
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiomes, 30 January 2023
Sec. Host and Microbe Associations
Volume 1 - 2022 | https://doi.org/10.3389/frmbi.2022.1055472
The vaginal microbiome exists in a dynamic state and its disruption, by diminution of Lactobacillus concentrations, can induce a state of microbial imbalance with significant health consequences, such as increased risk of sexually transmitted infection (STI) acquisition, preterm labor, and low birth weight babies. This delicate balance of microbes can be affected by many processes such as mechanical practices (i.e. douching) and hormonal changes: physiologic (i.e. menstrual cycle, menopause, puberty), pathologic (i.e. PCOS), and exogenous (i.e. contraceptives). Contraceptives fall into mechanical and hormonal categories, both of which prevent unintended pregnancy. The mechanical contraceptives of spermicides, diaphragms, and cervical caps alter the vaginal ecosystem, with spermicides being linked to an increased risk of vaginal dysbiosis. The impacts of Copper T intrauterine devices (Cu-IUDs) and hormonal contraceptives on the vaginal microbiome are contradictory. A better understanding and consensus of how contraceptive methods affect the vaginal microbiome is needed.
A complex relationship between microbes and their hosts has been well documented over the last two decades shaped by the advent of new technologies. High throughput sequencing has allowed scientists to identify microbial communities while studying their dynamic interactions through genomic, proteomic, transcriptomic, and metabolomic to further understand their intricate relationships (Pavlova et al., 2002; Antonio et al., 1999) Within the human reproductive system, there is a mutualistic relationship between host and microbes known as the vaginal microbiome. Disruption of this balance is known as dysbiosis. The vaginal microbiome considers the microbial composition of the host’s vagina, whose composition has historically been thought to be dominated by the Lactobacillus genus of gram-positive anaerobes in the state of normalcy (Burton and Reid, 2022). Recent advances in genome sequencing and metagenomic technologies have enabled scientists to observe regional and racial variations in vaginal microflora, questioning our concept of normalcy (Drell et al., 2013; Martínez-Peña et al., 2013; Pendharkar et al., 2013). While it is important to acknowledge this emerging information and the subjectivity of historical normalcy, the data remains unclear and does not provide strong evidence to redefine normalcy of the vaginal microbiome.
The vaginal microbiome exists as a dynamic environment. A multitude of factors influences this, including pregnancy (see 2.2), age (see 2.3), douching (see 2.5), menstruation (see 2.6), and contraception (see 3). Approximately 65% of women in the United States of America between the ages of 15-49 use a form of contraception at a given moment in time, and nearly all sexually active U.S. women have used a form of contraception at some point during their reproductive years (Center for Disease Control and Prevention, 2018; Kaiser Family Foundation, 2021). Given the high prevalence of contraceptives, it is necessary to understand the influence of contraceptives on alterations in vaginal microbiome and it is important to improve research on this topic to provide appropriate counseling for patients.
Vaginal dysbiosis is broadly defined by a decrease in Lactobacillus species population in the vagina. This causes an increase in pH, which is associated with negative health effects, such as bacterial vaginosis (BV), which has been linked to preterm labor and increased risk of acquiring sexually transmitted infections and vulvovaginal infections (Hillier et al., 1995; Brotman et al., 2010; Kalia et al., 2020; Ravel et al., 2021). The exact mechanism that causes vaginal dysbiosis is still unknown (van de Wijgert et al., 2014). To better understand dysbiotic states, it is imperative to know the composition of a healthy vaginal microbiome.
The vaginal microbiome is dominated by Lactobacillus, which produces lactic acid to create an acidic environment in the vagina (O'Hanlon et al., 2013; Tachedjian et al., 2017). This confers an innate immunity within the vagina to fight off pathogenic bacteria and to further allow Lactobacillus to proliferate. Such mechanisms include acidifying the bacterial cytoplasm leading to cell death and acting as a permeabilizer of gram-negative bacterial cell membranes (Alakomi et al., 2000).
The exact mechanism by which the vaginal microbiome is initially formed is unknown. It is hypothesized that individuals can be predisposed to dysbiosis through various means. One theory states that vaginal imbalance can be impacted within utero life. Findings from Collado et al. suggest that the fetal-maternal interface can be an important source of seeding for the gut microbiome, but its effects on the fetal vaginal microbiome have yet to be investigated (Collado et al., 2016).
Additionally, different birthing methods have been shown to impact the composition of the intestinal microbiome of the neonate. The intestinal microbiome of infants is dominated by maternal fecal bacteria, but neonates born via cesarean lack this exposure (Carlsson and Gothefors, 1975; Korpela et al., 2020; Song et al., 2021). The short- and long-term health consequences of this intervention are not fully understood.
A time frame that is marked by distinct changes in the vaginal microbiome is between pre-and post-puberty. Pre-pubescent individuals have a greater diversity of anaerobes, aerobes, and enteric bacteria. The onset of menarche is noted by Lactobacillus predominance, which is seen in most reproductive-aged women. This is likely due to the increase of estrogen which produces more intracellular glycogen supporting Lactobacillus growth (Porter et al., 2016). As the organ continues to develop, one of the ways in which the vagina maintains homeostasis is vaginal discharge. Vaginal discharge is a collection of mucus from the cervix, which is protective as it removes potentially pathogenic material as it travels down the vaginal canal. However, the self-cleaning properties are not a perfect defense and pathogenic bacteria can colonize and overgrow in the vagina.
BV is a state of dysbiosis characterized by foul-smelling vaginal odor and thin, gray-white vaginal discharge (Tachedjian et al., 2017). Although the inciting entity for BV remains controversial, it typically presents with a decreased population of Lactobacillus and high concentrations of anaerobic bacteria including Gardnerella vaginalis, Prevotella spp., Atopobium vaginae, Sneathia spp., and other BV-associated bacteria (BVAB) (Muzny et al., 2020). BV is the most common vaginal imbalance (Ahmadnia et al., 2016) affecting 21.2 million (29.2%) women in the United States aged 14-49 (Koumans et al., 2007). Adverse health outcomes of BV are associated with pre-term delivery of a low-birth-weight baby (Hillier et al., 1995), increased risk of acquiring sexually transmitted infections such as trichomonal, gonococcal, and chlamydial infections (O'Hanlon et al., 2013), and a controversial effect on fertility (Liversedge et al., 1999; Campisciano et al., 2017).
Although BV is one of the most common causes of dysbiosis, there are other mechanisms that can cause the imbalance. A mechanical mechanism that can cause dysbiosis in reproductive-age women is douching. Douching has been shown to decrease hydrogen peroxide producing Lactobacillus, therefore, increasing the vaginal pH (Ness et al., 2002; Beigi et al., 2005). Women with biologic vaginas and who douche regularly have a higher risk of developing BV (Ness et al., 2002).
Hormones can affect the bacterial composition of the vaginal microbiome. An example of a high hormonal state is Polycystic Ovary Syndrome (PCOS), with increased levels of testosterone and alterations in estrogen and progesterone levels. Women with PCOS were found to be colonized with a higher abundance of Mycoplasma and Prevotella with a lower prevalence of Lactobacillus crispatus (Hong et al., 2020).
A physiologic state that is characterized by hormonal fluctuations is the menstrual cycle. The two organs involved in the menstrual cycle are the uterus and ovaries. The uterus has three phases: menstrual, proliferative, and secretory while the ovaries have two: follicular and luteal. These cyclical phases of the two organs occur simultaneously, with the proliferative and follicular phases being dominated by estrogen and the secretory and luteal by progesterone. It should be noted that the menstrual cycle is usually 28 days but can vary among individuals (Baker and Driver, 2007).
Hormonal influences from the menstrual cycle have been documented to influence the vaginal microbiome as well. There is not a consensus on exactly which bacteria are affected during specific phases in the menstrual cycle. Some women’s microbiome remains stable throughout the menstrual cycle, and others experience a temporal shift in the dominance of bacterial populations during menses, which returns to normal once menses stop (Song et al., 2020; Critchley et al., 2020). Other studies report linear changes in bacterial populations throughout the menstrual cycle (Eschenbach et al., 2000).
Lactobacillus is a key player in the vaginal microbiome. Its predominance provides protection against an array of pathogens, and its disruption is associated with several diseased states. Discerning which practices are harmful or hurtful to its colonization provides insights to improve vaginal health. With the popularity of contraceptive use, it is important to understand how this practice can be impacted.
There are many methods of contraception that are often focused on preventing unintended pregnancies. Although a non-hormonal contraceptive pill (YCT529) for men has recently started clinical trials, currently available male contraception is conventionally focused on condoms and vasectomy. Options for persons with female reproductive organs are more comprehensive and include reversible, hormonal, mechanical, and permanent contraception. Choosing a method of contraception is often a personal decision including the individual’s subjective characteristics and the availability and usage of the method. While each method brings its own advantages and risks, there is a lack of discussion and understanding of the effects of contraceptives on the vaginal microbiome (Table 1).
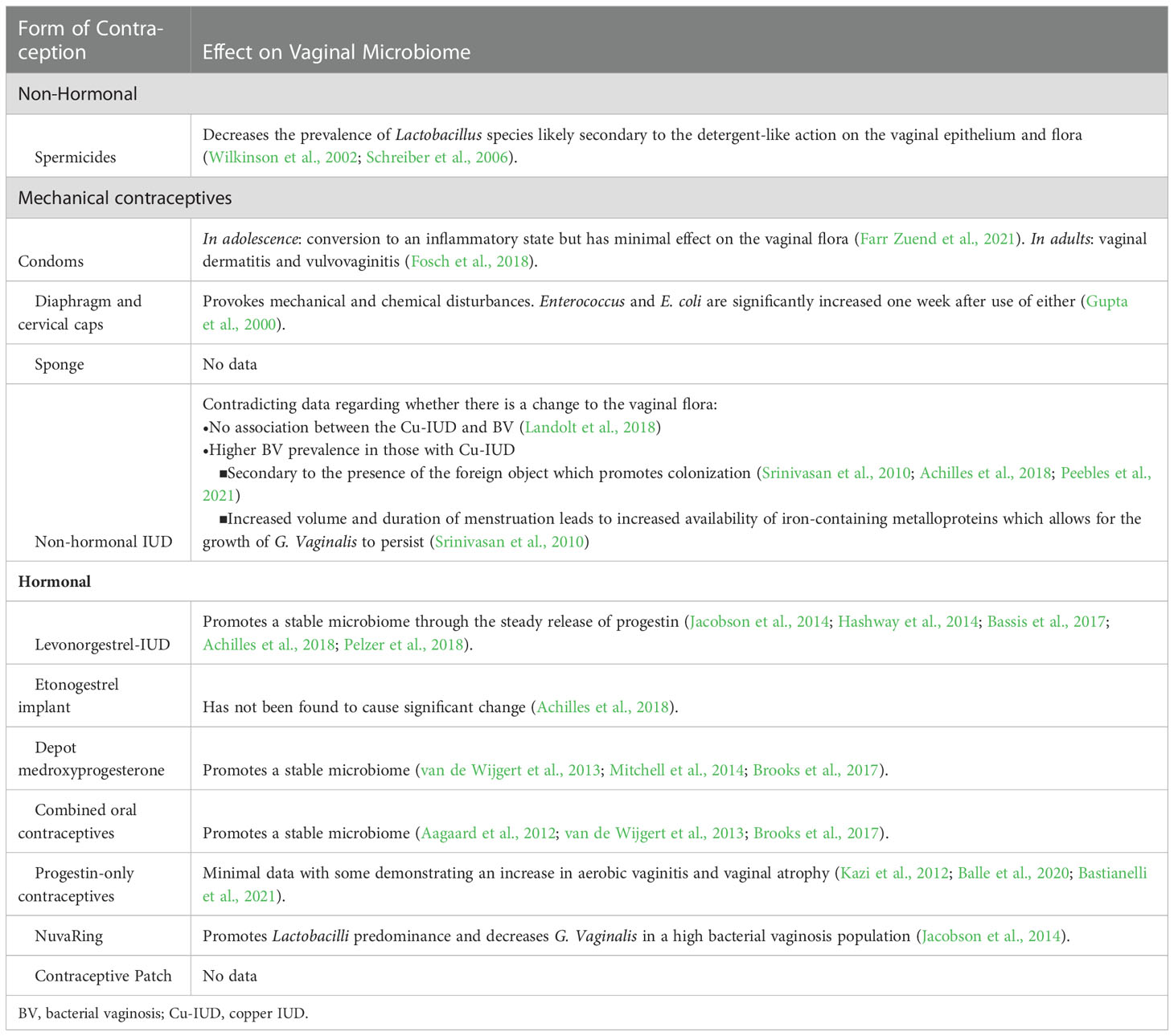
Table 1 Summary of the different forms of contraception and their known effects on the vaginal microbiome.
Mechanical contraception is often referred to as the barrier method. The diaphragm, cervical cap, male and female condoms, and spermicide form a physical block, preventing the fertilization of eggs by sperm. Often overlooked and under-emphasized is their influence on the vaginal microbiome.
Spermicides are sold as creams, films, foams, gels, or suppositories that are designed to kill sperm and/or block entry of sperm into the cervix. However, the product has been linked to vaginal microbiome alterations. The most common form of spermicide, Nonoxynol-9, significantly alters the vaginal microbiome leading to an apparent lack in Lactobacillus (Schreiber et al., 2006). This is a dose and exposure-dependent change, likely due to the nonspecific, detergent-like action on the vaginal epithelium and flora, enhancing the susceptibility of the space to vaginal irritation and allergic vaginitis. Additionally, the endothelial disruption may provide a viral entryway, as there is evidence of harm through genital lesions with Nonoxynol-9 use (Wilkinson et al., 2002). Despite the increased risk of dysbiosis and clinical injury, Nonoxynol-9 has continued to be sold as an over-the-counter (OTC) drug in creams, gels, foams, and condom lubricants for more than 30 years.
While the surfactant Nonoxynol-9 disrupts cell membranes, other spermicides impede sperm function in a less extreme fashion, though similar issues are reported. Cellulose sulfate impedes sperm penetration into cervical mucus but increases mucosal inflammation (Pellett Madan et al., 2015). Similar outcomes of the cellulose sulfate gel on an increased risk of HIV acquisition have also been reported when compared to the use of Nonoxynol-9 (Van Damme et al., 2008). Phexxi, a more recent addition to the spermicide market, is believed to have a gentler effect on the vaginal epithelium with its acid-based formulation. This acidic composition disrupts sperm and may also disrupt the vaginal microbial composition, reflecting the common side effects of yeast and urinary tract infections (Svoboda, 2020).
Male and female condoms are sheath barrier devices often made of latex that may be used in conjunction with lubricants or spermicides. The use of condoms has been associated with a conversion of the vaginal microbiome to an inflammatory state. In adolescent girls, condom use has been associated with changes in functional metabolic pathways in vaginal bacteria and inflammatory processes, but without significant changes in the overall vaginal microbiome (Farr Zuend et al., 2021). These functions are related to protein translation and fructose and mannose metabolism, which are important pathways for energy production by Lactobacillus. In adults, condom use is correlated with the presence of vaginal dermatitis, allergic and irritant vulvovaginitis, and inflammation, likely due to the influence of either the latex or the spermicide as some condoms come coated with spermicide (Fosch et al., 2018). The vulvar mucosa is susceptible to irritants and mechanical disturbances due to its composition of hormone-responsive nonkeratinized epithelium. Condom use may function as both a mechanical and chemical disturbance promoting vaginitis, an inflammatory state, which can further lead to microbiome disturbances.
Diaphragms and Cervical caps function as physical barriers by covering the cervix. Both contraceptive methods require the co-use of spermicides, and both have been shown to clearly alter the normal vaginal ecosystem. Enterococcus and E. coli species have been found to be significantly increased one week after initiation of either cervical caps or diaphragm use (Gupta et al., 2000). These methods may provoke both mechanical and chemical disturbances to the vaginal microbiome, but the exact mechanism is unknown.
Contraceptive sponges similarly cover the cervix and contain Nonoxynol-9 spermicide to prevent pregnancy. However, there is no available data on the effect of the sponge on the vaginal microbiome in humans although one brand remains available on the US market.
Mechanical contraception can cause both physical and irritant injury to the vaginal epithelium and mucosa which can consequently increase susceptibility to further damage.
Copper T intrauterine device (Cu-IUD) is a nonhormonal, metal device that is placed in the uterus and can reside there for up to 10 years as an effective form of birth control. Contradicting evidence has been reported regarding their association with vaginal dysbiosis. While Kancheva Landolt et al. (2018) reported there was no association between Cu-IUD use and BV, Peebles et al. (2021) reported a 1.28-fold increase in BV compared to those without or with another nonhormonal contraception. It should be noted that the study by Landolt et al. (2018) focused on a population in Thailand with a high BV prevalence among all their groups with or without contraception initiation. Another study executed in Zimbabwe also found a positive association between BV and Cu-IUD use, notable by increased colonization with the BV-associated microbiota G. vaginalis and A. vaginae (Achilles et al., 2018).
There are two predominating theories as to how Cu-IUDs impact the vaginal microbiome. The continued presence of the foreign object may promote colonization of BV-associated microbiota. However, the disruption to the vaginal microbiome that is seen with Copper IUDs is not similarly seen in hormonal IUD methods discussed in detail below, suggesting that this reaction is specific to the copper release. Alternatively, the influence of Cu-IUD initiation on the individual menstruation cycle may contribute to vaginal dysbiosis. A decrease in the predominant Lactobacillus species and increase in G. vaginalis often occurs during menstruation denoting a temporal variability in the microbiota of the human vagina (Srinivasan et al., 2010). As increased volume and duration of menstruation often accompanies Cu-IUD initiation, the growth of G. vaginalis may be allowed to persist to the point of dysbiosis due to the increased availability of iron-containing metalloprotein in erythrocytes (Figure 1).
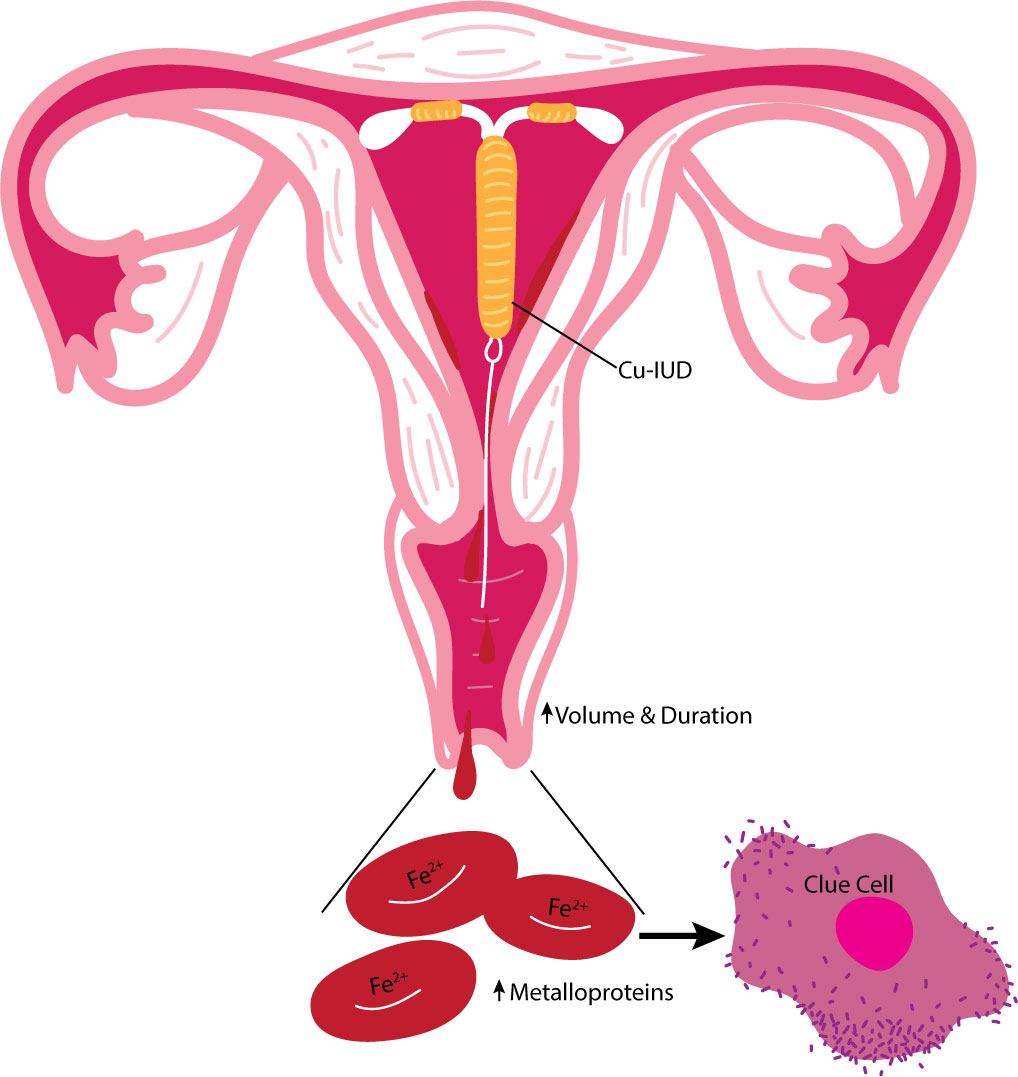
Figure 1 Summary of the proposed influence of the Cu-lUD on the vaginal environment. Cu-IUDs are associated with heavier menstrual cycles likely caused by copper's influence of vascular changes increasing the volume and duration of bleeding. The increased availability of iron-containing metalloproteins in erythrocytes due to heavy menstruation promotes colonization of BV associated microbiota such as G. vaginalis..
Hormonal contraceptives are a common form of birth control composed of a synthetic form of progestin with or without estrogen, which inhibits ovulation through suppression of the hypothalamic-pituitary-gonadal (HPG) axis. Although the delivery system may vary, different forms have similar effects, often accompanied by side effects indicating the hormones’ systemic capabilities.
To understand estrogen and progesterone’s impact on the vaginal microbiome, it is beneficial to understand changes to the system during a condition of decreased estrogen and progesterone production like menopause. During menopause, low hormone levels result in a decreased glycogen deposition on the vaginal epithelium, leading to less free glycogen availability for Lactobacilli nutrition. In comparison to a Lactobacillus-predominant vaginal microbiome in premenopausal American women (83%), post-menopausal women experience a statistically significant difference in the composition of the taxonomic distribution, decreasing to 54% (Brotman et al., 2014). A similar decrease of 63.2% to 23.7% was observed in Korean women when comparing pre- and post-menopausal taxonomic distributions, respectively (Kim et al., 2021). Post-menopausal vaginal microbiomes are additionally associated with increased diversity in microbe species and increased pH. Lactic acid from metabolized glycogen during reproductive age maintains an acidic vaginal pH of 3.8-4.2, suppressing the overgrowth of infectious organisms. This contrasts with postmenopausal women taking hormone replacement therapy (increased circulating estrogen) who are more likely to have majority vaginal Lactobacilli like those of reproductive age (Dahn et al., 2008).
Juxtaposing the low hormonal state of menopause, pregnancy posits a condition of high progesterone for further characterization of the hormonal influences on the vaginal microbiome. The vaginal microbiome signature in pregnancy is distinct from non-pregnant. Overall diversity and richness are reduced in pregnancy, with a dominance of Lactobacillus species iners, crispatus, jensenii, and johnsonii (Aagaard et al., 2012). Lactobacilli not only produce lactic acid, but they may also produce hydrogen peroxide (H2O2). which is bactericidal. L crispatus is a strong H2O2 producer. This activity of acidity and H2O2 production reflects an innate immune defense against pathogenic organisms and may be critical during pregnancy to prevent dysbiotic vaginal colonization and ascending infection.
Exogenous hormonal administration similarly saw an emergence of L. crispatus as the dominant phylotype in both the endometrial and the endocervical samples (Pelzer et al., 2018). In Pelzer et al, patients utilized a levonorgestrel-releasing IUD (hormonal IUD) for consistent release of progestin, and other studies have similarly corroborated that consistent hormonal dosing promotes a stable vaginal microbiome (Jacobson et al., 2014; Hashway et al., 2014; Achilles et al., 2018; Pelzer et al., 2018).
The use of combined oral contraceptives (COCs) (Aagaard et al., 2012) and depot-medroxyprogesterone acetate (DMPA, i.e. ‘the shot’) (Mitchell et al., 2014) have revealed similar results of a stable vaginal microbiome dominated by H2O2-producing bacteria of the Lactobacillus genus. COCs and DMPA impart exogenous hormones within the system and are associated with overall decreased BV-associated vaginal dysbiosis (van de Wijgert et al., 2013; Brooks et al., 2017). COC Etonogestrel implanted contraception has not been found to cause significant change (Achilles et al., 2018). There is minimal data on the effect of progesterone-only-pills (POP or ‘mini-pill’) on the vaginal microbiome, but some research has demonstrated no change in the BV rates, but increased rates of aerobic vaginitis and vaginal atrophy (Kazi et al., 2012; Balle et al., 2020). This is thought to be due to the increased bleeding that can occur with POPs compared to COC and hormonal IUDs (Bastianelli et al., 2021). The NuvaRing, an estrogen-containing vaginal ring, has been demonstrated to promote Lactobacilli predominance and decreasing G. vaginalis in a high-BV population (Crucitti et al., 2018). No data on the effect of the contraceptive patch on vaginal flora has been documented.
However, it is important to note that contradicting evidence has been reported regarding hormonal contraceptive use. Donders et al. reported COC and Hormonal IUD users had the same bacterial composition as non-contraceptive users (Donders et al., 2017). While Brooks et al. noted Hormonal IUD use was accompanied by several taxa typically associated with a dysbiotic vaginal microbiome including Prevotella (Brooks et al., 2017). The same study noted that women using DMPA and hormonal IUDs were no more or less likely to be colonized by H2O2-producing Lactobacillus than women using condoms (Brooks et al., 2017). Achilles et al. (2018) further found no significant changes in beneficial Lactobacillus species over 180 days after initiation of injectable (DMPA, norethisterone enanthate, or medroxyprogesterone acetate and Ethinyl estradiol) or implanted (levonorgestrel or etonogestrel) contraception. Additionally, a study conducted by Bassis et al. (2017) found that hormonal IUD had no effect on vaginal microbiome composition over a 12-month period.
Another concern noted with hormonal contraception is that COC use may increase vaginal candidiasis (van de Wijgert et al., 2013). Additionally, the age of the individual using COC influenced alterations in the vaginal microbiota, although Lactobacilli remained predominant in all age categories (Kazi et al., 2012). Despite the contrasting data, the majority of the evidence supports that hormonal contraception influences a vaginal microbiome of decreased diversity and pH predominated by H2O2-producing bacteria of the Lactobacillus genus (Brooks et al., 2017; Song et al., 2020; Balle et al., 2020) (Figure 2).
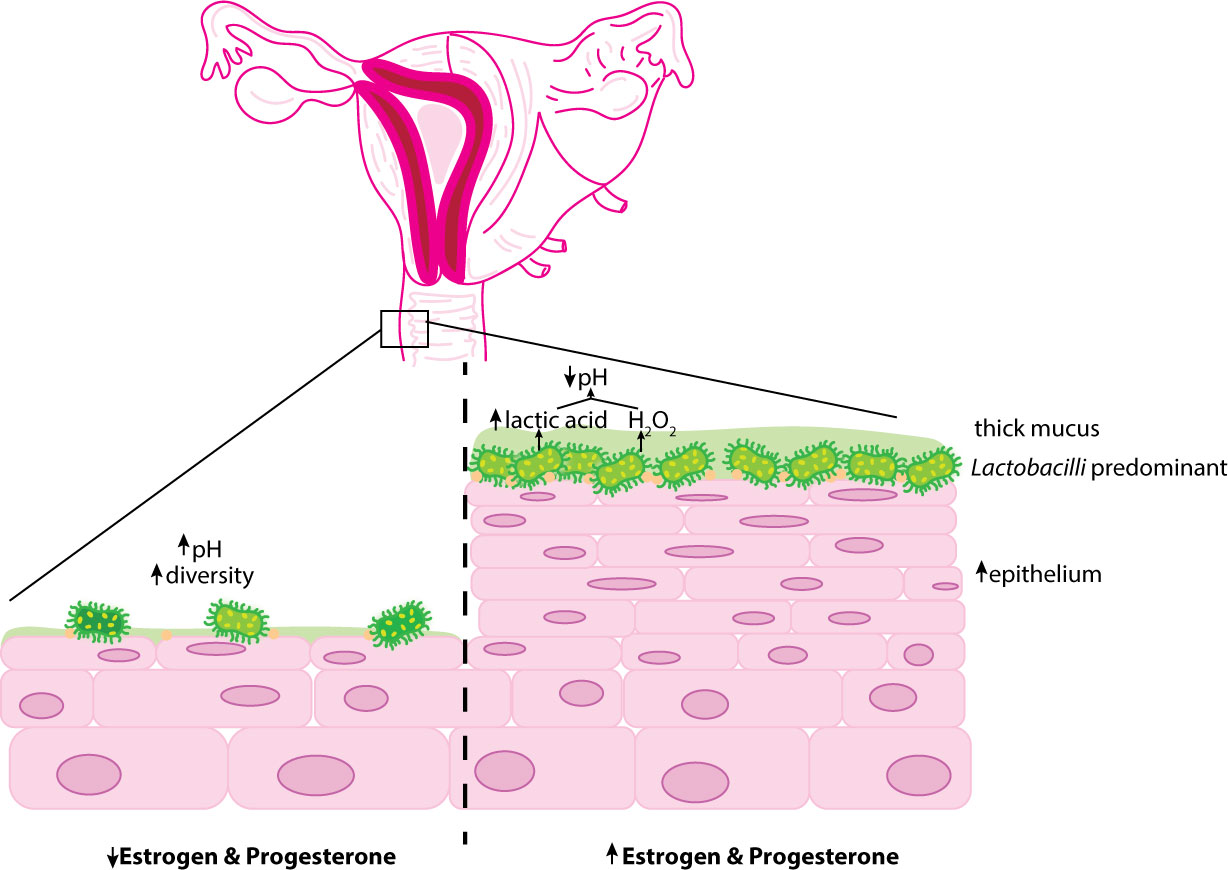
Figure 2 Overview ofthe influence of hormonal contraception on the vaginal epithelium. Estrogen and progesterone encourage thickening of the vaginal mucosa and glycogen deposition. Glycogen presence preferentially selects for glucose-fennenting microorganisms such as Lactobacilli. Lactobacilli metabolize glycogen into lactic acid and fonn H2O2 promoting an acidic environment. Decreased hormonal systems (prepubertal and postmenopausal) are associated with reduced glycogen deposition increasing the diversity of vaginal microbe species and vaginal pH.
The vaginal microbiome is an ever-adapting system that is predominated by Lactobacillus, which confers innate immunity by creating an acidic environment. When there is an imbalance, or dysbiosis, within the system it can lead to bacterial, viral, and fungal infection, vaginal atrophy, and vaginitis. Not only do alterations in weight, hormones, and pregnancy cause dysbiosis but external sources like douching, vaginal intercourse, and contraception impact the vaginal flora as well. With the high rate of contraception use in our population, it is vital to have a strong understanding of how these different methods may impact the vaginal microbiome.
Contraceptive counseling should be a shared decision-making process between patients and physicians considering patient history, physical exam findings, and patient desires from birth control that can then be used to arrive at the method that is best for the patient. Physicians counseling patients with recurrent infections or sensitivities may advise against spermicides, condoms, diaphragms, and cervical caps and recommend hormonal methods that may promote a more stable vaginal microbiome. Still, several areas require further understanding and research, including the impact of copper IUD and a consensus on the influence of various hormonal contraceptives on the vaginal microbiome. Better insight is needed to further understand the risks and benefits of each contraceptive option in relation to vaginal health so we can empower our patients to make informed decisions.
KB, CB, and NF wrote the manuscript. MF and AF supervised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aagaard K., Riehle K., Ma J., Segata N., Mistretta T. A., Coarfa C., et al. (2012). A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PloS One 7 (6), e36466. doi: 10.1371/journal.pone.0036466
Achilles S. L., Austin M. N., Meyn L. A., Mhlanga F., Chirenje Z. M., Hillier S. L. (2018). Impact of contraceptive initiation on vaginal microbiota. Am. J. Obstet Gynecol. 218 (6), 622.e1–622.e10. doi: 10.1016/j.ajog.2018.02.017
Ahmadnia E., Kharaghani R., Maleki A., Avazeh A., Mazloomzadeh S., Sedaghatpisheh T., et al. (2016). Prevalence and associated factors of genital and sexually transmitted infections in married women of Iran. Oman Med. J. 31 (6), 439–445. doi: 10.5001/omj.2016.88
Alakomi H. L., Skyttä E., Saarela M., Mattila-Sandholm T., Latva-Kala K., Helander I. M. (2000). Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66 (5), 2001–2005. doi: 10.1128/AEM.66.5.2001-2005.2000
Antonio M. A., Hawes S. E., Hillier S. L. (1999). The identification of vaginal lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J. Infect. Dis. 180 (6), 1950–1956. doi: 10.1086/315109
Baker F. C., Driver H. S. (2007). Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 8 (6), 613–622. doi: 10.1016/j.sleep.2006.09.011
Balle C., Konstantinus I. N., Jaumdally S. Z., Havyarimana E., Lennard K., Esra R., et al. (2020). Hormonal contraception alters vaginal microbiota and cytokines in south African adolescents in a randomized trial. Nat. Commun. 11, 5578. doi: 10.1038/s41467-020-19382-9
Bassis C. M., Allsworth J. E., Wahl H. N., Sack D. E., Young V. B., Bell J. D. (2017). Effects of intrauterine contraception on the vaginal microbiota. Contraception. 96 (3), 189–195. doi: 10.1016/j.contraception.2017.05.017
Bastianelli C., Farris M., Bianchi P., Benagiano G. (2021). The effect of different contraceptive methods on the vaginal microbiome. Expert Rev. Clin. Pharmacol. 14 (7), 821–836. doi: 10.1080/17512433.2021.1917373
Beigi R. H., Wiesenfeld H. C., Hillier S. L., Straw T., Krohn M. A. (2005). Factors associated with absence of H2O2-producing lactobacillus among women with bacterial vaginosis. J. Infect. Dis. 191 (6), 924–929. doi: 10.1086/428288
Brooks J. P., Edwards D. J., Blithe D. L., Fettweis J. M., Serrano M. G., Sheth N. U., et al. (2017). Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception. 95 (4), 405–413. doi: 10.1016/j.contraception.2016.11.006
Brotman R. M., Klebanoff M. A., Nansel T. R., Yu K. F., Andrews W. W., Zhang J., et al. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202 (12), 1907–1915. doi: 10.1086/657320
Brotman R. M., Shardell M. D., Gajer P., Fadrosh D., Chang K., Silver M. I., et al. (2014). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 21 (5), 450–458. doi: 10.1097/GME.0b013e3182a4690b
Burton J. P., Reid G. (2022). Evaluation of the bacterial vaginal flora of 20 postmenopausal women by direct (Nugent score) and molecular (polymerase chain reaction and denaturing gradient gel electrophoresis) techniques. J. Infect. Dis. 186 (12), 1770–1780. doi: 10.1086/345761
Campisciano G., Florian F., D'Eustacchio A., Stanković D., Ricci G., De Seta F., et al. (2017). Subclinical alteration of the cervical-vaginal microbiome in women with idiopathic infertility. J. Cell Physiol. 232 (7), 1681–1688. doi: 10.1002/jcp.25806
Carlsson J., Gothefors L. (1975). Transmission of lactobacillus jensenii and lactobacillus acidophilus from mother to child at time of delivery. J. Clin. Microbiol. 1 (2), 124–128. doi: 10.1128/jcm.1.2.124-128.1975
Center for Disease Control and Prevention (2018) Current contraceptive status among women aged 15-49: United states, 2015-2017. Available at: https://www.cdc.gov/nchs/products/databriefs/db327.htm#fig1.
Collado M. C., Rautava S., Aakko J., Isolauri E., Salminen S. (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 6, 23129. doi: 10.1038/srep23129
Critchley H. O. D., Babayev E., Bulun S. E., Clark S., Garcia-Grau I., Gregersen P. K., et al. (2020). Menstruation: science and society. Am. J. Obstet Gynecol. 223 (5), 624–664. doi: 10.1016/j.ajog.2020.06.004
Crucitti T., Hardy L., van de Wijgert J., Agaba S., Buyze J., Kestelyn E., et al. (2018). Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: A randomised, open-label longitudinal study in Rwandan women. PloS One 13 (7), e0201003. doi: 10.1371/journal.pone.0201003
Dahn A., Saunders S., Hammond J. A., Carter D., Kirjavainen P., Anukam K., et al. (2008). Effect of bacterial vaginosis, lactobacillus and premarin estrogen replacement therapy on vaginal gene expression changes. Microbes Infect. 10 (6), 620–627. doi: 10.1016/j.micinf.2008.02.007
Donders G., Bellen G., Janssens D., Van Bulck B., Hinoul P., Verguts J. (2017). Influence of contraceptive choice on vaginal bacterial and fungal microflora. Eur. J. Clin. Microbiol. Infect. Dis. 36 (1), 43–48. doi: 10.1007/s10096-016-2768-8
Drell T., Lillsaar T., Tummeleht L., Simm J., Aaspõllu A., Väin E., et al. (2013). Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PloS One 8 (1), e54379. doi: 10.1371/journal.pone.0054379
Eschenbach D. A., Thwin S. S., Patton D. L., Hooton T. M., Stapleton A. E., Agnew K., et al. (2000). Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 30 (6), 901–907. doi: 10.1086/313818
Farr Zuend C., Noël-Romas L., Hoger S., McCorriser S., Westmacott G., Marrazzo J., et al. (2021). Influence of dapivirine vaginal ring use on cervicovaginal immunity and functional microbiome in adolescent girls. AIDS. 35 (3), 369–380. doi: 10.1097/QAD.0000000000002751
Fosch S. E., Ficoseco C. A., Marchesi A., Cocucci S., Nader-Macias M. E. F., Perazzi B. E. (2018). Contraception: Influence on vaginal microbiota and identification of vaginal lactobacilli using MALDI-TOF MS and 16S rDNA sequencing. Open Microbiol. J. 12, 218–229. doi: 10.2174/1874285801812010218
Gupta K., Hillier S. L., Hooton T. M., Roberts P. L., Stamm W. E. (2000). Effects of contraceptive method on the vaginal microbial flora: a prospective evaluation. J. Infect. Dis. 181 (2), 595–601. doi: 10.1086/315267
Hashway S. A., Bergin I. L., Bassis C. M., Uchihashi M., Schmidt K. C., Young V. B., et al. (2014). Impact of a hormone-releasing intrauterine system on the vaginal microbiome: a prospective baboon model. J. Med. Primatol. 43 (2), 89–99. doi: 10.1111/jmp.12090
Hillier S. L., Nugent R. P., Eschenbach D. A., Krohn M. A., Gibbs R. S., Martin D. H., et al. (1995). Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. the vaginal infections and prematurity study group. N Engl. J. Med. 333 (26), 1737–1742. doi: 10.1056/NEJM199512283332604
Hong X., Qin P., Huang K., Ding X., Ma J., Xuan Y., et al. (2020). Association between polycystic ovary syndrome and the vaginal microbiome: A case-control study. Clin. Endocrinol. (Oxf). 93 (1), 52–60. doi: 10.1111/cen.14198
Jacobson J. C., Turok D. K., Dermish A. I., Nygaard I. E., Settles M. L. (2014). Vaginal microbiome changes with levonorgestrel intrauterine system placement. Contraception. 90 (2), 130–135. doi: 10.1016/j.contraception.2014.04.006
Kaiser Family Foundation (2021) Women's sexual and reproductive health services: Key findings from the 2020 KFF women's health survey. Available at: https://www.kff.org/womens-health-policy/issue-brief/womens-sexual-and-reproductive-health-services-key-findings-from-the-2020-kff-womens-health-survey/.
Kalia N., Singh J., Kaur M. (2020). Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical review. Ann. Clin. Microbiol. Antimicrob. 19 (1), 5. doi: 10.1186/s12941-020-0347-4
Kazi Y. F., Saleem S., Kazi N. (2012). Investigation of vaginal microbiota in sexually active women using hormonal contraceptives in Pakistan. BMC Urol 12 (22). doi: 10.1186/1471-2490-12-22
Kim S., Seo H., Rahim M. A., Lee S., Kim Y. S., Song H. Y. (2021). Changes in the microbiome of vaginal fluid after menopause in Korean women. J. Microbiol. Biotechnol. 31 (11), 1490–1500. doi: 10.4014/jmb.2106.06022
Korpela K., Helve O., Kolho K. L., Saisto T., Skogberg K., Dikareva E., et al. (2020). Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: A proof-of-Concept study. 2020. Cell. 183 (2), 324–334.e5. doi: 10.1016/j.cell.2020.08.047
Koumans E. H., Sternberg M., Bruce C., McQuillan G., Kendrick J., Sutton M., et al. (2007). The prevalence of bacterial vaginosis in the united states, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 34 (11), 864–869. doi: 10.1097/OLQ.0b013e318074e565
Landolt N. K., Chaithongwongwatthana S., Nilgate S., Teeratakulpisarn N., Ubolyam S., Apornpong T., et al. (2018). Use of copper intrauterine device is not associated with higher bacterial vaginosis prevalence in Thai HIV-positive women. AIDS Care 30 (11), 1351–1355. doi: 10.1080/09540121.2018.1450479
Liversedge N. H., Turner A., Horner P. J., Keay S. D., Jenkins J. M., Hull M. G. (1999). The influence of bacterial vaginosis on in-vitro fertilization and embryo implantation during assisted reproduction treatment. Hum. Reprod. 14 (9), 2411–2415. doi: 10.1093/humrep/14.9.2411
Martínez-Peña M. D., Castro-Escarpulli G., Aguilera-Arreola M. G. (2013). Lactobacillus species isolated from vaginal secretions of healthy and bacterial vaginosis-intermediate Mexican women: a prospective study. BMC Infect. Dis. 13, 189. doi: 10.1186/1471-2334-13-189
Mitchell C. M., McLemore L., Westerberg K., Astronomo R., Smythe K., Gardella C., et al. (2014). Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J. Infect. Dis. 210 (4), 651–655. doi: 10.1093/infdis/jiu176
Muzny C. A., Łaniewski P., Schwebke J. R., Herbst-Kralovetz M. M. (2020). Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 33 (1), 59–65. doi: 10.1097/QCO.0000000000000620
Ness R. B., Hillier S. L., Richter H. E., Soper D. E., Stamm C., McGregor J., et al. (2002). Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol. 100 (4), 765. doi: 10.1016/s0029-7844(02)02184-1
O'Hanlon D. E., Moench T. R., Cone R. A. (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS One 8 (11), e80074. doi: 10.1371/journal.pone.0080074
Pavlova S. I., Kilic A. O., Kilic S. S., So J.-S., Nader-Macias M. E., Simoes J. A., et al (2002). Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J. Appl. Microbiol. 92 (3), 451–459. doi: 10.1046/j.1365-2672.2002.01547.x
Peebles K., Kiweewa F. M., Palanee-Phillips T., Chappell C., Singh D., Bunge K. E., et al. (2021). Elevated risk of bacterial vaginosis among users of the copper intrauterine device: A prospective longitudinal cohort study. Clin. Infect. Dis. 73 (3), 513–520. doi: 10.1093/cid/ciaa703
Pellett Madan R., Dezzutti C. S., Rabe L., Hillier S. L., Marrazzo J., McGowan I., et al. (2015). Biomedical sciences working group and the MTN 004 protocol team. soluble immune mediators and vaginal bacteria impact innate genital mucosal antimicrobial activity in young women. Am. J. Reprod. Immunol. 74 (4), 323–332. doi: 10.1111/aji.12412
Pelzer E. S., Willner D., Buttini M., Huygens F. (2018). A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek. 111 (6), 933–943. doi: 10.1007/s10482-017-0992-6
Pendharkar S., Magopane T., Larsson P. G., de Bruyn G., Gray G. E., Hammarström L., et al. (2013). Identification and characterization of vaginal lactobacilli from south African women. BMC Infect. Dis. 13, 43. doi: 10.1186/1471-2334-13-43
Porter K. A., Turpin J., Begg L., Brown G., Chakhtoura N., Church E., et al. (2016). Understanding the intersection of young age, mucosal injury, and HIV susceptibility. AIDS Res. Hum. Retroviruses 32 (10-11), 1149–1158. doi: 10.1089/aid.2016.0206
Ravel J., Moreno I., Simón C. (2021). Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 224 (3), 251–257. doi: 10.1016/j.ajog.2020.10.019
Schreiber C. A., Meyn L. A., Creinin M. D., Barnhart K. T., Hillier S. L. (2006). Effects of long-term use of nonoxynol-9 on vaginal flora. Obstet Gynecol. 107 (1), 136–143. doi: 10.1097/01.AOG.0000189094.21099.4a
Song S. D., Acharya Kd, Zhu J. E., Deveney C. M., Walther-Antonio M. R. S., Tetel MJ and Chia N. (2020). Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. mSphere 5 (4), e00593-20. doi: 10.1128/mSphere.00593-20
Song S. J., Wang J., Martino C., Jiang L., Thompson W. K., Shenhav L., et al. (2021). Naturalization of the microbiota developmental trajectory of cesarean-born neonates after vaginal seeding. Med. (N Y). 2 (8), 951–964.e5. doi: 10.1016/j.medj.2021.05.003
Srinivasan S., Liu C., Mitchell C. M., Fiedler T. L., Thomas K. K., Agnew K. J., et al. (2010). Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PloS One 5 (4), e10197. doi: 10.1371/journal.pone.0010197
Svoboda E. (2020). Better birth control. Nature. 588 (7838), S166–S167. doi: 10.1038/d41586-020-03532-6
Tachedjian G., Aldunate M., Bradshaw C. S., Cone R. A. (2017). The role of lactic acid production by probiotic lactobacillus species in vaginal health. Res. Microbiol. 168 (9-10), 782–792. doi: 10.1016/j.resmic.2017.04.001
Van Damme L., Govinden R., Mirembe F. M., Guédou F., Solomon S., Becker M. L., et al. (2008). Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl. J. Med. 359 (5), 463–472. doi: 10.1056/NEJMoa0707957
van de Wijgert J. H., Borgdorff H., Verhelst R., Crucitti T., Francis S., Verstraelen H., et al. (2014). The vaginal microbiota: what have we learned after a decade of molecular characterization? PloS One 9 (8), e105998. doi: 10.1371/journal.pone.0105998
van de Wijgert J. H., Verwijs M. C., Turner A. N., Morrison C. S. (2013). Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 27 (13), 2141–2153. doi: 10.1097/QAD.0b013e32836290b6
Wilkinson D., Tholandi M., Ramjee G., Rutherford G. W. (2002). Nonoxynol-9 spermicide for prevention of vaginally acquired HIV and other sexually transmitted infections: systematic review and meta-analysis of randomised controlled trials including more than 5000 women. Lancet Infect. Dis. 2 (10), 613–617. doi: 10.1016/s1473-3099(02)00396-1
Keywords: contraception, lactobacilli, vaginal microbiome, dysbiosis, hormones
Citation: Bakus C, Budge KL, Feigenblum N, Figueroa M and Francis AP (2023) The impact of contraceptives on the vaginal microbiome in the non-pregnant state. Front. Microbiomes 1:1055472. doi: 10.3389/frmbi.2022.1055472
Received: 27 September 2022; Accepted: 28 December 2022;
Published: 30 January 2023.
Edited by:
Mingbang Wang, South China Hospital of Shenzhen University, ChinaReviewed by:
Xu Liu, Fudan University, ChinaCopyright © 2023 Bakus, Budge, Feigenblum, Figueroa and Francis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonia P. Francis, QW50b25pYS5raW1AaG1obi5vcmc=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.