
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Health Serv. , 02 August 2023
Sec. Implementation Science
Volume 3 - 2023 | https://doi.org/10.3389/frhs.2023.1233923
This article is part of the Research Topic Vaccines and Immunization Services During the Pandemic Era and Beyond View all 19 articles
 Grace Umutesi1*
Grace Umutesi1* Lynda Oluoch2
Lynda Oluoch2 Bryan J. Weiner1
Bryan J. Weiner1 Elizabeth Bukusi1,2
Elizabeth Bukusi1,2 Maricianah Onono2
Maricianah Onono2 Betty Njoroge2
Betty Njoroge2 Lucy Mecca3
Lucy Mecca3 Kenneth Ngure1,4
Kenneth Ngure1,4 Nelly R. Mugo1,2,†
Nelly R. Mugo1,2,† Ruanne V. Barnabas5,6,†
Ruanne V. Barnabas5,6,†
Background: Cervical cancer is the leading cause of cancer-related deaths among Kenyan women. Persistent infection with high-risk oncogenic Human papillomavirus (HPV) genotypes is a necessary cause of cervical cancer. HPV vaccines are safe, durable, and efficacious in preventing incident HPV infections. In Kenya, despite efforts to increase HPV vaccination, coverage remains low. We sought to assess: (1) barriers and facilitators of HPV vaccination from the perspective of adolescent girls and young women (AGYW), their guardians as well as stakeholders involved in HPV vaccine delivery, and (2) the acceptability of the single dose of the HPV vaccination among healthcare providers (HCPs).
Methods: Our study is nested within the KENya Single-dose HPV-vaccine Efficacy study (KEN SHE) that sought to test the efficacy of single-dose bivalent (HPV 16/18) and single-dose nonavalent (HPV 16/18/31/33/45/52/58/6/11) vaccination. We are conducting this study in Kiambu, Nairobi, and Kisumu counties. In these counties, we are interviewing stakeholders (n = ∼25), selected based on their role in HPV vaccination at the county and national levels. Interviews are audio recorded and conducted in English or Swahili. The semi-structured interview guides were designed based on: (1) the Theoretical Domains Framework (TDF) for AGYW and guardians and (2) the Consolidated Framework for Implementation Research (CFIR) for other stakeholders. The Theoretical Framework of Acceptability (TFA) was leveraged to design the survey administered to HCPs (n = ∼309) involved in HPV vaccination. We will develop a codebook based on emerging codes from the transcripts and constructs from the TDF and CFIR. Emerging themes will be summarized highlighting similarities and differences between and within the different stakeholder groups and counties. Descriptive statistics and a χ2 test will be used to assess the distribution of responses between the different sites and regression analysis will be used to assess factors associated with high acceptability of the single-dose strategy while controlling for confounding variables.
Discussion: Our study will describe key barriers and facilitators that affect HPV vaccination from the perspective of multiple stakeholders as well as insights on the perspective of HCPs towards the single-dose strategy to inform the designing of strategies to increase HPV vaccination uptake in Kenya and comparable settings.
Cervical cancer is the fourth leading cause of death among women (1). In 2020, the burden of new cases and deaths due to cervical cancer was concentrated in low-and-middle-income countries (LMIC), accounting for 90% of the global cancer incidence and mortalities (2). Sub-Saharan Africa bears a high prevalence of cervical cancer with a mortality rate of 94.1 per 100,000 in 2018 (2, 3). Invasive cervical cancer (ICC) is one of the few cancers with a known infectious etiology, and persistent infection with high-risk oncogenic human papillomavirus (HPV) is a necessary cause for ICC (4). However, HPV vaccines are safe, durable, and efficacious in preventing incident HPV infections that lead to cervical cancer (5, 6). The World Health Organization (WHO)'s Global Cervical Cancer Elimination Strategy has three pillars, one of which is achieving 90% HPV vaccination coverage for age-eligible girls. Unfortunately, the global HPV vaccination coverage for age-eligible adolescent girls remains low where it was estimated at 15% in 2019 (5, 7, 8).
In Kenya, cervical cancer is the leading cause of cancer-related deaths among women, resulting in approximately 3,400 deaths annually (9). In accordance with the recommendations from the WHO, a two-dose schedule for HPV vaccination was introduced in Kenya in 2019 targeting 10-year-old adolescent girls through facility-based delivery (10). At the time of the HPV vaccination launch, social mobilization, and community education efforts were conducted to raise awareness and ignite vaccine uptake (11, 12). Since the national introduction, the HPV vaccination coverage in Kenya has been suboptimal where 33% of eligible AGYW received the first doses in 2020 and this estimate increased to 77% in 2021 but only 31% of targeted AGYW had received 2 doses of the HPV vaccine in 2021 (9, 13). In Kenya, HPV vaccination coverage has been adversely impacted by delivery processes and vaccine hesitancy among healthcare providers (HCPs) and at the community level. Additionally, the global COVID-19 pandemic increased vaccine hesitancy at the community level, the lack of confidence among HCPs sparked their reluctance to promote HPV vaccination, and the lack of community engagement and education after the initial launch of the program resulted in knowledge gaps that fueled HPV vaccination refusals (11, 12, 14, 15).
These implementation-related challenges highlight the need for comprehensive evidence from stakeholders on factors that facilitate or impede the delivery and uptake of HPV vaccination. The overall objective of this study is to understand why HPV vaccination coverage remains low for AGYW in Kenya, despite evidence endorsing the crucial role of HPV vaccination in cervical cancer prevention. This study aims to generate knowledge on implementation drivers of HPV vaccination in Kiambu, Nairobi, and Kisumu County. Our specific aims are to (1) assess barriers and facilitators to HPV vaccination delivery in the three counties, and (2) assess the acceptability of the single dose of HPV vaccination among HCPs. Findings from this study will be shared with the Kenyan Ministry of Health and they will potentially contribute to informing the design of the national guideline for HPV vaccination and generate evidence for decision-makers.
This study is nested within the KENya Single-dose HPV-vaccine Efficacy study (KEN SHE, ClinicalTrials.gov number NCT03675256). The KEN SHE study is a randomized, multicenter, double-blind, three-arm, controlled trial that sought to test the efficacy of single-dose bivalent (HPV 16/18) and single-dose nonavalent (HPV 16/18/31/33/45/52/58/6/11) HPV vaccination compared with meningococcal vaccine among Kenyan women of 15–20 years of age (16). Interim data analysis done at 18 months of the KEN SHE study showed that the HPV vaccines were highly effective with a vaccine efficacy of 97.5% to prevent incident persistent HPV 16/18 infection.
We are leveraging a qualitative study design to assess barriers and facilitators to HPV vaccination delivery. Study participants are stakeholders involved in HPV vaccination delivery program at the national, county, sub-county, and community levels. Study participants are being selected based on their position and role in the delivery of HPV vaccination for AGYW in Kenya. They include:
(1) From the central government: staff from the National Immunization Program and the Reproductive Health Division at the Ministry of Health (MOH); staff from the Ministry of Education (MOE); national implementing partners (NGOs, advocacy groups, etc.)
(2) From the county level: the health minister or coordinator at county level, heads of county hospitals, other local implementing partners.
(3) From the delivery level: heads of hospitals where HPV vaccines are delivered, the nurse in charge of immunization at the health facility; healthcare frontline vaccine providers at facilities; clinical or medical officers where applicable; principals and teachers as well as opinion leaders (including religious leaders and area chiefs).
Adolescents, girls, and young women (AGYW) aged 10 years and older who were vaccinated and those who were not vaccinated along with their guardians, are also being interviewed to capture details on implementation drivers and barriers from the AGYW and guardians' perspective.
Study participants are purposefully sampled based on their involvement in the delivery of HPV vaccination. Eligible participants are enrolled and interviewed until saturation is reached for each participant category. We estimate that in each of the three counties, we will interview ∼5 participants from each category and 10 from the national level, however, more participants are being recruited and interviewed when saturation is not reached after interviewing the estimated number of participants.
The KEN SHE study team has engaged the Ministry of Health, Ministry of Education, and other relevant implementers at the national and county levels to recruit relevant study participants for interviews. Identification of eligible key informants is done in liaison with county managers and the target group includes teachers, religious leaders, and healthcare providers. Additionally, a list of key informants specific to each county is being supplemented with a list of AGYW and guardians (both vaccinated and unvaccinated). AGYW aged 10 years and older are being recruited from the parent study, health facilities, the community, schools, and colleges. The identification of unvaccinated AGYW is done by community health workers (CHWs) with experience in research recruitment, and who have been involved in the parent study, the KEN SHE trial. Parents or guardians approached for permission before speaking to AGYW and their consent is sought before interviewing AGYW under the age of 18. Informed assents are being obtained for all study participants before enrolment. All interviews are being held in an environment that is convenient for participants. This includes clinics for providers, offices for implementers and decision makers, churches, schools, or other community settings for opinion leaders, AGYW, and guardians.
To select constructs relevant to this study, we mapped out each construct from the Theoretical Domain Framework (TDF) and the Consolidated Framework for Implementation Research (CFIR) to the KEN SHE study setting (17–19). Constructs that matched the study setting and local context were leveraged to design the semi-structured interview guide to capture information on the selected TDF and CFIR constructs. We used the TDF to design the interview guide for AGYWs and their guardians. This approach ensured that the interview guide accounted for the characteristics of the implementation environment and determinants of behavior (Supplementary Appendix I). Additionally, the CFIR served as a guide to design the interview guide for opinion leaders and decision-makers since CFIR focuses more on aspects of the health system and it is more appropriate in contexts where the individual domain or determinants of behavior are less relevant (Supplementary Appendix II). This interview guide was also adapted from a CFIR-guided tool that was used in Mozambique for similar purposes (20). Both interview guides were pilot tested and refined prior to conducting the interviews with targeted study participants. Domains and constructs captured in each interview guide as well as sample questions are illustrated in Table 1.
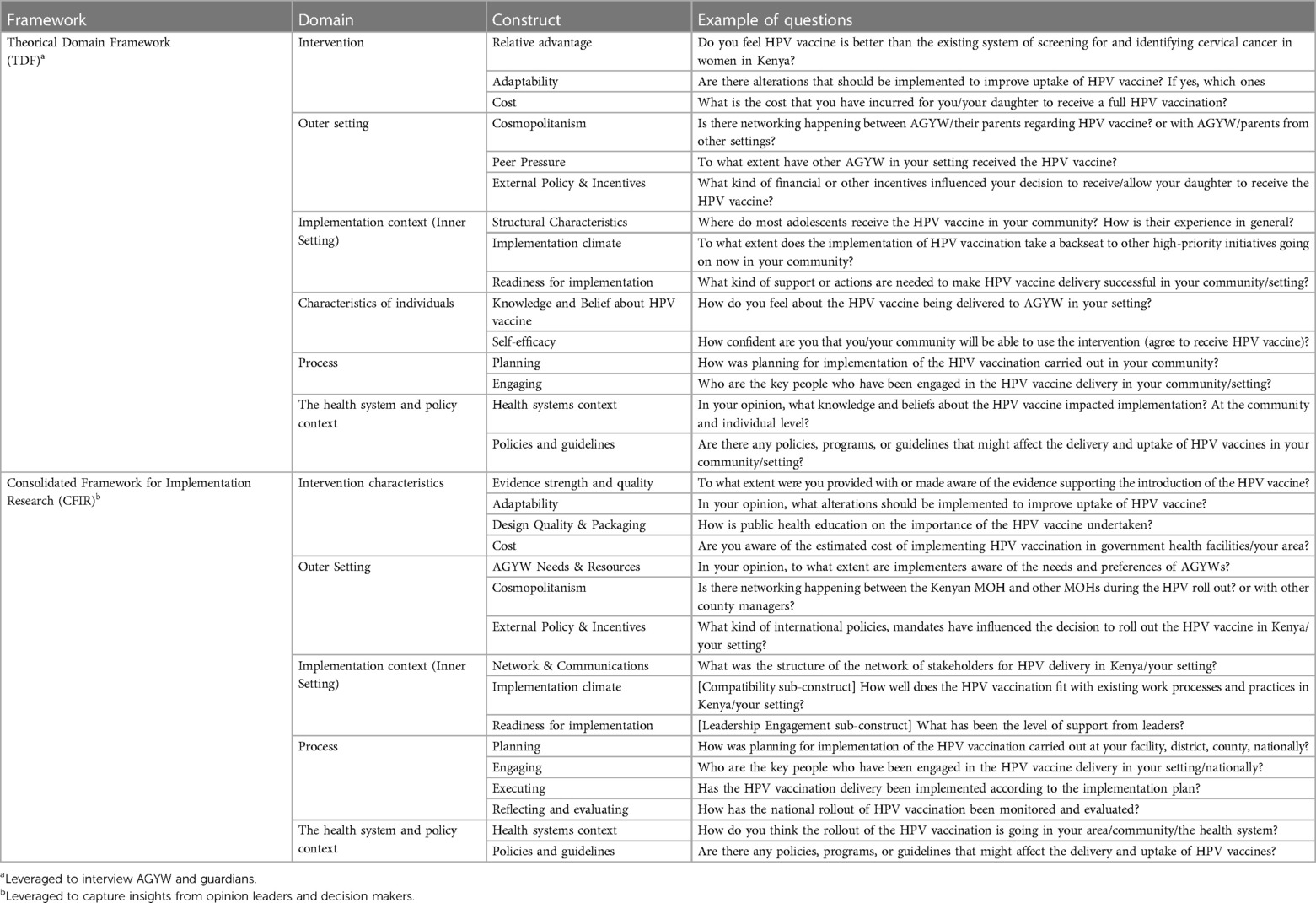
Table 1. Example of questions from the interview guides for AGYW, parents, opinion leaders and decision makers.
We will use constructs of the TDF and the adapted CFIR to guide the analysis of interview transcripts. A codebook will be developed based on emerging codes from the transcripts and the interview guide developed using TDF and CFIR. Once consensus on the codebook has been reached; the remaining interviews will be coded, and appropriate measures will be used to ensure that the coding approach is reliable. Regular coding checking will be performed to ensure that the coding strategy used is reliable. Field notes will be used to inform the interpretation of findings and all coding work will be done with Atlas.ti version 9. Participants' demographics will be summarized in a table detailing the distribution of key characteristics of each targeted group, the number of participants interviewed in total, and for each group. Emerging codes will be used to identify key themes that will be categorized within constructs and domains of the appropriate framework. Themes that do not fit within constructs and domains of the TDF or CFIR will also be listed as new insights that emerged from the interviews. We will summarize similarities and differences of key themes between and within groups.
We are using a concurrent mixed-methods study design to assess the acceptability of the single-dose strategy among healthcare providers. Study participants are healthcare providers involved in the HPV vaccination delivery at different levels of the health system. This includes nurses, clinical officers, pharmacists, pharmacy technicians, medical officers, and other relevant healthcare providers.
Based on the assumption that each health facility has at least one healthcare provider responsible for HPV vaccination with a total of 1,568 health facilities in the three counties (Kiambu: 364, Nairobi ∼1,000, Kisumu ∼200), we estimated the total number of healthcare providers responsible for HPV vaccination to be ∼1,568. Assuming that 50% of healthcare providers in the three countries are involved in HPV vaccination, at least 309 health healthcare providers will need to complete the 10-item survey to assess the acceptability of the reduced dose strategy among providers in the three counties with a 95% confidence interval that the real value is within a ± 5% of the survey results (Table 2).
The study coordinator collaborated with County Immunization Managers to map health facilities where providers are being recruited to participate in a survey that assesses their acceptability of the single-dose strategy. To minimize selection bias and obtain diverse perspectives, the survey link is being shared among providers at different levels of the healthcare system and via healthcare providers' WhatsApp groups to capture insights from providers at various levels of the health system, including private, public, and missionary health facilities, as well as different administrative levels such as county, sub-county, and health center.
We used the theoretical framework for acceptability (TFA) to design the survey focusing on questions that are relevant to Kenya and the KEN SHE study sites (21). Survey responses include qualitative data from free-text responses to the survey and quantitative data from selected responses to the survey.
We will employ constructs of the TFA to guide the analysis of text responses from the survey. A codebook will be developed inductively based on emerging codes from these responses and deductively from the TFA. Once consensus on the codebook has been reached, all text responses from the survey will be coded, and appropriate measures will be used to ensure that the coding approach is reliable. Participant demographics will be summarized in a table detailing the distribution of key characteristics, similar codes will be merged into key themes and categorized into domains of the TFA where applicable. Themes that do not fit within constructs and domains of the TFA will also be listed as new insights that emerged from the survey. All qualitative analyses will be conducted in Atlas.ti version 9. Additionally, descriptive statistics and a χ2 test will be used to assess the distribution of survey responses between the different sites. A multinomial logistic regression analysis will be used to assess the factors associated with the acceptability of the single-dose among healthcare providers controlling for confounding variables. All quantitative analyses will be performed in R-4.3.1. A joint display approach will be leveraged to mix qualitative and quantitative results to facilitate a mixed-method interpretation of findings.
Trained study staff are conducting all study procedures in private and protecting the privacy and confidentiality of study participants. Study-related information are being stored securely at the study clinic. Study records that contain names or other personal identifiers, such as the informed consent forms, are being maintained separately and securely with limited access. Forms, lists, and any other listings that link participant numbers to identifying information are being secured in a separate locked file area. Data collection and administrative forms are being identified only by coded numbers and kept secure, with access limited to authorized study staff. Audio-recorded interviews and interview transcripts are labeled with a study ID-specific site for each site and study participant (e.g.,: NBO/HCW/KI001—Nairobi/Healthcare worker/key informant#1) and stored securely. All study databases are being protected with password access systems and all datasets including interview transcripts and the survey responses will be stored in a password-protected SharePoint folder managed by the KEMRI team.
Informed written consents are being sought and obtained from all study participants and parents or guardians where applicable. For illiterate study participants, the consent form is being read out loud for them and their signature or thumbprint is being obtained for those who agree to participate in the study. All study participation is strictly voluntary, and participants can refuse study participation at any time.
All participants will go through an informed consent process and assent is being obtained from parents or guardians for all minor AGYW. All materials used in providing informed consent, including consent forms, were reviewed, and approved by the ethics committees.
Achieving WHO cervical cancer elimination goals requires understanding context-specific bottlenecks, factors that contribute to increasing HPV vaccination coverages, and uptake to protect as many women as possible from HPV infections and future cervical cancer incidences. Findings from this study will generate insights on barriers and facilitators that affect HPV vaccination from the perspective of a diversity of stakeholders at different levels of the health system (providers, opinion leaders, funders, implementers, AGYWs, and their parents) in Kenya. A variation of factors is expected between and within settings and other key characteristics of participants (gender, location of residence, level of training, role in the community, etc). Additionally, the acceptability of the single-dose strategy will vary depending on the providers' awareness of existing evidence on this new recommended vaccination schedule, their location, and level within the healthcare system. We do not expect the acceptability to vary among providers based on their demographic characteristics or education.
Insights from this study will contribute evidence to support the Kenya HPV vaccination delivery and uptake. Additionally, these findings will contribute to the development of setting-specific outreach, educational and training materials to disseminate evidence among different stakeholders which will contribute to increasing the HPV vaccination coverage in Kenya.
The study team is committed to public dissemination of results of the formative research to participants, local stakeholders and policy makers in Kenya, the global scientific community. Dissemination of study results will follow principles of good participatory practice. Results will be published in conference abstracts and peer-reviewed journals. Study results will be disseminated through presentations to local stakeholders and policymakers in Kenya, including the Ministry of Health.
This study involves human participants and it was approved by the Kenya Medical Research Institute's Scientific Ethics Review Unit (KEMRI - SERU, reference number/ID: KEMRI/SERU/CCR/0283/4546), the Massachusetts General Hospital's Institutional Review Board (IRB) (Protocol number: 2022P002863) and an IRB exemption was obtained from the University of Washington's Human Subject Division. Written informed consents to participate in this study were provided by the participants or their guardians where applicable.
All the listed author contributions include the conception and design, drafting the article or revising it critically for important intellectual content, and final approval of the version published. Regarding responsibility for overall content, the lead author, GU is the guarantor. GU and LO had the idea for this article and wrote the first draft. BJW, EB, MO, BN, LM, KN, RVB and NRM wrote additional section and suggested additional changes. All authors revised the article and approved the final version. As the guarantor, GU affirm that the manuscript provides an honest, accurate, and transparent account of the issues covered, that there are no important omissions, and that there are no discrepancies between what was planned and the final version. All authors contributed to the article and approved the submitted version.
This study was funded by the Bill and Melinda Gates Foundation (Grant no. OPP1188693). The funder had no role in study design, data collection and analysis, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Bill and Melinda Gates Foundation.
We are grateful to the committed team at the University of Washington, Seattle; Massachusetts General Hospital, Boston; and the Kenya Medical Research Team based in Thika, Nairobi and Kisumu, as well as the KEN SHE team for their collective work on this project.
RVB reports Regeneron Pharmaceuticals provided abstract and manuscript writing support outside this study. KN and NRM report research funding from the Merck Investigator Studies Program, outside this study. All reported funders were not involved in the study design, data collection, analysis, interpretation, the writing of this article, or the decision to submit it for publication.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2023.1233923/full#supplementary-material
1. Zhang X, Zeng Q, Cai W, Ruan W. Trends of cervical cancer at global, regional, and national level: data from the global burden of disease study 2019. BMC Public Health. (2021) 21(1):894. doi: 10.1186/s12889-021-10907-5
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Zur Hausen H. Papillomavirus infections: a major cause of human cancers. Infections Causing Human Cancer. Weinheim: Wiley–VCH. (2006):145–243. doi: 10.1002/9783527609314.ch5
5. Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and 1493 intraepithelial neoplasia in women. N Engl J Med. (2015) 372(8):711–23. doi: 10.1056/NEJMoa1405044
6. Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus 1495 type 16 vaccine. N Engl J Med. (2002) 347(21):1645–51. doi: 10.1056/NEJMoa020586
7. Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
8. PATH. Global HPV vaccine introduction overview (2022). Available at: https://www.path.org/resources/global-hpv-vaccine-introduction-overview/ (Accessed June 5, 2022).
9. World Health Organization. Kenya cervical cancer profile (2021). Available at: https://cdn.who.int/media/docs/default-source/country-profiles/cervical-cancer/cervical-cancer-ken-2021-country-profile-en.pdf?sfvrsn=5af61b0b_38&download=true (Accessed May 12, 2022).
10. World Health Organization. A global strategy for elimination of cervical cancer as a public health problem (2020). Available at: https://www.who.int/publications/i/item/9789240014107 (Accessed May 6, 2022).
11. Karanja-Chege CM. HPV Vaccination in Kenya: the challenges faced and strategies to increase uptake. Front Public Health. (2022) 10:802947. doi: 10.3389/fpubh.2022.802947
12. Kenya National Bureau of Statistics. Kenya demographic and health survey 2008–2009 (2010). Available at: https://dhsprogram.com/pubs/pdf/fr229/fr229.pdf (Accessed May 6, 2022).
13. Mwenda V, Jalang’o R, Miano C, Bor JP, Nyangasi M, Mecca L, et al. Impact, cost-effectiveness, and budget implications of HPV vaccination in Kenya: a modelling study. Vaccine. (2023) 41(29):4228–38. doi: 10.1016/j.vaccine.2023.05.019
14. Njuguna DW, Mahrouseh N, Isowamwen OV, Knowledge VO. Attitude and practice of main stakeholders towards human papillomavirus infection and vaccination in mombasa and tana-river counties in Kenya: a qualitative study. Vaccines. (2021) 9(10):1099. doi: 10.3390/vaccines9101099
15. JSI. Kenya’s HPV vaccine introduction (and JSI’s experiences). Available at: https://publications.jsi.com/JSIInternet/Inc/Common/_download_pub.cfm?id=24146&lid=3 (Accessed May 20, 2022).
16. Barnabas RV, Brown ER, Onono M, Bukusi EA, Njoroge B, Winer RL, et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE study): study protocol for a randomized controlled trial. Trials. (2021) 2(1):661. doi: 10.1186/s13063-021-05608-8
17. Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behavior change to investigate implementation problems. Implement Sci. (2017) 2(1):77. doi: 10.1186/s13012-017-0605-9
18. Consolidated Framework for Implementation Research. Available at: https://cfirguide.org/ (Accessed May 20, 2022).
19. Means AR, Kemp CG, Gwayi-Chore MC, Gimbel S, Soi C, Sherr K, et al. Evaluating and optimizing the consolidated framework for implementation research (CFIR) for use in low- and middle-income countries: a systematic review. Implement Sci. (2020) 15(1):17. doi: 10.1186/s13012-020-0977-0
20. Soi C, Gimbel S, Chilundo B, Muchanga V, Matsinhe L, Sherr K. Human papillomavirus vaccine delivery in Mozambique: identification of implementation performance drivers using the consolidated framework for implementation research (CFIR). Implement Sci. (2018) 13(1):151. doi: 10.1186/s13012-018-0846-2
Keywords: cervical cancer prevention, human papillomavirus vaccine, Kenya, consolidated framework for implementation research, theoretical domain framework, theoretical framework for acceptability, implementation science
Citation: Umutesi G, Oluoch L, Weiner BJ, Bukusi E, Onono M, Njoroge B, Mecca L, Ngure K, Mugo NR and Barnabas RV (2023) HPV vaccination in Kenya: a study protocol to assess stakeholders' perspectives on implementation drivers of HPV vaccination and the acceptability of the reduced dose strategy among providers. Front. Health Serv. 3:1233923. doi: 10.3389/frhs.2023.1233923
Received: 3 June 2023; Accepted: 20 July 2023;
Published: 2 August 2023.
Edited by:
Edina Amponsah-Dacosta, University of Cape Town, South AfricaReviewed by:
Pia Rausche, Bernhard Nocht Institute for Tropical Medicine (BNITM), Germany© 2023 Umutesi, Oluoch, Weiner, Bukusi, Onono, Njoroge, Mecca, Ngure, Mugo and Barnabas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grace Umutesi Z3JhY2UudW11dGVzQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.