- 1Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH, United States
- 2Medical Scientist Training Program, College of Medicine, The Ohio State University, Columbus, OH, United States
- 3Department of Pathology, College of Medicine, The Ohio State University, Columbus, OH, United States
- 4Department of Pathology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, United States
- 5Division of Hematology, Department of Internal Medicine, James Cancer Hospital and Solove Research Institute, The Ohio State University, Columbus, OH, United States
Introduction: Diffuse large B-cell lymphoma (DLBCL) is an aggressive form of non-Hodgkin lymphoma that accounts for approximately a quarter of all lymphomas in the United States. It is rare to have co-occurring T cell lymphoma with DLBCL as they often develop following treatment of DLBCL rather than concomitantly.
Case presentation: We report a 71-year-old male patient diagnosed with Epstein–Barr virus (EBV) -negative DLBCL with concurrent angioimmunoblastic T-cell lymphoma (AITL). A right inguinal lymph node biopsy demonstrated aggressive B-cell lymphoma consistent with DLBCL with the non-germinal center immunophenotype that was EBV-negative. Furthermore, abnormal T-cells with irregular nuclear contours were found in T cell receptor sequencing with monoclonal gamma and beta T cells. A bone marrow biopsy demonstrated occasional large atypical CD3+PD1+ T cells with corresponding identical T-cell receptor clones in the lymph node biopsy. Next-generation sequencing from the lymph node biopsy demonstrated dual inactivating TET2 mutations.
Conclusion: Composite DLBCL and AITL is a rare occurrence and the absence of EBV is even more so. Given the rare nature of having these two hematologic malignancies simultaneously, no standard of care exists. However, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is a regimen commonly used in both malignancies individually, and therefore this patient was treated using this approach achieving a partial remission after four cycles of therapy. Unfortunately, he developed refractory disease 1 month after completion of six cycles of R-CHOP.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive type of non-Hodgkin lymphoma accounting for approximately 25% of all lymphomas reported in the US (1, 2). DLBCL is a heterogeneous disease with multiple subtypes. The Hans algorithm differentiates DLBCL by cell of origin into either germinal center B-cell-like (GCB) lymphoma or non-germinal center B-cell-like lymphoma (3). In addition, aggressive lymphomas with genetic translocations of the BCL2, BCL6, or MYC loci (double or triple-hit DLBCL) are now commonly referred to as high-grade B-cell lymphoma (4). DLBCL can be associated with Epstein–Barr virus (EBV) infection, which has a worse prognosis than EBV-negative DLBCL (5).
T-cell lymphomas comprise less than 15% of non-Hodgkin lymphomas in the United States (6). While there are a variety of T-cell lymphomas, angioimmunoblastic T-cell lymphoma (AITL) represents one of the more common subtypes whose cells are thought to represent the malignant counterpart of T follicular helper (TFH) cells, and is now renamed as nodal TFH cell lymphoma, angioimmunoblastic type, in the most recent classification (4). TFH cells normally aid in the formation of germinal center B cells as one functional subset of CD4+ T cells (7). Additionally, AITL cells commonly express PD-1, CD10, BCL6, and ICOS. Genetically, AITL cells frequently have mutations affecting the TET2, IDH2, DNMT3A, and RHOA loci (8).
There have been multiple reports highlighting the concurrent development of T-cell lymphoma and DLBCL, often EBV+ (9–11). Here, we highlight a case of a patient diagnosed with EBV- DLBCL with concurrent T-cell lymphoma.
Case presentation
A 71-year-old male with a history of hypertension, hypothyroidism, and obstructive sleep apnea (OSA) presented with generalized weakness and dyspnea. Examination findings were significant for diffuse lymphadenopathy which developed rapidly over approximately 1 month. He reported a 10-pound unintentional weight loss, fevers, and night sweats. Lab results were notable for white blood count (WBC) of 12.2 x109/L, hemoglobin (Hgb) of 10.1 g/dL, and platelet count (Plt) of 19 x109/L. Platelets were noted to be above 200 x109/L approximately 2 months prior to his diagnosis. Initially, because of a concern for immune thrombocytopenic purpura (ITP) he was given 40mg dexamethasone daily for 4 days and received 1 dose of intravenous immunoglobin (IVIG) with no improvement in platelet count. The medications the patient was taking included levothyroxine and valsartan.
Positron emission tomography-computed tomography (PET-CT) (see Figure 1) demonstrated diffuse hypermetabolic lymphadenopathy prominent in the right axillary subpectoral lymph node (SUV 26.2) and right inguinal lymph node (SUV 12.6).
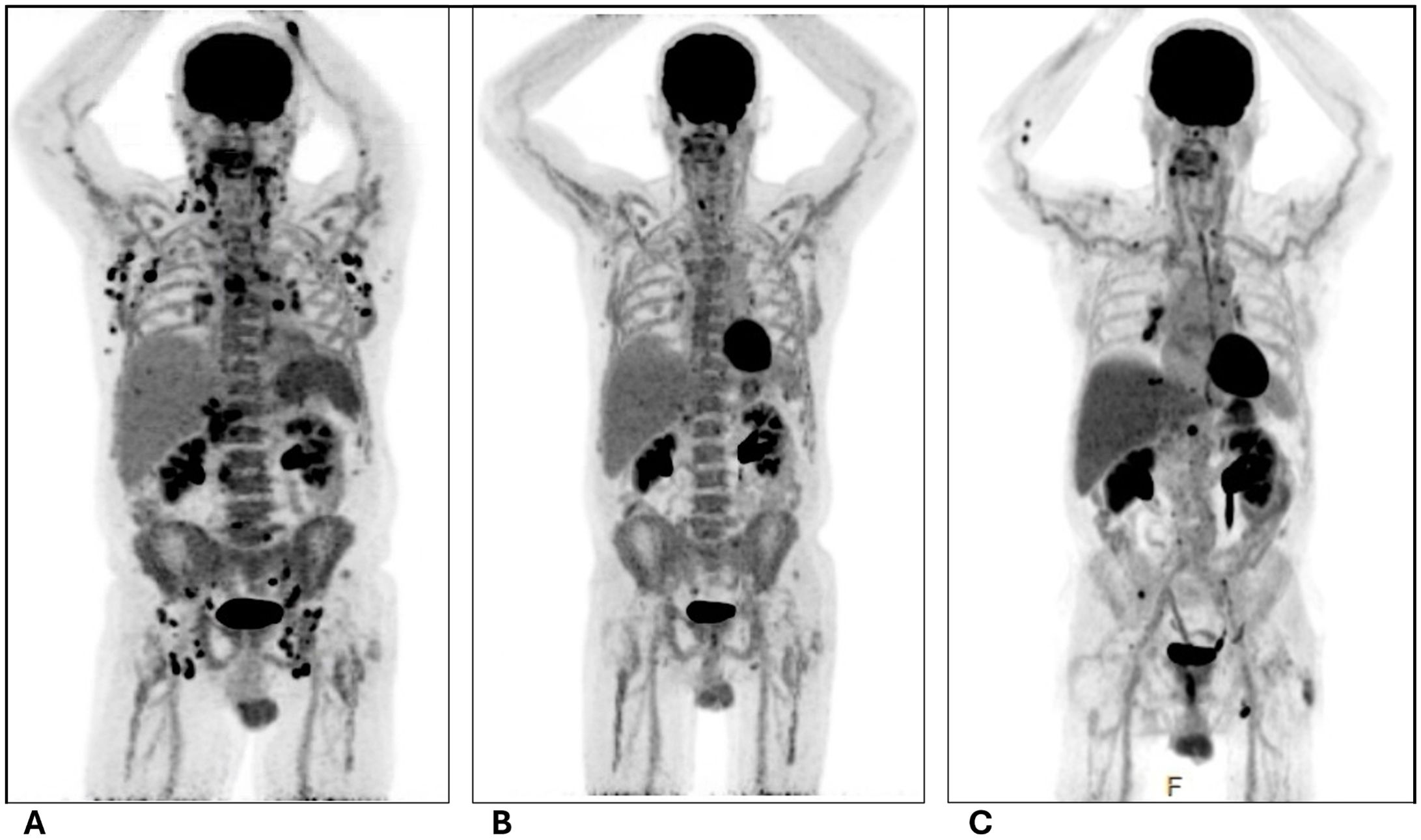
Figure 1. PET images of pre- and post-chemotherapy with R-CHOP. (A) Initial on admission; (B) After completion of 4 cycles of R-CHOP; (C) 1 month after completion of 6 cycles of R-CHOP. All images retrieved from patient chart with consent. R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
He underwent a bone marrow biopsy and a right inguinal lymph node biopsy. The bone marrow biopsy showed an atypical nodular T-cell infiltrate and predominantly polytypic plasmacytosis, presenting as multifocal plasma cell/histiocytic-rich lymphoid aggregates in markedly hypercellular, myeloid-predominant bone marrow (see Figure 2). The differential diagnoses of the initial biopsy findings included a reactive process to an infection or an autoimmune disease, Castleman disease, or a biclonal plasma cell neoplasm. Immunohistochemical (IHC) stains revealed medium to occasionally large-sized atypical CD3+PD1+ T cells with probable dim BCL6 positivity and were notably negative for CD10. There were also occasional small to medium-sized CD20+ B cells. HHV8 staining was negative with a rare EBV-encoded small RNA (EBER) positive lymphocyte population noted by in situ hybridization (ISH). EBV was also negative in a polymerase chain reaction (PCR) in blood samples. IgG IHC was positive in approximately 50% of the plasma cells with 5% positive for IgM IHC. Additionally, IgG4 IHC was negative.
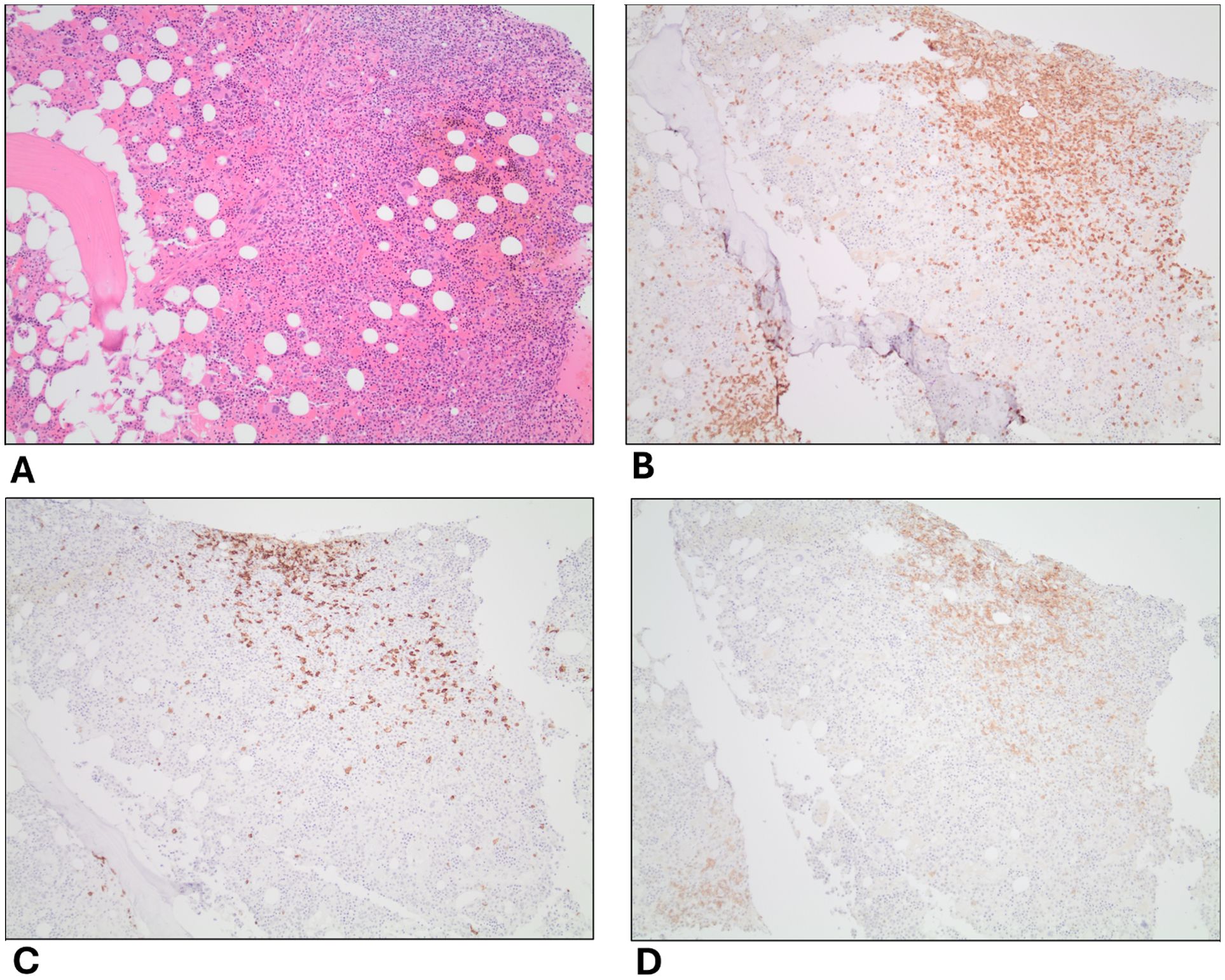
Figure 2. Histopathology of bone marrow biopsy. (A) H&E stain; (B–D) are IHC stains of CD3, CD20, and PD1 on T-cells, respectively. IHC, immunohistochemistry. 10X magnification.
The right inguinal lymph node biopsy demonstrated aggressive B-cell lymphoma consistent with diffuse large B-cell lymphoma with the non-germinal center immunophenotype that is EBV negative (see Figure 3). IHC staining revealed a high Ki-67 proliferation index of 70-80%. There were also atypical T cells intermixed. A T cell receptor (TCR) PCR revealed a monoclonal population for both TCR gamma (TCRG) and TCR beta (TCRB). An immunoglobulin heavy chain (IGH) PCR revealed a monoclonal B cell population. Next-generation sequencing revealed dual low-level TET2 inactivating variants, with a level and type most compatible with T-cell localization. These findings were suggestive of concurrent T-cell lymphoma, likely AITL based on CD3+, PD1+ T-cells with TET2 mutations. There were no rearrangements of BCL2, BCL6, or MYC.

Figure 3. Histopathology of right inguinal lymph node biopsy. (A) H&E stain; (B) EBER ISH; (C–F) are IHC stains of CD3, CD20, Ki67, and PD1 on T-cells, respectively. EBER, Epstein-Barr encoding region; ISH, in situ hybridization; IHC, immunohistochemistry. 20X magnification.
There is no standard of care for managing concurrent AITL and DLBCL. He was treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) given its clinical activity in both lymphoma subtypes (see Figure 4). The doses included rituximab 375mg/m2, cyclophosphamide 750mg/m2, doxorubicin 50mg/m2, vincristine 2mg, and prednisone 100mg. He tolerated the treatment well aside from some increased fatigue and mild nausea.
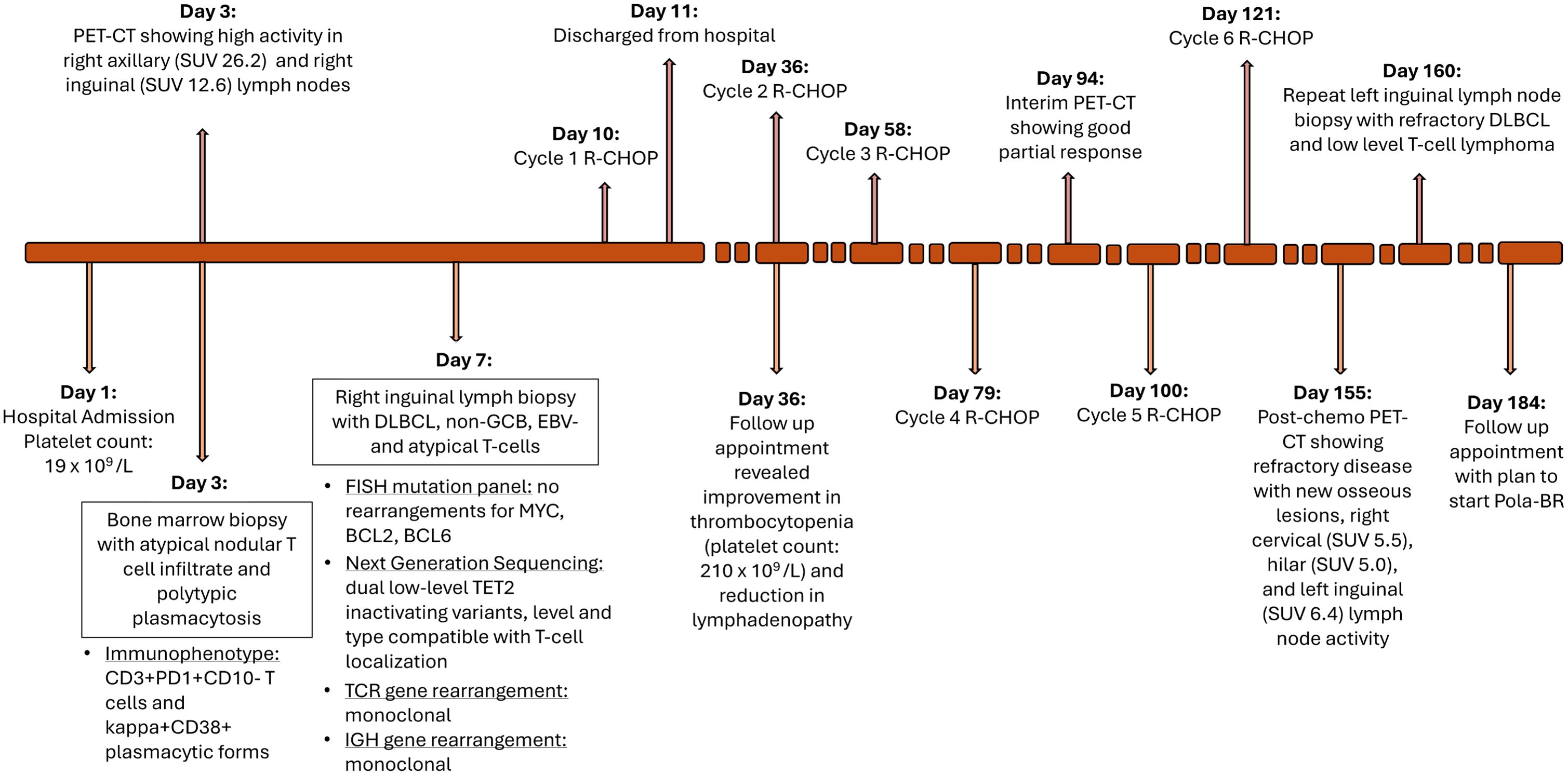
Figure 4. Timeline of diagnosis and treatment course. SUV, standardized uptake value; DLBCL, diffuse large B-cell lymphoma; GCB, germinal center B-cell like; EBV, epstein-barr virus; TCR, T-cell receptor; IGH, immunoglobulin heavy chain; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; Pola-BR, polatuzumab, bendamustine, rituximab.
He was seen for a follow-up 1 month later after completion of cycle 1, showing a reduction in lymphadenopathy and improvement in his thrombocytopenia (WBC 9.7 x109/L, Hgb 9.3 g/dL, Plt 210 x109/L). His interim PET scan after 4 cycles of R-CHOP demonstrated a very good partial response with only a small lymph node in the porta hepatis region with a low SUV uptake level of 4.8 (see Figure 1). He completed the 6 cycles of R-CHOP.
He underwent another PET-CT a month after the completion of his R-CHOP treatment regimen which unfortunately revealed refractory disease with multiple new hypermetabolic bone lesions in the ribs, vertebral bodies, and right posterior iliac bone, and new right cervical (SUV 5.5), hilar (SUV 5.0), and left femoral (SUV 6.4) lymphadenopathy (see Figure 1). A left inguinal lymph node biopsy revealed refractory DLBCL (which was Pax5+ and CD30+, with rare CD20+ cells) and clonal TCR gamma gene rearrangement (which was present on original diagnosis) indicated persistent concurrent low-level T-cell lymphoma. Thus, he will start Pola-BR (polatuzumab, bendamustine, and rituximab). This regimen was selected based on the activity and approval of Pola-BR in refractory DLBCL as well as the activity of bendamustine in T-cell lymphomas.
Discussion
We report an interesting case of composite B-cell and T-cell lymphoma. This is one of only a few cases reported with non-GCB, EBV-negative DLBCL with concurrent T-cell lymphoma, likely AITL. Typically, AITL can drive B-cell lymphoma through persistent activation of T-cells in the germinal center, reminiscent of their Tfh cell origins (12). These are often EBV-positive and of the GCB type, unlike in our patient.
The association between non-Hodgkin B-cell lymphoma and concurrent or subsequent T-cell lymphoma is well documented, but most cases are associated with EBV reactivation infections (9). There was a case of a patient with stage IIB DLBCL who was treated with R-CHOP and achieved complete remission but developed peripheral T-cell lymphoma (PTCL) a year later. Despite several different chemotherapies, the disease progressed. Another case report demonstrated a patient with grade IIA follicular lymphoma (FL) who was treated with R-CVP (rituximab, cyclophosphamide, vincristine, and prednisone) and achieved complete remission but developed AITL 2 years later. The mechanism of non-Hodgkin B-cell lymphoma and composite or subsequent T-cell lymphoma has been hypothesized to arise in individual cases through autoimmune conditions with or without accompanying therapies that impair immunity/T-cell surveillance, EBV reactivation infections, and rare germline variants that predispose to lymphoma. It has been speculated that multifocal epigenetic dysregulation through post-transcriptional mechanisms and/or mutations in epigenetic regulators such as the AITL-associated DNMT3A, IDH2, and TET2 genes underlie both B-cell and T-cell lymphomas. In this regard, EBV-associated malignancies are typically associated with a high degree of DNA hypermethylation, with response to hypomethylating therapies such as azacitidine or decitabine now added to standard R-CHOP treatment regimens in some cases (13, 14).
This case has several strengths, including the use of clinical, immunophenotypical, histological, and genetic approaches to define and classify these two malignancies. This multidisciplinary approach allowed the identification of the clonal T cell population that may have gone undetected without robust pathological analysis. Additionally, in our case, the patient at baseline had relatively few co-morbidities with no use of immunosuppressant medications or medications typically associated with thrombocytopenia, thus limiting the confounding bias when assessing hematological parameters.
Some weaknesses associated with our study include the relatively short follow-up time since the patient’s initial presentation not allowing for a complete assessment of his response to treatment. Additionally, although not typically performed, epigenetic profiling was not done to see if this patient would benefit from hypomethylating therapy in addition to R-CHOP.
Patient perspective
The patient continued to improve symptomatically since starting R-CHOP. He was seen 2 weeks after cycle 4 of 6 with an interim PET scan at that time which demonstrated a very good partial response with a solitary porta hepatis lymph node with residual FDG avidity and an SUV of 4.8. He completed his R-CHOP regimen over the next month which he tolerated well apart from fatigue and malaise 8 days following chemotherapy. He did require intermittent blood transfusions due to his Hgb level being less than 7.5 g/dL and his Plt level being less than 20 x 109/L. Unfortunately, he developed refractory disease following R-CHOP therapy and will start Pola-BR.
Conclusion
Although composite B-cell and T-cell lymphoma cases have been described before, DLBCL with EBV negativity and a non-GCB subtype in the setting of AITL is quite rare. Additionally, there is no well-studied optimal treatment for concurrent DLBCL and AITL. In our case, the patient was started on R-CHOP and initially demonstrated a very good clinical response but later developed refractory lymphoma, further highlighting the poor outcomes observed with composite lymphomas. Recognition of composite B-cell and T-cell lymphomas is critical for optimal therapy selection and prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
We obtained signed consent from the patient and this is the only requirement to publish case reports from the OSU IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
MS: Writing – original draft, Writing – review & editing. ML: Writing – original draft, Writing – review & editing. DJ: Writing – review & editing. NN: Writing – review & editing. JB: Writing – review & editing. JR: Writing – review & editing. TV: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ML was supported by grant F30CA250244.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid Malignancy statistics by World Health Organization subtypes. CA: A Cancer J Clin. (2016) 66:443–59. doi: 10.3322/caac.21357
2. Sarkozy C, Sehn LH. Management of relapsed/refractory DLBCL. Best Pract Res Clin Haematology. (2018) 31:209–16. doi: 10.1016/j.beha.2018.07.014
3. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. (2004) 103:275–82. doi: 10.1182/blood-2003-05-1545
4. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB de O, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
5. Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. Am J Hematology. (2018) 93:953–62. doi: 10.1002/ajh.25112
6. Phan A, Veldman R, Lechowicz MJ. T-cell lymphoma epidemiology: the known and unknown. Curr Hematol Malig Rep. (2016) 11:492–503. doi: 10.1007/s11899-016-0353-y
7. Gaulard P, de Leval L. Follicular helper T cells: implications in neoplastic hematopathology. Semin Diagn Pathology. (2011) 28:202–13. doi: 10.1053/j.semdp.2011.03.003
8. Fukumoto K, Nguyen TB, Chiba S, Sakata-Yanagimoto M. Review of the biologic and clinical significance of genetic mutations in angioimmunoblastic T-cell lymphoma. Cancer Science. (2018) 109:490–6. doi: 10.1111/cas.2018.109.issue-3
9. Jin X, Liu H, Li J, Xiao X, Yuan X, Chen P, et al. Composite B-cell and T-cell lymphomas: clinical, pathological, and molecular features of three cases and literature review. J Zhejiang Univ Sci B. (2023) 24:711–22. doi: 10.1631/jzus.B2300181
10. Wang E, Papavassiliou P, Wang AR, Louissaint A, Wang J, Hutchinson CB, et al. Composite lymphoid neoplasm of B-cell and T-cell origins: a pathologic study of 14 cases. Hum Pathol. (2014) 45:768–84. doi: 10.1016/j.humpath.2013.11.008
11. Suefuji N, Niino D, Arakawa F, Karube K, Kimura Y, Kiyasu J, et al. Clinicopathological analysis of a composite lymphoma containing both T- and B-cell lymphomas. Pathol Int. (2012) 62:690–8. doi: 10.1111/j.1440-1827.2012.02858.x
12. Chiba S, Sakata-Yanagimoto M. Advances in understanding of angioimmunoblastic T-cell lymphoma. Leukemia. (2020) 34:2592–606. doi: 10.1038/s41375-020-0990-y
13. Di Pietro A. Epstein–barr virus promotes B cell lymphomas by manipulating the host epigenetic machinery. Cancers. (2020) 12:3037. doi: 10.3390/cancers12103037
Keywords: diffuse large B cell lymphoma, angioimmunoblastic T cell lymphoma, Epstein-Barr virus, case report, non-germinal center B cell type, R-CHOP chemotherapy
Citation: Satoskar MA, Lordo MR, Jones DM, Nowacki NB, Brammer JE, Reneau JC and Voorhees TJ (2024) A patient with concurrent EBV-negative diffuse large B-cell lymphoma and angioimmunoblastic T-cell lymphoma: a case report. Front. Hematol. 3:1463487. doi: 10.3389/frhem.2024.1463487
Received: 12 July 2024; Accepted: 27 November 2024;
Published: 17 December 2024.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Luigi Rigacci, Campus Bio-Medico University Hospital, ItalyAda Maria Florena, University of Palermo, Italy
Copyright © 2024 Satoskar, Lordo, Jones, Nowacki, Brammer, Reneau and Voorhees. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Timothy J. Voorhees, VGltb3RoeS5Wb29yaGVlc0Bvc3VtYy5lZHU=
 Monika A. Satoskar
Monika A. Satoskar Matthew R. Lordo
Matthew R. Lordo Daniel M. Jones3,4
Daniel M. Jones3,4 Jonathan E. Brammer
Jonathan E. Brammer Timothy J. Voorhees
Timothy J. Voorhees