Healthcare-associated infections (HCAIs) occur in individuals while receiving medical care in a healthcare facility. These infections are often preventable. According to the U.S. Center for Disease Control and Prevention (CDC), around 1.7 million hospitalized patients acquire HCAIs per year while being treated for other health-related problems; one out of these seventeen infected patients die as a result of HCAIs (1), which is one of the top ten causes of death in the U.S. Around 7% of patients in high-income nations and 10% in emerging and developing nations acquire HCAIs, and 10% of those patients pass away (1). The rate of HCAIs is higher among intensive care unit (ICU) patients, mostly due to their immunocompromised status (2, 3). Of relevance, the higher risk of mortality among patients in the ICU is not only limited to their primary illness but is often amalgamated with HCAIs. The COVID-19 pandemic has highlighted the danger of HCAIs and the need for rigorous infection control measures in healthcare settings. A survey of 11,282 patients in various U.S. hospitals identified Clostridium difficile as the major cause of HCAIs (4). Another study on a large cohort found more than 2 million new patients developing HCAIs with antimicrobial resistance to Klebsiella pneumoniae and Acinetobacter species per year, in the European Union and European Economic Area (5).
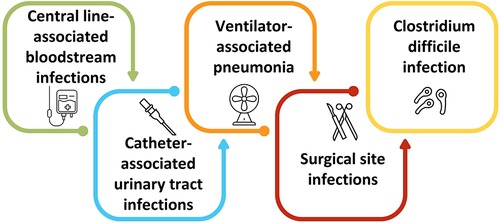
Bloodstream infection, urinary tract infection, surgical site infection, and pneumonia are identified as the most common causes of HCAIs (Figure 1) (6, 7). Bloodstream infections in ICU and hemodialysis centers are common, and around US$ 1.8 billion was spent in a decade in early 2000 to save more than 25,000 patients (8). Surgical site infection is a common postoperative complication with higher morbidity and mortality that comes with a financial burden to the patients and the care providers (9). Urinary tract infections, particularly catheter-induced infections, are among the most common causes of HCAIs, comprising around 40% of HCAIs, with higher fatality (10). Implants and prostheses can also induce HCAIs. The increased rate of HCAIs with higher numbers of antimicrobial resistance significantly burdens healthcare costs, particularly affecting low-resource countries more (11). Additionally, exacerbating antimicrobial resistance is another casualty of the COVID-19 pandemic (12–14). Although antibiotics are ineffective against viruses, including COVID-19, many COVID-19 patients have received antibiotics as a cautionary measure, causing an unnecessary use of antibiotics and the development of antimicrobial resistance, thereby making HCAIs more challenging to treat (15).
The available evidence suggests an association between COVID-19 and an increase in HCAIs. A primary concern during the COVID-19 pandemic is that patients were at a higher risk of acquiring the infection while receiving care in a healthcare facility. The proximity of infected healthcare individuals, the COVID-19 patients in healthcare settings, and the potential for healthcare worker-mediated transmission can increase HCAIs. A CDC analysis found a continued increase in HCAIs in U.S. hospitals during the pandemic in 2021; ventilator-associated events (VAEs) significantly increased across all types of infections (16). In a separate cross-sectional analysis of more than 5 million hospitalized patients between 2020 and 2022, the occurrence of catheter-associated urinary tract infection, central line-associated bloodstream infection, and methicillin-resistant Staphylococcus aureus bacteremia were found to be higher among the COVID-19 patients (17). The impact of HCAIs may vary depending on hospital practices and hospitalization period during the pandemic.
COVID-19 has also been shown to spread via bioaerosols (18). Bioaerosols are airborne particles that contain living organisms such as bacteria, viruses, and fungi (19). COVID-19 can be transmitted through bioaerosols generated when an infected person talks, coughs, or sneezes (20). Bioaerosols can be a limiting factor in reducing HCAIs, as they can spread infectious agents in various healthcare settings. Therefore, controlling bioaerosols can be an essential measure in limiting HCAIs. Proper ventilation, air filtration, hand hygiene, and toilet hygiene are some of the steps that can help reduce the concentration of bioaerosols and minimize the spread of infectious agents in healthcare settings. Proper ventilation can help decrease the concentration of bioaerosols in the air, while air filtration systems can remove bioaerosols from the air to lower the risk of microorganism transmission, including COVID-19 (21). Similarly, proper hand hygiene can help prevent the spread of infectious agents that may be present in bioaerosols, and adequate toilet hygiene and cleaning can help decrease the risk of transmission. A study found that flushing toilets, seeded with bacteria, can increase the bioaerosol concentration of a washroom to increase the spread of microorganisms (22). In the COVID-19 pandemic era, data-driven approaches to identifying the areas for improvement and implementing evidence-based practices to minimize the risk of developing HCAIs would better serve to protect patients. The COVID-19 pandemic highlighted the need for continuing education and training of infection prevention and control for healthcare workers to limits the spread of disease.
The CDC has provided guidelines for reducing HCAIs, covering primary infection prevention and control, and instructions for healthcare providers in specific settings to protect and provide safe care. The WHO advocates that all healthcare providers must wash their hands before dealing with patients. Effective hand hygiene is the most important practice to control HCAIs, which prevent the formation of colonies with multi-drug resistant pathogens (23). Poor hand hygiene compliance has shown to be one of the leading contributory factors to HCAIs, and it is estimated that improper hand hygiene by healthcare providers is responsible for about 40% of HCAIs in certain African countries (24). The WHO projected that maintenance of hand hygiene can reduce up to 50% of preventable illnesses acquired during healthcare delivery; a significant decrease in the rate of HCAIs was noted when hand hygiene compliance improved (25). As mentioned, a simple measure like handwashing is considered to be the single most effective action to stop the spread of infection, and such a measure becomes increasingly critical in the context of the COVID-19 pandemic. Furthermore, poor cleaning of the hospital surfaces is linked to HCAIs such as the transmission of the potentially fatal methicillin-resistant Staphylococcus aureus (26). Ongoing surveillance, education, and training of healthcare workers remain vital in reducing the incidence of HCAIs during the COVID-19 pandemic (27).
As stated, HCAIs constitute a significant health concern for both healthcare providers and recipients. During the COVID-19 pandemic, the rate of HCAIs is alarming and associated with prolonged hospitalizations, increasing morbidity and mortality (28). With evolving microorganisms and emerging microbial drug resistance, a dynamic change in healthcare practice would require ensuring hospital safety, reducing the occurrence of HCAIs, and minimizing the financial burden on individuals and society. Despite yearly spending between US$ 28 and US$ 45 billion for controlling HCAIs, around 90,000 patients die in the U.S. related to HCAIs (29, 30). The National Healthcare Safety Network found that COVID-19 patients are more vulnerable to HCAIs and require additional protective measures (17). During the COVID-19 pandemic, healthcare facilities must implement effective infection control policies and education initiatives to lower the risk of HCAIs and protect patients from harm (Box 1). Moreover, healthcare workers need to undergo training on infection prevention and control measures to minimize the risk of transmission to ensure patient safety and improve health outcomes. Healthcare facilities can provide safe and compassionate patient care (31), even amid the COVID-19 pandemic, by focusing on infection prevention and control.
Author contributions
MR: Writing – original draft, Writing – review & editing.
Funding
The author declares that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I want to express my sincere gratitude to Dr. Nuraly Akimbekov (Al-Farabi Kazakh National University, Kazakhstan) for drawing the illustration. I also thank Dr. Peace Uwambaye, Ms. Mythri Chittilla and Mr. M. Muhit Razzaque for providing useful suggestions. Information has been collected from online sources, including Google Scholar & ChatGPT.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Klevens RM, Edwards JR, Richards CL Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. (2007) 122(2):160–6. doi: 10.1177/003335490712200205
2. McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. (2011) 15(1):301. doi: 10.1186/cc9297
3. Massart N, Dupin C, Legris E, Legay F, Cady A, Fillatre P, et al. Prevention of ICU-acquired infection with decontamination regimen in immunocompromised patients: a pre/post observational study. Eur J Clin Microbiol Infect Dis. (2023) 42(10):1163–72. doi: 10.1007/s10096-023-04650-5
4. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. (2014) 370(13):1198–208. doi: 10.1056/NEJMoa1306801
5. Cassini A, Plachouras D, Eckmanns T, Abu Sin M, Blank HP, Ducomble T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. (2016) 13(10):e1002150. doi: 10.1371/journal.pmed.1002150
6. Askarian M, Yadollahi M, Assadian O. Point prevalence and risk factors of hospital acquired infections in a cluster of university-affiliated hospitals in Shiraz, Iran. J Infect Public Health. (2012) 5(2):169–76. doi: 10.1016/j.jiph.2011.12.004
7. Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections—an overview. Infect Drug Resist. (2018) 11:2321–33. doi: 10.2147/IDR.S177247
8. Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections–United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. (2011) 60(8):243–8.21368740
9. Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect. (2008) 70(Suppl 2):3–10. doi: 10.1016/S0195-6701(08)60017-1
10. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. (2003) 49(2):53–70. doi: 10.1067/mda.2003.7
11. Amin AN, Deruelle D. Healthcare-associated infections, infection control and the potential of new antibiotics in development in the USA. Future Microbiol. (2015) 10:1049–62. doi: 10.2217/fmb.15.33
12. Razzaque MS. Commentary: microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front Public Health. (2021) 8:629120. doi: 10.3389/fpubh.2020.629120
13. Razzaque MS. Exacerbation of antimicrobial resistance: another casualty of the COVID-19 pandemic? Expert Rev Anti Infect Ther. (2021) 19(8):967–71. doi: 10.1080/14787210.2021.1865802
14. Razzaque MS. Implementation of antimicrobial stewardship to reduce antimicrobial drug resistance. Expert Rev Anti Infect Ther. (2021) 19(5):559–62. doi: 10.1080/14787210.2021.1840977
15. Langford BJ, So M, Simeonova M, Leung V, Lo J, Kan T, et al. Antimicrobial resistance in patients with COVID-19: a systematic review and meta-analysis. Lancet Microbe. (2023) 4:e179–91. doi: 10.1016/S2666-5247(22)00355-X
16. Lastinger LM, Alvarez CR, Kofman A, Konnor RY, Kuhar DT, Nkwata A, et al. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. (2023) 44:997–1001. doi: 10.1017/ice.2022.116
17. Sands KE, Blanchard EJ, Fraker S, Korwek K, Cuffe M. Health care-associated infections among hospitalized patients with COVID-19, March 2020–March 2022. JAMA Netw Open. (2023) 6(4):e238059. doi: 10.1001/jamanetworkopen.2023.8059
18. Tedeschini E, Pasqualini S, Emiliani C, Marini E, Valecchi A, Laoreti C, et al. Monitoring of indoor bioaerosol for the detection of SARS-CoV-2 in different hospital settings. Front Public Health. (2023) 11:1169073. doi: 10.3389/fpubh.2023.1169073
19. Kim KH, Kabir E, Jahan SA. Airborne bioaerosols and their impact on human health. J Environ Sci (China). (2018) 67:23–35. doi: 10.1016/j.jes.2017.08.027
20. Guzman MI. An overview of the effect of bioaerosol size in coronavirus disease 2019 transmission. Int J Health Plann Manage. (2021) 36:257–66. doi: 10.1002/hpm.3095
21. Ereth MH, Fine J, Stamatatos F, Mathew B, Hess D, Simpser E. Healthcare-associated infection impact with bioaerosol treatment and COVID-19 mitigation measures. J Hosp Infect. (2021) 116:69–77. doi: 10.1016/j.jhin.2021.07.006
22. Knowlton SD, Boles CL, Perencevich EN, Diekema DJ, Nonnenmann MW, CDC Epicenters Program. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob Resist Infect Control. (2018) 7:16. doi: 10.1186/s13756-018-0301-9
23. Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. (2006) 6:641–52. doi: 10.1016/S1473-3099(06)70600-4
24. Engdaw GT, Gebrehiwot M, Andualem Z. Hand hygiene compliance and associated factors among health care providers in Central Gondar zone public primary hospitals, Northwest Ethiopia. Antimicrob Resist Infect Control. (2019) 8:190. doi: 10.1186/s13756-019-0634-z
25. Sickbert-Bennett EE, DiBiase LM, Willis TM, Wolak ES, Weber DJ, Rutala WA. Reduction of healthcare-associated infections by exceeding high compliance with hand hygiene practices. Emerg Infect Dis. (2016) 22(9):1628–30. doi: 10.3201/eid2209.151440
26. Otter JA, Yezli S, Salkeld JA, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control. (2013) 41(5 Suppl):S6–11. doi: 10.1016/j.ajic.2012.12.004
27. Wisniewski MF, Kim S, Trick WE, Welbel SF, Weinstein RA, Chicago Antimicrobial Resistance Project. Effect of education on hand hygiene beliefs and practices: a 5-year program. Infect Control Hosp Epidemiol. (2007) 28: 88–91. doi: 10.1086/510792
28. Fakhreddine S, Fawaz M, Hassanein S, Al Khatib A. Prevalence and mortality rate of healthcare-associated infections among COVID-19 patients: a retrospective cohort community-based approach. Front Public Health. (2023) 11:1235636. doi: 10.3389/fpubh.2023.1235636
29. Glance LG, Stone PW, Mukamel DB, Dick AW. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg. (2011) 146:794–801. doi: 10.1001/archsurg.2011.41
30. Stone PW. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res. (2009) 9:417–22. doi: 10.1586/erp.09.53
Keywords: COVID-19, healthcare-associated infections, infection prevention, patient safety, outcomes
Citation: Razzaque MS (2023) Healthcare-associated infections in the context of the pandemic. Front. Health Serv. 3:1288033. doi: 10.3389/frhs.2023.1288033
Received: 3 September 2023; Accepted: 10 November 2023;
Published: 28 November 2023.
Edited by:
Melissa Baysari, The University of Sydney, AustraliaReviewed by:
Bara Sarraj, Harold Washington College, United States© 2023 Razzaque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed S. Razzaque bXJhenphcXVlQGxlY29tLmVkdQ==; bXNyLm5hZ2FzYWtpQGdtYWlsLmNvbQ==
 Mohammed S. Razzaque
Mohammed S. Razzaque