- 1Department of Biological Sciences, Centre for Ecology, Evolution and Behaviour, School of Life Sciences and the Environment, Royal Holloway University of London, Egham, United Kingdom
- 2Consiglio Nazionale delle Ricerche, Istituto per lo studio degli Impatti Antropici e Sostenibilità in ambiente marino (CNR-IAS), Oristano, Italy
- 3Consiglio Nazionale delle Ricerche, Istituto d Biofisica (CNR-IBF), Pisa, Italy
Biological invasions of freshwater habitats are of increasing biological and economical concern, and both, salinity and parasites are considered to be key contributors to invasion success. Salinity, for example, influences the distribution of invasive mosquitofish (Gambusia holbrooki) and native killifish (Aphanius fasciatus) in Europe, with the latter now predominantly confined to high-salinity habitats. Here, we examined how salinity might affect female activity and preference for large and non-parasitized males in multiple populations of mosquitofish and killifish in Sardinia, Italy. We predicted that (1) females of both species would associate preferentially with larger and uninfected males, and that (2) female behavior in both species would be significantly influenced by salinity. We used dichotomous choice tests, in which we presented focal females with video animations of photos of the same male but differing in body size and presence/absence of an ectoparasite (Lernaea cyprinacea). We calculated female preference based on association time and quantified female inactivity as time spent in the central neutral zone during trials. Contrary to prediction 1, females did not prefer the large or uninfected male stimuli over their counterparts in any of the populations. However, while salinity did not significantly affect female preferences, it did significantly affect their activity, with mosquitofish becoming more inactive at higher salinities and killifish exhibiting the opposite pattern, matching prediction 2. These results suggest that salinity limits mosquitofish invasiveness by reducing their activity and thus provides a refuge for the Mediterranean killifish.
1 Introduction
Mounting evidence documents the negative impacts of human activities on biodiversity and ecosystem functioning (1, 2). For example, biological invasions, which are often caused by human action, are considered one of the main threats to global biodiversity, ranking third behind habitat fragmentation and habitat loss (3, 4). The introduction of invasive species can be damaging for several reasons, including that they may affect native species through predation, competition for resources and transmission of parasites (5, 6). Freshwater ecosystems are deemed particularly susceptible to the establishment of invading species (3). Additionally, the invasive success of an introduced non-native species may depend on factors such as the level of parasitism in those species, and their personality and behavioral traits, including mate choice (7, 8).
Sexual selection is widely recognized as one of the main evolutionary forces driving the development and refinement of traits that influence mating success (9, 10), and sexual selection can drive differences in sexual traits within and between native and invasive populations of the same species (11, 12). Mate choice is a multi-phase process that can occur before, during and after mating (13, 14). It typically involves the detection of signals and cues, their evaluation, decisions on who to mate with and different fitness consequences arising from mating (13, 14). For instance, choosers' preferences are partially dependent on their ability to process multiple cues and signals, including, but not limited to, visual [e.g., body size and coloration, (15, 16)] and olfactory stimuli [e.g., as often observed in insects; e.g., (17)]. Moreover, there is evidence that other cues such as parasite load can also directly affect mate choice, with choosers often preferring to associate with non-parasitized mating partners (13, 18, 19).
Mating preferences can vary among individuals, species and populations (14, 20, 21), and the strength and direction of these preferences are often shaped by environmental factors (22–24). These, including factors influenced by human activities, can affect the production and expression of traits relevant to mate choice, the transmission of information between potential mates, and the reception and processing of received information (25); for example, in fishes, pollution with synthetic hormones and hormone analogs has been shown to reduce the motivation to make informed mate choice decisions (26), turbidity has been shown to interfere with mate choice (27) and higher salinity has been demonstrated to negatively affect the desire to mate, activity levels during mate choice, and mating preferences [(24); for a negative influence of salinity on other aspects of fish activity, such as maximum swimming speed, see (28), for example]. Thus, by altering mating preferences and activity levels, environmental factors may influence the population viability and survival of a species (20, 29). Moreover, freshwater habitats around the world are getting saltier, a process termed freshwater salinization syndrome [e.g., (30, 31)]. How might salinization (and other human induced environmental change) influence mate choice and activity levels during mate choice? Since invasive species have often been found to be bolder and show more exploratory behavior compared to non-invasive species [e.g., (32, 33)], behavioral inactivity could influence pathways of species introductions, the likelihood of successful establishment in novel habitats and the level of impact on native organisms and ecosystems (34, 35)? Knowledge of these patterns is crucial to effectively predict to what extent climate change will impact species invasiveness and implement correct management protocols.
As a result of their broad distribution and capacity to tolerate a wide range of environmental conditions, eastern mosquitofish (Gambusia holbrooki) provide excellent model organisms for investigating environmental effects on female mate choice. They are native to North America but are, and have been, intentionally introduced worldwide as mosquito biocontrol agents, making them one of the most widely introduced aquatic species globally (36). Several studies have documented G. holbrooki introductions being responsible of the displacement and decline of native biota [reviewed in (37)], and competition from mosquitofish has been proposed as one of the main causes for the displacement of many Mediterranean fish species, like the Mediterranean banded killifish Aphanius fasciatus (38, 39). This cyprinodont fish is endemic to the central-eastern Mediterranean and is listed as a protected fauna species in the Annex II of the European Habitat Directive (92/43/CEE) and Annexes II and III of the Convention on the Conservation of European Wildlife and Natural Habitats. Like mosquitofish, killifish are capable of tolerating a wide range of temperatures and salinities [e.g., from freshwater to > 60‰; (39)]. Nonetheless, their distribution is now mostly confined to higher salinity waters due to the successful establishment of mosquitofish (39, 40). Therefore, it has been proposed that salinity may limit the invasive success of mosquitofish and that high salinity may act as a refuge for the native A. fasciatus (39, 40).
Both A. fasciatus and G. holbrooki display reverse sexual size dimorphism, with females being larger than males, but while males of A. fasciatus also differ from females in body and fin coloration (i.e., being generally more colorful), coloration of male and female G. holbrooki is very similar (41, 42). Mating behavior in A. fasciatus involves male courtship displays (43, 44) and the likelihood of successful mating has been proposed to be determined by both, aggressive interactions between males and female choice [e.g., through control on the latency to spawn; (45)]. Nonetheless, to our knowledge, no research has yet investigated female mate choice in this species. By contrast, mating in G. holbrooki is dominated by male coercion, where males approach females from behind and thrust their gonopodium (i.e., modified anal fin) into the females' genital pore (46–48). Although female cooperation is not necessary in this mating system, females can influence the likelihood that forced copulation attempts are successful by, for example, selecting a particular male and actively staying close to it, thus facilitating copulation and increasing the future reproductive success of the offspring (47, 49). Most studies on G. holbrooki have focused on evaluating the influence of male body size on female preference and females have often been reported to prefer larger males [e.g., (50, 51); but see (52, 53)]. However, while a recent study of Zhou et al. (24) has investigated the effects of salinity on female preference for larger males in a close relative, the western mosquitofish Gambusia affinis, no research has yet investigated the influence of parasites and salinity on female mating decisions in Gambusia holbrooki, despite these factors being documented as contributors to their invasiveness (39, 40, 54, 55). Thus, investigating how factors such as male body size and the presence of parasites influence female-mate choice in both species will substantially increase our understanding of their mating systems. Moreover, understanding how salinity influence mate-choice interactions in invasive mosquitofish and native killifish could provide more insight into mosquitofish invasiveness and establish whether or not salinity is a key determinant of successful mosquitofish invasions and their impact on killifish.
We examined how male body size and parasitism affect female mate preferences and activity patterns in G. holbrooki and the co-occurring native A. fasciatus, and how salinity influences the strength and direction of these behaviors. To do this, we sampled female G. holbrooki from four and female A. fasciatus from three distinct, allopatric populations in Sardinia, Italy. We investigated female mate preference using dichotomous choice tests, in which we presented focal females with computer animations of pictures of the same male, but differing in body size (i.e., large vs. small) and presence of parasites (i.e., infected vs. uninfected). Thus, we aimed to assess: (a) variation in female preferences and activity within and between populations of each species and between the two species and (b) the potential influence of changing salinity on female preferences and activity. Based on previous research, which we outlined above (24, 50, 51), and given that both species are known to withstand a wide range of salinities (36, 39), we predicted that: (i) during baseline experiments (i.e., trials examining initial female preferences within the context of each population's natural salinity) females of both species would associate preferentially with larger and non-parasitized males, and (ii) female activity and mate choice in both species would be significantly influenced by salinity, with mosquitofish showing decreased activity in higher salinity and killifish exhibiting the opposite pattern.
2 Materials and methods
2.1 Field sampling and housing conditions
Fieldwork was performed in September and October of 2021 in the central-western part of Sardinia, Italy. Adult specimens of G. holbrooki and A. fasciatus were collected with dip nets (~1–2 mm mesh size) from four and three sites, respectively (hereafter G1-G4 for sampling sites of mosquitofish and K1-K3 for sampling sites of killifish; Table 1; Figure 1; due to logistical constraints, an even sampling scheme was not possible). We measured the following parameters three to four times across consecutive days in situ at each site: Water temperature (°C) was measured using a Handy Polaris Probe (OxyGuard®, Denmark) and salinity (ppt) with a Handy Salinity Probe (OxyGuard®, Denmark).
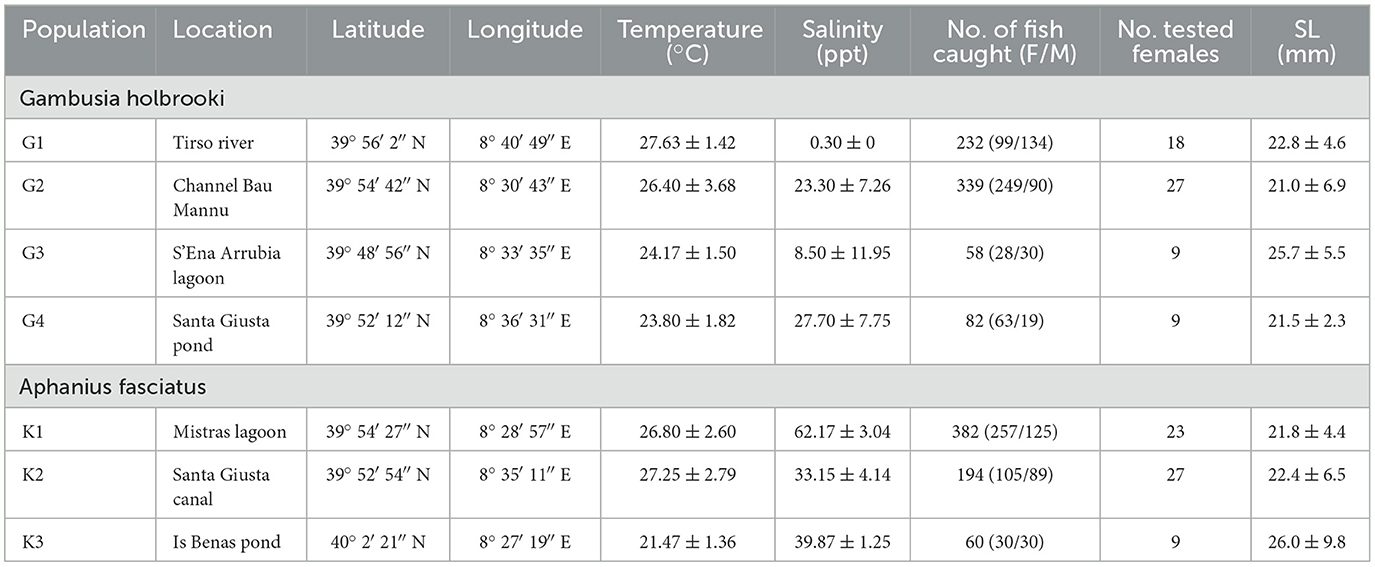
Table 1. List of Gambusia holbrooki and Aphanius fasciatus sampling sites, with site location (latitude and longitude), number of fish caught (F, females; M, males), number of tested females along with their standard length (SL), and mean values ± SD of water temperature and salinity measured in situ.

Figure 1. Map of the sampling sites of invasive mosquitofish (Gambusia holbrooki; i.e., G1–G4) and native killifish (Aphanius fasciatus; i.e., K1–K3) in Sardinia, Italy, with representative photos of each sample site at the day of sampling. The Map was generated using QGIS 3.2 (https://www.qgis.org/en/site/); photos taken by S. Pirroni and R. Riesch.
Immediately upon capture, fish were transported to the experimental facilities at CNR-IAS in Torregrande (Sardinia, Italy). In the laboratory, specimens of each species and population were sexed based on their respective sexual characteristics [modified anal fin in Gambusia, (48); bar patterning in Aphanius, (44)]. Specimens from each population and species were housed separately in mixed-sex groups (134.89 ± 57.86 total fish per tank) in large well-oxygenated housing tanks of ~148 L (44.4 × 37 × 90 cm) for a week. The tanks were provided with artificial vegetation to resemble their natural environments and ensure the animals' welfare. Furthermore, each tank was maintained at 25 ± 0.7°C and at a 9:15 h light:dark cycle photoperiod. Tanks were checked daily for the presence of ammonium, nitrate and nitrite, but values never reached thresholds of concern (i.e., ammonium was never above 0 ppm; nitrate was never >25 mg/L and nitrite was never > 0.2 mg/L). The salinity of each tank was kept as close as possible to the environmental values measured in the field. However, this was not possible for the housing tank with killifish from site K1 given that this site was characterized by extremely high salinity levels (>60 ppt). As artificial sea salt could not be obtained, these extremely high salinity levels were impossible to replicate in laboratory settings, and we, therefore, housed this populations in marine water (~36 ppt). All wild-caught specimens were daily fed ad libitum with commercial food flakes (Tetra) during the acclimation period. During this time, baseline mate-choice trials (i.e., trials performed at natural salinity levels for each population prior to salinity manipulation) were performed on nine females of each population of each species (i.e., 36 female G. holbrooki and 27 female A. fasciatus) at the same salinity level as their housing tanks. These females were subsequently removed from the setup and were not reused in any other part of the study.
2.2 Salinity acclimation protocol
After the initial acclimation period described above, we investigated salinity-dependent mate choice in two populations of each species (i.e., mosquitofish: G1 and G2; killifish: K1 and K2); logistical constraints again prevented us from doing this for all populations. Before the experiments, female and male specimens from these populations underwent a salinity acclimatization protocol, which consisted of an initial gradual adjustment of salinity in the housing tanks until the two desired experimental salinities were reached (i.e., 15 and 30 ppt), followed by 2 weeks of acclimation at those salinities in circulating systems. This protocol was not applied to all populations simultaneously but was done in two blocks. Specifically, the first block consisted of mosquitofish population G1 and killifish population K1, and the second block of mosquitofish population G2 and killifish population K2 (see details below).
2.2.1 Populations G1 and K1
Because fish from G1 and K1 were captured at sites with divergent levels of salinity (Site G1 was a river with a mean salinity of 0.3 ppt, while site K1 was a lagoon with a mean salinity of 62.2 ppt), the protocol consisted of reducing or increasing the salinity of each housing tank by 15 ppt each day until the two desired experimental salinities (i.e., 15 and 30 ppt, ±0.7 ppt) were reached. These experimental conditions and adaptation protocols were chosen given that both mosquitofish and killifish have been reported to withstand significant spatial-temporal fluctuations in salinity (41, 56), and that similar salinity changes with these and similar species, had not revealed any potential problems in previous settings (R. Riesch, personal observation). Once experimental salinities were obtained, fish were transferred into four independent indoor recirculating systems. Specifically, three replicates per species and salinity (15 and 30 ppt) were set up, each tank holding 40 L (26 × 30.7 × 50.5 cm) and 24 fish each (12 females and 12 males). Fish of each species were kept in these tanks, and under these conditions, for 2 weeks prior to the start of behavioral assays. Water temperature in the systems was maintained at 25 ± 0.7°C given the temperatures observed during sampling. Water temperature and salinity were monitored daily using a Handy Salinity Probe (OxyGuard®, Denmark). Furthermore, commercial food flakes were supplied ad libitum as a food source each day. Due to high mortality (90%) in G. holbrooki at 30 ppt within the first few minutes, female mosquitofish' mate choice preference and activity at 30 ppt could not be investigated.
2.2.2 Populations G2 and K2
Due to the high mortality of G1 mosquitofish at 30 ppt in block 1, the acclimation protocol for the fish for block 2 was slightly modified. Similar to block 1, fish from these populations were found in habitats with different levels of salinity (Site G2 was a canal with a mean salinity of 23.3 ppt, while site K2 was a lagoon with a mean salinity level of 33.15 ppt). Therefore, to avoid salinity stress (57), we adopted a protocol consisting of the gradual increasing or decreasing of salinity by 5 ppt every 2 days until the experimental conditions were reached. Fish of both species were then transferred into the 12 aquaria described above for block 1. Killifish mortality in the acclimatization tanks was ~14% whereas mosquitofish mortality was <5%.
2.3 Mate-choice experiments
2.3.1 Video animations
We investigated female preference for large and non-parasitized males in each sampled population of each species (G1-G4 and K1-K3) under different salinity settings. Specifically, we investigated female preference in nine females for each population within each testing condition. Female preference was assessed using 2D computer video animations as stimuli. The use of video animations is a validated technique to study female mate choice, and it has been previously applied in mate-choice studies on Gambusia spp. and other poecilids [e.g., (58–62)]. This technique allowed us to control and manipulate single male traits (i.e., male body size and parasitism) and test their effects on female preferences while keeping potential confounding factors (e.g., males' activity levels, boldness and length of gonopodium) constant. To generate animations, digital photographs were taken of individual males swimming in a narrow glass tank (20 × 20 × 3 cm) using a Nikon D70 digital camera. We took pictures of about three males from each population of mosquitofish. By contrast, because male killifish showed a high level of stress when transferred into the photo setup, we used only one picture of an original male taken in the lab and two pictures taken from the Web for this species. All fish photographed for video animations were not used in any of the experiments. Each resulting picture was then imported in Gimp [v2.10.24, (63)] to remove the background and only keep the image of the fish. Male size was manipulated at a later stage (see below), but to create parasitized males, the picture of an anchor worm (Lernaea cyprinacea) was pasted around the anal pore of a copy of each male's image. This freshwater parasite was chosen because it is a widely distributed common fish parasite (64, 65), it provides a clear visual cue and it was found on many collected specimens of G. holbrooki at our study sites (Supplementary Figure S1).
The resulting images were then animated using Adobe Animate 2021 (Adobe): The background of each picture was changed to white and the male size on the screen was digitally adjusted. Specifically, we created two pairs of animations: one pair showing two identical large males differing only in the presence or absence of L. cyprinacea and the other pair showing two identical males differing only in their size [i.e., one small (killifish SL: 18 ± 1.2 mm; mosquitofish SL: 14 ± 0.9 mm) and one large male (killifish SL: 27 ± 0.24 mm; mosquitofish SL: 19.6 ± 2.1 mm)]. The created animations were then converted into an mp4 file using Adobe Media Encoder 2021 (Adobe). Each animation was 130 s in duration and consisted of a male swimming vertically and horizontally, with invisible turns of 1 s. AVI video playbacks with infinite loops of these animations were created with Windows Video Editor (Microsoft).
2.3.2 Experimental procedure
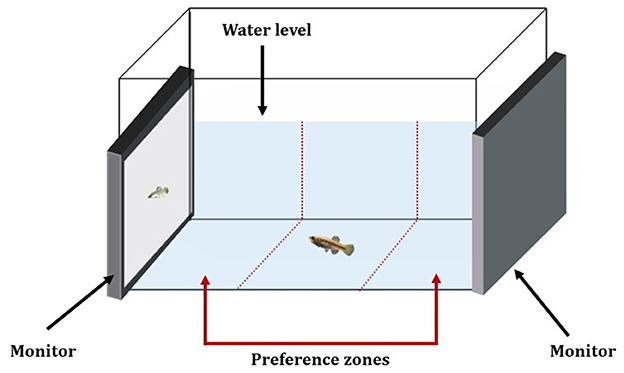
All mate-choice experiments were performed using the same experimental setup consisting of a 31L tank (50 × 30.4 × 30.2 cm filled to a depth of 20.5 cm), covered with white opaque material on all sides to reduce external disturbance. Water temperature was again maintained at 25 ± 0.7°C while salinity matched salinity of the tank of origin for the tested fish (i.e., baseline salinity, 15 or 30 ppt). During each mate-choice trial, a randomly chosen female was placed at the center of the experimental arena and two CHUWI 10.1-inch tablets were placed on either side of the tank (Figure 2). After a 5-min acclimation period, during which the fish could swim freely and explore the test tank, the screens started playing the video animations of the stimulus males (e.g., one screen playing videos of a stimulus male “infected with L. cyprinacea” and the opposite screen playing videos of the same individual, but without L. cyprinacea).
Female mating behavior was recorded using a GoPro Hero9 (60 fps), mounted above the tank in a position not visible to the animals and the time spent by the focal female in the compartment near each of the monitors (i.e., preference zone) was measured for an observation period of 5 min. To detect side biases, the video playbacks were then switched off and after 1 min, they were switched on again but now with the location of each video being reversed. The behavior of the focal female was again recorded for 5 min. This procedure was repeated twice for each fish (i.e., one time with the screens displaying parasitized and non-parasitized males and another time with screens displaying animations of larger vs. smaller males). Mate-choice preference was calculated based on the association time in seconds near each of the screens (the bottom of the experimental arena was divided into three equal-sized zones using VSDC Video Editor: one central neutral zone and two peripheral preference zones near the screens; Figure 2).
In addition to estimating female preference, female behavioral inactivity was recorded as the time the focal female spent in the central neutral zone during trials. After behavioral assays, the standard length (SL in mm), total length (TL in mm) and body mass (g) of each experimental subject were measured (Supplementary Table S1). Tested females were transferred into a new tank and were not re-used for any other procedure. To further reduce the risk of a tank effect, we used three replicates for each salinity treatment (i.e., three tanks at each level of salinity for each species) and tested a total of three females from each replicate (i.e., three females per tank) to investigate whether salinity influenced female mate choice preference. Furthermore, water in the experimental tank was replaced after every trial to avoid any potential confounding effects of chemical cues on fish behavior. After the completion of the experiments, all A. fasciatus were released back into the wild (depending on the opinion of the veterinarian) where they had been captured, while invasive G. holbrooki were sacrificed by percussive stunning in accordance with local guidelines (Art. 2, paragraph b of Legislative Decree No. 26 of 04/03/2014), and stored in 95% ethanol for potential future studies.
2.4 Statistical analyses
2.4.1 Do females exhibit a preference for large and non-parasitized males?
Following previous studies [e.g., (61, 66)] we excluded all trials from our analyses in which females exhibited a side bias; i.e., in which the focal females spent more than 85% of her total time during both 5-min trials in the same preference zone irrespective of which stimulus male was shown there. Trials in which females spent >50% of the total time in the preference zones were also discarded as females were considered not motivated to choose. For experiments with animations of large vs. small males, side biases occurred in 27 out of 122 total trials and only one trial was discarded due to low female response. For experiments with animations of parasitized vs. non-parasitized males, side biases occurred instead in 31 out of 122 trials and one trial was eliminated from the analyses due to low response. Furthermore, due to data loss from a faulty hard drive we could only analyse five out of nine females from K1 at 15 ppt.
First, we examined female preference separately for each population and testing condition (i.e., baseline, 15 and 30 ppt). This was done by comparing association times near both types of stimuli (i.e., large vs. small and parasitized vs. non-parasitized male) using paired t-tests.
Then, we investigated within- and between-species differences and the influence of salinity on the strength of preference (SOP). Each focal female's SOP for large vs. small and non-parasitized vs. parasitized male was calculated with the following equation:
SOP values ranged between from −1 (female spent all her time near small and parasitised males) to 1 (female spent all her time near large-bodied and non-parasitized males).
Analyses were conducted in two separate steps and once each for our two choice scenarios (i.e., once for large vs. small and once for parasitized vs. non-parasitized). First, because populations originated from habitats with drastically different salinities, we tested for differences in SOP between populations and species within the baseline salinity treatments. This would help us identify if the baseline salinity already had a significant influence on fish behavior. We therefore used SOP as the dependent variable in a two-way ANCOVA, for which species (A. fasciatus vs. G. holbrooki) and “population-nested-within-species” [hereafter: population(species)] were included as independent variables along with the interaction term of “SL-by-species”, and log10-transformed standard length (SL) was included as the covariate. If the interaction term had a p-value > 0.1, then it was subsequently removed from the final model. One ANCOVA model tested SOP within the context of a choice between a large and a small male, while a second ANCOVA model tested SOP within the context of a choice between a parasitized and a non-parasitized male of the same size.
The outcome of the above tests then determined how we planned to analyse mate choice quantified via SOP in the two experimental salinity treatments of 15 and 30 ppt. If we did not find any significant effects of “species” or “population(species)” in the baseline comparisons, then we constructed another ANCOVA with SOP as the dependent variable, SL as the covariate, and the independent variables were now “species”, “population(species)” and “salinity treatment” (15 vs. 30 ppt). We also initially added the interactions terms of “SL-by-species” and “salinity treatment-by-species”, but those were removed from the final model if p > 0.1. However, if we uncovered a significant effect in the initial (i.e., baseline) ANCOVA, we then implemented a mixed-effect ANCOVA on SOP with SL as the covariate, the independent variables now being “species” and “salinity treatment” (15 vs. 30 ppt), and the random effect being “baseline salinity”. We again initially added the interactions terms of “SL-by-species” and “salinity treatment-by-species”, but removed them from the final model if p > 0.1.
2.4.2 Does salinity influence female activity during mate choice?
To examine potential differences in female behavioral activity within and between species, and the potential influence of salinity, we applied the same statistical approach as outlined above for SOP (Section 2.4.1) also to inactivity time (i.e., time the focal female spent in the central neutral zone). Specifically, we again conducted these in two steps, because baseline salinities differed between species and populations. We therefore used “inactivity time” as the dependent variable in a two-way ANCOVA, for which “species” and “population(species)” were included as independent variables, and log10-transformed standard length (SL) was included as the covariate. The interaction term of “SL-by-species” was removed from the models when it was associated with a p-value > 0.1 and final models were refitted with the remaining parameters. One ANCOVA model tested inactivity within the context of a choice between a large and a small male, while a second ANCOVA model tested inactivity within the context of a choice between a parasitized and a non-parasitized male of the same size.
The outcome of the above tests then determined how we planned to analyse inactivity in the two experimental salinity treatments of 15 and 30 ppt. If we did not find any significant effects in the baseline comparisons, we constructed another ANCOVA with SOP as the dependent variable, SL as the covariate, and the independent variables were now “species”, “population(species)” and “salinity treatment” (15 vs. 30 ppt). We also initially added the interactions terms of “SL-by-species” and “salinity treatment-by-species”, but those were removed from the final model if p > 0.1. However, if we uncovered a significant effect of species in the initial ANCOVA, we then implemented a mixed-effect ANCOVA on inactivity, with SL as the covariate, the independent variables now being “species” and “salinity treatment” (15 vs. 30 ppt), and the random effect being “baseline salinity”. We again initially added the interactions terms of “SL-by-species” and “salinity treatment-by-species”, but those were removed from the final model if p > 0.1.
All t-tests were performed using the software R x60 3.5.1 (67), while all other analyses were performed using IBM SPSS Statistics VS 28.0.1.1 (IBM Corporation). Each model was applied after having checked model validation. Diagnostic plots were used to validate all models prior to consideration of estimated parameters.
3 Results
3.1 No female preference for large males and no influence of salinity
We did not find a significant difference in the amount of time females spent associating with the large vs. small male stimuli in any of the populations and treatment combinations for each species (all P ≥ 0.080; Table 2). However, when not considering testing conditions (i.e., baseline, 15 and 30 ppt treatment) separately, there was substantial individual variation among females within populations, with some females spending considerably more time near the large male stimulus while others exhibited either no preference or an opposite pattern (>65% of the total time considered as an indicator of a preference; Figure 3). For instance, of the total number of female mosquitofish from population G3, 29% exhibited a preference for the small male whereas 14% showed a preference for the opposite male stimulus, and 57% did not exhibit a preference for any of the stimuli (Figure 3).
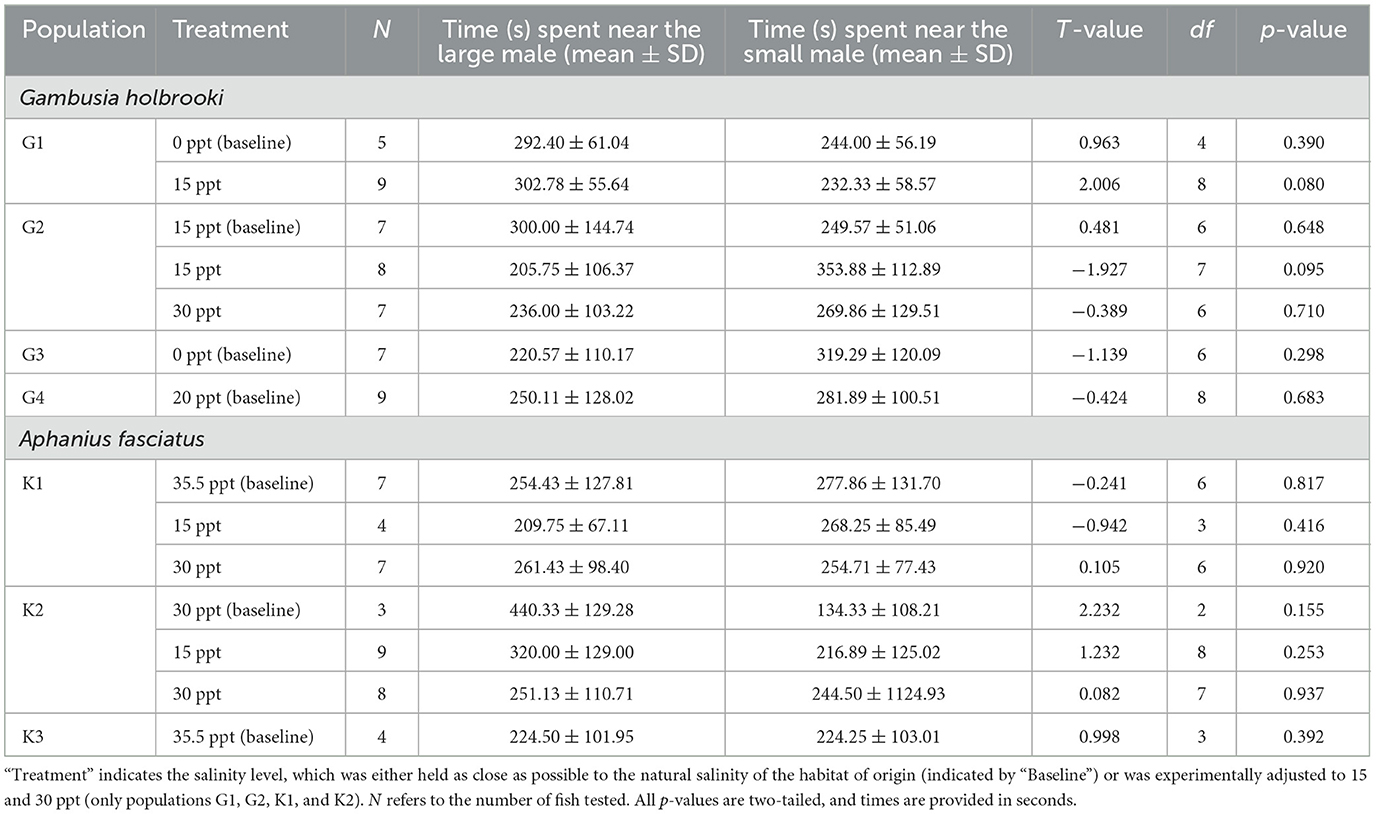
Table 2. Parameter estimates of paired t-tests investigating differences in association times near large and small males.
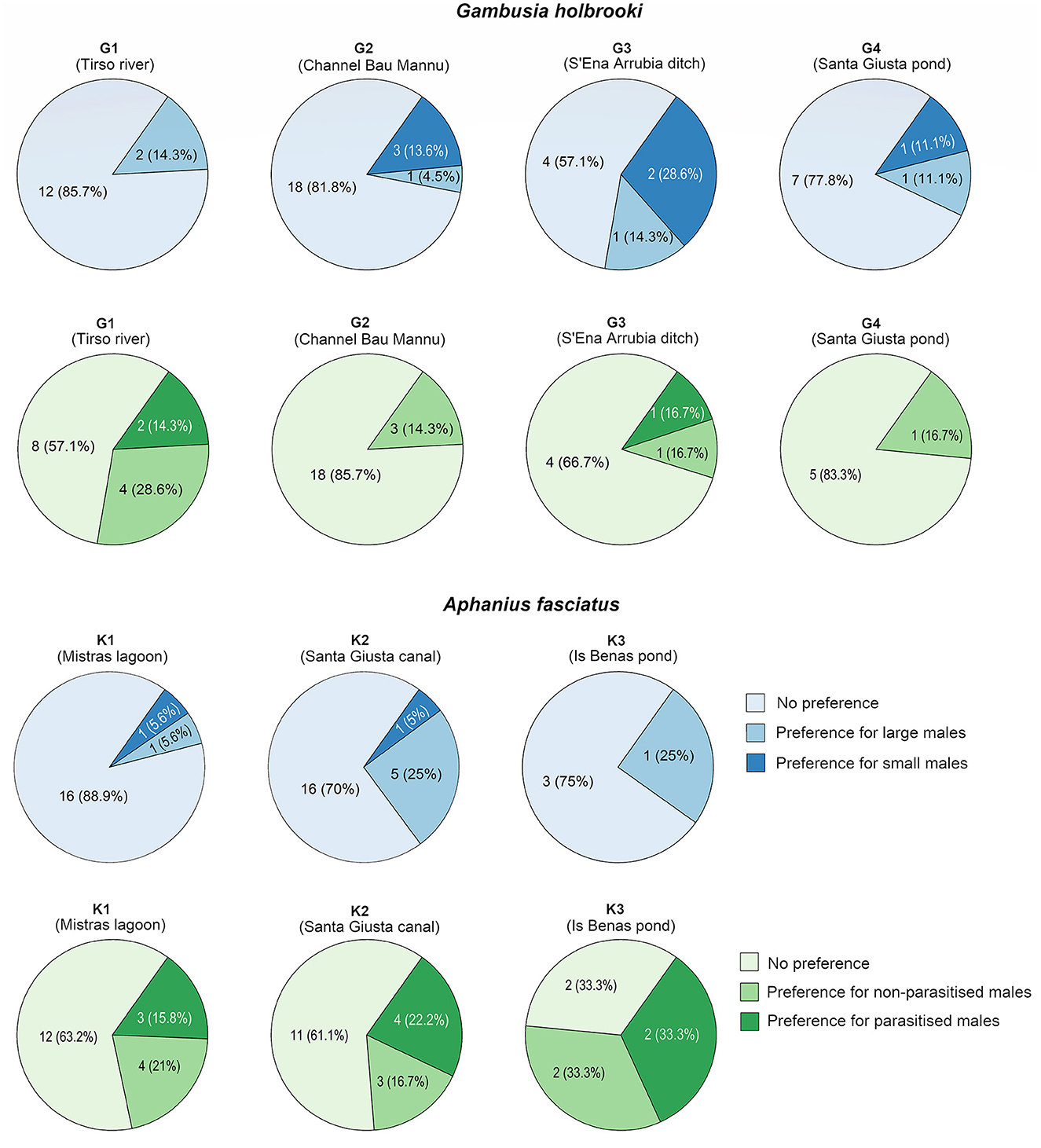
Figure 3. Individual-level variability in female choice (blue: large vs. small male; green: non-parasitized vs. parasitized male) for each mosquitofish (Gambusia holbrooki: G1–G3) and killifish (Aphanius fasciatus: K1–K3) population without considering testing conditions separately. Note that in these charts >65% of the total time spent associated with a male stimulus is considered indicative of a preference for that male. The name of the locations where these populations were sampled from is provided in brackets.
When only considering the baseline treatments, our nested ANCOVA testing for differences in SOP found no significant effects [e.g., SL: P = 0.802; population(species): P = 0.599], but indicated two non-significant trends, one for species (P = 0.056) and one for the interaction effect of “SL-by-species” (P = 0.078; Table 3A).

Table 3. Parameter estimates of nested ANCOVAs on baseline (i.e., natural salinity) treatments investigating the effects of species (Aphanius fasciatus vs. Gambusia holbrooki), “population nested within species” [population(species)], standard length (SL) and the interaction SL-by-species on female strength of preference (SOP) for (A) larger males and (B) non-parasitized males; and the effects of species, population(species), SL and the interaction SL-by-species on female inactivity during mate choice trials investigating preferences for (C) larger males and (D) non-parasitized males.
When testing females' responses to 15 and 30 ppt, the interaction terms of “SL-by-species” (P = 0.875) and “salinity treatment-by-species” (P = 0.262) were removed in a stepwise fashion from the nested ANCOVA. In the resulting final model, no term had a significant effect on SOP (species: P = 0.724; salinity treatment: P = 0.765; SL: P = 0.887; Table 4A).

Table 4. Parameter estimates of nested ANCOVAs on experimental salinity (i.e., 15 and 30 ppt) treatments investigating the effects of species (Aphanius fasciatus vs. Gambusia holbrooki), “population nested within species” [population(species)], salinity treatment, standard lengh (SL) and the interactions SL-by-species and salinity treatment-by-species on female strength of preference (SOP) for (A) larger males and (B) non-parasitized males; and the effects of species, population(species), salinity treatment, SL and the interactions SL-by-species and salinity treatment-by-species on female inactivity during mate choice trials investigating preferences for (C) larger males and (D) non-parasitized males.
3.2 No female preference for non-parasitized males and no influence of salinity
There was no effect of non-parasitized or parasitized male stimuli on female preference within any of the populations or treatment combinations for each species (Table 5). Again, there was large variance between individuals in association time within populations, with females exhibiting a preference for one of the stimuli on some occasions (Figure 3). For example, 33% of the female killifish from population K3 showed a preference for the non-parasitized male while 33% exhibited a preference for the parasitized male and 33% showed no preference (Figure 3).
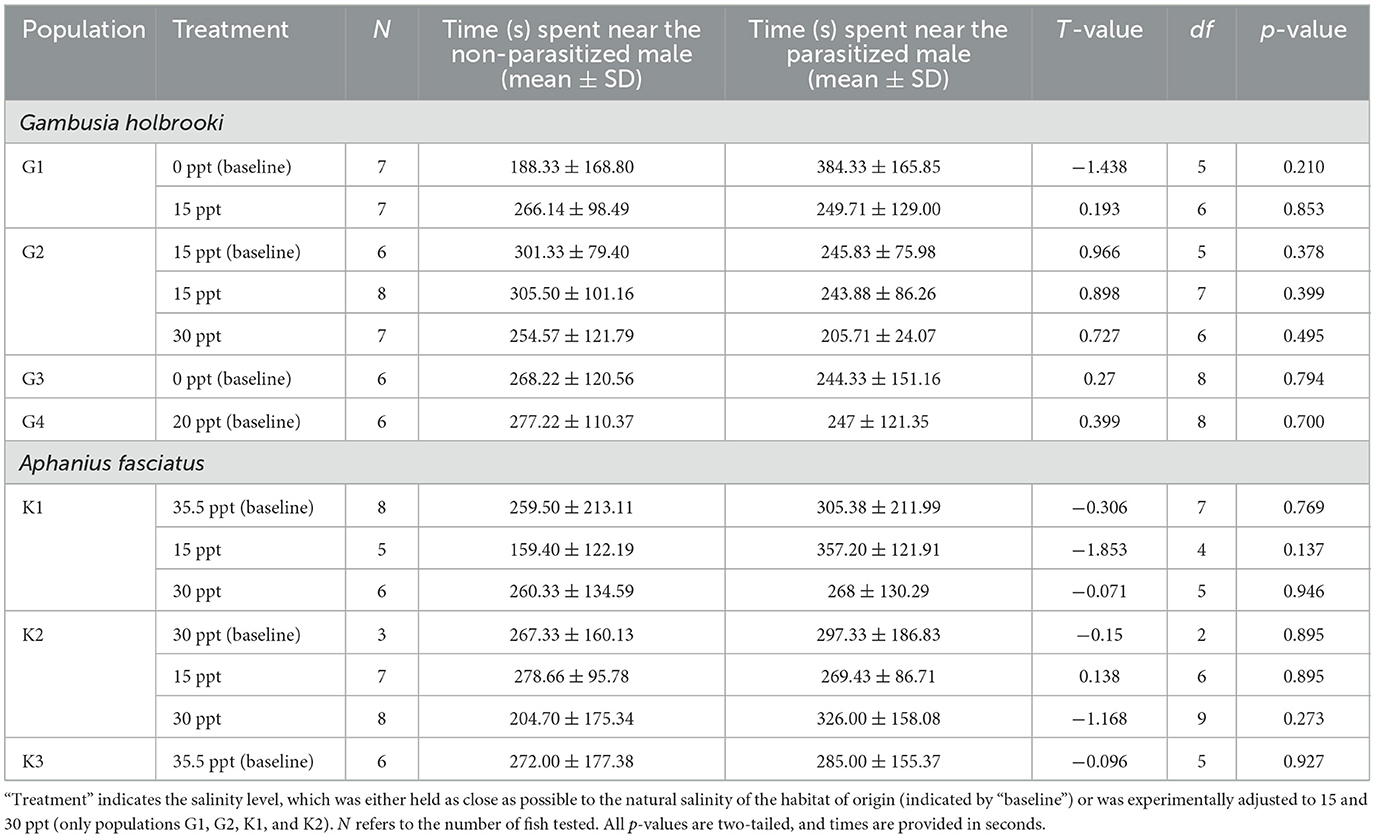
Table 5. Parameter estimates of paired t-tests investigating differences in female association times near parasitized and non-parasitized males.
When comparing the baseline treatments with each other, female SOP for non-parasitized males did not significantly differ between species (P = 0.692), between different populations of the same species [population(species): P = 0.695], and also not as a function of SL (P = 0.307; Table 3B). Furthermore, the interaction term “SL-by-species” had also not been significant (P = 0.235) and had been removed from the final model.
When testing females' SOP in 15 and 30 ppt, both interaction terms (“SL-by-species”: P = 0.887; “salinity treatment-by-species”: P = 0.762) were removed in a stepwise fashion from the nested ANCOVA. Nonetheless, no term had a significant effect on SOP in the final model (species: P = 0.078; salinity treatment: P = 0.762; SL: P = 0.145; Table 4B).
3.3 Salinity significantly affects female activity levels during some aspects of mate choice
Our nested ANCOVA on inactivity during baseline trials and when given a choice between a large and a small male did not reveal any significant effects (species: P = 0.340; population(species): P = 0.597; SL: P = 0.646; Table 3C), and the interaction term of “SL-by-species” had also been removed from the initial model due to lack of effect (P = 0.674).
We therefore applied a nested ANCOVA also to the inactivity data from females tested in 15 and 30 ppt. Again, the two interactions “SL-by-species” (P = 0.671) and “salinity treatment-by-species” (P = 0.219) were removed from the final model. In that final model, no significant effects were discovered [species: P = 0.271; population(species): P = 0.760; SL: P = 0.707; Table 4C].
Our nested ANCOVA on inactivity during baseline trials and when given a choice between a parasitized and a non-parasitized male, however, did reveal significant effects of species (P = 0.012) and “population(species)” (P = 0.019), while SL was not significant (P = 0.616; Table 3D), and the interaction term of “SL-by-species” had been removed from the final model (P = 0.835). Species differences were due to mosquitofish spending a greater amount of time inactive (estimated marginal means ± standard error: 73.89 ± 8.38 s) than killifish (37.14 ± 11.09 s). The effect highlighted by the nested term “population(species)” was driven by significant differences between some populations of mosquitofish, but not killifish. Specifically, G1 (estimated marginal means ± standard error: 29.92 ± 15.97 s) differed significantly in inactivity from G3 (113.37 ± 17.06 s; post-hoc Bonferroni-corrected pairwise comparison: P = 0.004) and G4 (94.77 ± 17.23 s; P = 0.040), while all other comparisons were not significant (P ≥ 0.108 in all cases).
However, when we attempted to therefore implement a mixed-effect ANCOVA as outlined in our methods, the model failed to reach convergence. We therefore applied another nested ANCOVA also to this inactivity data (qualitatively, both models, the mixed-effect and the nested ANCOVA, provided the same results). This model revealed significant effects on inactivity of “salinity treatment-by-species” (P = 0.004), “SL-by-species” (P = 0.002), species (P = 0.002), salinity treatment (P < 0.001), and SL (P = 0.016), while “population(species)” was not significant (P = 0.981; Table 4D). Specifically, while there were general differences between Gambusia and Aphanius, with the former spending more time inactive than the latter (estimated marginal means ± standard error: 127.32 ± 12.99 s and 74.07 ± 9.26 s, respectively), and general differences in inactivity between salinity treatments (i.e., 71.29 ± 8.96 s at 15 ppt compared to 130.10 ± 12.59 s at 30 ppt), the way of how the salinity treatment affected inactivity, differed between the species with Gambusia showing a much greater increase in inactivity compared to Aphanius (Figure 4A). Similarly, while there was an overall decrease in inactivity with SL (Figure 4B), this pattern only held true for Aphanius, while in Gambusia, larger females increased their inactivity (Figure 4C).
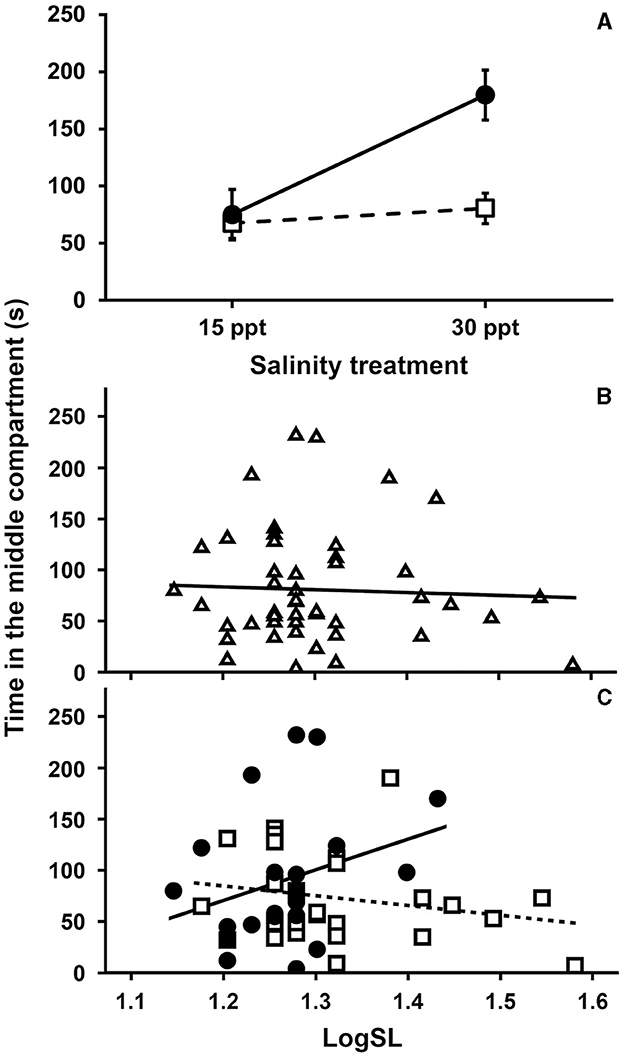
Figure 4. Visualization of significant effects from the ANCOVA on behavioral inactivity (i.e., time in the middle compartment) during mate-choice trials investigating female preference for non-parasitized males in mosquitofish (Gambusia holbrooki) and killifish (Aphanius fasciatus) populations; (A) the interaction effect of salinity treatment-by-species with filled circles and solid line representing G. holbrooki and empty squares and dotted line representing A. fasciatus; estimated marginal means +/− standard error (B) the effects of standard length (logSL); and (C) the interaction effect of “SL-by-species” with filled circles and solid line representing G. holbrooki and empty squares and dotted line representing A. fasciatus.
4 Discussion
We investigated how male body size and parasitism influence female behavioral activity and mating preferences in multiple populations of invasive eastern mosquitofish and native killifish. Furthermore, we examined how salinity influences the strength and direction of these behaviors. We did not find significant female preferences, significant effects of salinity on female preferences, or significant variation in preference between and within species. However, our analyses did reveal that salinity affected female activity during mate choice trials, with mosquitofish becoming less active at high salinities and killifish exhibiting the opposite pattern.
4.1 Female mosquitofish and killifish do not prefer to associate with larger males
Male body size is often considered an indirect signal of male dominance and a critical component of male fitness that can have direct benefits (e.g., increased fecundity and reduced predation risk) to the choosing female and/or confers indirect benefits to its offspring fitness and viability (13). Our first prediction stated that females of both species would exhibit a preference for large males. However, our analyses did not reveal a significant preference by females for larger males in either species. To our knowledge, this is the first study to investigate female mating decisions in Aphanius fasciatus. However, across mosquitofish more broadly, our findings contrast with those reported by several previous studies on mosquitofish [e.g., (51, 68)[ and other poeciliids [e.g., in Heterandria formosa: (69); in Xiphophorus nigrensis: (70)], but are congruent with a previous study of Bisazza and Marin (52) on another population of invasive G. holbrooki from Italy.
Our results, thus, suggest indifference of female mosquitofish and killifish toward male stimuli differing in body size but also that male body size might play only a small role in sexual selection for these species. In many poeciliids, large males court females while small males rely on sneaking to copulate (46). Such sexual harassment by small males can be highly costly to females [e.g., reduce their foraging efficiency; (71)], hence, resulting in females preferring to associate with large males to avoid the costs of harassment (46). In G. holbrooki, however, as males do not court females, but males of all sizes try to force copulation, the cost of associating with a large male could be equal to the cost of associating with a smaller male. This may result in females not exhibiting any preference, but rather associating with males apparently at random (i.e., potentially based on individual circumstances that might change from day to day). Furthermore, this could also explain why we found strong individual variability in mating preference within mosquitofish populations in our study. Such variation among females in their choosiness has also been documented before [e.g., (72–74)], indicating that it is important to distinguish between population- and individual-level preferences when interpreting the mating behavior of a species. With respect to A. fasciatus, a large male body size alone may not be a strong indicator of male dominance and benefits for the female but other traits such as number and span of the bars along the body flank could have a stronger influence in male mating success as suggested by Malavasi et al. (45) and observed in swordtails (75).
Furthermore, we cannot exclude that multiple-interacting factors drove the observed patterns. Personality traits also play an important role in female mating decisions and often affect male body size effects on female preferences (68, 74). For instance, Chen et al. (68) found an increasing female preference for larger males with increasing male boldness and activity levels in western mosquitofish, and other studies found female mating decisions to be influenced by social context (62, 76). Thus, we cannot exclude that our results may have been due to our specific setup (i.e., no additional conspecifics or cues on male personality).
We used computer animations to examine female mate-choice, therefore, it is possible that the lack of female preference for larger males in both species was the result of females not being able to discriminate animated male stimuli. However, we think this to be unlikely for two reasons. First, the use of animations for investigating mate-choice has been validated in multiple systems, including Poeciliidae [e.g., swordtails: (59, 77); guppies: (78); mollies: (72, 79); western mosquitofish: (24, 68)]. Second, we conducted several trial runs of this experimental setup using our laboratory stocks of G. holbrooki (an invasive population from southern Italy) at Royal Holloway, University of London, in the summer of 2021. These trial runs resulted in strong trends for preferences for (a) large males [t(8) = 1.095, p = 0.093] and (b) non-parasitized males [t(8) = 2.055, p = 0.070]. Third, while computer animations have not yet been applied to test mating decisions in A. fasciatus, the finding of high visual acuity in the congener species, A. sirhani (80), suggest that A. fasciatus should be able to discriminate between animated potential partners. Nonetheless, we acknowledge that a computer image may not allow size- or parasitization-perception as clearly as a real conspecific would be and so this is a potential limitation of the employed methods. Additionally, we used testing protocols with 2 × 5 min observation periods and video animations that are well-established for poeciliid fishes [e.g.,: (81–83); including previous studies used in our lab, such as (72, 84)], but we cannot completely rule out that this is not long enough, or that the rather uniform movement of the animated fish is not stimulating enough, to elicit a response for A. fasciatus.
4.2 Female mosquitofish and killifish do not prefer to associate with non-parasitized males
In addition to mating with large males, mating with non-parasitized males is also thought to have fitness benefits for choosing females (79). Thus, we predicted females of both species to exhibit a preference for non-parasitized males. However, contrary to our expectation, we did not find any population-level preference for non-parasitized vs. parasitized male stimuli. While we know of no other studies that have examined the influence of parasites on female mating preferences in both Aphanius and Gambusia [but see (18) for male mate choice in G. affinis], our results contrast with several previous studies in other Poeciliids (79, 85), where females preferred to mate with males that had no (or few) parasites over (more heavily) parasitized males.
Here, we digitally “infected” males with the ectoparasitic copepod (also called anchor worm), Lernaea cyprinacea. This parasite has a direct life cycle consisting of adult females releasing eggs onto the sediment, which hatch into non-parasitic nauplii that molt into parasitic copepods, attach to several parts of a fish host and undergo further metamorphosis. While attached to the host, this parasite penetrates fish skin and causes inflammation and lesions that might become necrotic or lead to secondary infections (65). Moreover, infection by this parasite often leads to a reduction in fish growth, fecundity and swimming abilities (64, 86). Fish in the wild are often able to reject these parasites even after penetration has occurred (87), so the observed lack of female responsiveness toward male stimuli differing in the parasites could be due to the fact females did not perceive this parasite to affect male reproductive state. Alternatively, we cannot rule out that females showed no preference because they did not observe any secondary infections (e.g., fungal infection) or other characteristics such as reduced swimming performance on the infected males in our video animations.
Furthermore, salinity has been documented to affect how well this parasite reproduces, with direct infection being significantly reduced at high salinities (65). This could explain why we did not find this parasite in any of the female specimens of A. fasciatus captured for this study (killifish were found in habitats often characterized by salinities >30 ppt) and a female preference for non-parasitized males in this species. However, we found anchor worms in almost all mosquitofish populations and a preference for uninfected males was not found even in the mosquitofish populations sampled in freshwater habitats (i.e., G1 and G3).
4.3 Salinity does not influence female preferences but activity levels during choice
In partial contrast with our prediction 2 (i.e., salinity effects on both female activity and mate-choice), our analyses did not reveal a significant effect of salinity on the strength and direction of female preferences in both species. However, congruent with this prediction, salinity significantly affected female activity levels, with mosquitofish being more active at lower salinities and killifish showing the opposite pattern. To our knowledge, this is the first study to investigate salinity effects on female preference and activity levels during mate choice in these species. Our results align with those of a recent study by Zhou et al. (24), who found female western mosquitofish (G. affinis) reduced their activity levels with increasing salinity. However, that study also found female G. affinis to prefer larger males only under freshwater and low-salinity conditions. Such context-dependent reduction in female activity during mate-choice has also been documented in invasive guppies, Poecilia reticulata (88). Specifically, the authors uncovered a reduction in female sexual activity and preference for male stimuli under predation threat. Together with our results, this suggests that the levels of female sexual activity in a species can be highly dependent on local habitat characteristics and environmental factors. In invasive species, changes in sexual activity may potentially be a way to adapt to novel environments. In this context, it is important to note that we only found a significant effect of salinity on inactivity in one of our two choice scenarios (i.e., when given the choice between a parasitized and a non-parasitized male) but that the underlying patterns of inactivity were the same in both settings (i.e., when choosing between a large and a small male, estimated marginal mean inactivity ± standard error for G. holbrooki at 15 ppt: 52.12 ± 12.66 s; at 30 ppt: 105.22 ± 23.54 s; A. fasciatus, at 15 ppt: 84.31 ± 14.94 s; 30 ppt: 95.83 ± 13.49 s). Nonetheless, we only measured inactivity within the context of mate choice and therefore call on future studies to investigate if this pattern holds also across other behavioral contexts.
Moreover, in our comparison of inactivity during baseline trials when females were given a choice between a parasitized and a non-parasitized male, we had found G. holbrooki to spend more time inactive than A. fasciatus, and that the G. holbrooki from G1 (habitat/baseline salinity of 0 ppt) spent less time being inactive than G. holbrooki from G3 (0 ppt) and G4 (20 ppt). Since baseline salinities between G. holbrooki and A. fasciatus were consistently greater in A. fasciatus, we cannot tease apart whether the species difference is based on taxon-specific differences or rather based on the salinity differences between the sampled habitats. However, the second effect on population differences in G. holbrooki partially supports the interpretation that salinity affects patterns of inactivity at least in G. holbrooki. However, since we also found significant differences between two habitats of the same salinity (i.e., 0 ppt), this also highlights the importance of testing multiple population replicates for certain environmental conditions, when trying to identify the impact of that environmental factor on certain organismal traits.
Observed salinity effects on female behavioral activity in our study species alone do not help explain mosquitofish invasiveness in Europe. However, taking these results together with those of another study we performed on the same populations in Sardinia (all authors, unpublished data) and a study of Alcaraz et al. (40), where mosquitofish food consumption and aggressiveness were significantly reduced at high salinities while killifish showed opposite patterns, this maps onto the current distribution patterns of invasive mosquitofish and native killifish in the Mediterranean. Hence, this suggests that salinity may limit the negative effects of invasive mosquitofish and that high-salinity habitats may act as a refuge for native killifish.
5 Conclusion
In conclusion, the results of this study suggest that male body size and parasitization with Lernea may play little role in the sexual decisions of invasive mosquitofish and native killifish females in Sardinia. In contrast, salinity appears to profoundly alter female sexual activity in both species and these effects also help explain their distribution patterns in other parts of Europe. Specifically, our findings suggest that while increasing salinization of freshwater habitats poses a serious global threat to ecosystem health and biodiversity (31), it may decrease the potential for freshwater invasive species such as G. holbrooki to spread in aquatic systems. Hence, salinization may reduce their impacts on native biota such as A. fasciatus (24).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The datasets generated and analyzed for this study can be found on Figshare (doi: 10.17637/rh.26067916).
Ethics statement
The animal study was approved by the Animal Welfare Ethical Review Board at Royal Holloway, University of London (RHUL-NRR-0038-2020). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SP: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. FL: Investigation, Writing – review & editing. JC: Investigation, Writing – review & editing. PD: Methodology, Resources, Writing – review & editing. MB: Methodology, Supervision, Writing – review & editing. SM: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing. RR: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by Royal Holloway University of London.
Acknowledgments
We thank A. Dunn and R. Thomas, who commented on the thesis version of this manuscript, and we thank the Regione Autonoma della Sardegna, Assessorato dell'Agricoltura e Riforma Agropastorale - Divisione Pesca e Acquacoltura for issuing a permit for fish collection (Autorizzazione alla pesca scientifica—Prot. No. 0018256 del 27/09/2021) and the Ministero della Salute, Direzione generale della sanità animale e dei farmaci veterinari for issuing the permit for the experiments (Autorizzazione alla sperimentazione Animale No. 138/2022-PR) in Sardinia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frish.2024.1455775/full#supplementary-material
References
1. Dornelas M, Chas JM, Gotelli NJ, Magurran AE, McGill BJ, Antão LH, et al. Looking back on biodiversity change: lessons for the orad ahead. Philos Transact R Soc B. (2023) 378:20220199. doi: 10.1098/rstb.2022.0199
2. Roswell M, Harrison T, Genung MA. Biodiversity-ecosystem function relationships change in sign and magnitude across the Hill diversity spectrum. Philos Transact R Soc B. (2023) 378:20220186. doi: 10.1098/rstb.2022.0186
3. Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, et al. Global biodiversity scenarios for the year 2100. Science. (2000) 287:1770–4. doi: 10.1126/science.287.5459.1770
4. United Nations Environment Programme (2007). The Global Environment Outlook 4 (GEO-4). Malta: Malta by Progress Press Ltd.
5. Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, et al. The population biology of invasive species. Annu Rev Ecol Syst. (2001) 32:305–32. doi: 10.1146/annurev.ecolsys.32.081501.114037
6. Gozlan RE, Britton JR, Cowx I, Copp GH. Current knowledge on non-native freshwater fish introductions. J Fish Biol. (2010) 76:751–86. doi: 10.1111/j.1095-8649.2010.02566.x
7. Chapple DG, Simmonds SM, Wong BBM. Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol. (2012) 27:57–64. doi: 10.1016/j.tree.2011.09.010
8. Chalkowski K, Lepczyk CA, Zohdy S. Parasite ecology of invasive species: conceptual framework and new hypotheses. Trends Parasitol. (2018) 34:655–63. doi: 10.1016/j.pt.2018.05.008
9. Fox RJ, Fromhage L, Jennions MD. Sexual selection, phenotypic plasticity and female reproductive output. Philos Transact R Soc B. (2019) 374:20180184. doi: 10.1098/rstb.2018.0184
10. Pilakouta N, Ålund M. Editorial: sexual selection and environmental change: what do we know and what comes next? Curr Zool. (2021) 67:293–8. doi: 10.1093/cz/zoab021
11. Ouyang X, Gao J, Xie M, Liu B, Zhou L, Chen B, et al. Natural and sexual selection drive multivariate phenotypic divergence along climatic gradients in an invasive fish. Sci Rep. (2018) 8:11164. doi: 10.1038/s41598-018-29254-4
12. Owen MA, Lahti DC. Rapid evolution by sexual selection in a wild, invasive mammal. Evolution. (2020) 74:740–8. doi: 10.1111/evo.13934
13. Schlupp I. Male Mate Choice, Female Competition, and Female Ornaments as Components of Sexual Selection. Oxford: Oxford University Press (2021).
14. Rosenthal GG, Ryan MJ. Sexual selection and the ascent of women: mate choice research since Darwin. Science. (2022) 375:eabi6308. doi: 10.1126/science.abi6308
15. Houde AE, Endler JA. Correlated evolution of female mating preferences and male color patterns in the guppy Poecilia reticulata. Science. (1990) 248:1405–8. doi: 10.1126/science.248.4961.1405
16. Cooper WE, Vitt LJ. Female mate choice of large male broad-headed skinks. Anim Behav. (1993) 45:683–93. doi: 10.1006/anbe.1993.1083
17. Riesch R, Muschick M, Lindtke D, Villoutreix R, Comeault AA, Farkas TE, et al. Transitions between phases of genomic differentiation during stick-insect speciation. Nat Ecol Evol. (2017) 1:0082. doi: 10.1038/s41559-017-0082
18. Deaton R. Effects of a parasitic nematode on male mate choice in a livebearing fish with a coercive mating system (western mosquitofish, Gambusia affinis). Behav Processes. (2009) 80:1–6. doi: 10.1016/j.beproc.2008.07.010
19. Beltran-Bech S, Richard F-J. Impact of infection on mate choice. Anim Behav. (2014) 90:159–70. doi: 10.1016/j.anbehav.2014.01.026
20. Riesch R, Schlupp I, Tobler M, Plath M. Reduction of the association preference for conspecifics in cave-dwelling Atlantic mollies, Poecilia mexicana. Behav Ecol Sociobiol. (2006) 60:794–802. doi: 10.1007/s00265-006-0223-z
21. Reuland C, Culbert BM, Devigili A, Kahrl AF, Fitzpatrick JL. Contrasting female mate preferences for red coloration in a fish. Curr Zool. (2020) 66:425–33. doi: 10.1093/cz/zoz052
22. Rosenthal GG. Mate Choice: The Evolution of Sexual Decision Making From Microbes to Humans. Princeton, NJ: Princeton University Press (2017).
23. Candolin U, Wong BBM. Mate choice in a polluted world: consequences for individuals, populations and communities. Philos Transact R Soc B. (2019) 374:20180055. doi: 10.1098/rstb.2018.0055
24. Zhou L, Liu K, Zhao Y, Cui L, Dong C, Wang Z, et al. Increasing salinization of freshwater limits invasiveness of a live-bearing fish: insights from behavioral and life-history traits. Environ Pollut. (2002) 308:119658. doi: 10.1016/j.envpol.2022.119658
25. Candolin U. Mate choice in a changing world. Biol Rev. (2019) 94:1246–60. doi: 10.1111/brv.12501
26. Wong BBM, Candolin U. Behavioral responses to changing environments. Behav Ecol. (2015) 26:665–73. doi: 10.1093/beheco/aru183
27. Sundin J, Berglund A, Rosenqvist G. Turbidity hampers mate choice in pipefish. Ethology. (2010) 116:713–21. doi: 10.1111/j.1439-0310.2010.01787.x
28. Swanson C. Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos). J Exp Biol. (1998) 201:3355–66. doi: 10.1242/jeb.201.24.3355
29. Seehausen O, van Alphen JJM. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav Ecol Sociobiol. (1998) 42:1–8. doi: 10.1007/s002650050405
30. Kaushal SS, Likens GE, Pace ML, Utz RM, Haq S, Gorman J, et al. Freshwater salinization syndrome on a continental scale. Proc Natl Acad Sci USA. (2018) 115:E574–83. doi: 10.1073/pnas.1711234115
31. Cunillera-Montcusí D, Beklioglu M, Cañedo-Argüelles M, Jeppesen E, Ptacnik R, Amorim CA, et al. Freshwater salinisation: a research agenda for a saltier world. Trends Ecol Evol. (2022) 37:440–53. doi: 10.1016/j.tree.2021.12.005
32. Rehage JS, Sih A. Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions. (2004) 6:379–91. doi: 10.1023/B:BINV.0000034618.93140.a5
33. Nordberg E, Denny R, Schwarzkopf L. Testing measures of boldness and exploratory activity in native versus invasive species: geckos as a model system. Anim Behav. (2021) 177:215–22. doi: 10.1016/j.anbehav.2021.05.013
34. Rahel FJ, Olden JD. Assessing the effects of climate change on aquatic invasive species. Conserv Biol. (2008) 22:521–33. doi: 10.1111/j.1523-1739.2008.00950.x
35. Hulme PE. Climate change and biological invasions: evidence, expectations, and response options. Biol Rev. (2017) 92:1297–313. doi: 10.1111/brv.12282
36. Carmona-Catot G, Magellan K, García-Berthou E. Temperature-specific competition between invasive mosquitofish and an endangered cyprinodontid fish. PLoS ONE. (2013) 8:e54734. doi: 10.1371/journal.pone.0054734
37. Pyke GH. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Ann Rev Ecol Evol Syst. (2008) 39:171–91. doi: 10.1146/annurev.ecolsys.39.110707.173451
38. Rincón PA, Correas AM, Morcillo F, Risueño P, Lobón-Cerviá J. Interaction between the introduced eastern mosquitofish and two autochthonous Spanish toothcarps. J Fish Biol. (2002) 61:1560–85. doi: 10.1111/j.1095-8649.2002.tb02498.x
39. Monti F, Marcelli M, Fastelli P, Fattorini N. Pushed to the edge: environmental factors drive ecological responses of Aphanius fasciatus when in sympatry with invasive Gambusia holbrooki. Aquat Conserv. (2021) 31:2547–59. doi: 10.1002/aqc.3600
40. Alcaraz C, Bisazza A, García-Berthou E. Salinity mediates the competitive interactions between invasive mosquitofish and an endangered fish. Oecologia. (2008) 155:205–13. doi: 10.1007/s00442-007-0899-4
41. Pyke GH. A review of the biology of Gambusia affinis and G. holbrooki. Rev Fish Biol Fish. (2005) 15:339–65. doi: 10.1007/s11160-006-6394-x
42. Lionetto MG, Zonno V, Schiavone R, Giordano ME, Barca A, Belmonte G, et al. The Mediterranean killifish Aphanius fasciatus (Valenciennes, 1821) (Teleostei: Cyprinodontidae) as a sentinel species for protection of the quality of transitional water environments: literature, insights, and perspectives. Water. (2023) 15:2721. doi: 10.3390/w15152721
43. Grech M, Schembri PJ. Observations on courtship and mating behaviour in Maltese populations of the killifish Apnonius fasciatus (Pisces: Cyprinodontidae). Cent Mediterranean Nat. (1993) 2:28–34.
44. Cavraro F, Zucchetta M, Torricelli P, Malavasi S. Sexual dimorphism of vertical bar patterning in the South European toothcarp Aphanius fasciatus. J Fish Biol. (2013) 82:1758–64. doi: 10.1111/jfb.12093
45. Malavasi S, Georgalas V, Cavraro F, Torricelli P. Relationships between relative size of sexual traits and male mating success in the Mediterranean killifish Aphanius fasciatus (Nardo, 1827). Mar Freshw Behav Physiol. (2010) 43:157–67. doi: 10.1080/10236244.2010.480837
46. Pilastro A, Benetton S, Bisazza A. Female aggregation and male competition reduce costs of sexual harassment in the mosquitofish Gambusia holbrooki. Anim Behav. (2003) 65:1161–7. doi: 10.1006/anbe.2003.2118
47. Wilson RS, Condon CH, Johnston IA. Consequences of thermal acclimation for the mating behaviour and swimming performance of female mosquito fish. Philos Transact R Soc B. (2007) 362:2131–9. doi: 10.1098/rstb.2007.2106
48. Macdonald J, Tonkin Z. A Review of the Impact of Eastern Gambusia on Native Fishes of the Murray-Darling Basin. Murray–Darling Basin Authority Publication, Canberra, Australia. 38/09 (2008).
49. Bisazza A, Vaccari AG, Pilastro A. Indirect female mate choice in a mating system dominated by male sexual coercion. Behav Ecol. (2000) 12:59–64. doi: 10.1093/oxfordjournals.beheco.a000379
50. McPeek MA. Mechanisms of sexual selection operating on body size in the mosquitofish (Gambusia holbrooki). Behav Ecol. (1992) 3:1–12. doi: 10.1093/beheco/3.1.1
51. Kahn AT, Mautz B, Jennions MD. Females prefer to associate with males with longer intromittent organs in mosquitofish. Biol Lett. (2010) 6:55–8. doi: 10.1098/rsbl.2009.0637
52. Bisazza A, Marin G. Male size and female mate choice in the eastern mosquitofish (Gambusia holbrooki: Poeciliidae). Copeia. (1991) 1991:730–5. doi: 10.2307/1446400
53. Bisazza A, Marin G. Sexual selection and sexual size dimorphism in the eastern mosquitofish Gambusia holbrooki (Pisces Poeciliidae). Ethol Ecol Evol. (1995) 7:169–83. doi: 10.1080/08927014.1995.9522963
54. Benejam L, Alcaraz C, Sasal P, Simon-Levert G, García-Berthou E. Life history and parasites of the invasive mosquitofish (Gambusia holbrooki) along a latitudinal gradient. Biol Invas. (2009) 11:2265–77. doi: 10.1007/s10530-008-9413-0
55. Ruiz-Navarro A, Moreno-Valcárcel R, Torralva M, Oliva-Paterna FJ. Life-history traits of the invasive fish Gambusia -holbrooki in saline streams (SE Iberian Peninsula): does salinity limit its invasive success? Aquat Biol. (2011) 13:149–61. doi: 10.3354/ab00360
56. Bertoli M, Giulianini PG, Chiti J, De Luca M, Pastorino P, Prearo M, et al. Distribution and biology of Aphanius fasciatus (Actinopterygii, Cyprinodontidae) in the Isonzo River Mouth (Friuli Venezia Giulia, northeast Italy). Turk J Fish Aquat Sci. (2019) 20:279–90. doi: 10.4194/1303-2712-v20_4_04
57. Kültz D. Physiological mechanisms used by fish to cope with salinity stress. J Exp Biol. (2015) 218:1907–14. doi: 10.1242/jeb.118695
58. Langerhans RB, Layman CA, DeWitt TJ. Male genital size reflects a tradeoff between attracting mates and avoiding predators in two live-bearing fish species. Proc Natl Acad Sci USA. (2005) 102:7618–23. doi: 10.1073/pnas.0500935102
59. Wong BBM, Rosenthal GG. Female disdain for swords in a swordtail fish. Am Nat. (2006) 167:136–40. doi: 10.1086/498278
60. Polverino G, Liao JC, Porfiri M. Mosquitofish (Gambusia affinis) preference and behavioral response to animated images of conspecifics altered in their color, aspect ratio, and swimming depth. PLoS ONE. (2013) 8:e54315. doi: 10.1371/journal.pone.0054315
61. Gierszewski S, Müller K, Smielik I, Hütwohl J-M, Kuhnert, K-D, Witte K. The virtual lover: variable and easily guided 3D fish animations as an innovative tool in mate-choice experiments with sailfin mollies-II. Validation. Curr Zool. (2017) 63:65–74. doi: 10.1093/cz/zow108
62. Sommer-Trembo C, Plath M, Gismann J, Helfrich C, Bierbach D. Context-dependent female mate choice maintains variation in male sexual activity. R Soc Open Sci. (2017) 4:170303. doi: 10.1098/rsos.170303
63. Mattis P, Kimball S. GIMP (version 2.10.24). GIMP Development Team (2021). Available at: https://www.gimp.org/ (accessed September, 2021).
64. Hassan M, Beatty S, Morgan D, Doupé R, Lymbery A. An introduced parasite, Lernaea cyprinacea L., found on native freshwater fishes in the south west of Western Australia. J R Soc West Aust. (2008) 91:149–53.
65. Hossain MMM, Ferdoushi J, Rupom AH. Biology of anchor worms (Lernaea cyprinacea). J Entomol Zool Stud. (2018) 6:910–7. doi: 10.22271/j.ento.2018.v6.i1m.3047
66. Hoysak DJ, Godin J-GJ. Repeatability of male mate choice in the mosquitofish, Gambusia holbrooki. Ethology. (2007) 113:1007–18. doi: 10.1111/j.1439-0310.2007.01413.x
67. R Core Team Development. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2019).
68. Chen B, Liu K, Zhou L, Gomes-Silva G, Sommer-Trembo C, Plath M, et al. Personality differentially affects individual mate choice decisions in female and male Western mosquitofish (Gambusia affinis). PLoS ONE. (2018) 13:e0197197. doi: 10.1371/journal.pone.0197197
69. Aspbury AS, Basolo AL. Repeatable female preferences, mating order and mating success in the poeciliid fish, Heterandria formosa. Behav Ecol Sociobiol. (2002) 51:238–44. doi: 10.1007/s00265-001-0443-1
70. Cummings M, Mollaghan D. Repeatability and consistency of female preference behaviours in a northern swordtail, Xiphophorus nigrensis. Anim Behav. (2006) 72:217–24. doi: 10.1016/j.anbehav.2006.01.009
71. Plath M, Makowicz AM, Schlupp I, Tobler M. Sexual harassment in live-bearing fishes (Poeciliidae): comparing courting and noncourting species. Behav Ecol. (2007) 18:680–8. doi: 10.1093/beheco/arm030
72. McCoy E, Syska N, Plath M, Schlupp I, Riesch R. Mustached males in a tropical poeciliid fish: emerging female preference selects for a novel male trait. Behav Ecol Sociobiol. (2011) 65:1437–45. doi: 10.1007/s00265-011-1154-x
73. Reding L, Cummings ME. Rational mate choice decisions vary with female age and multidimensional male signals in swordtails. Ethology. (2018) 124:641–9. doi: 10.1111/eth.12769
74. Sommer-Trembo C, Schreier M, Plath M. Different preference functions act in unison: mate choice and risk-taking behaviour in the Atlantic molly (Poecilia mexicana). J Ethol. (2020) 38:215–22. doi: 10.1007/s10164-020-00643-5
75. Morris MR, Elias JA, Moretz JA. Defining vertical bars in relation to female preference in the swordtail fish Xiphophorus cortezi (Cyprinodontiformes, Poeciliidae). Ethology. (2001) 107:827–37. doi: 10.1046/j.1439-0310.2001.00711.x
76. Schlupp I, Marler C, Ryan MJ. Benefit of male Sailfin of mating with heterospecific females. Science. (1994) 263:373–4. doi: 10.1126/science.8278809
77. Rosenthal GG, Evans CS. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc Natl Acad Sci USA. (1998) 95:4431–6. doi: 10.1073/pnas.95.8.4431
78. Herdegen-Radwan M. Can female guppies learn to like male colours? A test of the role of associative learning in originating sexual preferences. Proc R Soc B. (2022) 289:20220212. doi: 10.1098/rspb.2022.0212
79. Tobler M, Plath M, Burmeister H, Schlupp I. Black spots and female association preferences in a sexual/asexual mating complex (Poecilia, Poeciliidae, Teleostei). Behav Ecol Sociobiol. (2006) 60:159–65. doi: 10.1007/s00265-005-0152-2
80. Al-Adhami MA, Qar J, Alkhdour M. Ultrastructure of the outer retina in the killifish, Aphanius sirhani (Cyprinodontidae, Teleostei). Anal Biol. (2010) 32:39–46.
81. Sato A, Karino K. Use of digitally modified videos to examine female mate preference for orange spot coloration of males in the guppy, Poecilia reticulata. Ichthyol Res. (2006) 53:398–405. doi: 10.1007/s10228-006-0364-0
82. Fisher HS, Mascuch SJ, Rosenthal GG. Multivariate male traits misalign with multivariate female preferences in the swordtail fish, Xiphophorus birchmanni. Anim Behav. (2009) 78:265–9. doi: 10.1016/j.anbehav.2009.02.029
83. Bierbach D, Sassmannshausen V, Streit B, Arias-Rodriguez L, Plath M. Females prefer males with superior fighting abilities but avoid sexually harassing winners when eavesdropping on male fights. Behav Ecol Sociobiol. (2013) 67:675–83. doi: 10.1007/s00265-013-1487-8
84. Schlupp I, Riesch R, Tobler M, Plath M, Parzefall J, Schartl MA, et al. novel, sexually selected trait in poeciliid fishes: female preference for mustache-like, rostral filaments in male Poecilia sphenops. Behav Ecol Sociobiol. (2010) 64:1849–55. doi: 10.1007/s00265-010-0996-y
85. Kennedy CEJ, Endler JA, Poynton SL, McMinn H. Parasite load predicts mate choice in guppies. Behav Ecol Sociobiol. (1987) 21:291–5. doi: 10.1007/BF00299966
86. Welicky RL, De Swardt J, Gerber R, Netherlands EC, Smit NJ. Drought-associated absence of alien invasive anchorworm, Lernaea cyprinacea (Copepoda: Lernaeidae), is related to changes in fish health. Int J Parasitol. (2017) 6:430–8. doi: 10.1016/j.ijppaw.2017.01.004
87. Shields RJ, Goode RP. Host rejection of Lernaea cyprinacea L. (Copepoda). Crustaceana. (1978) 35:301–7. doi: 10.1163/156854078X00457
Keywords: video animations, female mate choice, sexual selection, parasites, Gambusia holbrooki, Aphanius fasciatus
Citation: Pirroni S, Leggieri F, Cuccuru J, Domenici P, Brown MJF, Marras S and Riesch R (2024) Salinity limits mosquitofish invasiveness by altering female activity during mate choice. Front. Fish Sci. 2:1455775. doi: 10.3389/frish.2024.1455775
Received: 27 June 2024; Accepted: 06 September 2024;
Published: 25 September 2024.
Edited by:
Sébastien Alfonso, Université de Nice Sophia Antipolis, FranceReviewed by:
Tatiana Colchen, Université d'Angers, FranceMing-Yih Leu, National Dong Hwa University, Taiwan
Copyright © 2024 Pirroni, Leggieri, Cuccuru, Domenici, Brown, Marras and Riesch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rüdiger Riesch, UnVkaWdlci5SaWVzY2hAcmh1bC5hYy51aw==
†These authors share senior authorship
 Sara Pirroni
Sara Pirroni Francesca Leggieri
Francesca Leggieri Jessica Cuccuru2
Jessica Cuccuru2 Paolo Domenici
Paolo Domenici Stefano Marras
Stefano Marras Rüdiger Riesch
Rüdiger Riesch