- 1Department of Genomic Health, Research Institute, Geisinger, Danville, PA, United States
- 2Heart and Vascular Institute, Geisinger, Danville, PA, United States
- 3Geisinger Commonwealth School of Medicine, Geisinger College of Health Sciences, Geisinger, Scranton, PA, United States
- 4Department of Population Health Sciences, Research Institute, Geisinger, Danville, PA, United States
- 5Family Heart Foundation, Pasadena, CA, United States
- 6Prevention Research Center in St. Louis, Brown School, Washington University in St. Louis, St. Louis, MO, United States
- 7Department of Surgery (Division of Public Health Sciences), Alvin J. Siteman Cancer Center, Washington University School of Medicine, Washington University in St. Louis, St. Louis, MO, United States
- 8University of Health Sciences and Pharmacy in St. Louis, St. Louis, MO, United States
- 9Division of Endocrinology, Metabolism and Lipid Research, John T. Milliken Department of Internal Medicine, Washington University School of Medicine in St. Louis, Washington University in St. Louis, St. Louis, MO, United States
- 1023andMe, Sunnyvale, CA, United States
Introduction: Familial hypercholesterolemia (FH) is a common inherited cholesterol disorder that, without early intervention, leads to premature cardiovascular disease. Multilevel strategies that target all components of FH care including identification, cascade testing, and management are needed to address gaps that exist in FH care. We utilized intervention mapping, a systematic implementation science approach, to identify and match strategies to existing barriers and develop programs to improve FH care.
Methods: Data were collected utilizing two methods: a scoping review of published literature, related to any component of FH care, and a parallel mixed method study using interviews and surveys. The scientific literature was searched using key words including “barriers” or “facilitators” and “familial hypercholesterolemia” from inception to December 1, 2021. The parallel mixed method study recruited individuals and families with FH to participate in either dyadic interviews (N = 11 dyads/22 individuals) or online surveys (N = 98 respondents). Data generated from the scoping review, dyadic interviews, and online surveys were used in the 6-step intervention mapping process. Steps 1–3 included a needs assessment, development of program outcomes and creation of evidence-based implementation strategies. Steps 4–6 included program development, implementation, and evaluation of implementation strategies.
Results: In steps 1–3, a needs assessment found barriers to FH care included underdiagnosis of the condition which led to suboptimal management due to a myriad of determinants including knowledge gaps, negative attitudes, and risk misperceptions by individuals with FH and clinicians. Literature review highlighted barriers to FH care at the health system level, notably the relative lack of genetic testing resources and infrastructure needed to support FH diagnosis and treatment. Examples of strategies to overcome identified barriers included development of multidisciplinary care teams and educational programs. In steps 4–6, an NHLBI-funded study, the Collaborative Approach to Reach Everyone with FH (CARE-FH), deployed strategies that focused on improving identification of FH in primary care settings. The CARE-FH study is used as an example to describe program development, implementation, and evaluation techniques of implementation strategies.
Conclusion: The development and deployment of evidence-based implementation strategies that address barriers to FH care are important next steps to improve identification, cascade testing, and management.
1. Introduction
Familial hypercholesterolemia (FH) is a common inherited cholesterol disorder (prevalence 1 in 250) which leads to premature cardiovascular disease when left untreated (1, 2). Patients with a pathogenic variant in an FH gene have triple the risk for atherosclerotic cardiovascular disease (ASCVD) when compared to those without a genetic variant at any low-density lipoprotein-cholesterol (LDL-C) level, due to lifelong exposure (3). Diagnosis is often made in middle-aged adults, after experiencing premature ASCVD (4). Event rates for an FH patient with prevalent ASCVD are 5-fold higher compared to those with no prior ASCVD (5). Treatment beginning in adolescence lowers the risk for ASCVD before age 40 years from about 25% to <1% (6, 7). Although diagnostic criteria and treatment guidelines exist, data from patient registries show that FH remains underdiagnosed and undertreated for decades (4, 8, 9).
Since FH is a disease that runs in families, it is imperative that family communication and cascade testing occur so that at-risk family members are notified of their risk and have the option to undergo testing for FH. Preliminary data from the MyCode Genomic Screening and Counseling Program at Geisinger showed that probands who received FH results had approximately three living at-risk first-degree relatives that should be notified of this diagnosis and their risk; however, cascade testing had only occurred for approximately 3.5% of those relatives. Strategies have been deployed in practice to address barriers for each component of FH care: identification, cascade testing, and management (10, 11). Such efforts include improving data monitoring, sending electronic notifications to clinicians, development of new clinical teams, etc. However, in the United States the identification gap has only been improved from 10% to 30% of people being diagnosed with FH and cascade testing efforts have been suboptimal (12, 13).
To date, a systematic implementation approach has not been taken to improve FH care. One method to systematically develop implementation strategies uses both intervention (14) and implementation mapping (15), and includes diverse stakeholder perspectives to inform and improve care (14, 16). The six-steps of intervention mapping build toward developing an intervention and its evaluation (14). The six-steps are: (1) needs assessment, (2) specifying change objectives, (3) selecting theory-based intervention methods and practical applications, (4) producing the program, (5) specifying implementation plans, and (6) generating an evaluation plan (14). Implementation mapping expands upon intervention mapping to add strategies to improve adoption, implementation, and maintenance. When a systematic approach has been applied in other health contexts, such as depression, there has been improvement in care (17, 18). In this paper, we describe a systematic adapted intervention (19) and implementation mapping approach, to identify and match implementation strategies to barriers to improve FH care.
2. Materials and methods
2.1. FH care
A comprehensive care approach for individuals and families with FH involves three components: identification of patients, cascade testing of at-risk family members, and effective lipid management of the affected individuals. Identification occurs when a patient meets clinical diagnostic criteria and/or has an identified disease-causing variant in one of the genes associated with FH. Cascade testing includes risk notification and testing of at-risk relatives for FH. Management is the clinical care path established by the clinicians and an individual patient with FH to reduce their cardiovascular event risk. Management is based on the application of evidence-based guidelines (1).
2.2. Data collection
Data on key determinants of FH care related to identification, cascade testing, and management including barriers and facilitators, attitudes, and perspectives were collected using two methods: (1) a scoping review of published literature, and (2) a mixed methods study using interviews and surveys.
2.2.1. Scoping review
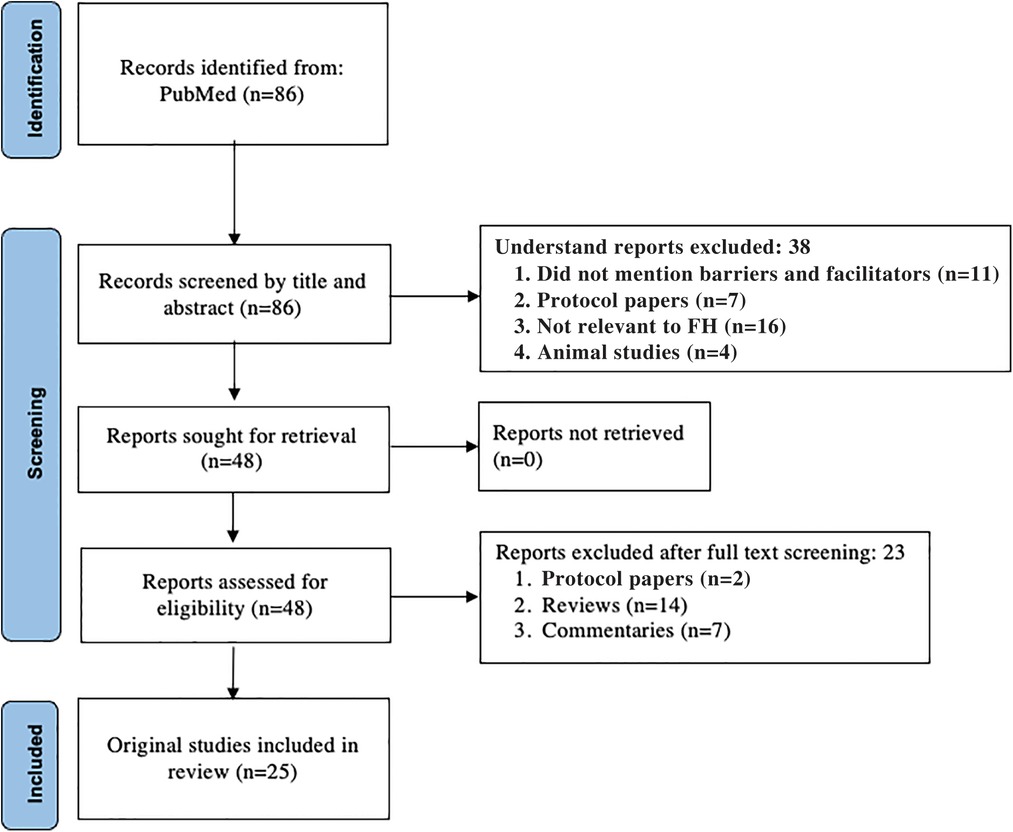
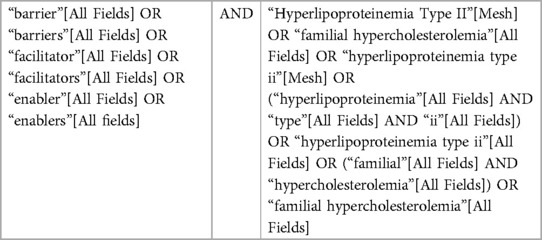
A scoping review was performed to identify published literature related to any component of FH care. PubMed was searched using key words including “barriers” or “facilitators” and “familial hypercholesterolemia” from inception to December 1, 2021 (Table 1). Articles were excluded if they were not relevant to FH, not relevant to a component of FH care including identification, cascade testing, or management, or if the publication type was a narrative review, commentary, protocol-only, nonhuman, or were not published in the English language. This initial search resulted in a total of 86 potential articles; 25 articles were included in the analysis after the exclusion criteria were applied during abstract and full-text review (Figure 1). Articles were then categorized by component of FH care.
2.2.2. Interviews and surveys
The mixed methods study recruited individuals and families with FH from Geisinger and the Family Heart Foundation to participate in either dyadic interviews (n = 11 dyads/22 individuals) or online surveys (n = 98 respondents). FH diagnosis was assigned by self-report or confirmed by genetic testing for those that participated in the MyCode Community Health Initiative at Geisinger (20). Two spouses participated in the dyadic interviews because they were the FH patient's caregiver and active in their care as well as communication with the family about FH. Dyadic phone interviews included the participant with an FH diagnosis and the family member they recruited to take part in the in-depth interview. Participants who completed interviews received a $20 Amazon gift card. Invitations to complete the online survey were sent via email to individuals identified through Geisinger and the Family Heart Foundation's databases, as well as via social media posts to the Family Heart Foundation's private groups. Snowball sampling was utilized by allowing survey respondents to invite their family members to complete a separate but similar version of the online survey. Survey participants were asked if they were the first person to be diagnosed with FH in their family. Survey respondents recruited from Geisinger were entered into a raffle to win one of five $50 Amazon gift cards. This recruitment strategy enabled us to have a sample of participants representing diverse diagnostic odysseys (i.e., journey to identification) and clinical experiences related to FH.
The interview guide and survey questions were developed through study team collaboration with the intent to elicit responses from participants about their experiences communicating about FH within their family (e.g., how did your family learn about FH? How does your family talk about FH?) (21, 22). Before interviews, participants were asked to review three family communication strategies and share their feedback on how to (re)design these strategies during the interviews (e.g., How can we improve the family letter? How would you want us to reach out to family members for a direct contact program?). Surveys were broken into three sections in which examples or additional information for each of the three family communication strategies was displayed and participants were asked open-ended questions about each strategy (e.g., How can we improve the family letter? How can we improve the chatbot? How would you want us to reach out to family members for a direct contact program?). The interview guide was tested with one dyad, iterated upon, and then deployed for all interviews thereafter (Supplementary File S1). Three separate versions of the survey were created for individuals with FH from Geisinger, individuals with FH from the Family Heart Foundation, and family members (Supplementary Files S2–S4). The combination of methods enabled triangulation of qualitative findings to capture the breadth and depth of experiences (23).
Audio-recorded dyadic phone interviews were transcribed, de-identified, and checked for accuracy before analysis. Open-ended survey responses were exported from the survey platform, de-identified, and checked for accuracy by ensuring there was only one IP address per response before inclusion in the full data set.
2.3. Procedures
This study deployed a modified version of intervention and implementation mapping (Table 2).
2.3.1. Steps 1–3
Data generated from the scoping review, dyadic interviews, and online surveys were used to inform intervention mapping steps 1–3 (19). A scoping review was conducted to perform a needs assessment and uncovered reported barriers and facilitators to FH care in the literature for step 1. Two medical students and a senior researcher evaluated inclusion of the articles in abstract and full text screening in duplicate. Data from the scoping review was compared to conducted dyadic interviews (interviews with individuals with FH and their families) and online surveys to create a complete list of barriers and facilitators (step 1). Data from step 1 was used to develop program outcomes (step 2). In step 2, behaviors that promote better FH care were analyzed at the individual, clinician, and health system level. Determinants of those behaviors were extracted and ranked based on their changeability and importance. The ranking was performed by sending a survey to the study team that consists of FH researchers, advocates, and individuals with FH. Step 3 deviated from the original steps of intervention mapping, in that instead of selecting behavioral change interventions, implementation strategies were selected. This change occurred because evidence-based guidelines for FH care exist, and the purpose of the study was to improve guideline translation, so development of implementation strategies was necessary. Results from steps 1–2 were mapped to the Expert Recommendations for Implementing Change (ERIC), a compilation of evidence-based implementation strategies generated by implementation experts (step 3) (24). The ERIC compilation was chosen because the concepts from steps 1 and 2 more closely aligned with this list of strategies.
2.3.2. Steps 4–6
To demonstrate steps 4–6 that include program development, implementation and evaluation, we provide an example of an FH program that was developed from the data generated in steps 1–3. This example includes a description of a funded study that is deploying implementation strategies to improve identification of FH in primary care.
3. Results
3.1. Demographics
3.1.1. Data from the scoping review
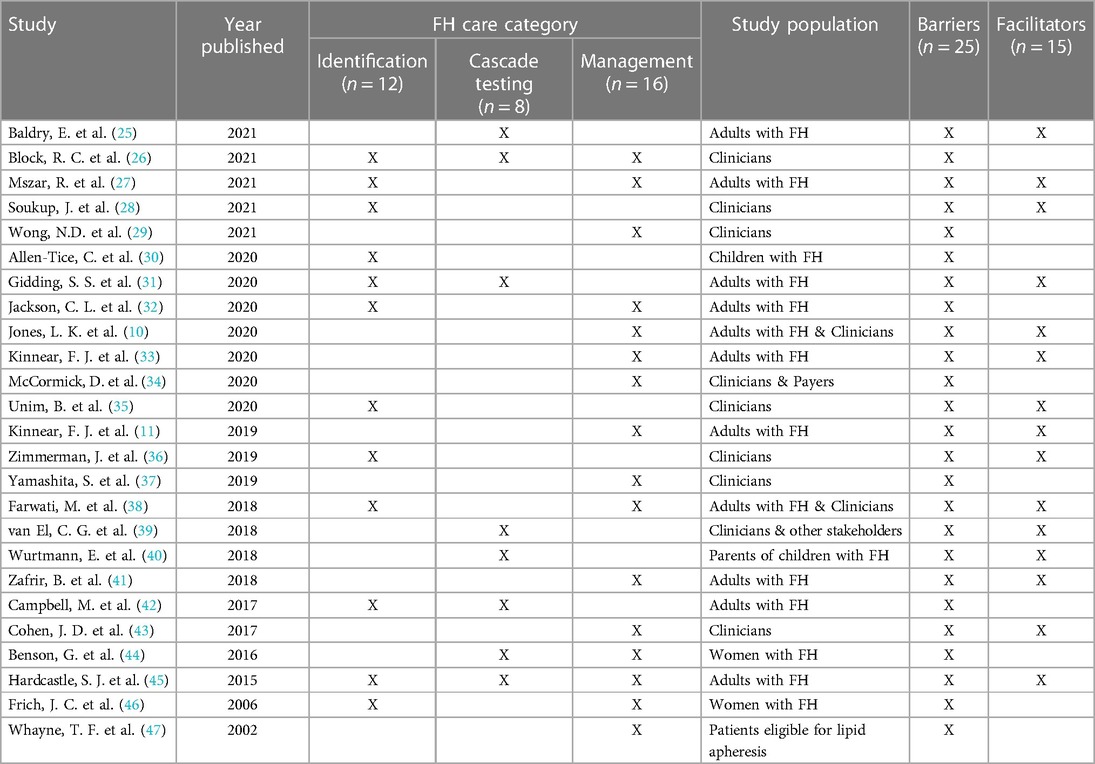
A total of 25 studies were included from a scoping review of the literature (Table 3). These studies were published between 2002 and 2021 and mention at least one component of FH care: 12 referenced identification, 8 referenced cascade testing, and 16 referenced management with several addressing more than one component. Only two studies addressed all three components of care (26, 45). Most studies reported on barriers and facilitators to FH care.
3.1.2. Data from interviews and surveys
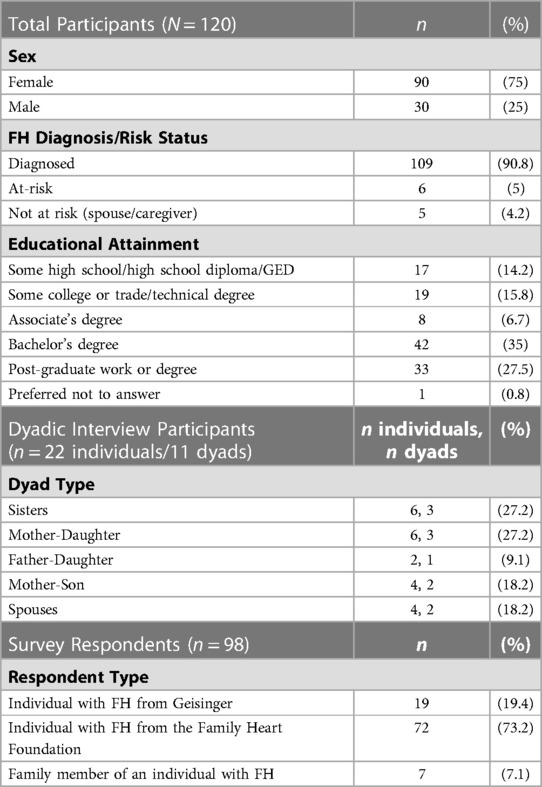
A total of 120 participants completed a dyadic interview or survey. Eleven family dyads (n = 22 individuals) were interviewed between July and August 2020. Detailed demographic information is available in Table 4.
3.2. Step 1: needs assessment
3.2.1. Summary of implementation problems identified
Data extracted from the scoping review of the literature, interviews, and surveys illuminated the barriers related to caring for individuals with FH and their families. These challenges can be categorized into three areas: identification, cascade testing, and management. Identification of FH has been a known problem worldwide with only 10%–30% of individuals estimated to have been diagnosed with FH (12, 13). Under-identification of FH has resulted in part from lack of a universally accepted definition for FH. To date, FH can be diagnosed via the presence of clinical criteria such as, high cholesterol levels and presence of family history with or without physical exam features. Multiple clinical screening tools exist, but there is not a gold standard. Alternatively, individuals can have a genetic diagnosis of FH by having a disease-causing variant in one of the genes associated with FH. There are also biological differences on how the ultimate health outcome, cardiovascular disease, presents in men as compared to women. Age-related differences in cholesterol levels exist due to the presence of other factors that affect lipid values over time, including environmental factors and diet. Limited screening during childhood has made it more difficult to prevent premature heart disease in early adulthood. Although, lipid screening in children is more discriminatory because children have not developed other risk factors thus if a high level of LDL-C is detected it is more likely to represent FH. By screening in childhood, it is also more likely to find an undiagnosed parent. Limitations due to privacy, family dynamics, geography, and other health and non-health-related concerns have presented family communication and cascade testing challenges. After identification and diagnosis, individuals with FH often receive suboptimal treatment. Women and children are less likely to be treated than adult men (48). Management of FH often requires daily combination lipid lowering therapy for life which can make adherence difficult.
3.2.2. Barriers and facilitators influencing behaviors and environmental conditions
Barriers and facilitators were categorized into three levels: individual-, clinician-, and health system-level (Table 5).
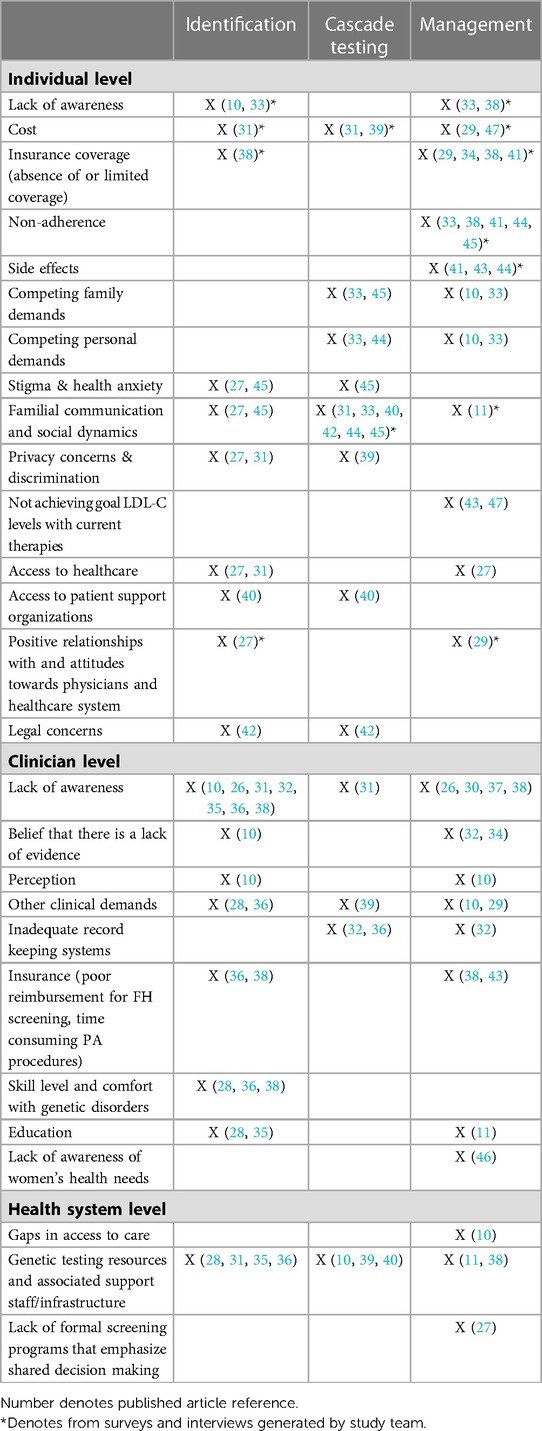
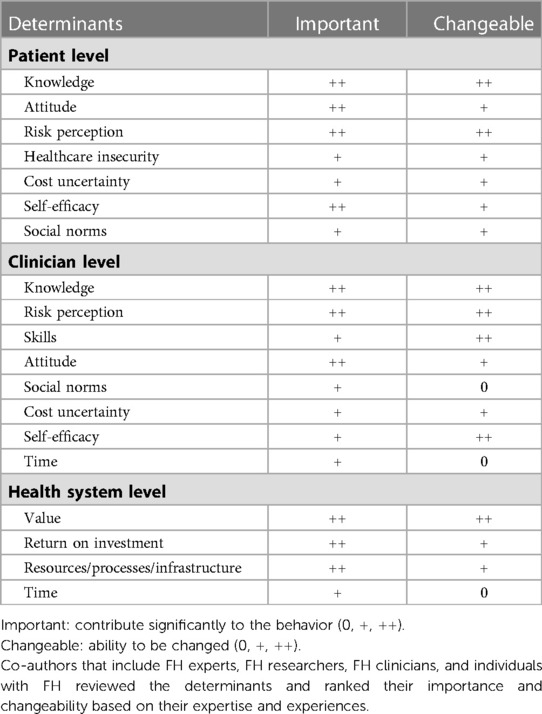
Table 5. Description of behaviors influencing FH care identified through published and unpublished literature.
3.2.2.1. Individual level
Barriers to FH identification, cascade testing, and management at the individual level include a lack of awareness of FH, which limits patients' ability to access testing and treatments. Additionally, interview and survey participants described ambivalent attitudes as another potential barrier to FH testing and treatments. Specifically, participants discussed how they or family members believed FH was not a serious condition or diagnosis, was not distinct from elevated cholesterol due to lifestyle, and the sense that high cholesterol is “the norm” in their family and to be expected. Participants described these attitudes as potentially undermining medical information about FH and as reducing likelihood they or their family members would feel a need to identify their high cholesterol FH or change current health management for high cholesterol. Next, while the cost of genetic testing is generally decreasing, financial concerns are still cited as a barrier to patient identification and family cascade testing, including confusion around the availability of insurance coverage for all types of testing for FH. Treatment costs and insurance coverage concerns also represent barriers when it comes to treatment(47), particularly with newer (brand-only) FDA-approved treatments, such as the proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies and use of procedures such as low-density lipoprotein (LDL) apheresis. Treatment-related side effects, especially those attributed to statins, were also reported as a barrier to FH management. Monotherapy with statins is often unable to provide sufficient LDL cholesterol (LDL-C) lowering and has been cited as a barrier in the management of FH.
Competing personal and family demands may also prevent individuals with FH from communicating with their families about cascade testing and preventing them from prioritizing their health (e.g., adhering to treatments and lifestyle modifications). Similarly, some individuals with FH report difficulty contacting family members for cascade testing due to social and intra-familial communication dynamics (e.g., patients who no longer communicate with some or all family members). Individuals with FH and their families report a fear of a loss of privacy of their genetic information if they were to be tested, or discrimination from insurance companies if they were to receive a genetic diagnosis of FH. While there are laws that protect health information and prohibit the use of genetic information by health insurers and most employers, many individuals are unaware of such protections or do not trust that these laws will protect their information.
Finally, it has also been noted that stigma and health anxiety may prevent FH patients and their families from getting tested (i.e., FH patients do not want to be diagnosed with a serious medical condition). Limited access to healthcare and lack of patient support groups have also been reported as factors that may impede FH identification, cascade testing, and management.
Participants described experiences in which clinicians gave incorrect information such as suggesting that high LDL-C levels were acceptable without further treatment options, cascade testing was not necessary for at-risk relatives, or exhibiting poor interpersonal interactions to scare individuals about their FH-related health risks. These experiences created a barrier related to trust in clinicians and the healthcare system that participants explained complicated their ability to undergo testing for FH and/or receive appropriate treatment recommendations.
3.2.2.2. Clinician level
At the clinician level, lack of awareness of FH as a specific genetic condition has also been cited as a barrier to testing in the index case, cascade testing, and management. Some clinicians believe that there is a lack of evidence to support FH identification and treatment, and a lack of FH-related education has been cited as a barrier to FH identification, cascade testing, and management among clinicians. Some clinicians (e.g., primary care clinicians) may also not feel comfortable with identifying genetic disorders in general, which hampers FH index patient identification. Clinicians may also feel that competing clinical demands (e.g., other health issues they need to cover with patients in short visits), affect their ability to initiate FH identification, cascade testing, and management. Inadequate record keeping systems also impede clinicians' ability to detect index cases with FH and initiate cascade testing. Clinicians also cite a lack of reimbursement as a limiting factor in FH identification and treatment. Finally, cascade testing is seen as difficult by some due to clinicians' legal concerns about making direct contact with a proband's family members.
3.2.2.3. Health system level
At the health system level, access to care, particularly access to specialists, has been cited as a barrier to FH management. Similarly, organized FH screening programs that would facilitate FH identification and cascade testing are lacking. Healthcare systems, in general, may not have the infrastructure and resources (e.g., lipid-management specialists, genetic counseling programs, etc.) necessary to meet the needs of the FH population.
3.2.3. Step 2: program outcomes and objectives
3.2.3.1. Behaviors that promote better FH care
3.2.3.1.1. Individual level
The level of individual patient and family knowledge of FH and its genetic basis potentially impacts FH identification. Individuals with FH felt that learning of their condition, and its specific genetic basis, is important and will prompt them to communicate the result with their at-risk relatives. They felt this would likewise prompt their relatives to undergo testing for FH and improve both their and their relatives' adherence to management recommendations. In addition, individuals can encourage screening when discussing the FH result with their at-risk relatives. Individuals who understand the importance of taking their medications as prescribed are often more willing to discuss medication-related side effects with their clinicians.
3.2.3.1.2. Clinician level
Behaviors that affect identification include knowledge and implementation of guideline recommendations to screen for and identify FH. It is important for clinicians to understand that earlier identification is key to preventing future cardiovascular disease. Clinicians can also promote and facilitate communication between the individual with FH and their at-risk relatives. Clinicians have a key role in understanding and recommending appropriate treatment options and intensify treatment regimens.
3.2.3.1.3. Health system level
Health systems may help to improve components of FH care by implementing protocols that make screening for FH easy for both the initial patient identified and their at-risk relatives, allow clinicians including primary care clinicians, specialists, and other healthcare clinicians to share responsibilities for FH care and remove barriers to ordering of testing and medications for FH. A health system's central laboratory could adopt language on lipid results prompting the clinician to consider FH when an LDL-C is found to be over 190 mg/dl.
3.2.3.5. Determinants
Based on the barriers and facilitators identified through the mixed methods study, determinants are ranked by their ability to be addressed (changeable) and their contribution to the behavior (important) at the individual, clinician, and health system level (Table 6). The most mentioned determinants across all levels of care were knowledge, attitude, and risk perception. These determinants will serve as priority topics for development of implementation outcomes.
3.2.4. Step 3: implementation strategies mapped to program outcomes and objectives
Implementation strategies that can be deployed to help translate the evidence into clinical practice are detailed in Table 7. These implementation strategies were mapped to program outcomes and objectives and were standardized using the ERIC compilation. These strategies may provide a guide that can be used to develop tailored implementation strategies for a specific component of FH care. Most of these implementation strategies can be defined to address each component of FH care.
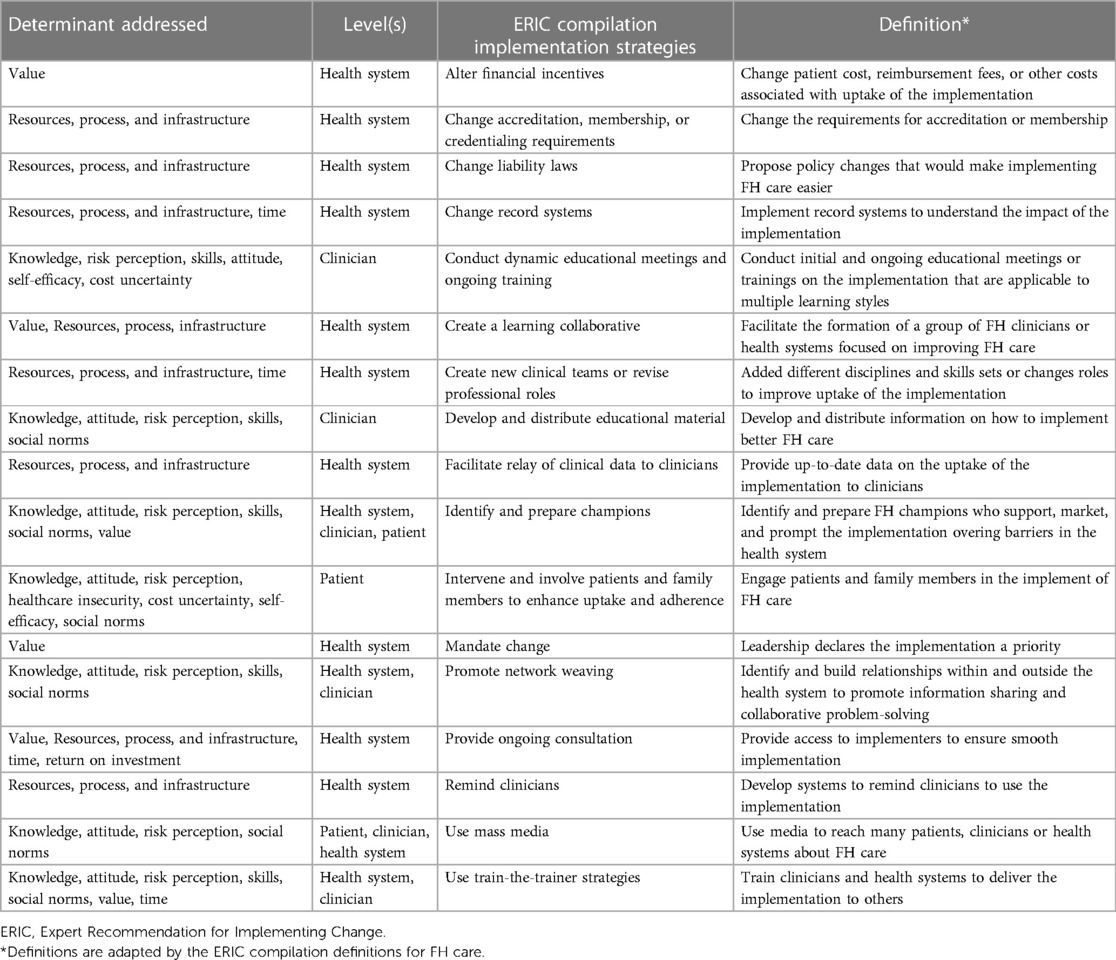
Table 7. Examples of implementation strategies that address determinants that relate to all components of FH care.
To ensure success and sustainability after deployment of the implementation strategies, it is important to develop a plan to obtain ongoing feedback from all stakeholders involved, reexamine the implementation outcomes, and provide assistance at the level of the patient, family, and clinician up to the health system, to help improve utility of the implementation strategies. These strategies will provide information on whether the implementation should be altered based on external and internal factors.
3.3. Example of a current FH program with an implementation and evaluation plan
Steps 4–6 were satisfied by designing CARE-FH (Collaborative Approach to Reach Everyone with FH), a clinical trial of implementation strategies, is funded by National Heart Lung and Blood Institute, and based on findings from steps 1–3.The goal of CARE-FH is to improve FH identification in primary care (49).
3.3.1. Step 4: design the intervention
Steps 1–3 determined that there was a gap in translating evidence-based guidelines related to screening for FH into practice. The needs assessment found that screening for FH is recommended but not routinely performed. The decision was made that the evidence-based guidance would be based on the 2018 AHA/ACC/Multi-society Cholesterol Guidelines and the 2020 Expert Consensus Genetic Testing Statement (1, 50). These evidence-based guidelines were used to generate the diagnostic screening algorithm for clinicians to screen for FH in the study (Figure 2).
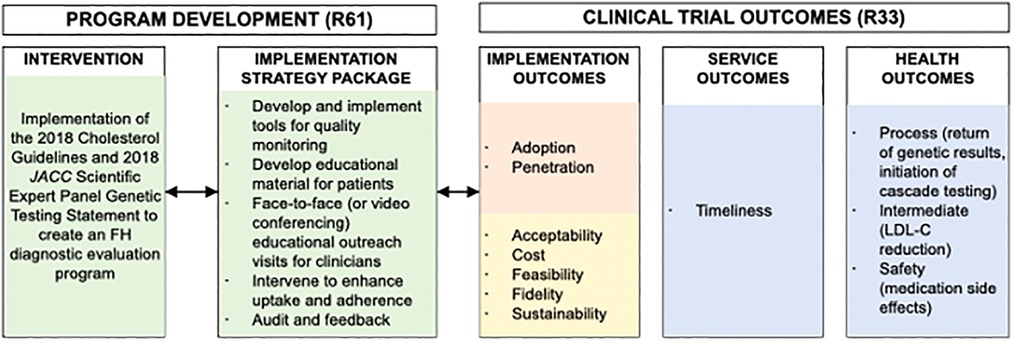
Figure 2. Adapted conceptual model of implementation research for the CARE-FH study. Reproduced under the CC-BY license from Jones LK et al. (49).
3.3.2. Step 5: create an implementation approach
The CARE-FH study team selected implementation strategies relevant to FH identification from Table 7. These implementation strategies included: conduct dynamic educational meetings and ongoing training, develop and distribute educational material, intervene and involve patients and family members to enhance uptake and adherence, remind clinicians, and facilitate relay of clinical data to clinicians. The proposed comprehensive multi-level implementation strategy package was based on the ERIC compilation (Table 8). These implementation strategies were tailored to the Geisinger primary care practice using a 1-year pre-implementation phase where surveys, contextual inquiries, and pilot testing of the strategies was conducted.
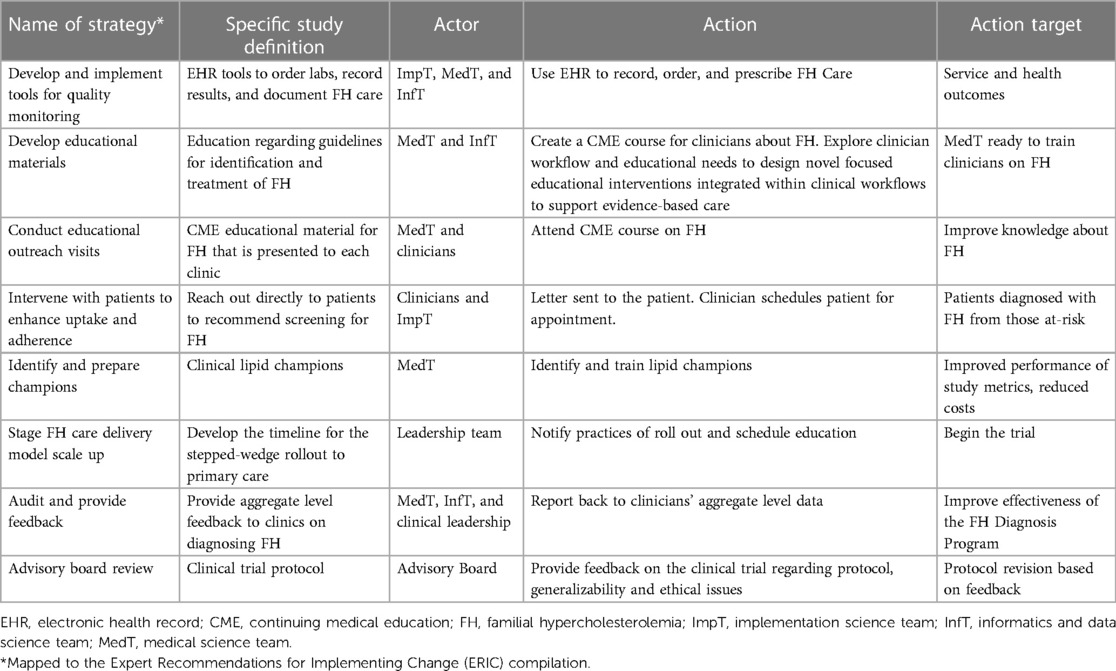
Table 8. CARE-FH study proposed multi-level implementation strategies. Reproduced under the CC-BY license from Jones LK et al. (49).
3.3.3. Step 6: develop an evaluation plan
An evaluation plan was developed using the Conceptual Model for Implementation Research and included the following implementation outcomes: adoption, penetration, acceptability, feasibility, fidelity, sustainability, and cost (51, 52). The study team has adapted this model to CARE-FH (Figure 3). The evaluation plan also includes service and health outcomes are detailed in Table 9.
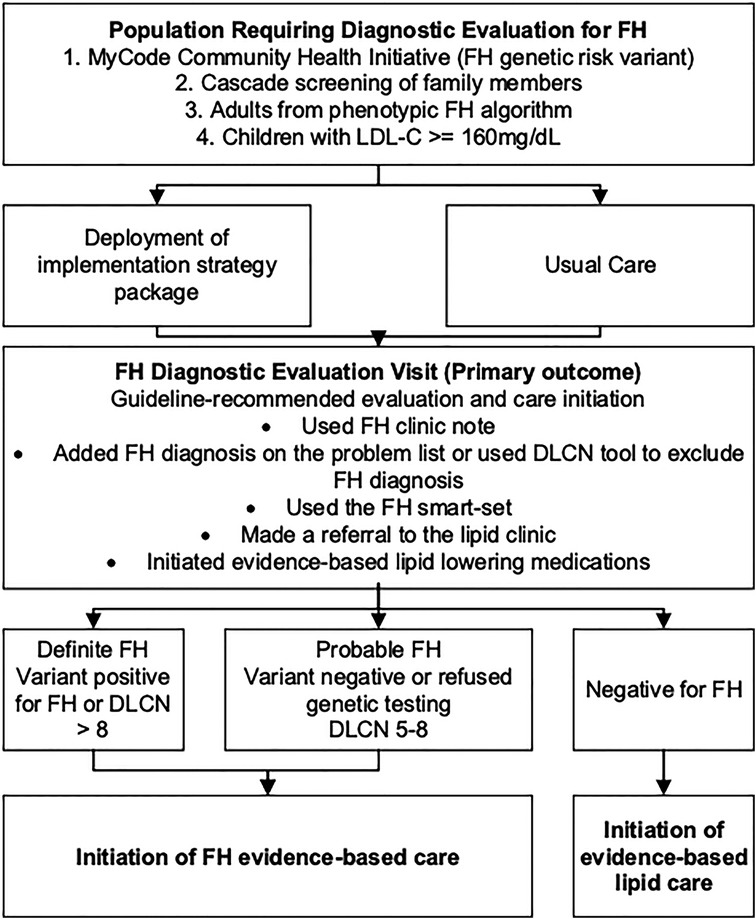
Figure 3. CARE-FH study diagnostic evaluation plan. Reproduced under the CC-BY license from Jones LK et al. (49).
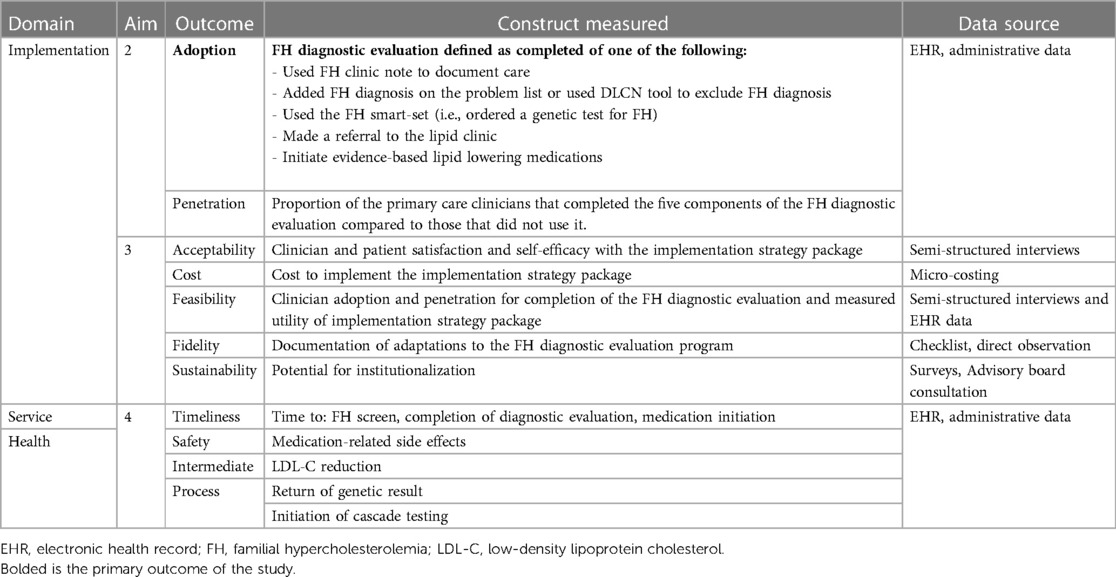
Table 9. CARE-FH study evaluation plan. Reproduced under the CC-BY license from Jones LK et al. (51).
4. Discussion
The application of principles from implementation science to the field of FH has been discussed in many recent articles including original research, reviews, commentaries, and guidelines (53–63). By leveraging implementation science, which aims to close the large gap from knowledge generation to implementation into practice, we can improve every component of FH care.
Intervention mapping provides a systematic process for developing evidence-based implementation strategies to improve FH care. Through steps 1–3 in this process, we identified barriers and facilitators to three components of FH care: identification, cascade testing, and management. We also found that barriers and facilitators may not be one-dimensional and exist at the patient, clinician, and health system levels. To overcome these barriers, we need to develop an implementation strategy package that addresses each level and component of FH care. We have provided a list of implementation strategies specific to FH care that others can adapt to their local context. For steps 4–6, we highlighted an example of a currently funded study, CARE-FH, that is using implementation strategies based on evidence to improve FH identification. This systematic evaluation using intervention mapping allowed us to develop implementation strategies and allows other teams to replicate and/or adapt these strategies by other teams. The FH specific strategies that were developed in this study can be tailored by others to their specific context to improve care.
To date, there have been many explorations of barriers and facilitators to FH care, but few have developed strategies to address them that can then be implemented into practice and subsequently evaluated (10). Previous studies focused on only one component of FH care or at one level (patient, clinician, or health system) (53, 64). By addressing these barriers for components of FH care at multiple levels, we can more thoroughly address the problems faced by individuals with FH and their families.
Due to the limited evidence on implementation strategies to improve FH care, two recent review articles have retrospectively mapped interventions from previous studies to a compilation of implementation strategies (53, 65). This work has facilitated the use of a common language for naming and describing implementation strategies. By having a common nomenclature, it becomes easier to tailor implementation strategies for specific contexts such as FH care. From these two articles, we know that only certain implementation strategies, including assess for readiness and identify barriers and facilitators, develop and organize quality monitoring systems, create new clinicals teams, facilitate relay of clinical data to providers, and involve patients and family members, have been tested in practice for FH care (53). There is a need to deploy other implementation strategies listed in compilations such as promote network weaving, create a learning collaborative, change liability law, among others (53, 65). In addition, implementation strategies need to be explained using a standardized reporting method so they can be replicated in the future (53, 65, 66).
Some cholesterol and FH guidelines have started to include sections on how to help improve the translation of their guidelines into practice (1, 60–63, 67). However, these guidelines are not formatted in such a way as to promote their translation and implementation in the clinic setting (54). A recent editorial provides a framework to help facilitate the translation of evidence-based recommendations with implementation recommendation to create clinical practice guidelines that can then be implemented and evaluated in local contexts (55).
4.1. Limitations
An important limitation is that not all health systems, clinicians, or patients will have the ability to implement strategies that affect multiple levels or multiple components of FH care. It will be important to identify strategies that are relevant to specific health contexts and the needs of particular health systems. This project only reported implementation strategies that we have found important for our work in our health care context, but other strategies might arise or need to be adapted. Another limitation is that this study only reported on the ERIC compilation of strategies that were relevant for the implementation phase of a study and not those that are important for pre-implementation work. Steps 1 and 2 of intervention mapping include strategies relevant for pre-implementation, including conducting a needs assessment, identifying barriers and facilitators, and assessing readiness of the organization to implement the evidence-based practice. Barriers and facilitator data collected from FH patients was supplemented with the literature to account for broader perspectives. Additional pre-implementation strategies that should be considered prior to implementation include developing evaluative and iterative strategies (e.g., developing and organizing quality monitoring systems) and adapting and tailoring strategies to the local context.
5. Conclusions
Using a systematic, evidence-based, multilevel approach to the development of implementation strategies, implementation recommendations, and evaluation is imperative to success in changing practice and care for individuals with FH. This study provides an overview of one evidence-based approach to accomplish this task: intervention mapping. The implementation strategies developed as part of this report can be utilized by others to improve FH care and learnings from the highlighted study can facilitate near-term deployment into practice as well as evaluation of both clinical and implementation outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The Geisinger Institutional Review Board determined that this project was not a systematic investigation designed to develop or contribute to generalizable knowledge as defined at 45 CFR 46.102(1), and was therefore not research. All procedures performed in studies involving human participants were in accordance with the ethical standard of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Participants agreed verbally to participate in interviews and in writing to complete surveys.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LJ, EC, GC, NW, AB, GR, MM, AR, AS. The first draft of the manuscript was written by LJ. All authors contributed to the article and approved the submitted version.
Funding
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R61HL161775, K12HL137942 and R01HL148246. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The documents in the Supplementary Material have been published before in the following study: Campbell-Salome G, Jones LK, Walters NL, Morgan KM, Brangan A, Ladd IG, et al. Optimizing communication strategies and designing a comprehensive program to facilitate cascade testing for familial hypercholesterolemia. BMC Health Serv Res. (2023) 23(1):340. doi: 10.1186/s12913-023-09304-y. PMID: 37020233; PMCID: PMC10074725.
Conflict of interest
LJ is a consultant for Novartis Corporation. SG is a consultant for Esperion. AG has received research funding from Amgen, Sanofi, Novartis, Arrowhead, IONIS, Regeneron, New Amsterdam, Esperion, and Pfizer and is a consultant for IONIS, New Amsterdam, and Regeneron. AS is an employee and has stockholder in 23andMe and an Advisor to Nest Genomics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2023.1104311/full#supplementary-material.
References
1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139(25):e1082–143. doi: 10.1161/CIR.0000000000000625.30586774
2. Abul-Husn N, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, et al. Genetic identification of familial hypercholesterolemia within a single U.S. Health care system. Science (New York, NY). (2016) 354(6319):aaf7000. doi: 10.1126/science.aaf7000
3. Khera AV, Won H-H, Peloso GM, Lawson KS, Bartz TM, Deng X, et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. (2016) 67(22):2578–89. doi: 10.1016/j.jacc.2016.03.520
4. deGoma EM, Ahmad ZS, O'Brien EC, Kindt I, Shrader P, Newman CB, et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH registry. Circ Cardiovasc Genet. (2016) 9(3):240–9. doi: 10.1161/CIRCGENETICS.116.001381
5. Duell PB, Gidding SS, Andersen RL, Knickelbine T, Anderson L, Gianos E, et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis. (2019) 289:85–93. doi: 10.1016/j.atherosclerosis.2019.08.007
6. Watts GF, Gidding SS, Mata P, Pang J, Sullivan DR, Yamashita S, et al. Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol. (2020) 17(6):360–77. doi: 10.1038/s41569-019-0325-8
7. Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, et al. 20-Year Follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. (2019) 381(16):1547–56. doi: 10.1056/NEJMoa1816454
8. Pérez de Isla L, Alonso R, Mata N, Fernández-Pérez C, Muñiz O, Díaz-Díaz JL, et al. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART registry (spanish familial hypercholesterolemia cohort study). Circulation. (2017) 135(22):2133–44. doi: 10.1161/CIRCULATIONAHA.116.024541
9. Vallejo-Vaz AJ, De Marco M, Stevens CAT, Akram A, Freiberger T, Hovingh GK, et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries—the EAS familial hypercholesterolaemia studies collaboration (FHSC). Atherosclerosis. (2018) 277:234–55. doi: 10.1016/j.atherosclerosis.2018.08.051
10. Jones LK, Sturm AC, Seaton TL, Gregor C, Gidding SS, Williams MS, et al. Barriers, facilitators, and solutions to familial hypercholesterolemia treatment. PLoS One. (2020) 15(12):e0244193. doi: 10.1371/journal.pone.0244193
11. Kinnear FJ, Wainwright E, Perry R, Lithander FE, Bayly G, Huntley A, et al. Enablers and barriers to treatment adherence in heterozygous familial hypercholesterolaemia: a qualitative evidence synthesis. BMJ Open. (2019) 9(7):e030290. doi: 10.1136/bmjopen-2019-030290
12. MacDougall DE MM, Ahmed CD, Rader DJ, Knowles JW, Wilemon KA, Myers KD. Diagnosis of familial hypercholesterolemia: A work in progress. Louisville, KY: American Society for Preventive Cardiology (2022).
13. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European atherosclerosis society. Eur Heart J. (2013) 34(45):3478–90. doi: 10.1093/eurheartj/eht273
14. Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav. (1998) 25(5):545–63. doi: 10.1177/109019819802500502
15. Fernandez ME, Ten Hoor GA, Van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. (2019) 7:158. doi: 10.3389/fpubh.2019.00158
16. Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res. (2017) 44(2):177–94. doi: 10.1007/s11414-015-9475-6
17. Jabbour M, Curran J, Scott SD, Guttman A, Rotter T, Ducharme FM, et al. Best strategies to implement clinical pathways in an emergency department setting: study protocol for a cluster randomized controlled trial. Implement Sci. (2013) 8:55. doi: 10.1186/1748-5908-8-55
18. Zwerver F, Schellart AJ, Anema JR, Rammeloo KC, van der Beek AJ. Intervention mapping for the development of a strategy to implement the insurance medicine guidelines for depression. BMC Public Health. (2011) 11:9. doi: 10.1186/1471-2458-11-9
19. Eldredge LKB, Markham CM, Ruiter RA, Fernández ME, Kok G, Parcel GS. Planning health promotion programs: an intervention mapping approach. San Francisco: John Wiley & Sons (2016).
20. Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, et al. The geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med. (2016) 18(9):906–13. doi: 10.1038/gim.2015.187
21. Campbell-Salome G, Jones LK, Masnick MF, Walton NA, Ahmed CD, Buchanan AH, et al. Developing and optimizing innovative tools to address familial hypercholesterolemia underdiagnosis: identification methods, patient activation, and cascade testing for familial hypercholesterolemia. Circ Genom Precis Med. (2021) 14(1):e003120. doi: 10.1161/CIRCGEN.120.003120
22. Campbell-Salome G, Walters NL, Ladd IG, Sheldon A, Ahmed CD, Brangan A, et al. Motivating cascade testing for familial hypercholesterolemia: applying the extended parallel process model for clinician communication. Transl Behav Med. (2022) 12(7):800–9. doi: 10.1093/tbm/ibac018
23. Thurmond VA. The point of triangulation. J Nurs Scholarsh. (2001) 33(3):253–8. doi: 10.1111/j.1547-5069.2001.00253.x
24. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci. (2015) 10(1):1–14. doi: 10.1186/s13012-015-0209-1
25. Baldry E, Redlinger-Grosse K, MacFarlane I, Walters ST, Ash E, Steinberger J, et al. Outcomes from a pilot genetic counseling intervention using motivational interviewing and the extended parallel process model to increase cascade cholesterol screening. J Genet Couns. (2021) 31(1):164–75. doi: 10.1002/jgc4.1466
26. Block RC, Bang M, Peterson A, Wong ND, Karalis DG. Awareness, diagnosis and treatment of heterozygous familial hypercholesterolemia (HeFH)—results of a US national survey. J Clin Lipidol. (2021) 15(5):682–89. doi: 10.1016/j.jacl.2021.09.045
27. Mszar R, Buscher S, McCann D, Taylor HL. Self-Efficacy, perceived barriers to care, and health-promoting behaviors among franco-Americans across cardiovascular risk factors: a cross-sectional study. Am J Health Promot. (2021) 35(5):703–7. doi: 10.1177/0890117120982412
28. Soukup J, Zierhut HA, Ison HE. Universal cholesterol screening among pediatric primary care providers within California and Minnesota: a qualitative assessment of barriers and facilitators. J Pediatr. (2021) 233:175–82.e2. doi: 10.1016/j.jpeds.2021.02.065
29. Wong ND, Bang M, Block RC, Peterson ALH, Karalis DG. Perceptions and barriers on the use of proprotein subtilisin/kexin type 9 inhibitors in heterozygous familial hypercholesterolemia (from a survey of primary care physicians and cardiologists). Am J Cardiol. (2021) 152:57–62. doi: 10.1016/j.amjcard.2021.04.034
30. Allen-Tice C, Steinberger J, Murdy K, Zierhut H. Pediatric cholesterol screening practices in 9- to 11-year-olds in a large midwestern primary care setting. J Clin Lipidol. (2020) 14(2):224–30. doi: 10.1016/j.jacl.2020.01.013
31. Gidding SS, Sheldon A, Neben CL, Williams HE, Law S, Zhou AY, et al. Patient acceptance of genetic testing for familial hypercholesterolemia in the CASCADE FH registry. J Clin Lipidol. (2020) 14(2):218–23.e2. doi: 10.1016/j.jacl.2020.02.001
32. Jackson CL, Ahmad Z, Das SR, Khera A. The evaluation and management of patients with LDL-C ≥ 190 mg/dl in a large health care system. Am J Prev Cardiol. (2020) 1:100002. doi: 10.1016/j.ajpc.2020.100002
33. Kinnear FJ, Wainwright E, Bourne JE, Lithander FE, Hamilton-Shield J, Searle A. The development of a theory informed behaviour change intervention to improve adherence to dietary and physical activity treatment guidelines in individuals with familial hypercholesterolaemia (FH). BMC Health Serv Res. (2020) 20(1):27. doi: 10.1186/s12913-019-4869-4
34. McCormick D, Bhatt DL, Bays HE, Taub PR, Caldwell KA, Guerin CK, et al. A regional analysis of payer and provider views on cholesterol management: pCSK9 inhibitors as an illustrative alignment model. J Manag Care Spec Pharm. (2020) 26(12):1517–28. doi: 10.18553/jmcp.2020.26.12.1517
35. Unim B, De Vito C, Hagan J, Villari P, Knoppers BM, Zawati M. The provision of genetic testing and related services in Quebec, Canada. Front Genet. (2020) 11:127. doi: 10.3389/fgene.2020.00127
36. Zimmerman J, Duprez D, Veach PM, Zierhut HA. Barriers to the identification of familial hypercholesterolemia among primary care providers. J Community Genet. (2019) 10(2):229–36. doi: 10.1007/s12687-018-0383-3
37. Yamashita S, Masuda D, Arai H, Matsuzawa Y. Cultural barriers in the treatment of dyslipidemia: a survey of Japanese physician attitudes. J Atheroscler Thromb. (2019) 26(2):154–69. doi: 10.5551/jat.44677
38. Farwati M, Kumbamu A, Kochan DC, Kullo IJ. Patient and provider perspectives on a decision aid for familial hypercholesterolemia. J Pers Med. (2018) 8(4):35. doi: 10.3390/jpm8040035
39. van El CG, Baccolini V, Piko P, Cornel MC. Stakeholder views on active cascade screening for familial hypercholesterolemia. Healthcare (Basel). (2018) 6(3):108. doi: 10.3390/healthcare6030108
40. Wurtmann E, Steinberger J, Veach PM, Khan M, Zierhut H. Risk communication in families of children with familial hypercholesterolemia: identifying motivators and barriers to cascade screening to improve diagnosis at a single medical center. J Genet Couns. (2018).30109451
41. Zafrir B, Jubran A. Lipid-lowering therapy with PCSK9-inhibitors in the real-world setting: two-year experience of a regional lipid clinic. Cardiovasc Ther. (2018) 36(5):e12439. doi: 10.1111/1755-5922.12439
42. Campbell M, Humanki J, Zierhut H. A novel approach to screening for familial hypercholesterolemia in a large public venue. J Community Genet. (2017) 8(1):35–44. doi: 10.1007/s12687-016-0285-1
43. Cohen JD, Cziraky MJ, Jacobson TA, Maki KC, Karalis DG. Barriers to PCSK9 inhibitor prescriptions for patients with high cardiovascular risk: results of a healthcare provider survey conducted by the national lipid association. J Clin Lipidol. (2017) 11(4):891–900. doi: 10.1016/j.jacl.2017.04.120
44. Benson G, Witt DR, VanWormer JJ, Campbell SM, Sillah A, Hayes SN, et al. Medication adherence, cascade screening, and lifestyle patterns among women with hypercholesterolemia: results from the WomenHeart survey. J Clin Lipidol. (2016) 10(4):937–43. doi: 10.1016/j.jacl.2016.03.012
45. Hardcastle SJ, Legge E, Laundy CS, Egan SJ, French R, Watts GF, et al. Patients’ perceptions and experiences of familial hypercholesterolemia, cascade genetic screening and treatment. Int J Behav Med. (2015) 22(1):92–100. doi: 10.1007/s12529-014-9402-x
46. Frich JC, Malterud K, Fugelli P. Women at risk of coronary heart disease experience barriers to diagnosis and treatment: a qualitative interview study. Scand J Prim Health Care. (2006) 24(1):38–43. doi: 10.1080/02813430500504305
47. Whayne TF Jr., Zielke JC, Dickson LG, Winters JL. State of the art treatment of the most difficult low density lipoprotein (LDL) cholesterol problems: lDL apheresis. J Ky Med Assoc. (2002) 100(12):535–8.12522946
48. Balla S, Ekpo EP, Wilemon KA, Knowles JW, Rodriguez F. Women living with familial hypercholesterolemia: challenges and considerations surrounding their care. Curr Atheroscler Rep. (2020) 22(10):1–13. doi: 10.1007/s11883-020-00881-5
49. Jones LK, Williams MS, Ladd IG, Cawley D, Ge S, Hao J, et al. Collaborative approach to reach everyone with familial hypercholesterolemia: CARE-FH protocol. J Pers Med. (2022) 12(4):606. doi: 10.3390/jpm12040606
50. Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, et al. Clinical genetic testing for familial hypercholesterolemia: jACC scientific expert panel. J Am Coll Cardiol. (2018) 72(6):662–80. doi: 10.1016/j.jacc.2018.05.044
51. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38(2):65–76. doi: 10.1007/s10488-010-0319-7
52. Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. (2009) 36(1):24–34. doi: 10.1007/s10488-008-0197-4
53. Jones LK, Brownson RC, Williams MS. Applying implementation science to improve care for familial hypercholesterolemia. Curr Opin Endocrinol Diabetes Obes. (2022) 29(2):141–51. doi: 10.1097/MED.0000000000000692
54. Jones LK, Sturm AC, Gionfriddo MR. Translating guidelines into practice via implementation science: an update in lipidology. Curr Opin Lipidol. (2022) 33(6):336–41. doi: 10.1097/MOL.0000000000000835
55. Sarkies MN, Jones LK, Gidding SS, Watts GF. Improving clinical practice guidelines with implementation science. Nat Rev Cardiol. (2022) 19(1):3–4. doi: 10.1038/s41569-021-00645-x
56. Pang J, Sullivan DR, Hare DL, Colquhoun DM, Bates TR, Ryan JD, et al. Gaps in the care of familial hypercholesterolaemia in Australia: first report from the national registry. Heart Lung Circ. (2021) 30(3):372–9. doi: 10.1016/j.hlc.2020.07.012
57. Pang J, Sullivan DR, Harada-Shiba M, Ding PY, Selvey S, Ali S, et al. Significant gaps in awareness of familial hypercholesterolemia among physicians in selected Asia-pacific countries: a pilot study. J Clin Lipidol. (2015) 9(1):42–8. doi: 10.1016/j.jacl.2014.09.011
58. Birnbaum RA, Horton BH, Gidding SS, Brenman LM, Macapinlac BA, Avins AL. Closing the gap: identification and management of familial hypercholesterolemia in an integrated healthcare delivery system. J Clin Lipidol. (2021) 15(2):347–57. doi: 10.1016/j.jacl.2021.01.008
59. Pang J, Chan DC, Hu M, Muir LA, Kwok S, Charng MJ, et al. Comparative aspects of the care of familial hypercholesterolemia in the “ten countries study”. J Clin Lipidol. (2019) 13(2):287–300. doi: 10.1016/j.jacl.2019.01.009
60. Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, et al. Integrated guidance on the care of familial hypercholesterolaemia from the international FH foundation. Eur J Prev Cardiol. (2015) 22(7):849–54. doi: 10.1177/2047487314533218
61. Watts GF, Sullivan DR, Hare DL, Kostner KM, Horton AE, Bell DA, et al. Integrated guidance for enhancing the care of familial hypercholesterolaemia in Australia. Heart Lung Circ. (2021) 30(3):324–49. doi: 10.1016/j.hlc.2020.09.943
62. National Institute for Health and Care Excellence: Guidelines. Familial hypercholesterolaemia: identification and management. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2019 (2019).
63. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and European atherosclerosis society (EAS). Eur Heart J. (2020) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
64. Jones LK, Walters N, Brangan A, Ahmed CD, Gatusky M, Campbell-Salome G, et al. Acceptability, appropriateness, and feasibility of automated screening approaches and family communication methods for identification of familial hypercholesterolemia: stakeholder engagement results from the IMPACT-FH study. J Pers Med. (2021) 11(6):587. doi: 10.3390/jpm11060587
65. Jones LK, Tilberry S, Gregor C, Yaeger LH, Hu Y, Sturm AC, et al. Implementation strategies to improve statin utilization in individuals with hypercholesterolemia: a systematic review and meta-analysis. Implement Sci. (2021) 16(1):40. doi: 10.1186/s13012-021-01108-0
66. Moise N, Cené CW, Tabak RG, Young DR, Mills KT, Essien UR, et al. Leveraging implementation science for cardiovascular health equity: a scientific statement from the American heart association. Circulation. (2022) 146(19):e260–78. doi: 10.1161/CIR.0000000000001096
Keywords: familial hypercholesterolemia, implementation science, intervention mapping, identification, cascade testing, treatment, management, cholesterol
Citation: Jones LK, Calvo EM, Campbell-Salome G, Walters NL, Brangan A, Rodriguez G, Ahmed CD, Morgan KM, Gidding SS, Williams MS, Brownson RC, Seaton TL, Goldberg AC, McGowan MP, Rahm AK and Sturm AC (2023) Designing implementation strategies to improve identification, cascade testing, and management of families with familial hypercholesterolemia: An intervention mapping approach. Front. Health Serv. 3:1104311. doi: 10.3389/frhs.2023.1104311
Received: 21 November 2022; Accepted: 6 April 2023;
Published: 28 April 2023.
Edited by:
Per Nilsen, Linköping University, SwedenReviewed by:
Jane Lewis, Centre for Evidence and Implementation, United KingdomAldo Grefhorst, Amsterdam University Medical Center, Netherlands
© 2023 Jones, Calvo, Campbell-Salome, Walters, Brangan, Rodriguez, Ahmed, Morgan, Gidding, Williams, Brownson, Seaton, Goldberg, Mcgowan, Rahm and Sturm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laney K. Jones ljones14@geisinger.edu
 Laney K. Jones
Laney K. Jones Evan M. Calvo1,3
Evan M. Calvo1,3 Gemme Campbell-Salome
Gemme Campbell-Salome Marc S. Williams
Marc S. Williams Ross C. Brownson
Ross C. Brownson Terry L. Seaton
Terry L. Seaton Alanna K. Rahm
Alanna K. Rahm