- 1Ifremer, Unité HMMN, Laboratoire RHBL, Boulogne-sur-Mer, France
- 2Ifremer, CCEM Contamination Chimique des Écosystèmes Marins, Nantes, France
- 3Department of Environmental Management and Pollution, Faculty of Environmental Management, Nigeria Maritime University, Okerenkoko, Delta, Nigeria
- 4Laboratoire de Biologie Marine, Institut Fondamental d'Afrique Noire Cheikh Anta Diop, Université Cheikh Anta Diop, Dakar, Senegal
- 5Groupe de Recherche Biotechnologies Appliquees and Bioprocedes Environnementaux, Ecole Superieure Polytechnique, Universite Cheikh Anta Diop de Dakar, Dakar, Senegal
Introduction: The genus Pseudotolithus, commonly called croaker, is represented by the three main species found in West African waters.
Methods: In total, 934 individuals were sampled in 8 locations in Senegal, Ghana, and Nigeria. Several tests identified using sectioned otolith as the best method for determining the croakers' age. The marginal increment analysis validated the age data.
Results: The relationship between total length and total weight was significant for all species. This relationship is significantly different according to the geographical area. There was significant sexual dimorphism in P. senegalensis and P. typus, and the reproduction period presented a significant effect on the body length-weight relationship of P. senegalensis. The von Bertalanffy growth model constrained by t0 = 0 was the best fit for the observed age data from the three species in the sampled countries. The asymptotic length TL∞ was estimated from 26.6 cm for P. elongatus on the Nigerian coast to 42.2 cm for P. senegalensis on the Senegalese coast. The growth rate K ranged from 0.58 for P. senegalensis to 1.12 for P. typus.
Discussion: The growth was significantly different among the three sampled countries for P. senegalensis. Conversely, there was no significant difference of growth between specimens of P. typus from Nigeria and Ghana. Finally, there was no significant sexual dimorphism in the growth of P. senegalensis in Senegalese waters. This new information on the biology of these three croaker species is essential for the future assessment and management of these commercial species in West African waters.
1 Introduction
The Gulf of Guinea, a West African body of water, is defined as the oceanic waters from Senegal to Angola. Approximately 40% of the West African population lives in the coastal areas, and consequently, the fisheries industry is an important economic sector and plays a role in the food security of Africa (1–3). However, these fisheries are existentially threatened by climate change impacts and anthropic pressures, such as pollution and unsustainable fishing practices (4). Among the commercial species, the first fish family caught by artisanal fisheries is Sciaenidae, which includes croakers, drums, maigres, and weakfishes (5, 6). The genus Pseudotolithus, commonly called croakers, is an abundant fish group that includes several very important commercial species, especially in the Gulf of Guinea, where it accounts for ~40% of the value of commercial landings (1, 7–9). P. senegalensis, P. elongates, and P. typus are three species widely distributed along the West African coast from Senegal in the north to Angola in the south (5). Since 1994, the total catches of Pseudotolithus have been decreasing, potentially because of overfishing by industrial fishing fleets (1, 3). Growth studies were initially carried out from 1960 to 1971 (7, 10–13), but since then, only two studies have followed the growth of the mainly commercial fish in the Gulf of Guinea (3, 6). Consequently, the objective of this study is to evaluate, with good accuracy, the growth of Pseudotolithus species in West African waters according to the physiological and/or geographical factors, especially for P. senegalensis, to provide biological data, an essential preliminary step for stock assessment.
2 Materials and methods
2.1 Fish sampling
In total, 934 individuals belonging to the three studied croaker species (Pseudotolithus elongatus, senegalensis, and typus) were sampled in eight different locations in three countries of West Africa. The three croaker species (210 individuals of P. typus, 174 individuals of P. senegalensis, and 48 individuals of P. elongatus) were sampled in January 2022 in Nigeria (three locations: Ibeno Beach, Ibeno, Akwa Ibom State; Ogulagha, Warri, Delta State; and Makoko, Lagos Island, Lagos State) and Ghana [three locations: Apam Beach, Apam, and Elmina Beach Market, Cape Coast (both in the Central Region); and Albert Bosomtwi Sam Fishing Harbor, Sekondi-Takoradi; Figure 1]. Another 502 individuals of P. senegalensis were obtained between August 2015 and July 2016 in the fish markets of Joal and Mboro (Senegal).
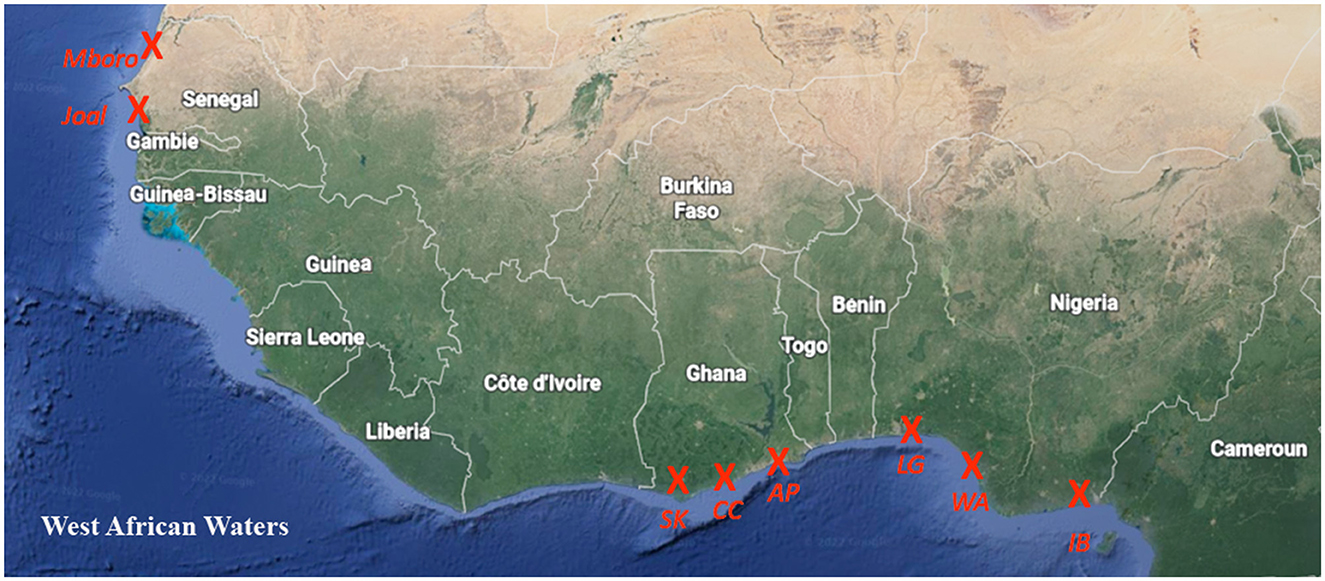
Figure 1. Sampling countries and locations of croaker species from Senegal to Nigeria in the Gulf of Guinea (Atlantic Ocean). The three croaker species were sampled in Nigeria (IB, Ibeno Beach; WA, Warri; LG, Lagos Island), in Ghana (AP, Apam Beach; CC, Cape Coast; SK, Sekondi-Takoradi), and in Senegal (Mboro, Joal).
All individuals were analyzed in the laboratory to measure, with high precision, individual parameters: total length (TL, measured to the nearest millimeter) and total weight (WT, measured to the nearest gram). The macroscopic observation of the gonads was applied to evaluate the sex and sexual maturity stage. Finally, both sagittal left and right otoliths were extracted from the cranial cavity, cleaned with water, and stored dry before being shipped to Ifremer (Boulogne sur mer, France).
2.2 Total length/total weight relationship
All individual data of biological parameters were graphically observed to identify and delete the potential outliers. For each species, to estimate the allometric relationships between total length/total weight, a base-10 logarithm was fitted to data:
where a is the intercept and b is the slope named the growth coefficient (14–16). To test the effect of the explanatory variables {sex [Se], sampling country [Co], sampling location [Lo], and reproduction period [Re, defined from November to March; (17)]} on the relationship between total length and total weight, a completed generalized linear model was applied for each species:
With the interaction between each factor and the length (sex: Log TL:S, geographical area: Log TL:Co, and Log TL:Co:Lo, reproduction period: Log TL:Re, and the physiological state by sex: Log TL:Re:Se). When a factor showed a significant effect (p < 0.05) on the TL–WT relationship, the analysis was realized for each factor modality.
To understand the difference in the TL−−WT relationship, the condition factor, Le Cren's (14) index, Kn (i.e., fish showing the high value of Kn are heavy for their length), has been used:
The difference between this observed growth coefficient for each fish species and the theoretical value (b = 3.0) was tested with a one-sample t-test.
2.3 Aging method
As there has been no previous direct growth study for these species, calibrating the tests of the aging method was necessary. Whole and sectioned otoliths were tested to analyze the growth ring patterns. The image processing was carried out using the Icy image analysis system (18). Finally, to limit interpretation bias, each image was independently analyzed by two expert readers, and after this step, the images presenting a difference in age were discussed to increase precision, which, for this study, is defined as the reproducibility of repeated measurements on the otoliths or another calcified structure (19). Alternating translucent and opaque bands were visible in whole otoliths. It was assumed that each annual growth ring consisted of one opaque ring and one translucent band. The age was defined by age group; for example, a fish in age group 0 lived between 1 and 364 days (i.e., between hatching and before 1 year), as recommended by international expert groups (19–21).
2.4 Age validation
No growth validation studies (i.e., mark–recapture of wild individuals, captive rearing of either chemically labeled fish of unknown age or known age, etc.) had not been applied to these species, marginal increment analysis (MIA) was used. This technique assesses the periodicity of increments in calcified structures (22). MIA is a quantitative approach that relies on measuring the size of the increment under formation [named the marginal increment (MI)] as the distance between the last growth ring and the edge of the calcified structures. The relative measure of the MI is given by
where RO is the otolith radius measured from its focus to the edge, Rn is the distance between the focus and the last growth ring n, and Rn−1 is the distance between the focus and the last-but-one growth ring n−1. If growth rings are formed annually, the MI will thus exhibit an intra-annual periodic pattern that can be observed by plotting the MI against the date of origin, that is, the month at which the specimen was captured. The MI was measured for each specimen using the otolith and plotted against the catch month. A sinusoidal regression of MI against the month of capture m with a period of 12 months was used to test for the annual periodicity of growth ring formation after linearization:
so that
with
Linear regression was used to statistically validate an intra-annual pattern in the growth rings. The classical assumptions of the linear models (normality, homoscedasticity, and absence of trends in the residuals) were verified before to realise the growth models.
2.5 Growth model estimation
To optimize the growth model from the sampled individuals, the length at age (TLt) was back-calculated from the Fraser–Lee procedure (23) after checking the significant linear relationship between fish and otolith lengths:
where TLt is the length at age at age t, TLc is total length at capture, TLbi is length at the biological intercept, Rt is otolith radius at age t, Ro is otolith radius at capture, and Rbi is otolith radius at the biological intercept.
The growth patterns were described according to four different growth models:
- the unconstrained von Bertalanffy model (24):
- the von Bertalanffy model with forced t0 = 0:
- the Gompertz (25) model:
- the logistic model (26):
where TL1, TLt, and TL∞ are, respectively, the length at age 1, the length at age t, and the asymptotic length and K is the rate at which the asymptote is reached, also called the growth coefficient. The best growth model was the bias-corrected form of the Akaike information criterion [AICc; i.e., gave the smallest value of AICc; (27, 28)]. The AICc balances the trade-off between the fit quality and the number of used parameters (29) while accounting for small-sample bias and is defined as
where n is the sample size, k is the total number of parameters of the model, and TL is its likelihood.
Fish growth was estimated using the growth performance index [ϕ; (30)]:
The growth performance index was preferred for growth comparison rather than comparing TL∞ and K individually. These two parameters of the growth model are correlated (31).
To test the growth variation according to the different effects of the selected species, the sampling country, and sexual dimorphism (difference between the females and males) on the back-calculated length, four mixed-effects models were fitted, with the age and the interaction of age/species, age/country, and age/sex as fixed effects. Random effects were used to account for variability due to each individual, which could present several back-calculated lengths for all ages. Finally, the significance of back-calculated length at 5% was tested by likelihood ratio tests.
Statistical analyses were performed using the following packages in the statistical environment R (40): ggplot2 (32), and car (33).
3 Results
3.1 Morphological parameters
Measured total length and total weight for all species ranged, respectively, from 16.0 to 58.8 cm and from 20.7 to 1,720.3 g (Table 1). Important differences were observed in weight for the same total length among the three croaker species, with P. senegalensis having the highest weight and P. elongatus having the lowest (Figure 2). Among the three croaker species, all showed a significant correlation (p < 0.05) between total length and total weight (Table 1). The body parameters from the length–weight relationships showed that the initial growth coefficient a varied from 0.005 to 0.162, and the growth coefficient b ranged from 2.142 ± 0.211 (for P. elongatus) to 3.132 ± 0.031 (for P. typus). For P. elongatus and P. senegalensis, the specimens had thin, elongated bodies (i.e., b < 3.0), while P. typus have thicker bodies (i.e., b > 3.0). The geographical and physiological effects on the relationship between total length and weight were also analyzed. For the geographical effect, two geographical scales (i.e., at the country level and, after, the location within the country) were used and for all species, the geographical effect was significant showing the difference among the sampling countries and between the locations within the country too (Table 1). The second effect was the physiological effect with two different levels (i.e., between the males and the females, between the reproduction and the sexual rest period, and, finally, the interaction between these two levels). The TL–WT relationship showed a significant difference between males and females for P. senegalensis and P. typus (p < 0.05), while for P. elongatus, there was no significant difference. The second level of the physiological effect was tested only for P. senegalensis, which presented the monthly sampling for 1 year. For this species, the TL–WT relationship changed significantly between the reproduction period and the sexual rest period. Finally, this modification during the year depends on the sex analyzed (Table 1).
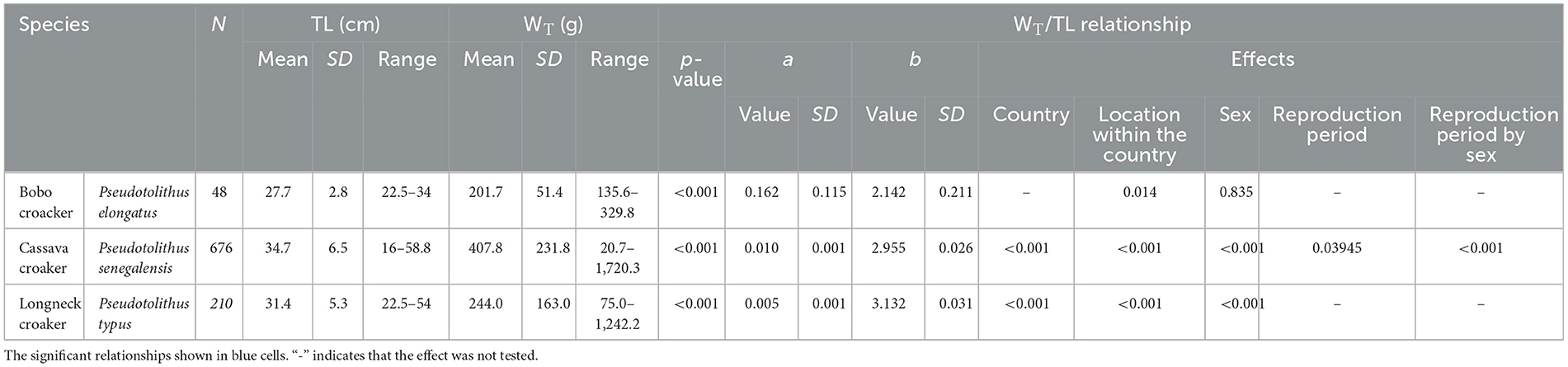
Table 1. Relationships between total length (TL; cm) and total weight (WT; g; the significance of this relationship, number of individuals, details of each measurement, parameters a and b, and the p-value of each potential effect).
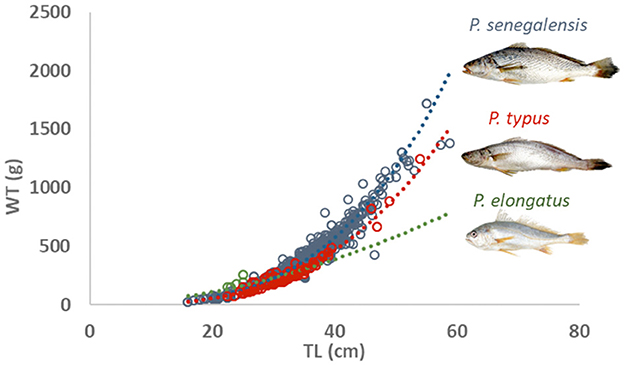
Figure 2. Total length/total weight relationship of the croaker species from the western coast of Africa.
The fitness of each fish species according to sex (females vs. males), geographic factor (Senegal, Ghana, and Nigeria and, for the sampling country, the mean value was estimated for each location), and the reproduction period (active vs. sexual rest) was followed from the condition factor (K; Supplementary Table S2). Among the three croaker species, only P. typus showed sexual dimorphism, with the mean condition factor of females being smaller than that for males. For the geographical factor, there were some differences for each species between the sampling countries and/or locations. However, no trend was observed for all species from the eastern part to the western part of the Gulf of Guinea.
3.2 Growth parameters
The whole sagittal otolith and the sectioned otoliths of the same individuals were tested and the aging data showed that the best otolith preparation method was the sectioned otolith. These preparations were analyzed under transmitted light microscopy to count the growth increments along the longest growth axis. The individuals' ages ranged between 1 and 7 years. Before realizing the first growth analysis, applying the aging validation method is very important. The MI analysis of P. senegalensis allowed a significant intra-annual variation in their growth (p < 0.001; Figure 2, upper panel) to be detected. From November to March, the growth of marginal increments is maximal, and from April to October, it is reduced, revealing an annual rhythm in growth ring formation (Figure 3). For P. elongatus and P. typus, the available data were sampled during 2 or 3 months. Consequently, the MIA was applied only to P. senegalensis.
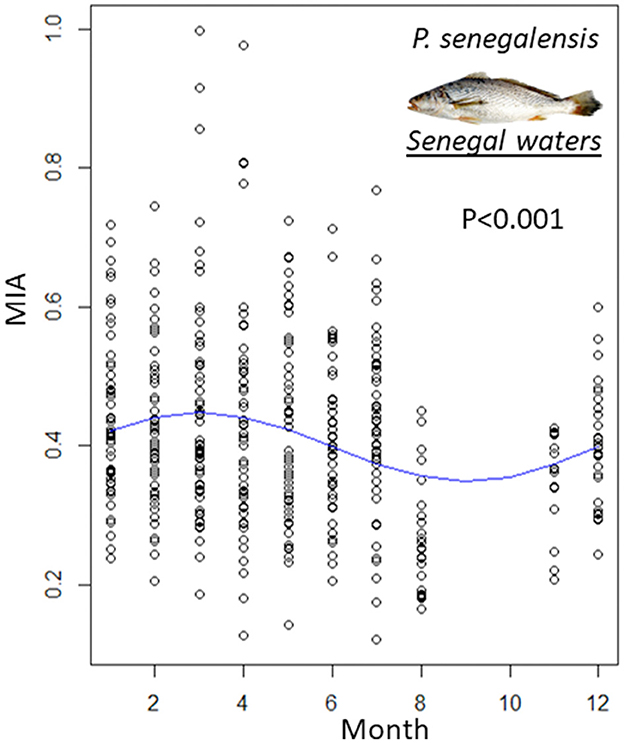
Figure 3. Marginal increment analysis (MIA) per sampling month (blue solid line; mean ± standard deviation) of P. senegalensis sampled in Senegalese waters. The continuous blue curve represents the fit of a sinusoidal regression of marginal increments per month for 12 months to test for annual periodicity.
For the three croaker species (P. elongatus, P. senegalensis, and P. typus), the age range was 1–7 years, except for P. typus, which had only one specimen aged to 4 years (Figure 4). The von Bertalanffy growth model constrained with t0 = 0 was the best fit for the observed age data from the three species in the sampled countries (Senegal, Ghana, and Nigeria; Table 2). The asymptotic length TL∞ varied from 26.6 cm for P. elongatus along the Nigeria coast to 42.2 cm for P. senegalensis along the Senegal coast (Table 2). The rate at which the asymptotic length was reached, K, ranged from 0.58, for P. senegalensis, to 1.12, for P. typus. Consequently, for these croaker species, the growth performance index (ϕ) varied from 2.73 to 3.24 (Table 2). Comparisons of the growth curves of these three species in West African waters showed that, among these species, growth was very different (Figure 4A). For one species, P. senegalensis, the results of mixed-effects models showed that the growth of one species could be significantly different among the samples from the three countries, whereas for the P. typus specimens from Nigeria and Ghana, the difference in their growth was not significant (Figure 4). Finally, the growth difference between males and females was tested for P. senegalensis from Senegalese waters, and no significant effect of sexual dimorphism on the growth of this species was found (p = 0.251; Figure 4).
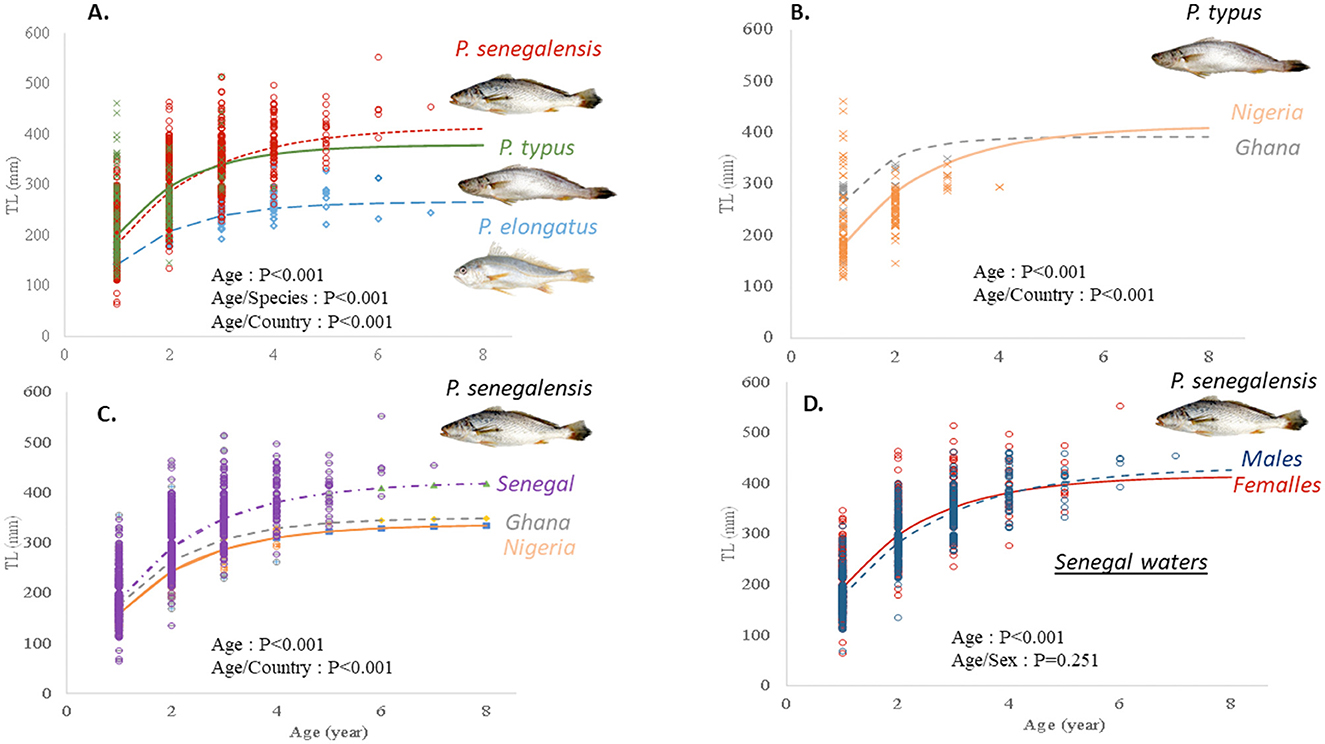
Figure 4. Growth curves of the croaker species in the Gulf of Guinea fitted to individual length at age. (A) Comparison of growth curves among three croaker species; (B) Comparison of the growth curves of P. typus between the Nigeria and Ghana samples; (C) Comparison of the growth curves of P. senegalensis from Senegal to Nigeria; (D) Comparison of growth curves of P. senegalensis in Senegalese waters between females and males. For each growth data set, the probability of each factor or the interaction between two factors tested according to the mixed-effects model is presented. MIA, marginal increment analysis.
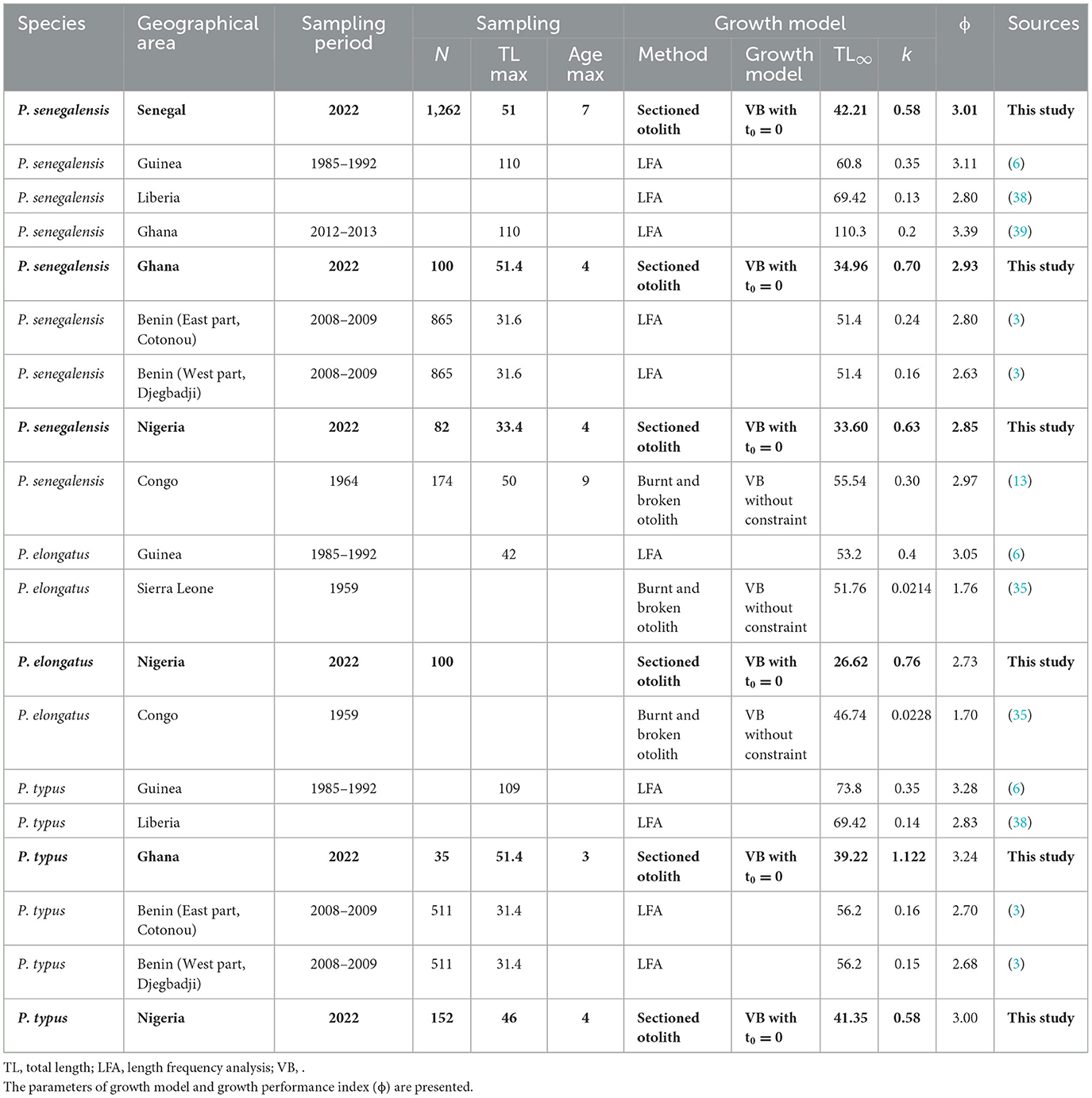
Table 2. Biogeographic comparison of the biological parameters of croaker species with the details of sampling [number of samples, observed maximum total length (TL), and age] of the growth model (model used with the type of method being length frequency analysis, LFA, for otoliths and its preparation technique).
4 Discussion
Age and growth data were analyzed for three croakers (Pseudotolithus) species in the western part of Africa around the Gulf of Guinea from Senegal to Nigeria. First, the total length and total weight relationship (LWR) were analyzed and showed significant differences among the species and within each species, with geographical and physiological (i.e., males vs. females or sexual rest vs. reproduction period) factors influencing significantly the LWR of the croaker species, a result similar to other species (34). This morphological information on the fish body can be used to evaluate the parameters, such as growth and mortality, of other fisheries. This knowledge of factors that modify the LWR is essential to precisely determine the individual composition of all catches used in the stock assessment.
Age data were measured from the sagittal otolith; after testing different preparation methods, the highest accuracy of the age data was obtained from the sectioned otoliths. The results from the transversal section of the sagittal otoliths allowed better observation of the growth rings, especially close to the edge, compared to whole otoliths or burnt and broken otoliths as used in previous studies [(13, 35); without preliminary tests to evaluate the bias due to different preparation methods of otoliths for the age data]. Previous growth studies on croaker species were realized from only the proxy of the length frequency analysis (LFA). Using whole or broken otoliths is a preparation method that underestimates the age of the old fish. Similarly, extracting the old-age classes is difficult using the LFA approach (21). Consequently, comparing this new growth study of croakers in the western part of Africa with the previous studies is difficult without introducing the bias due to the method used to calculate the growth parameters. The annual periodicity of our age growth estimation from the sectioned otoliths was validated from the MIA for P. senegalensis in the waters of Senegal. For P. elongatus and P. typus, the data were sampled during 2 or 3 months only. Consequently, these available data are well-distributed throughout the year. They could be enlarged in the future. However, the MIA for P. senegalensis validated the annual periodicity of age growth estimation. In the same ecosystem and with species that are very close phylogenetically, the age validation for a species (P. senegalensis) should be transferable to those of other species belonging to the same genus Pseudotolithus, commonly called croakers. This step is necessary to evaluate the precision of the age data, especially in cases in which prior knowledge of age is lacking. For age, MIA, which assesses the periodicity of increments in calcified structures such as otoliths, is the most widely used indirect validation method (21, 22). This indirect method could be confirmed in the future by direct validation methods such as mark–recapture of wild individuals. The second step for optimize the growth analysis used the back-calculation method of the length at age, especially in the first life stages as juveniles, and tested the statistical precision of the several growth models (36). This was not the case for previous studies (13, 35). It was an important approach for the croaker species, for which obtaining an efficient number of specimens for all length classes in all geographical areas is not easy. Finally, the growth model type used is the last step to optimize the biological analysis within one species or among several species. Among different classical growth models, the von Bertalanffy model, with t0 equalling 0, was the best growth model that fit the data for all the croakers. In the future, the sampling effort of fish must be carried out to validate the choice of the best model for each species, as these data are important to adjust the different models. The previous studies used another von Bertalanffy model [i.e., without constraint; (13, 35)]. Consequently, the growth difference among species or within species between this study and the previous works could be related to not only environmental differences (e.g., temperature fluctuations, food availability and composition, etc.) but also the difference in age acquisition methods and the statistical approach used (Table 2).
To compare growth, the growth performance index could be a good tool. The growth performance index for P. senegalensis showed a longitudinal gradient, with the highest values from the western part (i.e., Senegal) and decreasing toward the eastern part (i.e., Benin and Nigeria; Table 2). This growth pattern indicates that P. senegalensis growth rates decrease significantly from the western part to the eastern part of the gulf (Figure 4C). It was possible that environmental conditions (i.e., habitats) and/or high fishing pressures (37) explain this higher growth rate result. The observed longitudinal growth gradient for this species must be confirmed in the future using data available from several years of sampling in all locations/countries to validate this trend. However, this trend was not confirmed for the two other species (P. elongatus and P. typus). In addition, the geographical gradient of growth depends on the analyzed species. For P. elongatus, during 1959, there were few studies, and environmental factors and/or methodology bias could explain the very low values observed in Sierra Leone and Congo (35) compared to other studies. For P. typus, there was only one previous study on the Benin coast that used the LFA approach (3), but in our study, there was no clear trend between individuals from Ghana and Nigeria.
5 Conclusion
The growth of three croaker (Pseudotolithus) species in the Atlantic waters of Africa, from the Senegalese coast in the north to the Nigerian coast in the south, showed the same growth patterns, in particular, fast growth during the first 4 years, followed by a decrease in the growth rate. Comparing the growth rate among the croaker species, in the same age classes, P. elongatus showed the smaller growth rate than the other species. Finally, for P. senegalensis in the Senegal waters, no significant effect of the sexual dimorphism on the growth for the same age classes was evidenced. These biological data are essential to provide a stock assessment of these very important commercial species in the western part of Africa.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study was conducted in accordance with the local legislation and institutional requirements. All specimens were from landings commercial fishing boats with commercial species. Ethical review and approval were waived for this study because the fish were obtained from fisheries and were already dead when the calcified structures were extracted.
Author contributions
KM: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AE: Conceptualization, Data curation, Investigation, Writing – original draft. KD: Conceptualization, Data curation, Investigation, Writing – review & editing. CM: Formal analysis, Investigation, Methodology, Writing – review & editing. YA: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. NA: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AD: Writing – review & editing. MD: Formal analysis, Methodology, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was carried out as part of the MOPGA4 fellowship funded by the French Ministry of Europe and Foreign Affairs in collaboration with the French Ministry of Higher Education, Research and Innovation. This work is part of the Graduate School IFSEA that benefits from grant ANR-21-EXES-0011 operated by the French National Research Agency, under France 2030 program. This work was supported too by the Fonds d'Impulsion de la Recherche Scientifique et Technique (FIRST) of Senegalese Ministry of Higher Education (Senegal data), Research and Innovation and the Institut Français de Recherche et d'Exploitation de la Mer and the ULCO University (doctoral support to N. Andrialovanirina, 2021–2024).
Acknowledgments
We thank all the fishermen and colleagues who helped us in the field and the reviewers for their comments and suggestions. The authors would especially like to thank Kirsteen MacKenzie for valuable help in editing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frish.2024.1498784/full#supplementary-material
References
2. Garcia S, Rosenberg AAA. Food security and marine capture fisheries: characteristics, trends, drivers and future perspectives. Philos Trans R Soc. (2010) 365:2869–80. doi: 10.1098/rstb.2010.0171
3. Sossoukpe E, Nunoo FKE, Ofori-Danson PK, Fiogbe ED, Dankwa HR. Growth and mortality parameters of P. senegalensis and P. typus (Sciaenidae) in nearshore waters of Benin (West Africa) and their implications for management and conservation. Fish Res. (2013) 137:71–80. doi: 10.1016/j.fishres.2012.08.020
4. Okafor-Yarwood I. Pollution, fisheries and food. In:Ruth R, Ankita G, Emily W, , editors. Security in the Gulf of Guinea. Symposium: ‘Transnational Food Security', Transnational Legal Theory Vol. 9, 2018, Forthcoming, TLI Paper 5/2018 (2018).
5. Edwards AJ, Anthony CG, Abohweyere PO. A revision of Irvine's marine fishes of tropical West Africa. Darwin Initiative Rep. (2001) 2:157.
6. Sidibé A. Les ressources halieutiques démersales côtières de la Guinée. Exploitation, biologie et dynamique des principales espèces de la communauté à Sciaenidae (Ph.D. thesis), ENSAR, Rennes France (2003). 320 p.
7. Bayagbona EO. Age determination and the Bertalanffy growth parameters of Pseudotolithus typus and Pseudotolithus senegalensis using the “burnt otolith technique.” In: Actes Symposium Océanographie et Ressources Halieutiques Atlantique Tropical, UNESCO. Abidjan (1969). p. 349–59.
8. Sossoukpe E. Ecological Studies on Pseudotolithus spp (Sciaenidae) in Benin (West Africa) Nearshore Waters: Implications for Conservation and Management (Ph.D. thesis), University of Ghana, Legon (2011). 219 p.
9. Ekperusi AO, Bely N, Pollono C, Mah, é K, Munschy C, Aminot Y. Prevalence of per- and polyfluoroalkyl substances (PFASs) in marine seafood from the Gulf of Guinea. Chemosphere (2023) 335:139110. doi: 10.1016/j.chemosphere.2023.139110
10. Collignon J. Contribution à la connaissance des otolithus des côtes d'Afrique équatoriale. Bull. Inst. Etudes Centr. (1960) 19–20: 55–84.
11. Longhurst AR. Synopsis of biological data on West African croakers (Pseudotolithus typus, P. senegalensis and P elongatus). FAO Fish Synop. (1966) 35:50.
12. Poinsard F, Troadec JP. Détermination de l'âge par la lecture des otolithes chez deux espèces de Sciaenidés Ouest-africains (Pseudotolithus senegalensis (C.V.) et P. typus (BLKR). J Cons Per Int Exp Mer. (1966) 30:291−307. doi: 10.1093/icesjms/30.3.291
13. Troadec JP. Biologie et dynamique d'un Sciaenidae africain, Pseudotolithus typus. Scientifique Centre de Recherche Océanographique. (1971) 2:1–125.
14. Le Cren ED. The length–weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol. (1951) 20:201–19. doi: 10.2307/1540
15. Ricker WE. Computation and interpretation of the biological statistics of fish populations. Bull Fish Res Bd Can. (1975) 191:1–382.
16. Froese R. Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichth. (2006) 22:241–53. doi: 10.1111/j.1439-0426.2006.00805.x
17. Chao LN, Trewavas E. Sciaenidae. In:Quero JC, Hureau JC, Karrer C, Post A, Saldanha L, , editors. Check-list of the Fishes the Eastern Tropical Atlantic (CLOFETA). JNICT, Lisbon; SEI, vol. 2. Paris: UNESCO (1990). p. 813–26.
18. De Chaumont F, Dallongeville S, Chenouard N, Hervé N, Pop S, Provoost T, et al. Icy: an open bioimage informatics platform for extended reproducible research. Nat Methods. (2012) 9:690–6. doi: 10.1038/nmeth.2075
19. Chilton DE, Beamish RJ. Age determination methods for fishes studies by the groundfish program at the pacific biological station. Can Spec Publ Fish Aquat Sci. (1982) 60:1–102.
20. Panfili J, de Pontual H, Troadec JP, Wright PJ. Manual of fish sclerochronology. Ifremer IRD Brest. (2002) 1:464.
21. Vitale F, Worsøe Clausen L, NiChonchúir G. Handbook of fish age estimation protocols and validation methods. ICES Coop Res Rep. (2019) 346:1–180. doi: 10.17895/ices.pub.5221
22. Campana S. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol. (2001) 59:197–242. doi: 10.1111/j.1095-8649.2001.tb00127.x
23. Campana SE. How reliable are growth back-calculations based on otoliths? Can. J Fish Aquat Sci. (1990) 47:2219–27. doi: 10.1139/f90-246
24. Von Bertalanffy L. A quantitative theory of organic growth (Inquiries on growth laws II). Hum Biol. (1938) 10:181–213.
25. Gompertz B. On the nature of the function expressive of the law of human mortality and on a new mode of determining the value of life contingencies. Philos Trans R Soc Lond. (1825) 115:515–85.
26. Verhulst PF. Notice sur la loi que la population poursuit dans son accroissement. Corresp Math Phys. (1838) 10:113–21.
27. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. (1974) 19:716–23. doi: 10.1109/TAC.1974.1100705
29. Pauly D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy's Growth Formula. Kiel: University of Kiel and Institut für Meereskunde (1979).
30. Pauly D, Munro JL. Once more on the comparison of growth in fish and invertebrates. int cent living aquat resour manage. Fishbyte. (1984) 2:1–21.
31. Sparre P, Ursin E, Venema SC. Introduction to Tropical Fish Stock Assessment. Part 1: Manual. Roma, Italy: FAO Fisheries Technical Paper (1987).
33. Fox J, Weisberg S. Thousand Oaks CA: Sage (2019). Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accesses November 25, 2024).
34. Roos D, Taconet J, Gentil C, Brisset B, Evano H, Aumond Y, et al. Variation of the relationships between lengths and weights applied to 123 fish species observed at Réunion Island (Indian Ocean). Afr J Mar Sci. (2022) 44:171–80. doi: 10.2989/1814232X.2022.2075936
35. Le Guen JC. Dynamiques des populations de Pseudotolithus (Fonticulus) elongatus (Bowd. 1825) - Poissons Sciaenidae. Cahiers ORSTOM Sér Océanogr. (1971) 1:3–84.
36. Wilson JA, Vigliola L, Meekan MG. The back-calculation of size and growth from otoliths: Validation and comparison of models at an individual level. J Exp Mar Biol Ecol. (2009) 368:9–21. doi: 10.1016/j.jembe.2008.09.005
37. Marty L, Rochet MJ, Ernande B. Temporal trends in age and size at maturation of four north Sea gadid species: cod, haddock, whiting and Norway pout. Mar Ecol Prog Ser. (2014) 497:179–97. doi: 10.3354/meps10580
38. Wehye AS, Ofori-Danson PK, Lamptey AM. Population dynamics of Pseudotolithus senegalensis and Pseudotolithus typus and their implications for management and conservation within the coastal waters of Liberia. Fish Aqua J. (2017) 8:201. doi: 10.4172/2150-3508.1000201
39. Okyere I, Blay J. Assessment of the fishery, growth and mortality characteristics of the cassava croaker, Pseudotolithus senegalensis (Sciaenidae) from coastal waters of Ghana. Reg. Stud. Mar Sci. (2020) 39:101425. doi: 10.1016/j.rsma.2020.101425
40. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2022). Available at: https://www.R-project.org/ (accesses November 25, 2024).
Keywords: Senegal, Ghana, Nigeria, age, otolith, growth model, aging validation study
Citation: Mahe K, Ekperusi AO, Diouf K, Munschy C, Aminot Y, Andrialovanirina N, Dussuel A and Diop M (2024) Growth assessment of croakers (Pseudotolithus species) in West African waters. Front. Fish Sci. 2:1498784. doi: 10.3389/frish.2024.1498784
Received: 19 September 2024; Accepted: 18 November 2024;
Published: 11 December 2024.
Edited by:
Felipe Amezcua, National Autonomous University of Mexico, MexicoReviewed by:
Jorge Payán Alejo, Universidad Autonoma de Sinaloa, MexicoGorgonio Ruiz-Campos, Universidad Autónoma de Baja California, Mexico
Copyright © 2024 Mahe, Ekperusi, Diouf, Munschy, Aminot, Andrialovanirina, Dussuel and Diop. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelig Mahe, a2VsaWcubWFoZUBpZnJlbWVyLmZy
 Kelig Mahe
Kelig Mahe Abraham O. Ekperusi2,3
Abraham O. Ekperusi2,3 Catherine Munschy
Catherine Munschy Mamadou Diop
Mamadou Diop