- 1Rollins School of Public Health, Emory University, Atlanta, GA, United States
- 2Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, United States
- 3Georgia Center for Oncology Research and Education, Atlanta, CO, United States
- 4Georgia Cancer Specialists, Atlanta, GA, United States
- 5Department of Obstetrics and Gynecology, Augusta University, Augusta, GA, United States
- 6Piedmont HealthCare, Columbus, GA, United States
- 7Cancer Health Equity Institute, Morehouse School of Medicine, Atlanta, GA, United States
- 8Emory University, Atlanta, GA, United States
The Georgia Center for Oncology Research and Education (Georgia CORE) and the Georgia Society of Clinical Oncology (GASCO) held a one-day summit exploring opportunities and evidence-based interventions to address disparities in cancer clinical trials. The purpose of the summit was to identify clear and concise recommendations aimed at decreasing clinical trial accrual disparities in Georgia for rural and minority populations. The summit included expert presentations, panel discussions with leaders from provider organizations throughout Georgia, and breakout sessions to allow participants to critically discuss the information presented. Over 120 participants attended the summit. Recognizing the need for evidence-based interventions to improve clinical trial accrual among rural Georgians and persons of color, summit participants identified four key areas of focus that included: improving clinical trial design, providing navigation for all, enhancing public education and awareness of cancer clinical trials, and identifying potential policy and other opportunities. A comprehensive list of takeaways and action plans was developed in the four key areas of focus with the expectation that implementation of the strategies that emerged from the summit will enhance cancer clinical trial accrual for all Georgians.
Introduction
In 2022, the Georgia Center for Oncology Research and Education (Georgia CORE) and the Georgia Society of Clinical Oncology (GASCO) held a one-day summit exploring opportunities and evidence-based interventions to address disparities in cancer clinical trials. The summit included expert presentations, panel discussions with leaders from provider organizations throughout Georgia, and breakout sessions to allow participants to critically discuss the information presented.
Participants at the summit sought to build upon recent work by offering clear and concise recommendations aimed at decreasing clinical trial accrual disparities in Georgia. Under-enrollment of minority populations in cancer trials has been an ongoing challenge in cancer research, and it is ultimately detrimental to all people who would benefit from more well-studied cancer treatments (1, 2). Reduced minority participation raises questions about “the generalizability of results for clinical decision making and contributes to persistent racial disparities in cancer outcomes.” (1) Additionally, clinical trials provide access to advanced treatments, and increasing minority enrollment helps address health disparities caused by structural problems (3).
Since patients typically do not choose to enroll at different rates due to skin color, gender or other patient demographics, most disparities in clinical trial accrual are structural in nature, and prospective patients are too often not given opportunities to access cutting edge medicine due to trial availability or geography (3–5). Accrual disparities may also arise as unexpected consequences of trial design (3, 4, 6). Finally, bias and social determinants of health play a role in accrual disparities, and intentional efforts may be required to address existing inequalities to facilitate greater access for patients locked out of trial participation due to circumstances beyond their control (3, 4, 7, 8). By increasing trial availability in rural communities, loosening unnecessary eligibility requirements, and resourcing trial infrastructure, clinical trial access could expand to more than 75% of cancer patients, as opposed to the 5% now participating (3).
Recognizing the need for evidence-based interventions to improve clinical trial accrual among rural Georgians and persons of color, summit participants identified four key areas to focus on:
(a) Improving clinical trial design.
(b) Providing navigation for all.
(c) Enhancing public education and awareness of cancer clinical trials.
(d) Identifying potential policy and other opportunities.
Key stakeholders are encouraged to further develop and enact recommendations in these four areas with the expectation that the set of combined strategies emerging from the summit will enhance cancer clinical trial accrual for Georgians. (Table 1).
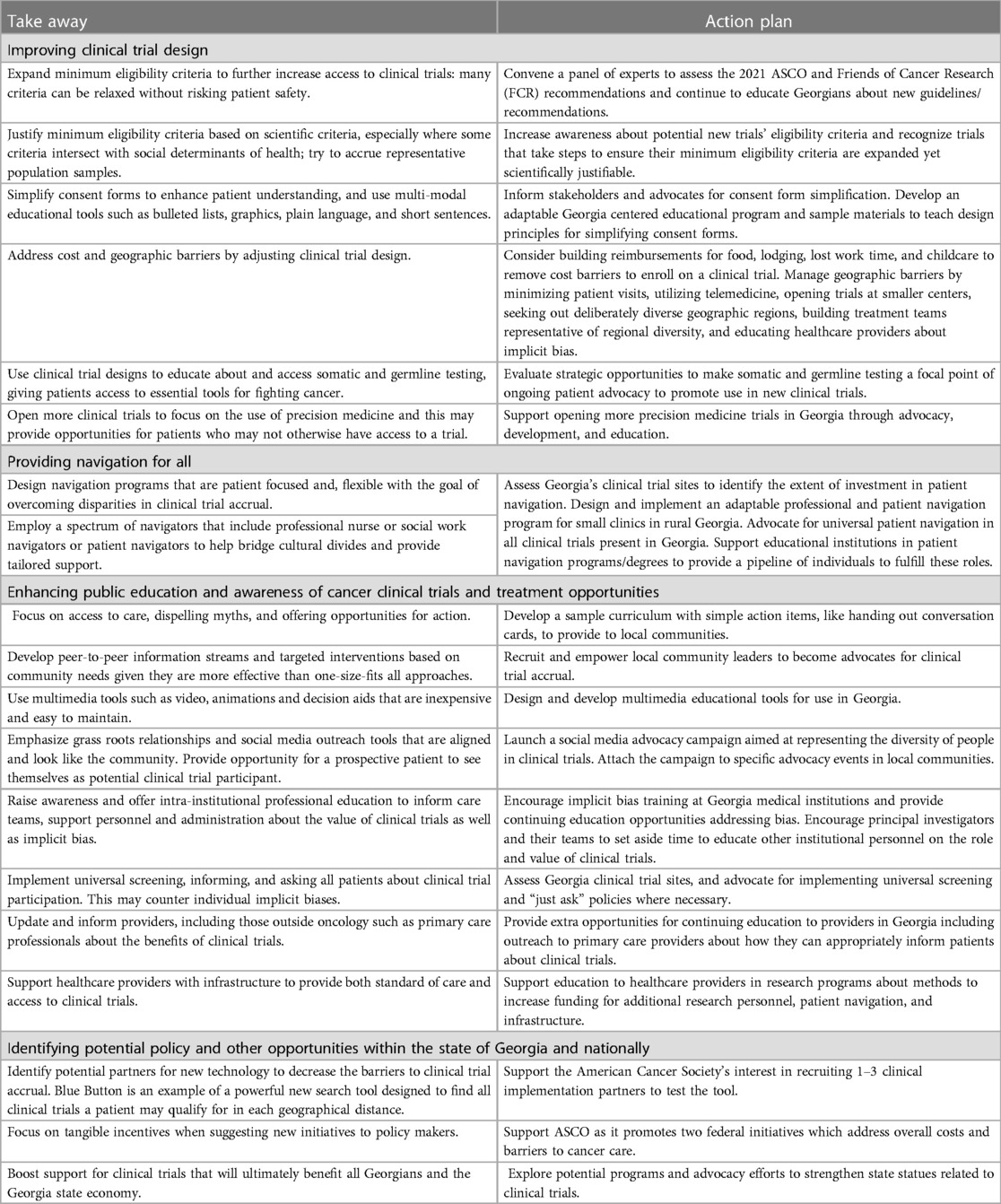
Table 1. Comprehensive Key Takeaways and Action Plans from Georgia's 2022 Summit on Disparities in Clinical Trial Accrual.
Cancer care in Georgia
Georgia's modern cancer care landscape began to take shape in 2001 with a new initiative aimed at using tobacco settlement funds to move the state from the 4th quartile nationally to the 1st (9, 10). The initiative coordinated public and private investors with academic and community healthcare institutions: regional cancer coalitions formed addressing frontline opportunities such as screening and prevention. The 2001 initiative also launched the state's first Comprehensive Cancer Control Plan which would later be revised to create a “living” document allowing for continuous adjustment as contextual elements change (10).
Progress has been made; however, Georgia still has work to do to become a national leader in both cancer treatment and research (11). An estimated 63,170 Georgians will be diagnosed with cancer in 2024, and 18,740 Georgians will die from cancer this year (12). Georgia has an elevated incidence of cancer, with an age adjusted incidence rate of 463.8 per 100,000 per year, compared with the national average, 442.3 per 100,000 per year (13). Data on clinical trial accrual disparities at the state level can be hard to come by, but trial accrual disparities are well documented at the national level (3, 14, 15).
There is little evidence to suggest that Georgia differs markedly from the national pattern, and if it does, evidence of other treatment disparities imply that trial accrual disparities would exceed national averages. In Georgia, age adjusted incidence rates for black patients are 462.3 per 100,000 per year with white patients at 485.1 per 100,000 per year; however, a look at age adjusted mortality rates reveals an inverse relationship with Black patients dying at a rate of 166.6 per 100,000 per year as opposed to 155.1 for white patients (13). Notably, disparities in overall mortality rates have narrowed over the past 20 years, but they have not disappeared, nor do they reflect the relative incidence of cancer for their respective populations. Black men have a higher lifetime probability of developing and dying from prostate cancer, averaging 40.5 age adjusted deaths in Georgia per 100,000 per year as opposed to white men whose rates are 16.6 deaths per 100,000 per year in Georgia (13).
Furthermore, rural populations in Georgia bear the brunt of the state's cancer burden. 71.1% of Georgia's population, living in 149 of 159 counties, are medically underserved according to state defined criteria, and nearly 54% (85/159) of Georgia's counties are classified as rural based on the 2013 Rural-Urban Continuum Codes (16, 17). Georgia's cancer mortality hotspots are concentrated in the eastern Piedmont to Coastal Plain, southwestern rural Georgia, and northern rural Georgia (16). Hotspot counties generally have a higher proportion of non-Hispanic black adults, older adults, greater poverty, limited access to healthy food, and more rurality (16). For all cancers, age adjusted mortality rates were higher in hotspot counties (16). Differences in outcomes ascribed to rurality are likely related to healthcare access, and when clinical trial data was used to evaluate patient outcomes, rural and urban patients faired similarly, indicating that access to uniform treatment strategies can resolve geographically related disparities in cancer outcomes (6).
Potential solutions to overcome existing accrual disparities
Improving clinical trial design
Several promising action items emerged from summit discussions to improve trial design: expanding minimum eligibility criteria, expanding access to precision medicine, and using trial design to proactively address systemic barriers to enrollment.
There is a growing consensus that minimum eligibility criteria are frequently too narrow. A recent series of reports by the American Society of Clinical Oncology (ASCO) and the Friends of Cancer Research (FCR) reviewed common minimum eligibility criteria, finding many to be unnecessarily restrictive at the cost of significantly reducing eligible populations, accrual rates, and excluding historically marginalized populations (18–24). Many typical criteria particularly exclude black patients from clinical trial participation and are often not medically justifiable (25, 26). With excluded patients typically receiving standard of care treatments, several studies have found that patients’ lack of tolerability of standard of care cannot be used to justify excluding them from clinical trials. Patients found to be ineligible, most often because of advanced age and heart disease, go on to tolerate, and even improve, with standard of care treatment, and they are more likely to die from the disease than complications resulting from treatment (27, 28).
Precision medicine provides new opportunities to capture more representative cross sections of patients in the accrual process. Genomic and transcriptomic profiling have proven useful for improving therapy recommendations and patient outcomes, but access to precision medicine, particularly genetic counseling and germline and somatic testing, may be limited by age, ethnicity, and insurance status (29–31). One option for expanding access to genetic counseling and testing is streamlining processes, such as training non-geneticist clinicians to be able to initiate genetic testing, and aiming to implement universal testing while working with existing resources and keeping costs down (29, 32). Expanding access to somatic and germline testing through trial design could be especially useful for addressing barriers related to trial availability. One value of genomic biomarkers is that they may be used in the selection of active immunotherapy or gene directed therapy for patients whose tumor type would not otherwise be individually studied (33). Patients typically ineligible for trials due to availability, if they have a rare or otherwise understudied tumor type for example, may become eligible as trials open that seek cross sections of the population based on genomic biomarkers rather than tumor type.
Finally, trials can be designed to explicitly address common systemic barriers to participation. Major barriers include location and cost. Costs not traditionally covered by insurance, including food, lodging, lost work time, and childcare, may impose significant hurdles to trial participation to families with limited incomes; however, reimbursement opportunities combined with navigation can improve clinical trial accrual (34, 35). Geography also has a major impact on patient costs as it is correlated with travel time, cost, and cultural difference. Overcoming geographic barriers may include efforts to minimize the number of patient visits, open trials at smaller centers, use more telemedicine, seek out geographic diversity, and diversify teams to match the demographics of a given region (36, 37).
Providing navigation for all
Clinical trial navigation rates extremely high as the most important tool for trial accrual and retention. Several studies have found both professional and lay navigators to be an indispensable resource for educating prospective patients, boosting patient satisfaction, and accruing and maintaining patient participation (3, 38–43). Cancer is one of the most disorienting experiences a person can experience, and navigation helps orient patients toward effective treatments. Professional navigators are particularly helpful in assisting patients with overcoming barriers to participation by employing specialized knowledge to identify open trial opportunities, arranging communication, referrals, service arrangements, and proactive education (42). Lay, also referred to as nonclinical, navigators help to bridge gaps in education, cultural experience, and even language (38–41, 44). Expanded navigation has even proven useful in addressing cancer screening disparities in the state of Delaware, and there is every reason to believe it is an indispensable tool for addressing clinical trial accrual disparities (45, 46).
Enhancing public education and awareness of cancer clinical trials
One theme that emerges when examining solutions to ending disparities in trial accrual is that patients’ prior education affects their ability to advocate for themselves and make optimal decisions. Likewise, providers make decisions about which patients to try to accrue for clinical trials, and bias, conscious or unconscious, can affect decision making. As such, a robust general education program on clinical trials and implicit bias training for providers may be valuable tools for addressing disparities in clinical trial accrual. Additionally, messaging and education should focus on motivating providers to advocate for clinical trials. By simply educating every patient and their families, screening them, and asking them to participate in open trials, biases may be circumvented.
Identifying potential policy and other opportunities
Policy at all levels may be used to address areas where education gaps, as well as explicit and implicit bias, may affect clinical trial accrual. Advocates for reducing clinical trial disparities should understand that race is a factor that principal investigators, healthcare providers, and staff may not explicitly consider when accruing patients (47). Choosing not to consider race may fit a practice of moving away from historic patterns of systematized discrimination based on race in the United States, but it comes at the risk of failing to recognize the legacy of barriers implemented with that same systematized discrimination. St. Jude Children's Hospital is a particularly helpful example of one opportunity to combat bias through policy since pediatric oncology, a historically clinical trial centric field, offers almost all patients opportunities to participate in clinical trials. St. Jude's staff, despite a lack of exposure to implicit bias training and a measured preference for high socioeconomic status white individuals, did not differ in recommending patients for trial participation (7). They defaulted to the cultural norms of the field by simply asking everyone. Knowing that acceptance rates do not differ all that much, just asking every single patient could impact accrual disparities (3).
Barriers to implementing proposed solutions in Georgia and elsewhere
As with many complex problems, improving clinical trial accrual requires addressing multiple factors at different levels of existing healthcare infrastructure. Some solutions are beyond the reach of Georgia, and they will require the collaboration of federal agencies. For example, ordinary Georgians cannot control whether the Centers for Medicare and Medicaid Services (CMS) will pay for patient navigation services, services that may help those seeking a clinical trial as a part of their cancer treatment. CMS is to be applauded for recent efforts to add patient navigation to billable services in the physician fee schedule (48). Further such efforts are needed, but progress is being made.
Many factors are within the control of Georgia's residents, and collaborative efforts can begin to address barriers that exist at the state and local level. Until 2023, the law in Georgia lacked clarity about whether reimbursements for clinical trial patients’ expenses could be considered undue inducements for participation in trials (49, 50). At the time of enactment, Georgia was only the seventh state to codify in law that covering trial participation expenses is legal (49). Legal ambiguity can have a negative effect on innovation designed to address the barriers many people must overcome to participate in clinical trials. Likewise, recent guidance by the FDA acknowledges the human factor in improving clinical trial accrual. They note that many components of eligibility criteria are used as templates across trials without strong clinical or scientific justification (51). Templates are useful because they may save unnecessary labor or guide work. Unfortunately, disrupting the status quo can be challenging and it is often easier to maintain certain processes, ignoring critical reflection on the evidence to support changes, such as eligibility criteria.
Stakeholders seeking to improve clinical trial accrual must keep the human factor in mind. It is not enough to just teach people about the need for equitable accrual. Managers and public health authorities must be able to provide the resources for transition to a better system. People respond well to tools and systems that are user friendly. That means investments that will pay off are updates to existing infrastructure and tools, such as default templates that everyone uses, that smooth the experience of patients and researchers by enabling efficient usage of resources. New incentives may be helpful such as expanded funding tied to equitable accrual requirements. Administrators must also evaluate procedures and processes at the local level. Each organization will need self-examination and proactive planning if this effort is to succeed. Otherwise, organizational inertia will slow or prevent improvements in clinical trial accrual.
Conclusion
Reducing disparities in clinical trial accrual is a relatively young field of study, and the evidence-based literature for interventions is limited. Georgians are committed to expanding the opportunities to intervene to provide increased access to critical and lifesaving cancer care. One major lesson that emerged from Georgia's summit is that everyone can play a part ranging from advocacy to policy making, and to direct trial design. The potential for significant progress, particularly through changes to trial design alone, is cause for hope. The progress that has been made is worth celebrating as the risk of dying from cancer continues to drop at an accelerated pace and public funding mechanisms are aligned to help those who struggle the most with the cost of cancer (52, 53). Georgia is ready to continue the fight.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. BC: Conceptualization, Project administration, Validation, Writing – review & editing. SB: Conceptualization, Project administration, Writing – review & editing. LD: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. LG: Conceptualization, Writing – review & editing. SG: Conceptualization, Writing – review & editing. AP: Conceptualization, Supervision, Validation, Writing – review & editing. BR: Conceptualization, Validation, Writing – review & editing. CS: Conceptualization, Formal Analysis, Investigation, Validation, Writing – review & editing. SG-M: Conceptualization, Formal Analysis, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Acknowledgments
Parts of this manuscript have previously been released as a report on Georgia CORE's and GASCO's one day summit Addressing Disparities in Cancer Clinical Trials. The whole report is available at: https://d15yi9gnq6oxdl.cloudfront.net/assets/pdfs/Disparities_Summit_2022_Report.pdf (H J) (54).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Oyer RA, Hurley P, Boehmer L, Bruinooge SS, Levit K, Barrett N, et al. Increasing racial and ethnic diversity in cancer clinical trials: an American society of clinical oncology and association of community cancer centers joint research statement. J Clin Oncol. (2022) 40(19):2163–71. doi: 10.1200/JCO.22.00754
2. Duma N, Vera Aguilera J, Paludo J, Haddox CL, Gonzalez Velez M, Wang Y, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract. (2018) 14(1):e1–e10. doi: 10.1200/JOP.2017.025288
3. Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. JNCI J Natl Cancer Inst. (2019) 111(3):245–55. doi: 10.1093/jnci/djy221
4. Eskander MF, Gil L, Beal EW, Li Y, Hamad A, Oppong B, et al. Access denied: inequities in clinical trial enrollment for pancreatic cancer. Ann Surg Oncol. (2022) 29(2):1271–7. doi: 10.1245/s10434-021-10868-4
5. Brundage MD. Revisiting barriers to clinical trials accrual. JNCI J Natl Cancer Inst. (2021) 113(3):219–20. doi: 10.1093/jnci/djaa156
6. Unger JM, Moseley A, Symington B, Chavez-MacGregor M, Ramsey SD, Hershman DL. Geographic distribution and survival outcomes for rural patients with cancer treated in clinical trials. JAMA Netw Open. (2018) 1(4):e181235. doi: 10.1001/jamanetworkopen.2018.1235
7. Graetz DE, Madni A, Gossett J, Kang G, Sabin JA, Santana VM, et al. Role of implicit bias in pediatric cancer clinical trials and enrollment recommendations among pediatric oncology providers. Cancer. (2021) 127(2):284–90. doi: 10.1002/cncr.33268
8. Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. (2013) 28(11):1504–10. doi: 10.1007/s11606-013-2441-1
9. Georgia Cancer Info. History of Georgia CORE. Available online at: https://www.georgiacancerinfo.org/georgia-core-history.aspx (Accessed December 13, 2022).
10. Mealor R, Canterbury K, Paris N, Irby S, Johnson N. Georgia On my mind: one state’s unified, comprehensive approach to cancer treatment. Oncol Issues. (2008) 23(3):34–43. doi: 10.1080/10463356.2008.11883417
11. Leveraging the Tobacco Master Settlement Agreement to Fight Cancer in Georgia: 5 Actions to Save More Lives. Georgia Center for Oncology Research and Education; (2019).
12. American Cancer Society | Cancer Facts & Statistics. American Cancer Society | Cancer Facts & Statistics. Available online at: http://cancerstatisticscenter.cancer.org/ (accessed March 01, 2024).
13. State Cancer Profiles > Quick Profiles/Incidence Rates 2015-2019. Available online at: https://statecancerprofiles.cancer.gov/quick-profiles/index.php?statename=georgia (accessed March 01, 2024).
14. Loree JM, Anand S, Dasari A, Unger JM, Gothwal A, Ellis LM, et al. Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol. (2019) 5(10):e191870. doi: 10.1001/jamaoncol.2019.1870
15. Green AK, Trivedi N, Hsu JJ, Yu NL, Bach PB, Chimonas S. Despite the FDA’s five-year plan, black patients remain inadequately represented in clinical trials for drugs: study examines FDA’s five-year action plan aimed at improving diversity in and transparency of pivotal clinical trials for newly-approved drugs. Health Aff (Millwood). (2022) 41(3):368–74. doi: 10.1377/hlthaff.2021.01432
16. Moore JX, Tingen MS, Coughlin SS, O'Meara C, Odhiambo L, Vernon M, et al. Understanding geographic and racial/ethnic disparities in mortality from four major cancers in the state of Georgia: a spatial epidemiologic analysis, 1999–2019. Sci Rep. (2022) 12(1):14143. doi: 10.1038/s41598-022-18374-7
17. Veitinger JK, Kerber AS, Gabram-Mendola SGA, Liu Y, Durham LM, Durrence D, et al. Screening for individuals at risk for hereditary breast and ovarian cancer: a statewide initiative, Georgia, 2012–2020. Am J Public Health. (2022) 112(9):1249–52. doi: 10.2105/AJPH.2022.306932
18. Harvey RD, Mileham KF, Bhatnagar V, Brewer JR, Rahman A, Moravek C, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-friends of cancer research washout period and concomitant medication work group. Clin Cancer Res. (2021) 27(9):2400–7. doi: 10.1158/1078-0432.CCR-20-3855
19. Harvey RD, Bruinooge SS, Chen L, Garrett-Mayer E, Rhodes W, Stepanski E, et al. Impact of broadening trial eligibility criteria for patients with advanced non–small cell lung cancer: real-world analysis of select ASCO- friends recommendations. Clin Cancer Res. (2021) 27(9):2430–4. doi: 10.1158/1078-0432.CCR-20-3857
20. Giantonio BJ. Eligibility in cancer clinical research: the intersection of discovery, generalizability, beneficence, and justice. Clin Cancer Res. (2021) 27(9):2369–71. doi: 10.1158/1078-0432.CCR-21-0085
21. Kim ES, Uldrick TS, Schenkel C, Bruinooge SS, Harvey RD, Magnuson A, et al. Continuing to broaden eligibility criteria to make clinical trials more representative and inclusive: aSCO–friends of cancer research joint research statement. Clin Cancer Res. (2021) 27(9):2394–9. doi: 10.1158/1078-0432.CCR-20-3852
22. Magnuson A, Bruinooge SS, Singh H, Wilner KD, Jalal S, Lichtman SM, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-friends of cancer research performance Status work group. Clin Cancer Res. (2021) 27(9):2424–9. doi: 10.1158/1078-0432.CCR-20-3868
23. Osarogiagbon RU, Vega DM, Fashoyin-Aje L, Wedam S, Ison G, Atienza S, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO–friends of cancer research prior therapies work group. Clin Cancer Res. (2021) 27(9):2408–15. doi: 10.1158/1078-0432.CCR-20-3854
24. Spira AI, Stewart MD, Jones S, Chang E, Fielding A, Richie N, et al. Modernizing clinical trial eligibility criteria: recommendations of the ASCO-friends of cancer research laboratory reference ranges and testing intervals work group. Clin Cancer Res. (2021) 27(9):2416–23. doi: 10.1158/1078-0432.CCR-20-3853
25. Duggal M, Sacks L, Vasisht KP. Eligibility criteria and clinical trials: an FDA perspective. Contemp Clin Trials. (2021) 109:106515. doi: 10.1016/j.cct.2021.106515
26. Riner AN, Girma S, Vudatha V, Mukhopadhyay N, Skoro N, Gal TS, et al. Eligibility criteria perpetuate disparities in enrollment and participation of black patients in pancreatic cancer clinical trials. J Clin Oncol. (2022) 40(20):2193–202. doi: 10.1200/JCO.21.02492
27. Khurana A, Mwangi R, Nowakowski GS, Habermann TM, Ansell SM, LaPlant BR, et al. Impact of organ function–based clinical trial eligibility criteria in patients with diffuse large B-cell lymphoma: who gets left behind? J Clin Oncol. (2021) 39(15):1641–9. doi: 10.1200/JCO.20.01935
28. Karim S, Xu Y, Kong S, Abdel-Rahman O, Quan ML, Cheung WY. Generalisability of common oncology clinical trial eligibility criteria in the real world. Clin Oncol. (2019) 31(9):e160–6. doi: 10.1016/j.clon.2019.05.003
29. Borno HT, Odisho AY, Gunn CM, Pankowska M, Rider JR. Disparities in precision medicine—examining germline genetic counseling and testing patterns among men with prostate cancer. Urol Oncol Semin Orig Investig. (2021) 39(4):233.e9–233.e14. doi: 10.1016/j.urolonc.2020.10.014
30. Kwon DHM, Borno HT, Cheng HH, Zhou AY, Small EJ. Ethnic disparities among men with prostate cancer undergoing germline testing. Urol Oncol Semin Orig Investig. (2020) 38(3):80.e1–7. doi: 10.1016/j.urolonc.2019.09.010
31. Rodon J, Soria JC, Berger R, Miller WH, Rubin E, Kugel A, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. (2019) 25(5):751–8. doi: 10.1038/s41591-019-0424-4
32. Selvarajah S, Schrader K, Kolinsky M, Rendon RA, El Hallani S, Fleshner NE, et al. Recommendations for the implementation of genetic testing for metastatic prostate cancer patients in Canada. Can Urol Assoc J. (2022) 16(10):321–32. doi: 10.5489/cuaj.7954
33. Fountzilas E, Tsimberidou AM, Vo HH, Kurzrock R. Clinical trial design in the era of precision medicine. Genome Med. (2022) 14(1):101. doi: 10.1186/s13073-022-01102-1
34. Borno HT, Lin TK, Zhang S, Skafel A, Lalanne A, Dornsife D, et al. Accelerating cancer clinical trial recruitment through a financial reimbursement program integrated with patient navigation: an interrupted time series analysis. J Cancer Policy. (2021) 30:100305. doi: 10.1016/j.jcpo.2021.100305
35. Borno HT, Zhang L, Zhang S, Lin TK, Skafel A, Nieves E, et al. Implementation of a multisite financial reimbursement program in cancer clinical trials integrated with patient navigation: a pilot randomized clinical trial. JCO Oncol Pract. (2022) 18(6):e915–24. doi: 10.1200/OP.21.00328
36. Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, et al. Barriers to accrual and enrollment in brain tumor trials. Neuro-Oncol. Published online June 7 (2019) 21:noz104. doi: 10.1093/neuonc/noz104
37. Reihl SJ, Patil N, Morshed RA, Mehari M, Aabedi A, Chukwueke UN, et al. A population study of clinical trial accrual for women and minorities in neuro-oncology following the NIH revitalization act. Neuro-Oncol. (2022) 24(8):1341–9. doi: 10.1093/neuonc/noac011
38. Vuong I, Wright J, Nolan MB, Eggen A, Bailey E, Strickland R, et al. Overcoming barriers: evidence-based strategies to increase enrollment of underrepresented populations in cancer therapeutic clinical trials—a narrative review. J Cancer Educ. (2020) 35(5):841–9. doi: 10.1007/s13187-019-01650-y
39. Fouad MN, Acemgil A, Bae S, Forero A, Lisovicz N, Martin MY, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract. (2016) 12(6):556–63. doi: 10.1200/JOP.2015.008946
40. Guadagnolo BA, Boylan A, Sargent M, Koop D, Brunette D, Kanekar S, et al. Patient navigation for American Indians undergoing cancer treatment: utilization and impact on care delivery in a regional healthcare center. Cancer. (2011) 117(12):2754–61. doi: 10.1002/cncr.25823
41. Clair McClung E, Davis SW, Jeffrey SS, Kuo MC, Lee MM, Teng NNH. Impact of navigation on knowledge and attitudes about clinical trials among Chinese patients undergoing treatment for breast and gynecologic cancers. J Immigr Minor Health. (2015) 17(3):976–9. doi: 10.1007/s10903-013-9901-x
42. Cartmell KB, Bonilha HS, Simpson KN, Ford ME, Bryant DC, Alberg AJ. Patient barriers to cancer clinical trial participation and navigator activities to assist. Adv Cancer Res. (2020) 146:139–66. doi: 10.1016/bs.acr.2020.01.008
43. Sae-Hau M, Disare K, Michaels M, Gentile A, Szumita L, Treiman K, et al. Overcoming barriers to clinical trial participation: outcomes of a national clinical trial matching and navigation service for patients with a blood cancer. JCO Oncol Pract. (2021) 17(12):e1866–78. doi: 10.1200/OP.20.01068
44. Garcia-Alcaraz C, Roesch SC, Calhoun E, Wightman P, Mohan P, Battaglia TA, et al. Exploring classes of cancer patient navigators and determinants of navigator role retention. Cancer. (2022) 128(S13):2590–600. doi: 10.1002/cncr.33908
45. Noguchi Y. Delaware is Shrinking Racial Gaps in Cancer Death. Its Secret? Patient Navigators. NPR. Available online at: https://www.npr.org/sections/health-shots/2022/03/07/1084317639/delaware-is-shrinking-racial-gaps-in-cancer-death-its-secret-patient-navigators Published March 7, 2022. (Accessed December 13, 2022).
46. Cancer Incidence and Mortality in Delaware, 2013-2017. Delaware Department of Health and Social Services Division of Public Health. (2021).
47. Niranjan SJ, Martin MY, Fouad MN, Vickers SM, Wenzel JA, Cook ED, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. (2020) 126(9):1958–68. doi: 10.1002/cncr.32755
48. CMS Includes Reimbursement for Oncology Patient Navigation in CY24 Medicare Physician Fee Schedule Proposal. American Cancer Society Cancer Action Network. Published July 14, 2023. Available online at: https://www.fightcancer.org/releases/cms-includes-reimbursement-oncology-patient-navigation-cy24-medicare-physician-fee-schedule (Accessed December 2, 2023).
49. Reardon D. New Law Will Reimburse Cancer Patients for their Participation in Trials. Published May 2, 2023. Available online at: https://www.atlantanewsfirst.com/2023/05/02/new-law-will-reimburse-cancer-patients-their-participation-trials/ (Accessed February 8, 2024).
50. Watson, Tillery, Hufstetler, Robinson. Senate Bill 223.; 2023. 23 LC 33 9402 S. B. 223 - 1 Georgia.gov Available online at: https://gov.georgia.gov/document/2023-signed-legislation/sb-223/download (accessed March 01, 2024).
51. Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs for Industry. U.S. Department of Health and Human Services Food and Drug Administration. (2020). Available online at: https://www.fda.gov/media/127712/download (accessed March 01, 2024).
52. 2022 Cancer Facts & Figures Cancer | Cancer Death Rate Drops. Available online at: https://www.cancer.org/latest-news/facts-and-figures-2022.html (Accessed January 13, 2023).
53. Lujan BR. H.R.913 - 116th Congress (2019-2020): CLINICAL TREATMENT Act. Published January 30, 2019. Available online at: http://www.congress.gov/ (Accessed January 13, 2023).
54. Hoin J. Addressing Disparities in Cancer Clinical Trials. 2022 Summit: A Recap and Roadmap to more Equitable Accrual. Available online at: https://d15yi9gnq6oxdl.cloudfront.net/assets/pdfs/Disparities_Summit_2022_Report.pdf (accessed March 01, 2024).
Keywords: cancer care access, clinical trials, disparities, clinical trial accrual, navigation
Citation: Hoin JA, Carthon BC, Brown SJ, Durham LM, Garrot LC, Ghamande SA, Pippas AW, Rivers BM, Snyder CT and Gabram-Mendola SGA (2024) Addressing disparities in cancer clinical trials: a roadmap to more equitable accrual. Front. Health Serv. 4:1254294. doi: 10.3389/frhs.2024.1254294
Received: 6 July 2023; Accepted: 27 February 2024;
Published: 8 March 2024.
Edited by:
Soumya J. Niranjan, University of Alabama at Birmingham, United StatesReviewed by:
Camille Ragin, Fox Chase Cancer Center, United States© 2024 Hoin, Carthon, Brown, Durham, Garrot, Ghamande, Pippas, Rivers, Snyder and Gabram-Mendola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheryl Gordon Ann Gabram-Mendola c2dhYnJhbUBlbW9yeS5lZHU=
 Jon A. Hoin1
Jon A. Hoin1 Brian M. Rivers
Brian M. Rivers Sheryl Gordon Ann Gabram-Mendola
Sheryl Gordon Ann Gabram-Mendola