- 1School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom
- 2Acute Leukemia Advocates Network (ALAN), Leukemia Patient Advocates Foundation, Bern, Switzerland
- 3Quality of Life Monitoring Unit, Saint Petersburg State University Hospital, Saint Petersburg, Russia
- 4DIELNET SrL, Reggio Calabria, Italy
- 5IFC-CNR Institute of Clinical Physiology, Reggio Calabria, Italy
- 6Chronic Lymphocytic Leukaemia (CLL) Advocates Network, Leukemia Patient Advocates Foundation, Bern, Switzerland
- 7CML Advocates Network, Leukemia Patient Advocates Foundation, Bern, Switzerland
- 8Hematology Unit, Grande Ospedale Metropolitano Bianchi Melacrino Morelli, Reggio Calabria, Italy
Background: Disease-specific factors associated with decreased quality of life (QoL) in patients with leukemia have not been studied in a large-scale, global, observational study.
Methods: This cross-sectional study used the validated Hematological Malignancy Patient Reported Outcomes (HM-PRO) questionnaire to assess the impact of leukemia subtype, age, sex, and years living with the disease on QoL of patients with leukemia.
Results: Overall, 2,628 patients responded: 45.7% had chronic lymphocytic leukemia (CLL), 34.0% had chronic myeloid leukemia (CML), 11.8% had acute myeloid leukemia (AML), and 3.5% had acute lymphoblastic leukemia (ALL). HM-PRO scores differed significantly between leukemia subtypes (p<0.001); patients with ALL reported the worst outcomes. Women had significantly worse scores than men (p<0.001). HM-PRO scores were inversely correlated both with age (ρ= –0.24, p<0.001) and years living with the disease (ρ= –0.14, p<0.001).
Conclusion: Patients reported the greatest concerns over their future treatment and future health, as well as concerns over dying and being a burden to others. Patients need access to support services, such as the availability of a clinical psychologist as part of the hematology team, to provide support with the emotional aspects of a leukemia diagnosis, especially for patients with acute leukemia subtypes reporting the lowest mean QoL scores.
1 Introduction
Leukemia (the production of abnormal leukocytes) subtypes can be broadly broken down into acute or chronic, based on the speed of proliferation; and myeloid or lymphoid, based on where the cells originate (1). The predominant subtypes of leukemia are acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML) and chronic lymphocytic leukemia (CLL) (1). CLL is the most common subtype of leukemia, with approximately 4.1 cases/100,000 adults, resulting in approximately 4,500 deaths per year in the United States (US) (2). AML is the second most common form of leukemia, making up approximately 1.1% of cancer diagnoses, but around 1.9% of cancer deaths (3). AML most frequently occurs in adults, causing symptoms of bone marrow failure and organ infiltration (3). CML accounts for approximately 15% of all diagnoses of leukemia in adults (1–2 cases/100,000 adults). Functional cure is common; however, this requires lifelong observation and continued treatment (4). ALL is most common in children aged 1–4 years, although it also affects adults; approximately 60% of the 6000 cases reported annually in the US are reported in patients <20 years old (5). The pediatric survival rate of ALL is >90% (5). Patient populations and prognosis are highly variable between these predominant subtypes, particularly between acute and chronic etiologies. This would have an impact on the choice of treatment since treatment options are also very varied (6–8).
It is well documented that quality of life (QoL) is impacted by leukemia diagnosis and treatment (9–14). Previous studies have reported that age and sex are significantly associated with QoL scores in patients with leukemia (14, 15), whereas in the general population age has not been found to independently reduce QoL (16). However, a more recent study has reported that sex is often associated with QoL in the general population (17). The Duration of disease has also been found to negatively impact QoL in other chronic conditions (18–20). It is therefore evident that the impact of age, sex, or years living with the disease on QoL, as well as how this varies across all four main subtypes of leukemia has not previously been studied in a large-scale observational study within a global population.
The Acute Leukemia Advocates Network (ALAN) is an independent global network of patient organizations, aiming to use patient advocacy to improve outcomes of patients with acute leukemia. Similarly, the CLL Advocates Network (CLLAN) and the CML Advocates Network (CMLAN) aim to improve patient outcomes for patients with CLL and CML, respectively.
The aim of this study therefore was to assess how QoL (measured by the validated Hematological Malignancy Patient Reported Outcomes [HM-PRO] instrument (21–23)) varied for patients with AML, ALL, CML, or CLL, as well as whether QoL (HM-PRO scores) is influenced by patients’ age, sex, or years living with the disease.
2 Materials and methods
2.1 Study design and ethics
We conducted a cross-sectional study of patients with leukemia in European, African, Asian, North American, and South American countries between 18 September 2021 and 07 January 2022. This study was conducted in accordance with the Declaration of Helsinki and International Society for Pharmacoepidemiology (ISPE) guidelines for Good Clinical and Pharmacoepidemiology Practice (GPP). The study protocol and informed consent form were reviewed and approved by each participating patient support group in their respective country. The ALAN Network based in Switzerland, was responsible for coordination and the contract research organization, IQVIA (UK & Ireland Healthcare and Government Division) was responsible for data management and analysis for the study. Patients were provided with written information explaining the study design and were asked to provide consent electronically before participating in the study. Adequate protections were taken to maintain the confidentiality of their responses. Data were managed in compliance with the General Data Protection Regulation and any regulations regarding management of personal data required by participants’ respective country of residence.
The STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) Checklist (24) was implemented in the design and reporting of the study (Supplementary Table 1).
2.2 Study assessment tools
The study questionnaire was developed based on an existing patient pathway experience questionnaire (14). The ALAN Network also agreed to include a number of questions relating to the COVID-19 pandemic, as well as the validated established Hematological Malignancy Patient Reported Outcomes (HM-PRO) instrument (21–23). The HM-PRO contains items specific to QoL aspects impacted by hematological malignancies; these include physical, social, and emotional health, eating and drinking, symptoms, and side effects (21–23). The HM-PRO consists of two parts, assessing impact (Part A) and signs and symptoms (Part B) of hematological malignancies. Both scales have linear scoring systems ranging from 0 to 100, with higher scores representing greater (negative) impact on QoL and symptom burden. The HM-PRO recall period for Part A is “at the moment” (i.e., at present, today) and for part B is the last three days. A minimal clinically important difference (MCID) for HM-PRO in patients with hematological malignancies is 6.2 according to standard error of measurement (25). Overall, the study assessment tool consisted of 200 questions (some with sub questions, across 16 sections, including demographic information). This analysis reports on Part A of the patient questionnaire.
The data included socio-demographics, disease and treatment profile, and QoL. The results reported in this manuscript are those relevant to the study aims: to assess whether age and years living with the disease, are correlated to the HM-PRO scores of patients with a) chronic and b) acute leukemia.
2.3 Content validity and cognitive debriefing
The HM-PRO, as part of its original development in English language underwent extensive psychometric testing including content validity (21–23). Content validity is identified as the most important step of psychometric testing of a newly developed PRO instrument by FDA in their PRO Guidelines documents (26). The 10 translations of the HM-PRO were carried out by MAPI Research Trust following the established standard approach outlined in the ISPOR PRO instrument cross-cultural adaptation document (27). This process encompassed two forward and backward translations, consolidation at the end of each step and then arbitration by the developers of the original HM-PRO, followed by content validity (cognitive debriefing) involving patient with hematological malignancy. On finalization of cross-cultural adaption of the HM-PRO to the 10 languages, respective translation certificates were issued by the MAPI Research Trust.
2.4 Study participants and procedure
This was a global study; participants were recruited through national patient support groups (i.e. members of ALAN, CLLAN, and CMLAN Networks), via email, social media, and newsletters. The questionnaires were made available in ten languages: Chinese, English, French, German, Hebrew, Italian, Korean, Portuguese (Brazilian), Russian, and Spanish. These official certified translations were used in all 76 countries. The study was of 16 weeks duration (18 September 2021 and 07 January 2022) and the patients were asked to only complete the sections that were relevant to their leukemia subtype. It was determined that a sample size of at least 350 patients would be sufficient to address the study objective based on 5% margin of error and a 95% confidence interval.
2.5 Statistical analysis
For all questions (with the exception of those asked in the form of “tick all that apply”) the percentage responses were calculated after excluding those respondents who did not answer that particular question.
The overall number of evaluable responses for questions which were presented in the form of “tick all that apply” was determined by the number of respondents eligible to respond. Missing responses were those where any eligible respondents chose not to select any options. Additionally, where applicable, scores have been recalculated to exclude non-specific responses (such as don’t know/can’t remember), or responses indicating that the question was not applicable to the participant’s circumstances. Where responses were missing, percentages are based on the number of patients who answered that question. Age was calculated by subtracting birth year from 2021; years living with the disease (disease duration) was calculated by subtracting year of diagnosis from 2021-.
Since the HM-PRO Part A scores were not normally distributed (right skewed), non-parametric statistical methods were used for the analysis. Two sample Wilcoxon rank-sum, or Kruskal-Wallis rank tests, were used to test for differences in scores between groups. Chi-squared (χ2) test was used to test differences between categorical variables.
The Spearman’ rank correlation coefficient (ρ) was used to determine the strength of relationships (and partial correlation) between the scores, patient age and years living with the disease (disease duration); an absolute value of 0.7 or greater indicated a strong relationship, 0.4 -0.7, a moderate relationship and between 0.2 and 0.4 a weak relationship.
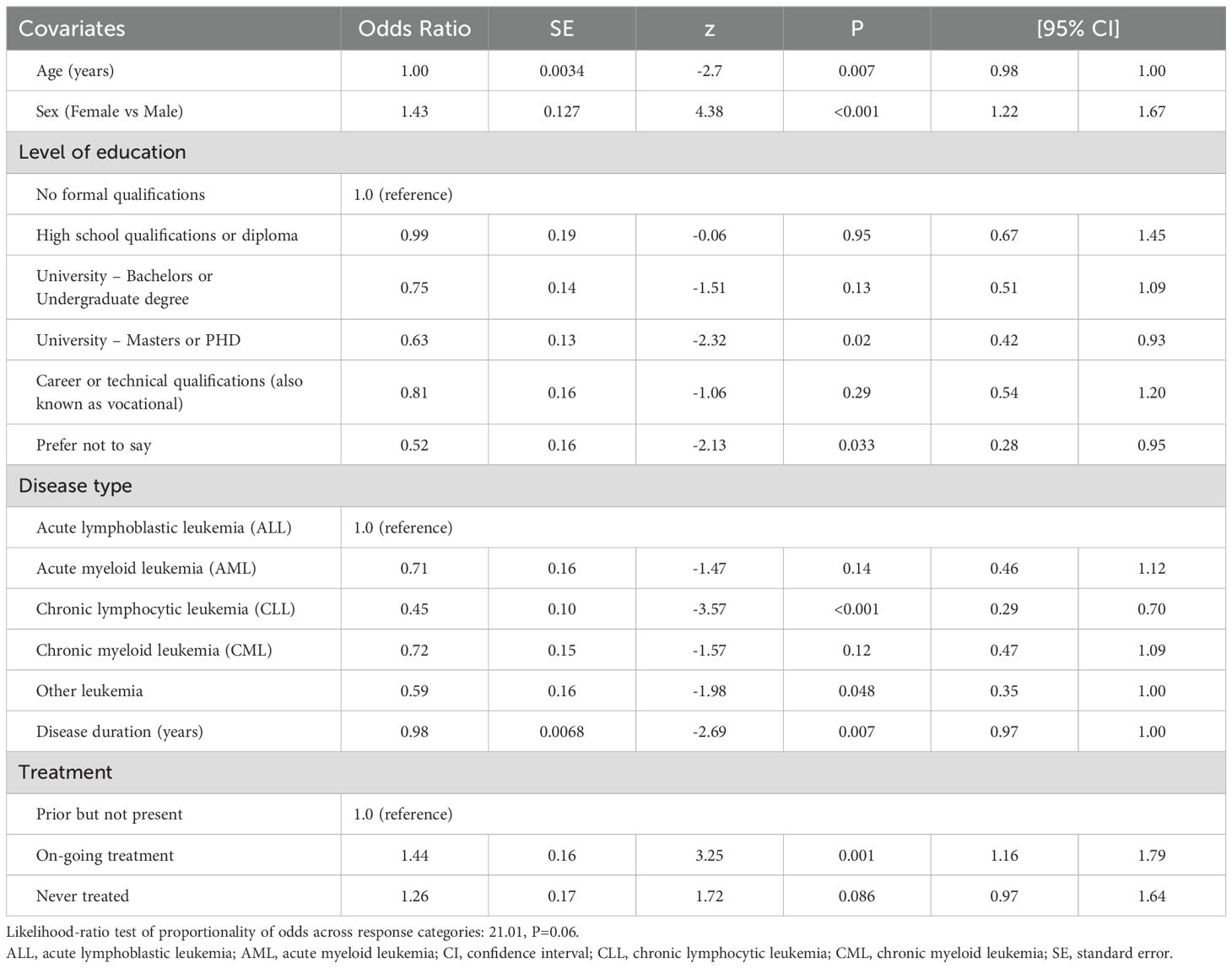
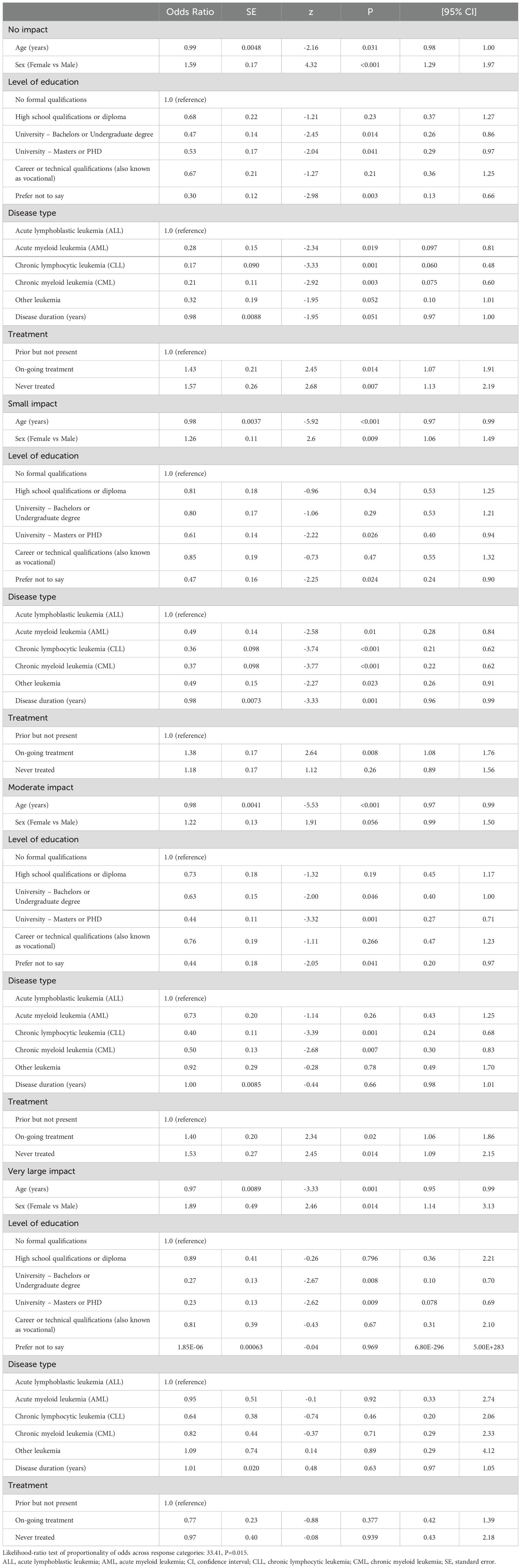
The HM-PRO domain score (physical behavior score [PB SCORE], emotional behavior score [EB SCORE], social well-being score [SW SCORE], eating and drinking [ED SCORE]), and total HM-PRO [PARTA SCORE] were represented in score banding (no impact=0-7, small impact=8-25, moderate impact=26-41, very large impact = 42-74, extremely large impact=75-100) (28). The independent correlates of ordinal dependent variables (namely, PB SCORE, EB SCORE, SW SCORE, ED SCORE, and PARTA SCORE) were identified by preliminary testing the proportionality of odds associated to all covariates across response categories in multiple regression models. The proportionality assumption was tested by the likelihood-ratio (LR) test (29) considering as null hypothesis that there was no difference in the regression coefficients linking each covariate to the levels of the outcome variable (that is, no covariate has a disproportionate effect on a specific level of the dependent variable). If the null hypothesis is accepted (P value of LR test >0.05), an ordinal logistic regression was fitted for each outcome variable. In these models, the odds ratio indicates how much increases the odds of being in a higher level of the outcome variable, given that all the other variables in the model are held constant. If the null hypothesis is rejected (P value of LR test ≤0.05), different logistic models were fitted to describe the relationship between covariates and each pair of outcome categories appropriately grouped. For example, the PART - A SCORE categories are numbered 1, 2, 3, 4, and 5. The first panel of odds ratios can be interpreted as those from a binary logistic regression where the dependent variable is recoded as 1 vs 2 + 3 + 4 + 5. The second panel of odds ratio can be interpreted as those from a binary logistic regression where the dependent variable is recoded 1 + 2 vs 3 + 4 + 5, etc. Thus, in this analysis odds ratio are interpreted as in standard binary logistic models where categories of outcome variable are collapsed into two categories. Positive coefficients mean that higher values on the covariates make higher values on the dependent variable more likely. In these models, data were expressed as odds ratio (OR), standard error (SE), z value, P value, and 95% confidence intervals. Missing answers were random and were excluded for these tests. The probability of type I error was set at p <0.05. Stata 11 (29) was used for data processing and analysis.
3 Results
3.1 Socio-demographic characteristics of the study participants
Overall, 2,628 patients aged 16 and over completed the questionnaires and were included in the analysis.
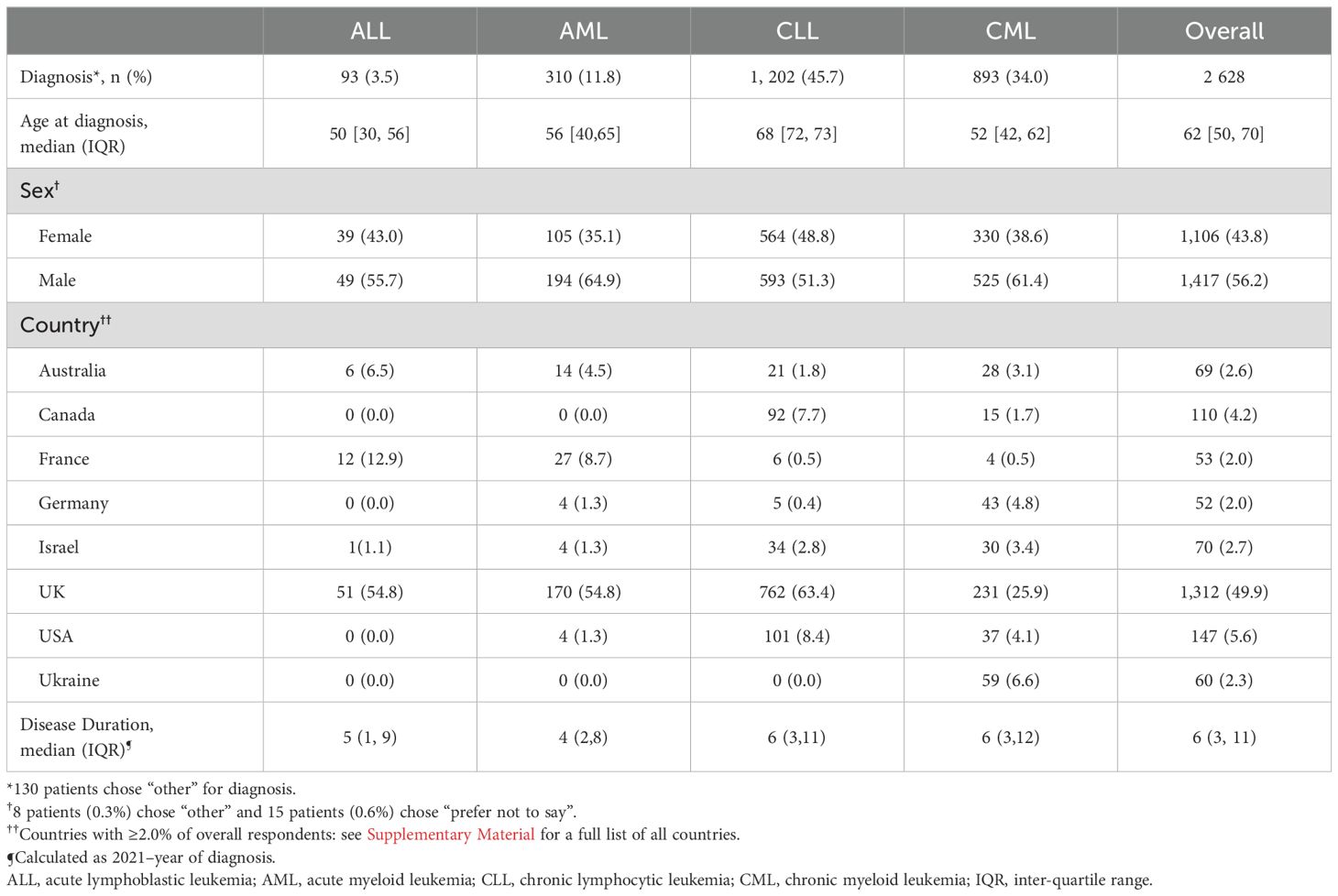
The most common diagnosis was CLL (1,202 patients [45.7%]), while 893 (34.0%) patients had a diagnosis of CML, 310 (11.8) patients had AML, and 93 (3.5%) patients had ALL (Table 1). A further 130 patients (5.0%) responded “other” for diagnosis type.
The questionnaire was completed by patients from 76 countries. The majority of patients were from the UK (1,312 patients, 49.9%), USA (147, 5.6%), Canada (110, 4.2%), Israel (70, 2.7%), Australia (69, 2.6%), France (53, 2.0%), Ukraine (60, 2.3%) and Germany (52 patients, 2.0%) (Table 1). All other countries had <50 respondents combined (<2.0% of all respondents).
More patients were female (1,417, 56.2%) overall, and across all subtypes. The proportions differed significantly between the subtypes (p<0.001) with e.g., 51% of men having CLL, compared with 41.8% of women.
A total of 2,526 patients reported their age (median 62, IQR [50, 70]). Only 18 (0.73%) were aged under 35. Age differed significantly between the subtypes (p<0.001) with ALL patients on average younger, and CLL older (Table 1).
Date of diagnosis was reported by 2,495 respondents. Disease duration ranged from 0 to 63 years (Table 1 shows the breakdown by subtypes). Four respondents (all with chronic disease) gave their year of diagnosis as their year of birth, so these, together with the respondent (with CLL) who provided no year of birth but reported a (implausible) duration of 63 years, were excluded from analysis of duration of disease. Disease duration differed significantly (p<0.001) across leukemia subtypes; with medians [IQR]; 5 [1,9] for ALL; 4 [2,8] for AML; 6 [3,11] for CLL; and 6 [3,12] for CML.
3.2 Overall HM-PRO scores and relationships with age, sex, and duration of disease
With the exclusions applied, overall, there were 2,552 evaluable HM-PRO scores, with a median score of 22.3. Scores differed significantly between leukemia subtypes (p<0.001) Patients with ALL reported the worst outcomes across all four leukemia subtypes (Figure 1). Additionally, women (median=24.6) experienced significantly (p<0.001) higher impact on their QoL than men (median=19.7).
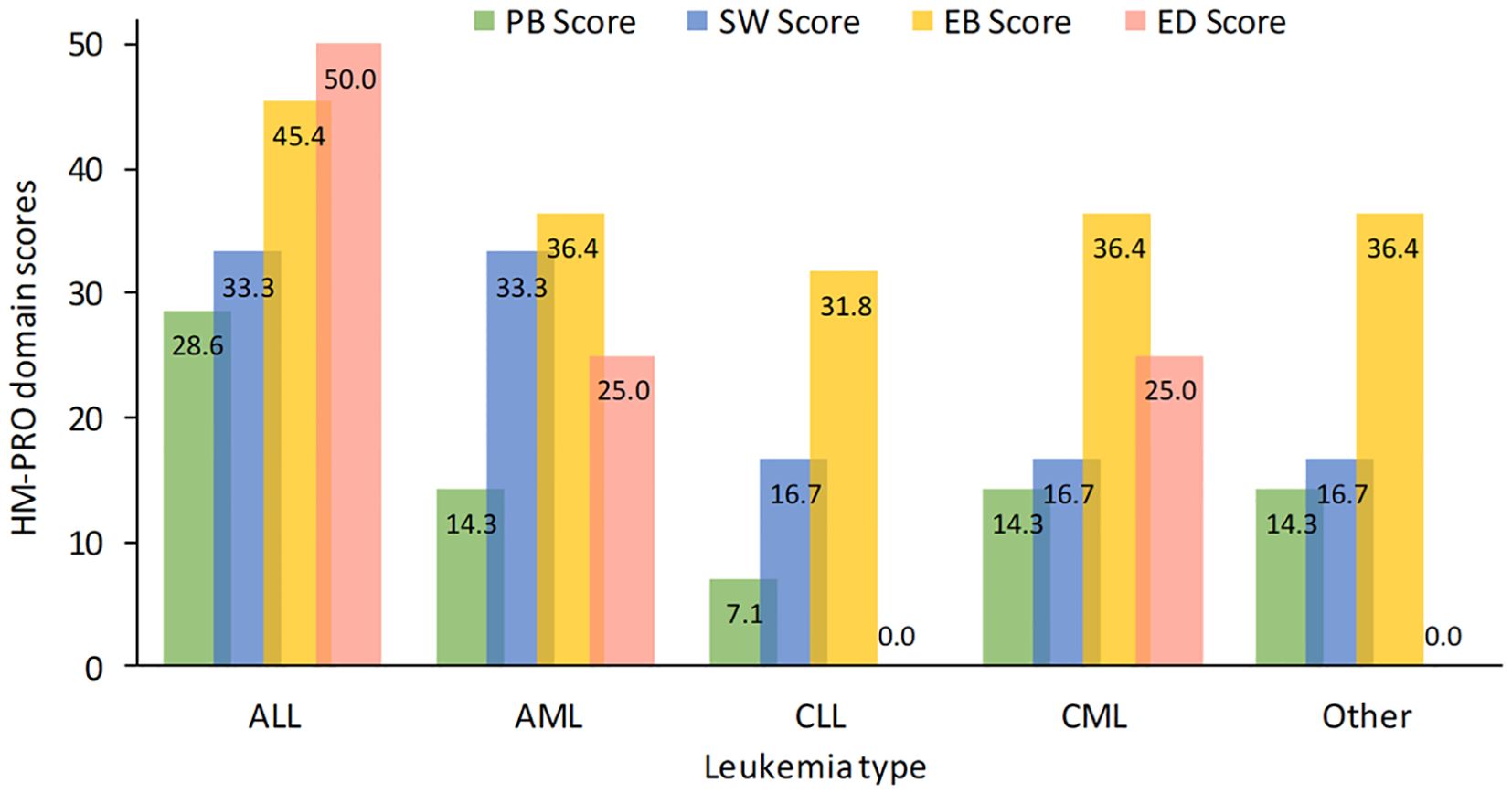
Figure 1. HM-PRO quality of life domain scores for leukemia subtype. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; HM-PRO, hematological malignancies patient reported outcomes.
Of the 2,552 respondents, 2,494 reported their age (median=62 years) and 2,432 reported a valid year of diagnosis (median=6 years). Overall, 2,383 patients with an HM-PRO score had reported their age and had a valid year of diagnosis. Both age (ρ= –0.24, p<0.001) and years living with the disease (ρ= –0.14, p<0.001) were significantly negatively correlated (weakly) with HM-PRO scores. Investigation of partial correlation coefficients, between the scores with both age and disease duration, indicated that disease duration was independently associated with the HM-PRO scores, although this association was even weaker when HM-PRO scores were adjusted for age (age: ρ= –0.21, p<0.001), years living with the disease (ρ= –0.08, p<0.001).
3.3 The HM-PRO domain score and relationships between leukemia sub-types
3.3.1 Physical behavior
The majority of patients reported no difficulties with walking (1,378 patients, 60.87%), self-care (1,953 patients, 83.1%), leaving the house (1,852 patients, 79.6%), traveling (1,686 patients, 72.7%) work or studies (1,133 patients, 59.5% of those the question applied to) or going on holiday (1,303 patients, 51.7%) (Supplementary Figure 1). In contrast, the majority of patients reported some difficulty with physical activity/sports; either a little (820 patients, 35.1%) or a lot (547 patients, 23.4%).
The overall physical behavior score (with 7 items, median=15.3) showed that there was a significant difference between responses for different leukemia subtypes (p<0.001), with ALL having the worse scores (median [IQR]=28.6 [8.3, 50]) compared to the other groups (14.3 or less).
3.3.2 Social well-being
The majority of patients reported no difficulty with socializing (1,260 patients, 56.3%) or personal relationships (1,612 patients, 69.9%), while approximately half of patients reported problems with their sex life (969 patients, 50.0% of respondents for that question). Overall, most patients reported similar responses across all subtypes, however more patients with ALL reported problems with personal relationships (45 patients, 49.9%) (Supplementary Figure 2).
The overall social well-being score (with 3 items, median=16.7) showed that there was a significant difference between the subtypes (p<0.001), with ALL and AML having the worst scores with median of 33.3 vs 16.7 for the CLL, CML and “Other”.
3.3.3 Emotional behavior
The majority of patients reported no concerns over people judging them (1,461 patients, 61.5%). However, approximately half worried about their appearance (33.1% a little; 14.2% a lot), reported feeling distressed (1,181 patients, 49.6%), or did not feel confident (1,234 patients, 52.3%; 38.91% a little and 13.45% a lot). Most patients worried about being a burden to others (1,500 patients, 66.5%), reported feeling anxious (1,631 patients, 67.0%), worry about dying (1,500, 62.1%), but of these 1,091 reported only worrying about it a little. The majority reported change in their sleeping patterns (1,632 patients, 67.6%), difficulty concentrating (1,501 patients, 62.2%) and worry about treatment (1,513 patients, 63.9%). Almost all patients reported worrying about their future health (2,143 patients, 87.4%) (Supplementary Figure 3).
The overall emotional behavior score (with 11 items, median=36.4) showed that there was a significant difference between the subtypes (p<0.001), with ALL having the worst median score of 45.5 vs 36.4 for AML, CML, and “Other”, and 31.8 for CLL.
When asked about how their overall emotional well-being had changed since diagnosis, most patients (1,223 patients, 48.2%) reported feeling anxious or depressed since their diagnosis, with 126 patients (5.0%) reporting they had continually felt depressed (Supplementary Figure 3). Similarly, most patients (1,643, 77.5% of those for whom it was applicable) reported that their leukemia diagnosis had not caused any problems with their relationship with their spouse or partner.
3.3.4 Eating and drinking
Most patients had no trouble with their appetite (1,485 patients, 67.6%), while around half reported their eating or drinking habits had changed (1,219 patients, 50.6% and 1,093 patients, 48.2%, respectively) (Supplementary Figure 4). The overall eating and drinking score (3 items, median=25.0) differed significantly between subtypes (p<0.001), with CLL patients least affected.
3.4 The impact of sociodemographic and leukemia subtypes on QoL
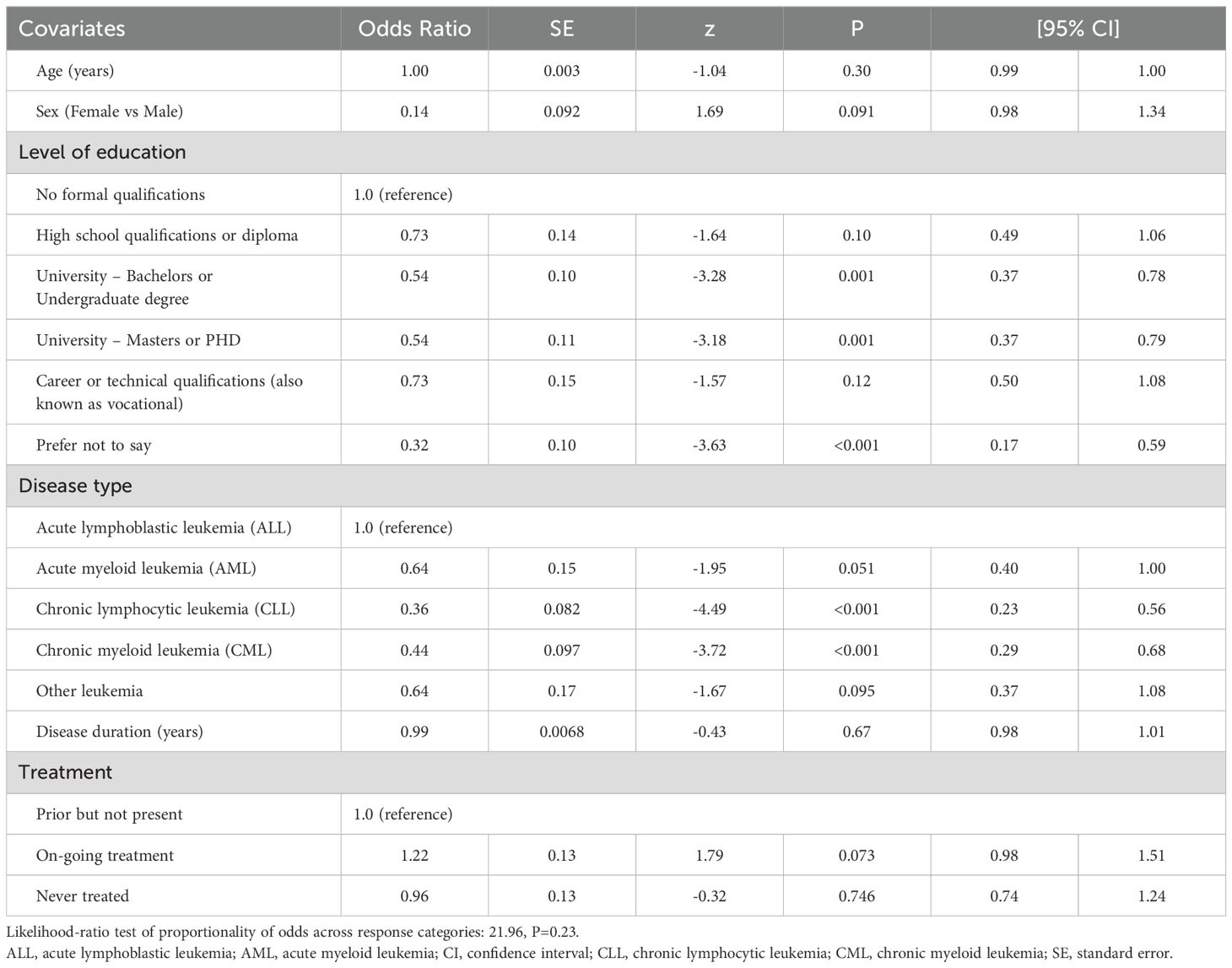
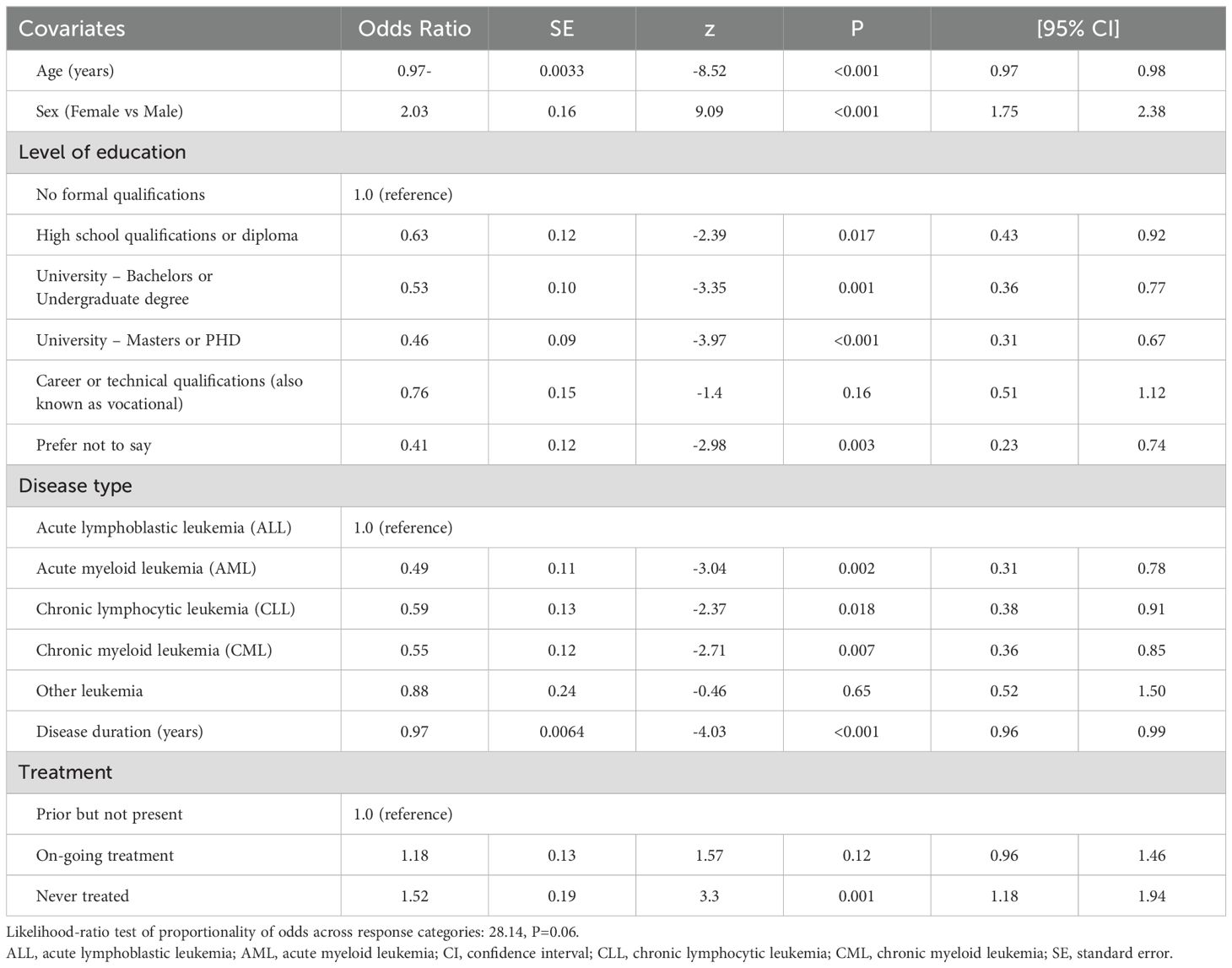
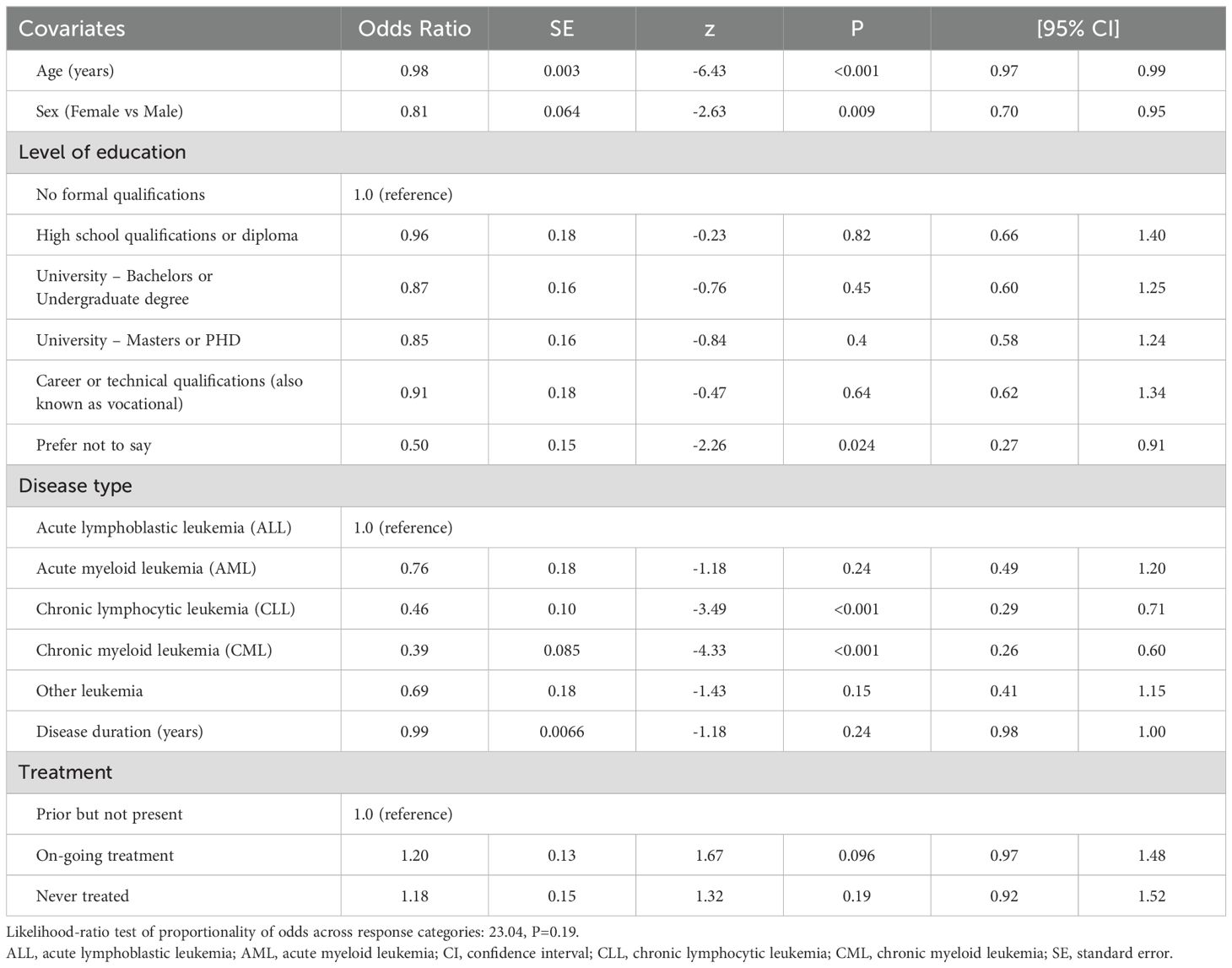
The independent correlates of PB SCORE, EB SCORE, SW SCORE, ED SCORE, and PARTA SCORE are detailed in Tables 2–6. In ordinal logistic regression analyses, age and sex were both significantly related to EB SCORE, SW SCORE, and ED SCORE (Tables 3-5) but not to PB SCORE (Table 2). Age was coherently associated in an inverse fashion to EB SCORE, SW SCORE, and ED SCORE whereas female sex was related directly to EB SCORE and ED SCORE and inversely to SW SCORE. Overall, patients who achieved a higher level of education had a lower odds ratio of being in a higher category of PB SCORE, EB SCORE, and ED SCORE when compared to those with no formal qualification (Tables 2, 3, 5). No association was found between scholarity and SW SCORE (Table 4). Among disease types, patients affected by CLL had a lower odds ratio of being in a higher category of PB SCORE, EB SCORE, SW SCORE, and ED SCORE (Tables 2-4) when compared to those with acute lymphoblastic leukemia and this was also true for patients with chronic myeloid leukemia although this latter did not attain the statistical significance for the ED SCORE (Table 5). The duration of disease was significantly and inversely related to EB SCORE (Table 3) and ED SCORE (Table 5) but not to PB SCORE (Table 2) and SW SCORE (Table 4). Never treated patients and patients on ongoing treatment had higher odds ratios of being in a higher category of EB SCORE (Table 3) and ED SCORE (Table 5), respectively, when compared to those previously treated. The associations between age, sex, level of education, disease type, disease duration, and treatment with PARTA SCORE are reported in Table 6 (see Methods-Statistical Analysis for more details).
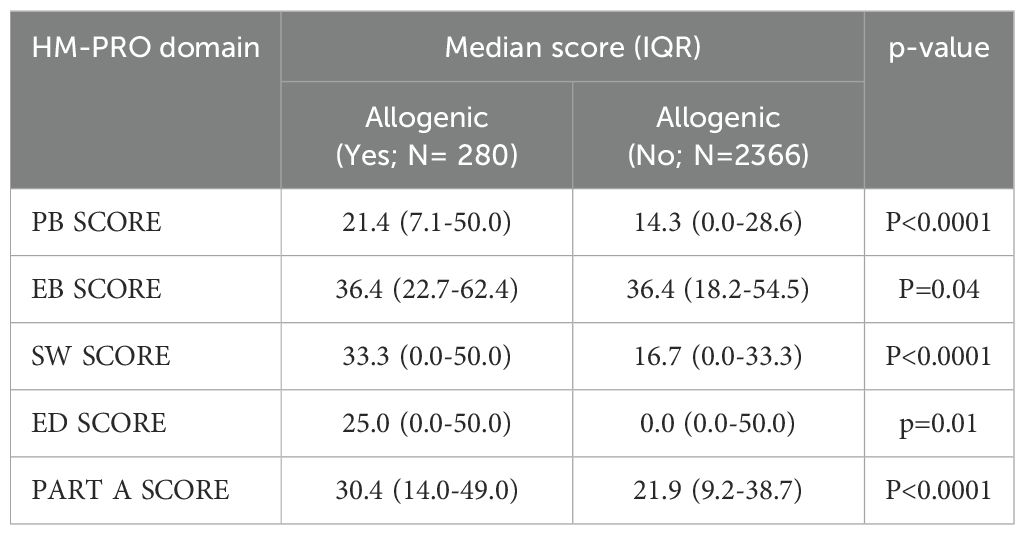
3.5 The impact of treatment type and leukemia subtypes on QoL
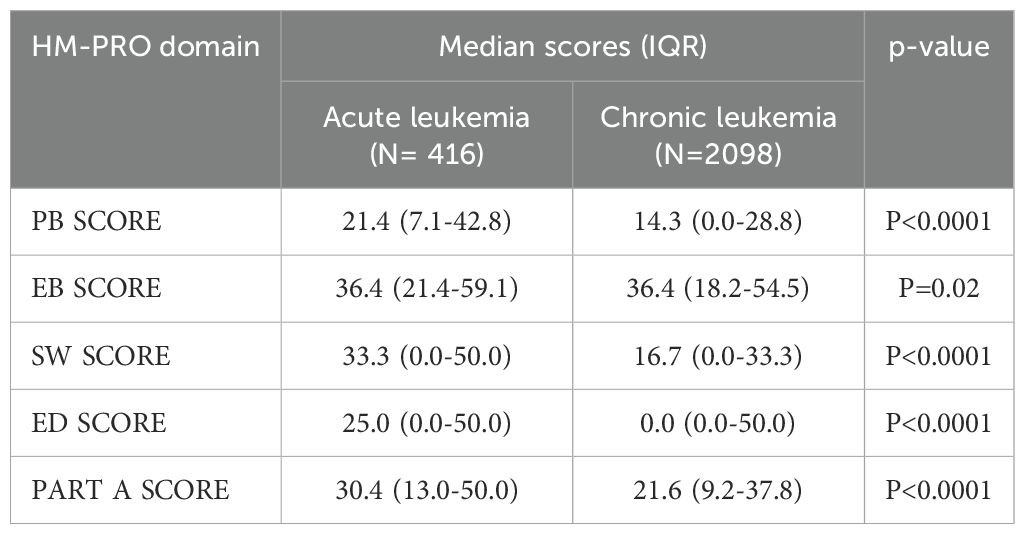
In an additional sub analysis, we evaluated whether the acute or chronic leukemia subtypes and type of treatment could have an impact on HM-PRO domain scores. When considering patients with acute leukemias compared to patients with chronic leukemias, we observed a difference in all HM-PRO domains which reached the MCID, with the exception of EB-SCORE (Table 7). We next examined whether different treatment types could impact upon patient QoL. No clinically significant differences were observed across all HM-PRO domain scores when patients were stratified by oral vs intravenous treatment or in those receiving Bruton’s tyrosine kinase (BTK) inhibitors vs other treatment (only in patients affected by CLL). However, in patients who underwent allogenic transplantation, we observed marked statistically significant (and clinically important) higher scores (worse QoL) in all HM-PRO domains compared to patients who did not undergo allogenic transplantation (with the exception of EB SCORE) (Table 8). Furthermore, when categorizing patients according to time since allogenic transplantation, patients <1 year after allogenic transplant were observed to have higher domain scores (i.e. lower QoL) compared to 1-5 years and >5 years after transplant, with higher scores seen across SW, ED and PART A SCORE domains (Figure 2). This was not observed when comparing QoL scores in acute leukemia patients who received and did not receive allogenic transplantation.
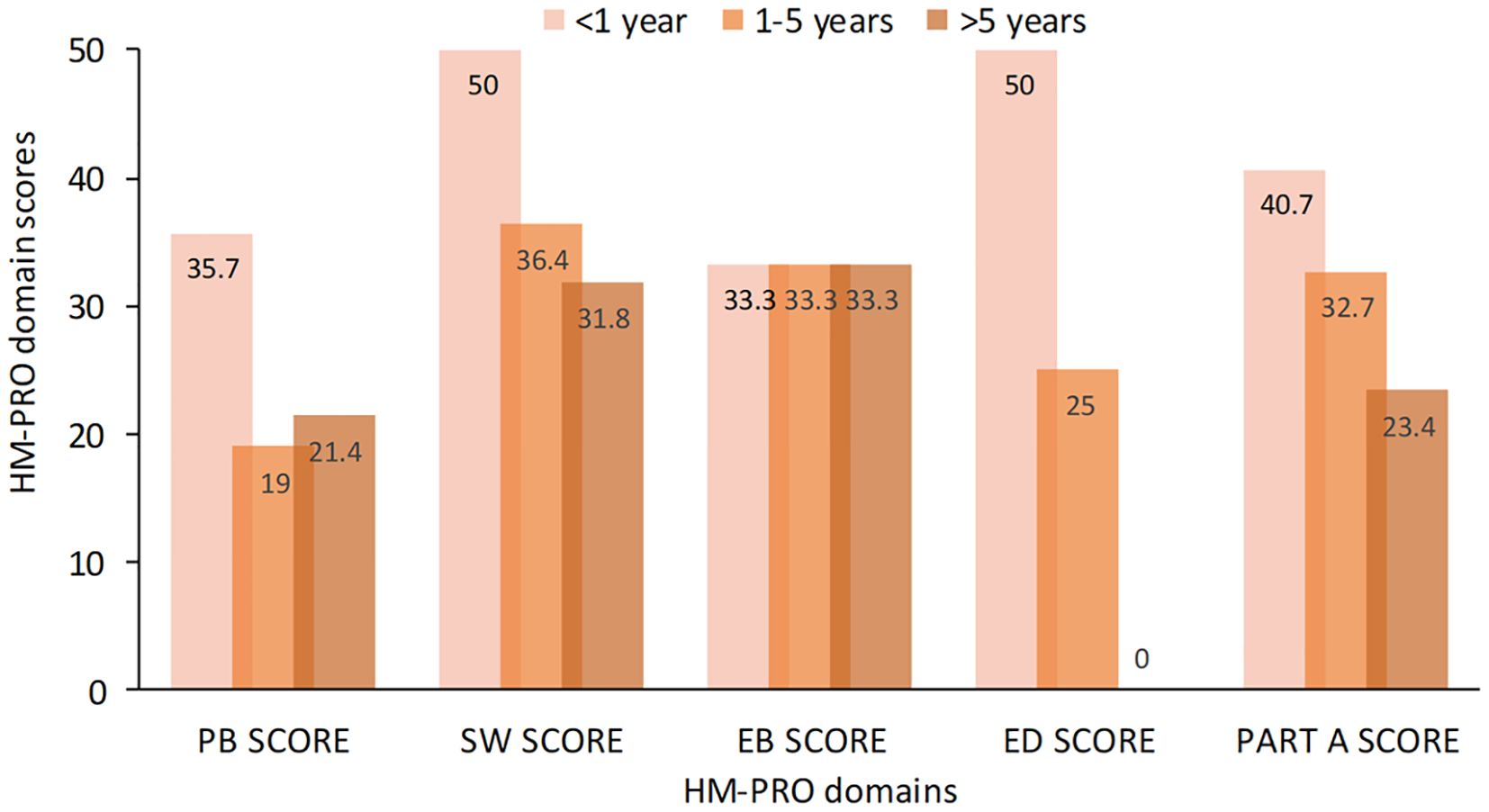
Figure 2. HM-PRO quality of life domain scores at different time intervals post allogenic transplantation. HM-PRO, hematological malignancies patient reported outcomes.
4 Discussion
We report on the largest ever global study of QoL in over 2,600 patients with leukemia, describing the impact of subtypes of leukemia, disease duration, age, and sex on patient-reported QoL. The study utilized a global advocacy network partnership to ascertain, through a validated PRO measure for patients with hematological malignancies, which aspects of physical, social, and emotional health are most impacted in patients with ALL, AML, CLL and CML.
Significant differences between responses for different leukemia subtypes were observed for both physical, social and emotional well-being, as well as eating and drinking. Overall, patients with ALL reported the worst impact on QoL. Additionally, women had significantly higher HM-PRO Part A scores (i.e., lower QoL) than men.
Overall, the majority of respondents reported few physical problems or problems with eating and drinking. However, patients with ALL reported more problems with physical behavior and eating and drinking than across the other leukemia subtypes. Conversely, patients with CLL generally reported the least difficulties with aspects of physical behavior. Problems with social well-being were reported more frequently than problems with physical behavior, across all leukemia subtypes; again, patients with ALL were more likely to report problems with social well-being than the other leukemia subtypes; patients with chronic leukemia (CML or CLL) were least likely to report problems with social well-being.
Consistent with the physical and social components of the HM-PRO, patients with ALL reported more problems with emotional aspects related to leukemia; patients with CLL reported less emotional problems. Overall, the biggest emotional issue for patients with leukemia was worrying about their future health – across all subtypes, around half of patients reported having some worries around their future health, while another one-third of patients reported they worried “a lot” about their future health. Slightly fewer patients reported concerns about dying; patients with ALL reported the most concerns. Notably, worrying “a lot” about treatment was reported most frequently by patients with CML (possibly due to the lifelong requirement for observation and continued treatment in CML (4)), whereas for all other questions, patients with ALL were most likely to report worrying “a lot”. Patients tended to report feeling depressed or anxious more often since their diagnosis, however 20% of patients reported feeling more positive overall since their diagnosis. A positive correlation between impact of treatment and diagnosis and reduced QoL have previously been reported in patients with acute leukemia, as have feelings of isolation and reduced abilities to carry out physical or enjoyable activities, and lower QoL scores in women (14).
We also evaluated the relationship between different types of treatment and QoL in leukemia patients. While no clinically significant differences were observed based on route of administration (i.e., oral vs intravenous therapies) or between BTK inhibitors and other treatments, patients who underwent allogeneic transplantation experienced statistically and clinically worse QoL, particularly within the first year post-transplant, compared to those who did not undergo transplantation. QoL was also observed to be better in patients with increased time since undergoing allogeneic transplantation. Indeed, data from longitudinal studies support our observations where moderate impairments are often observed early on but generally improve and return to pre-transplant levels by day 100. Several studies report that over 60% of patients experience good to excellent QoL within the first 1 to 4 years following hematopoietic cell transplantation (30). In patients with acute leukemia who underwent allogenic transplantation, we did not observe a significant impact on QoL, that may be attributed to various factors such as subsequent therapy and smaller number of cases involved (N=416).
Of the additional variables that were considered (disease duration, age, and sex), all appeared to be (weakly) related to the HM-PRO scores, with older patients being slightly more likely to report a better QoL. Living with the disease for longer was also associated with better QOL, but this was mainly confounded by the patients’ age. This is consistent with a previous study in patients with acute leukemia, which reported that both younger age and female sex, as well as lower income were significantly negatively associated with QoL (14). In contrast, other studies have reported worsening QoL in older patients; however, this may be confounded by worsening mobility and general health in older populations (28).
Differences in treatment regimens between leukemia subtypes may partially explain the differing reports of physical impact of leukemia; for example, “watch and wait” (clinical observation without therapy) is the standard of care for many patients with CLL (7); meaning that patients often do not have physical symptoms from either the disease, or from associated treatments, and will subsequently report little impact on physical abilities. In contrast, not receiving treatment for an incurable blood cancer diagnosis has previously been reported to result in patients experiencing similar increases in depression and anxiety, and decreased QoL, to those undergoing active treatment (31, 32). In addition, previous studies have shown correlations between QoL with the type of carcinoma, length of hospital stays required, depression and severity of symptom burden (33).
Additionally, patients diagnosed with acute leukemia (ALL) are more likely to be younger, which has previously been found to be associated with worse QoL (14); teenagers and young adults may be more socially conscious of their appearance and how the disease or their treatment impacts on their social life and ability to study, work, or travel. They may also feel more isolated from their healthy peers, and have more concerns over the future, given the length of time they may be living with, or in remission from leukemia – long-term cancer survivors have reported impacts on QoL up to 26 years after a cancer diagnosis (34). Fertility issues in patients with hematologic malignancies may also contribute to lower QoL (35), particularly in younger patients – in a study of 406 patients aged under 40 who received a hematopoietic stem cell transplant, one-third reported concerns regarding their ability to have children (36).
Overall, patients with leukemia reported the greatest concerns over their future treatment and future health, as well as concerns over dying and being a burden to others. The lowest mean QoL scores were reported by patients with acute leukemia subtypes, as compared to chronic leukemia patients, across physical, eating and drinking, social, and emotional QoL components; patients with ALL were impacted more than those with AML. It is important that patients fully understand their diagnosis and the treatment regimens they may follow, and what impact this may have on their future health. Additionally, patients need access to support services, such as the availability of a clinical psychologist as part of the hematology team, to provide support with the emotional aspects of a leukemia diagnosis.
Limitations of the study include the use of a convenience sample which was only available through online platforms (i.e. social networks, newsletters, and email). This recruitment method may have introduced selection bias, as only a subset of the target population likely participated, and their participation may have been influenced by their QoL. Additionally, there are significant differences in the clinical characteristics, disease progression, and treatment strategies between acute and chronic leukemias, raising concerns about the reliability of findings from smaller subgroups, such as patients with ALL and AML.
In addition, due to the online method of recruitment, the response rate could not be assessed. Respondents were self-selected, participated on a voluntary basis, and were recruited through patient organizations and while participants from 76 countries were involved, the UK accounted for nearly half of the total cohort (1,312 out of 2,628 patients; 49.9%) and therefore may not reflect the perspectives of all leukemia patients. In addition, this disproportionate representation could result in bias, given the potential differences in patient management, caregiver support, and healthcare systems across countries. These variations, particularly in the absence of standardized treatment protocols, may limit the generalizability of the findings across different healthcare settings.
Furthermore, being only available online could have introduced limitations to accessibility by factors such as region and socioeconomic status. In addition, younger patients may be more likely to engage with online options for completing questionnaires. However, 40% of respondents were over 65 years old, suggesting traditional barriers to online questionnaires may not have been observed in this study.
Other limitations of the study were primarily caused by various confounders, that we will attempt to address here. One concern could be the accuracy of the collected data being an online study, that is in terms of those who completed the study were indeed patients with leukemia. We are however confident that those who entered the study were alerted by their respective country patient support group who otherwise would not have known about the study. In addition, it is likely that due to the vast number of countries taking part in the study, this could have introduced bias regarding comprehension of the questionnaire and therefore affected the accuracy of the data. However, we are confident that this would have been minimized due to the simplicity of the questionnaire items and their brevity as well as the fact that translatability and universality was considered in their initial development. Surprisingly, a very low number of random missing items supports such notion. This, no doubt, was an ambitious study requiring tremendous number of resources and good will on the part of the various countries. Nevertheless, it is hoped that the outcomes reported here create an awareness of the scale of leukemia burden around the globe and the importance of incorporation of patients’ voice in treatment decision making.
5 Conclusion
This global study, involving over 2,600 leukemia patients, examined factors affecting their QoL using the HM-PRO questionnaire. Key concerns for patients centered on future treatments, health, and fears of dying or becoming a burden. Acute leukemia subtypes had the lowest QoL scores, with acute lymphoblastic leukemia patients being more impacted than those with acute myeloid leukemia. This study highlights the urgent need for support services, including access to clinical psychologists, to address emotional challenges and improve patient QoL, suggesting changes in how clinicians manage leukemia patients.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statement
This study did not require Ethics Committee approval. This was an online survey. Patients were informed of the research and freely agreed to participate in the study. All patients signed an informed consent. All personal data was blinded to protect patients’ privacy. The database did not contain sensitive data, according to the data protection law. The study did not increase the frequency of hospital visits. Patients were free to participate in the survey and not obliged to continue in the study and were allowed to stop answering the questions at any time. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SN: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ZP-N: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. TI: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. GI: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. GT: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. NY: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NS: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. KH: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MR: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DC: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. LP: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. ENO: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this review project was provided by the Acute Leukemia Advocates Network.
Acknowledgments
Medical writing support was provided by Nicola Illingworth, Medwrite Pharma Ltd and funded by Quality Health Ltd.
Conflict of interest
SS reports an educational grant from GSK and CIRS Centre for Innovative Regulatory Science; speaking consultancy from Otsuka; and conference travel support from the EHA European Hematology Association; and is a joint copyright holder of the HM-PRO. EO reports consultancy and advisory board membership for Janssen; consultancy and advisory board membership for Daiichi; consultancy, advisory board and speaker bureau membership for Alexion; consultancy and royalties for Ryvu; advisory board and speaker bureau membership for BMS; speaker bureau membership and royalties for Novartis; speaker bureau membership for Amgen; speaker bureau membership for Sobi; royalties for Halia Therapeutics; and is a joint copyright holder of the HM-PRO. Author GI was employed by DIELNET SrL.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2024.1502166/full#supplementary-material
References
1. Chennamadhavuni A, Lyengar V, Mukkamalla SKR, Shimanovsky A. Leukemia, in: StatPearls (2022). Treasure Island (FL: StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK560490/ (Accessed March 3, 2023).
2. Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. (2019) 94:1266–87. doi: 10.1002/ajh.25595
3. Stubbins RJ, Francis A, Kuchenbauer F, Sanford D. Management of acute myeloid leukemia: A review for general practitioners in oncology. Curr Oncol. (2022) 29:6245–59. doi: 10.3390/curroncol29090491
4. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. (2020) 95:691–709. doi: 10.1002/ajh.25792
5. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. (2013) 381. doi: 10.1016/S0140-6736(12)62187-4
6. Smith G, Apperley J, Milojkovic D, Cross NCP, Foroni L, Byrne J, et al. A British Society for Haematology Guideline on the diagnosis and management of chronic myeloid leukaemia. Br J Haematology. (2020) 191:171–93. doi: 10.1111/bjh.16971
7. Walewska R, Parry-Jones N, Eyre TA, Follows G, Martinez-Calle N, McCarthy H, et al. Guideline for the treatment of chronic lymphocytic leukaemia. Br J Haematology. (2022) 197:544–57. doi: 10.1111/bjh.18075
8. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
9. Shanafelt TD, Bowen D, Venkat C, Slager SL, Zent CS, Kay NE, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol. (2007) 139:255–64. doi: 10.1111/j.1365-2141.2007.06791.x
10. Korol EE, Wang S, Johnston K, Ravandi-Kashani F, Levis M, van Nooten F. Health-related quality of life of patients with acute myeloid leukemia: A systematic literature review. Oncol Ther. (2017) 5:1–16. doi: 10.1007/s40487-016-0039-6
11. Fardell JE, Vetsch J, Trahair T, Mateos MK, Grootenhuis MA, Touyz LM, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: A systematic review. Pediatr Blood Cancer. (2017) 64. doi: 10.1002/pbc.26489
12. Vetsch J, Wakefield CE, Robertson EG, Trahair TN, Mateos MK, Grootenhuis M, et al. Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: a systematic review. Qual Life Res. (2018) 27:1431–43. doi: 10.1007/s11136-018-1788-5
13. Trask PC, Cella D, Powell C, Reisman A, Whiteley J, Kelly V. Health-related quality of life in chronic myeloid leukemia. Leuk Res. (2013) 37:9–13. doi: 10.1016/j.leukres.2012.09.013
14. Pemberton-Whiteley Z, Nier S, Geissler J, Wintrich S, Verhoeven B, Christensen RO, et al. Understanding quality of life in patients with acute leukemia, a global survey. J Patient Cent Res Rev. (2023) 10:21–30. doi: 10.17294/2330-0698.1951
15. Wang C, Yan J, Chen J, Wang Y, Lin YC, Hu R, et al. Factors associated with quality of life of adult patients with acute leukemia and their family caregivers in China: a cross-sectional study. Health Qual Life Outcomes. (2020) 18:8. doi: 10.1186/s12955-020-1269-8
16. Netuveli G, Blane D. Quality of life in older ages. Br Med Bull. (2008) 85:113–26. doi: 10.1093/bmb/ldn003
17. Nolte S, Liegl G, Petersen MA, Aaronson NK, Costantini A, Fayers PM, et al. General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the Unites States. Eur J Cancer. (2019) 107:153–63. doi: 10.1016/j.ejca.2018.11.024
18. van Rhijn BD, Smout AJPM, Bredenoord AJ. Disease duration determines health-related quality of life in adult eosinophilic esophagitis patients. Neurogastroenterol Motil. (2014) 26:772–8. doi: 10.1111/nmo.12323
19. Knowles SR, Keefer L, Wilding H, Hewitt C, Graff LA, Mikocka-Walus A. Quality of life in inflammatory bowel disease: A systematic review and meta-analyses—Part II. Inflammatory Bowel Dis. (2018) 24:966–76. doi: 10.1093/ibd/izy015
20. Tański W, Szalonka A, Tomasiewicz B. Quality of life and depression in rheumatoid arthritis patients treated with biologics – A single centre experience. Psychol Res Behav Manage. (2022) 15:491–501. doi: 10.2147/PRBM.S352984
21. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Development of a novel hematological Malignancy specific patient-reported outcome measure (HM-PRO): content validity. Front Pharmacol. (2020) 11:209. doi: 10.3389/fphar.2020.00209
22. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Reliability of a novel hematological Malignancy specific patient-reported outcome measure: HM-PRO. Front Pharmacol. (2020) 11:571066. doi: 10.3389/fphar.2020.571066
23. Goswami P, Oliva EN, Ionova T, Else R, Kell J, Fielding AK, et al. Hematological Malignancy specific patient-reported outcome measure (HM-PRO): construct validity study. Front Pharmacol. (2020) 11:1308. doi: 10.3389/fphar.2020.01308
24. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
25. Goswami P, Oliva EN, Ionova T, Salek S. Responsiveness and the minimal clinically important difference for HM-PRO in patients with hematological Malignancies. Blood. (2018) 132:2294. doi: 10.1182/blood-2018-99-117094
26. FDA. Core Patient-Reported Outcomes in Cancer Clinical Trials (2021). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/core-patient-reported-outcomes-cancer-clinical-trials (Accessed January 15, 2024).
27. Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. (2005) 8:94–104. doi: 10.1111/j.1524-4733.2005.04054.x
28. Goswami P, Oliva EN, Ionova T, Salek S. Translating the science of patient reported outcomes into practice: meaningfulness of HM-PRO scores in patients with hematological Malignancies. Blood. (2018) 132:4860. doi: 10.1182/blood-2018-99-117180
29. Stata Bookstore. Regression Models for Categorical Dependent Variables Using Stata . Available online at: https://www.stata.com/bookstore/regression-models-categorical-dependent-variables/ (Accessed January 19, 2024).
30. Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. (2009) 114:7. doi: 10.1182/blood-2008-10-182592
31. Evans J, Ziebland S, Pettitt AR. Incurable, invisible and inconclusive: watchful waiting for chronic lymphocytic leukaemia and implications for doctor-patient communication. Eur J Cancer Care (Engl). (2012) 21:67–77. doi: 10.1111/j.1365-2354.2011.01278.x
32. Molica S. Quality of life in chronic lymphocytic leukemia: a neglected issue. Leuk Lymphoma. (2005) 46:1709–14. doi: 10.1080/10428190500244183
33. Firkins J, Hansen L, Driessnack M, Dieckmann N. Quality of life in “chronic” cancer survivors: a meta-analysis. J Cancer Surviv. (2020) 14:504–17. doi: 10.1007/s11764-020-00869-9
34. Drageset J, Sandvik RK, Eide LSP, Austrheim G, Fox M, Beisland EG. Quality of life among cancer inpatients 80 years and older: a systematic review. Health Qual Life Outcomes. (2021) 19:98. doi: 10.1186/s12955-021-01685-0
35. Loren AW. Fertility issues in patients with hematologic Malignancies. Hematology. (2015) 2015:138–45. doi: 10.1182/asheducation-2015.1.138
Keywords: leukemia, patient-centered care, patient experience, patient-reported outcomes, quality of life
Citation: Salek S, Nier S, Pemberton-Whiteley Z, Ionova T, Ianni G, Tripepi G, York N, Schroeter N, Huntley K, Rynne M, Costello D, Pecova L and Oliva EN (2025) Impact of leukemia subtype and demographics on patient quality of life in 76 countries: a cross-sectional study. Front. Hematol. 3:1502166. doi: 10.3389/frhem.2024.1502166
Received: 26 September 2024; Accepted: 30 December 2024;
Published: 21 January 2025.
Edited by:
Alexandra Smith, University of York, United KingdomReviewed by:
Matilde Scaldaferri, AOU Città della Salute e della Scienza di Torino, ItalyGiovangiacinto Paterno, University of Rome Tor Vergata, Italy
Copyright © 2025 Salek, Nier, Pemberton-Whiteley, Ionova, Ianni, Tripepi, York, Schroeter, Huntley, Rynne, Costello, Pecova and Oliva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sam Salek, bS5zLnNhbGVrQGhlcnRzLmFjLnVr; c3NzYWxlazUyQGdtYWlsLmNvbQ==
 Sam Salek
Sam Salek Samantha Nier2
Samantha Nier2 Tatyana Ionova
Tatyana Ionova Kathryn Huntley
Kathryn Huntley Esther Natalie Oliva
Esther Natalie Oliva