
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Health Serv. , 06 September 2022
Sec. Implementation Science
Volume 2 - 2022 | https://doi.org/10.3389/frhs.2022.968175
This article is part of the Research Topic Perspectives and Opinions in Health Services View all 14 articles
 Justin Knox1,2,3*
Justin Knox1,2,3* Geoffrey M. Curran4,5
Geoffrey M. Curran4,5In a recent editorial published in Science (1), Proctor and Geng argue that COVID-19 has made evident that we need to more formally prioritize implementation research, the study of the uptake of evidence-based interventions, to ensure that our nation's health discoveries are fully realized. Relatedly, Dr. Francis Collins, head of the National Institutes of Health, recently acknowledged that we have underinvested in research on human behavior, as evidenced by the 60 million eligible Americans who have not been vaccinated against COVID-19 despite the widespread availability of safe and effective vaccines. While Proctor and Geng propose a new lane for science, made possible through significant changes to NIH-funding priorities, we argue that methodological approaches within implementation research also need prioritization. To build on their metaphor, in addition to building a new lane for science, we could also merge lanes to improve science.
One example of this would be greater use of hybrid effectiveness-implementation designs (2), which blend elements of clinical effectiveness and implementation research to foster more rapid translational science gains. Had COVID-19 vaccine efficacy trials used hybrid designs to assess implementation challenges and efficacy, we could have anticipated some of the implementation barriers that emerged sooner. For example, the trials could have assessed the potential reach of the COVID-19 vaccines, surveying those who refused to participate in the trials, which would have shown that a sizable proportion of Americans would be vaccine-hesitant. We also could have captured covid-specific vaccine hesitancy concerns and created vaccine hesitancy mitigation strategies more specifically and rapidly. Table 1 provides a more detailed description of the implementation data that could have been collected had COVID-19 vaccine efficacy trials used hybrid designs. While we lament this missed opportunity, we note that we could correct this moving forward. For example, we could use hybrid effectiveness-implementation designs more frequently in future vaccines and other clinical trials.
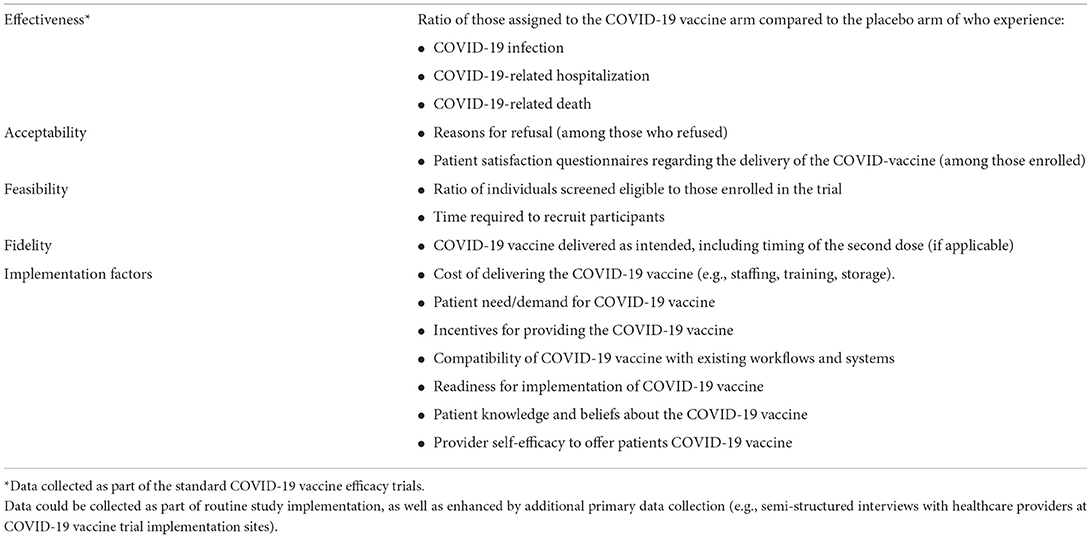
Table 1. Examples of data that could be collected as part of a hybrid effectiveness-implementation study on COVID-19 vaccination.
Further application of innovative approaches like hybrid effectiveness-implementation designs could supplement the actions called for by Proctor and Geng.
Conceptualization and writing—original draft: JK. Writing—review and editing: JK and GC. All authors contributed to the article and approved the submitted version.
This study was supported by National Institutes of Health grant K01AA028199 (JK) and UL1TR003107 (GC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Proctor EK, Geng E. A new lane for science. Science. (2021) 374:659. doi: 10.1126/science.abn0184
Keywords: hybrid implementation-effectiveness designs, COVID-19, vaccines, vaccine hesitancy, implementation science
Citation: Knox J and Curran GM (2022) Merging lanes for science. Front. Health Serv. 2:968175. doi: 10.3389/frhs.2022.968175
Received: 13 June 2022; Accepted: 08 August 2022;
Published: 06 September 2022.
Edited by:
Ann Catrine Eldh, Linköping University, SwedenReviewed by:
Nick Sevdalis, King's College London, United KingdomCopyright © 2022 Knox and Curran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin Knox, anJrMjExNUBjb2x1bWJpYS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.