013) 155:1451–63. doi: 10.1016/j.cell.2013.11.024
318. Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. (2020) 367:8429. doi: 10.1126/science.aaw8429
319. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. (2014) 158:705–21. doi: 10.1016/j.cell.2014.05.052
320. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. (2009) 27:358–68. doi: 10.1055/s-0029-1237424
321. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. (2014) 156:84–96. doi: 10.1016/j.cell.2013.12.016
322. Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. (2000) 74:3321–9. doi: 10.1128/JVI.74.7.3321-3329.2000
Keywords: pregnancy, maternal inflammation, infections, fetal development, craniofacial development
Citation: Bhagirath AY, Medapati MR, de Jesus VC, Yadav S, Hinton M, Dakshinamurti S and Atukorallaya D (2021) Role of Maternal Infections and Inflammatory Responses on Craniofacial Development. Front. Oral. Health 2:735634. doi: 10.3389/froh.2021.735634
Received: 03 July 2021; Accepted: 10 August 2021;
Published: 06 September 2021.
Edited by:
Nagihan Bostanci, Karolinska Institutet (KI), SwedenReviewed by:
Roger M. Arce, University of Texas Health Science Center at Houston, United StatesCopyright © 2021 Bhagirath, Medapati, de Jesus, Yadav, Hinton, Dakshinamurti and Atukorallaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devi Atukorallaya, RGV2aS5BdHVrb3JhbGxheWFAdW1hbml0b2JhLmNh
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.