- 1Alexion, AstraZeneca Rare Disease, Boston, MA, United States
- 2Genesis Research Group, Hoboken, NJ, United States
- 3Division of Pediatric Allergy, Immunology, and Bone Marrow Transplantation, University of California, San Francisco, San Francisco, CA, United States
- 4Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
Background: Thrombotic microangiopathy (TMA) associated with hematopoietic stem cell transplantation (HSCT-TMA) is a serious post-transplant complication. Diagnosis is difficult due to overlapping symptoms with other conditions and a lack of universally adopted diagnostic criteria.
Methods: This retrospective, observational study investigated HSCT-TMA incidence between July 2009–August 2020 using the TriNetX US Electronic Medical Record database. Patients who underwent autologous or allogeneic HSCT procedures and had conditioning agents were stratified as follows: confirmed TMA (≥1 hemolytic uremic syndrome (HUS)/TMA diagnosis code), suspected TMA [no HUS/TMA code but met modified published Cho (adult) or Jodele (pediatric) diagnostic criteria (further information in main text), and non-TMA (met neither criteria). Baseline demographics, clinical characteristics and outcomes, and all-cause unadjusted healthcare resource utilization (HCRU) within 12-months of HSCT, were assessed. Statistical comparisons were against the non-TMA cohort (p<0.05).
Results: The study included 16,809 adults and 901 pediatrics. Of these, 125 adults (0.7%) and 30 pediatrics (3.3%) had confirmed TMA, 3029 (18.0%) adults and 94 (10.4%) pediatrics had suspected TMA; 13,655 (81.2%) adults and 777 (86.2%) pediatrics met non-TMA criteria. Confirmed and suspected TMA incidences were higher after allogeneic HSCT in adults. In pediatrics, confirmed TMA incidence was higher following autologous transplantation, and suspected TMA higher after allogeneic transplantation. Confirmed and suspected TMA patients had significantly higher Charlson Comorbidity Indexes pre-HSCT and more post-HSCT complications. In adults with confirmed and suspected TMA, mortality estimates within 12-months of HSCT were significantly higher compared to non-TMA patients, and numerically higher in pediatrics. All confirmed and suspected TMA patients had significantly more ER visits, inpatient stays and ICU admissions. HCRU within 12-months of HSCT was higher in all confirmed TMA patients; ≤0.1% of patients with suspected TMA, and 25.6–50.0% of patients with confirmed TMA, received complement inhibitors.
Conclusions: Our results demonstrate that incidence of HSCT-TMA in the real world, as per billing codes, is low compared with historical literature. However, a proportion of suspected TMA cases, based on diagnosis criteria, share similarly poor outcomes and HCRU. HSCT-TMA is likely underdiagnosed, or under-coded, in real world practice. Our study highlights the need for greater vigilance to this severe complication.
Scope statement
This study evaluates real-world incidence and outcomes of HSCT-TMA in adult and pediatric patients using a large EMR database, and our findings indicate that despite not having a diagnosis recorded as a billing code, many HSCT recipients retrospectively met published HSCT-TMA diagnostic criteria and could have had suspected TMA. We believe our study fits well into the mission and scope of Frontiers in Hematology as our findings emphasize the need for standardization of diagnostic criteria for this severe complication of TMA following HSCT, in order to accurately identify patients who are missed in current clinical practice, and who share similarly poor clinical outcomes to those patients with diagnosed TMA.
Introduction
Thrombotic microangiopathies (TMA) are a group of disorders characterized by consumptive thrombocytopenia, microangiopathic hemolytic anemia and tissue/organ injury related to endothelial damage and dysfunction (3–5). TMA occurring after hematopoietic stem cell transplantation (HSCT) is termed HSCT-TMA, or transplant associated TMA (TA-TMA) (6, 7). While HSCT-TMA often occurs within a few weeks of transplantation, later occurrence has been reported (2, 6). Recent studies have estimated global HSCT-TMA incidence rates of around 6–20% following HSCT procedures, with higher frequencies after pediatric HSCT; HSCT-TMA also appears more common following allogeneic HSCT than autologous HSCT (8). However, research and determination of the true incidence and impact of HSCT-TMA have been hampered by the lack of uniform diagnostic criteria and screening practices (7, 9–13). In addition, HSCT-TMA shares many clinical characteristics with atypical hemolytic uremic syndrome (aHUS, a form of complement-mediated TMA) which may further complicate diagnosis (14, 15).
Despite recognition of TMA as a known post-HSCT complication associated with poor outcomes, its identification in routine clinical practice is challenging due to overlapping features with other post-HSCT complications, including conditioning regimen toxicities, drug adverse effects, hepatic sinusoidal obstruction syndrome (SOS)/venoocclusive disease (VOD), infections, and graft-versus-host-disease (GvHD) (13). This often leads to delayed or missed HSCT-TMA diagnoses. Additionally, there is no single test to confirm the suspicion of HSCT-TMA diagnosis; while biopsies are particularly useful in determining a diagnosis of HSCT-TMA, they may not always be feasible early after HSCT (9, 16). As such, diagnosis mostly relies on application of clinical criteria in the appropriate clinical context (9, 16). Although many sets of clinical diagnostic criteria/algorithms have been proposed to aid in the diagnosis of HSCT-TMA, none have been well validated. Before the harmonized diagnostic criteria were published in 2023, the two most commonly used criteria were the Cho et al. and Jodele et al. criteria in adult and pediatric patients, respectively (Tables 1, 2) (1, 17). The harmonized criteria, proposed by experts from HSCT societies across the globe, are based on modifications of the Jodele et al. criteria, however optimal adoption of this harmonized approach remains to be determined (8, 17). The current literature suggests that HSCT-TMA may be under-recognized and underdiagnosed in clinical practice, further highlighting inconsistencies in diagnosis protocols, a lack of awareness and screening, and the need for standardized criteria (8, 12, 17).
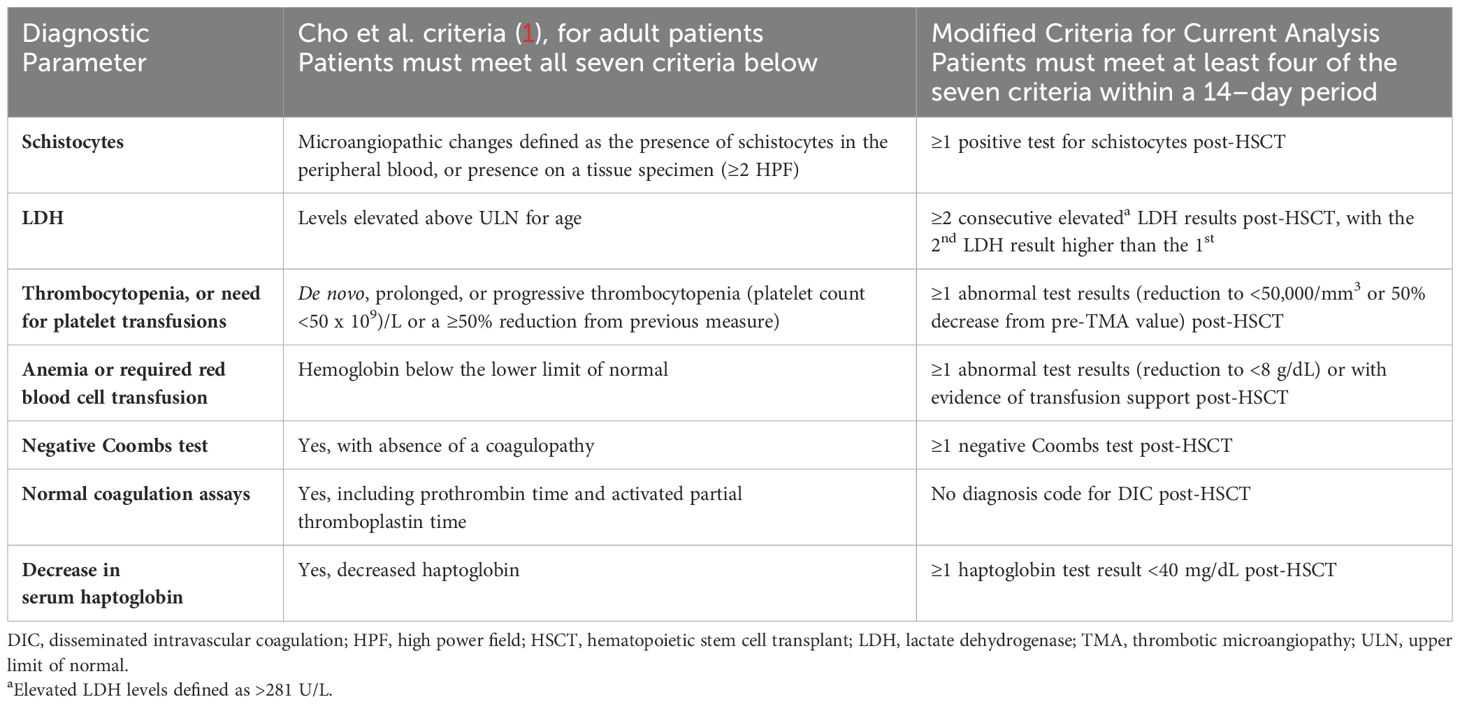
Table 1 Established HSCT-TMA diagnostic criteria from the literature and modifications used in this analysis to identify “suspected TMA” cases in adult patients.
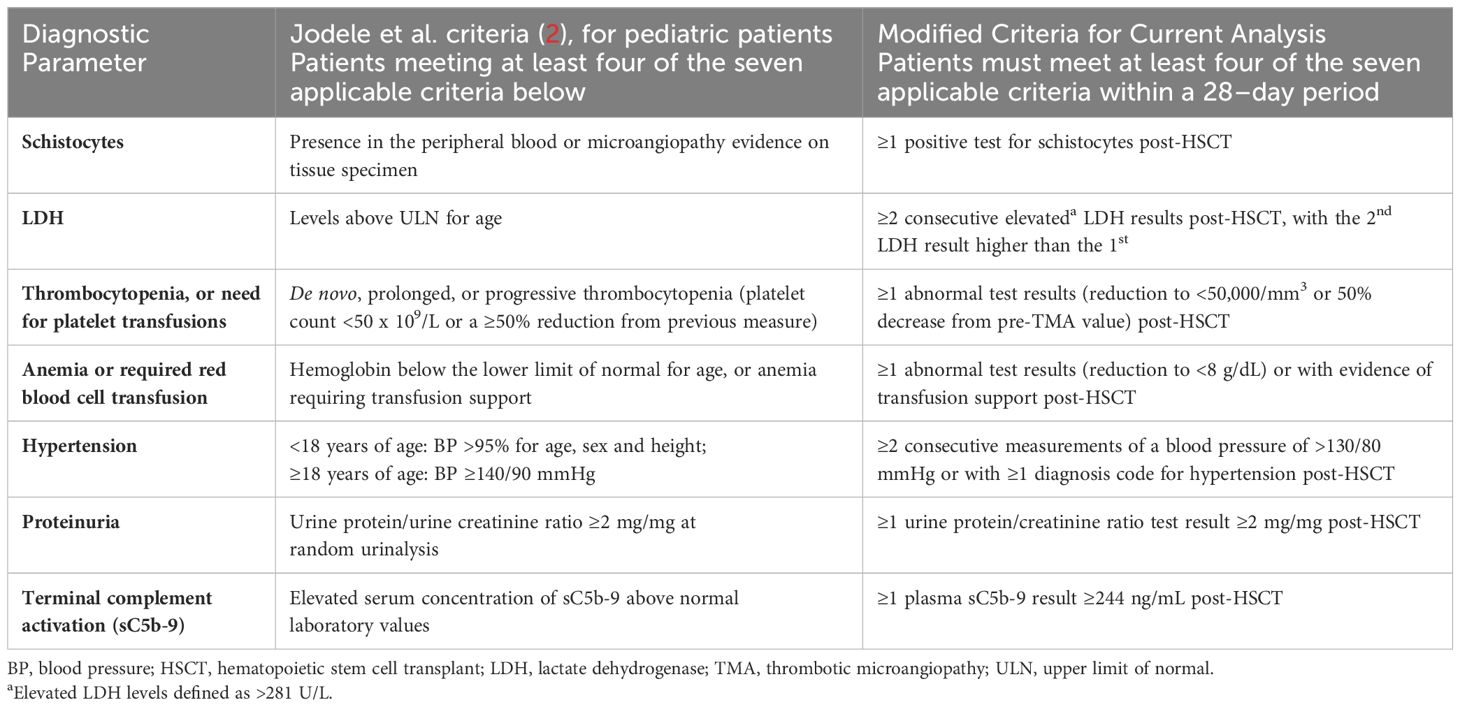
Table 2 Established HSCT-TMA diagnostic criteria from the literature and modifications used in this analysis to identify “suspected TMA” cases in pediatric patients.
Using data from a large modern electronic medical record (EMR) database in the US, this study aimed to assess the incidence of diagnosed (based on the presence of ICD diagnosis codes) and suspected (based on application of modified published criteria) HSCT-TMA cases in a real-world setting, and determine the real-world impact of HSCT-TMA on clinical outcomes and unadjusted all-cause healthcare resource utilization (HCRU).
Methods
This was a retrospective, observational study conducted using the TriNetX US EMR database. The TriNetX database is administered by TriNetX, LLC, an organization which offers paid for access to de-identified real-world patient data from large networks of healthcare organizations in the US and contains information derived mostly from large academic medical institutions, many of which are adult acute-care facilities. Data include demographic and clinical data such as diagnoses, procedures, laboratory assessments and medication histories. Due to the nature of the TriNetX database, and as the study is a retrospective assessment of de-identified EMR data, no institutional review board assessment was sought for this study.
Study population
The study period for this analysis was 01 July 2009 through 31 August 2020. Patients in the TriNetX database who had an allogeneic or autologous HSCT procedure code and received a conditioning agent ≤15 days prior to, or ≤5 days after, the HSCT procedure code date were considered to have had a HSCT and were included in this analysis. A time window was utilized to account for the real-world nature of the data collected for inclusion in this database, including the possibility of delays in date entry/recording. No specific exclusion criteria were applied. The presence of at least one conditioning agent code, appearing concurrently with a HSCT ICD code, was required to verify that the patient underwent HSCT at the time the data were entered into the database; this excluded patients who may, for example, have had a transplant in one network and moved to a different center for follow up. A full list of conditioning agents is presented in the Supplement.
Baseline was defined as the six months on or before the index date, unless otherwise specified; the index date was defined as the first date a patient had a HSCT procedure code recorded, and the minimum follow-up duration was 12 months post-index date. Patients who underwent more than one HSCT, based on ICD codes, had each analyzed separately.
This analysis assessed the incidence of HSCT-TMA by defining three distinct cohorts: confirmed TMA, suspected TMA, and non-TMA. Confirmed TMA included patients with a recorded diagnosis of hemolytic uremic syndrome (HUS; ICD 9: 283.11/ICD 10: D59.3) or TMA (ICD 9: 446.6/ICD 10: M31.1) within 12 months of HSCT; no specific diagnosis code(s) for HSCT-TMA were available during our study period. Suspected TMA included patients who did not have a recorded diagnosis code for HUS/TMA but who met modified Cho et al. (adults) or Jodele et al. (pediatric) criteria for diagnosis of HSCT-TMA (Tables 1, 2) within 12 months of HSCT (1, 17). Due to the limited availability of certain lab test results, modifications were made to ensure diagnostic criteria were applicable to data available in the TriNetX database. Published diagnostic criteria and any modifications which were made to address potential limitations of retrospectively analyzing real-world data are reported in detail in Tables 1, 2. Patients were included in the non-TMA cohort if they did not have a diagnosis code for HUS/TMA and did not meet the modified TMA diagnosis criteria. Adult (≥18 years) and pediatric (<18 years) patients, based on age at transplantation, were analyzed separately (Figure 1). The list of diagnostic codes used to identify and classify TMA patients in this analysis, alongside the proportion of patients with each recorded code, are included in the Supplement.
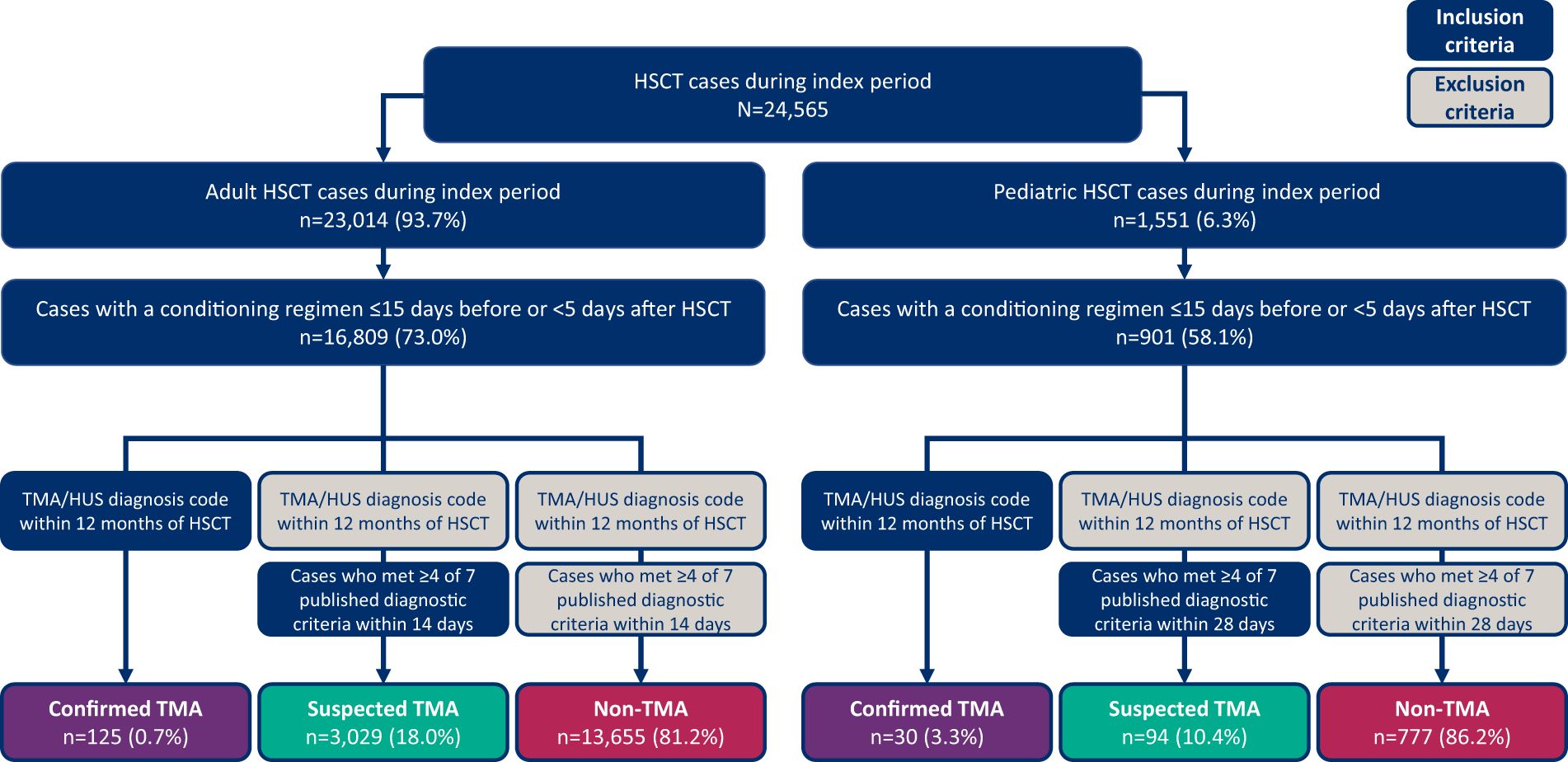
Figure 1 Patient identification and selection process diagram. HSCT, hematopoietic stem cell transplant; HUS, hemolytic uremic syndrome; TMA, thrombotic microangiopathy.
Patient demographics and clinical characteristics
Demographics and clinical characteristics of each of the three HSCT-TMA cohorts were described. Additional variables such as type and reason for HSCT, along with other post-transplant complications within six months of the procedure, such as GvHD, gastrointestinal (GI) bleeding, respiratory failure, acute and chronic renal injury, dialysis requirement, and renal transplantation were also assessed. Age at TMA diagnosis was defined as the patient’s age on the encounter date in patients with a TMA diagnosis code (confirmed TMA patients), or the earliest date at which the modified published criteria were met (suspected TMA patients). The Charlson Comorbidity Index (CCI) was calculated based on ICD codes recorded for each patient at baseline; haptoglobin level, oxygen/intubation status, dialysis requirement and renal transplantation requirement were also assessed. As date of death is not available, and only age at death is available, in the TriNetX database, we were restricted to estimating one year mortality from HSCT (index date), based on the patient’s age at HSCT relative to their age of death. The lower end of the estimated mortality at one year was restricted to patients whose age at death was the same as their age at HSCT (index), while the maximum estimated mortality included patients whose age at death was one year older than their age at HSCT (index). Patients with age of death ≥2 years after HSCT were not included in the mortality calculations; missing data, i.e. no age at death, was considered as no death. Further detail on the variables above are described in the footnotes of Table 3.
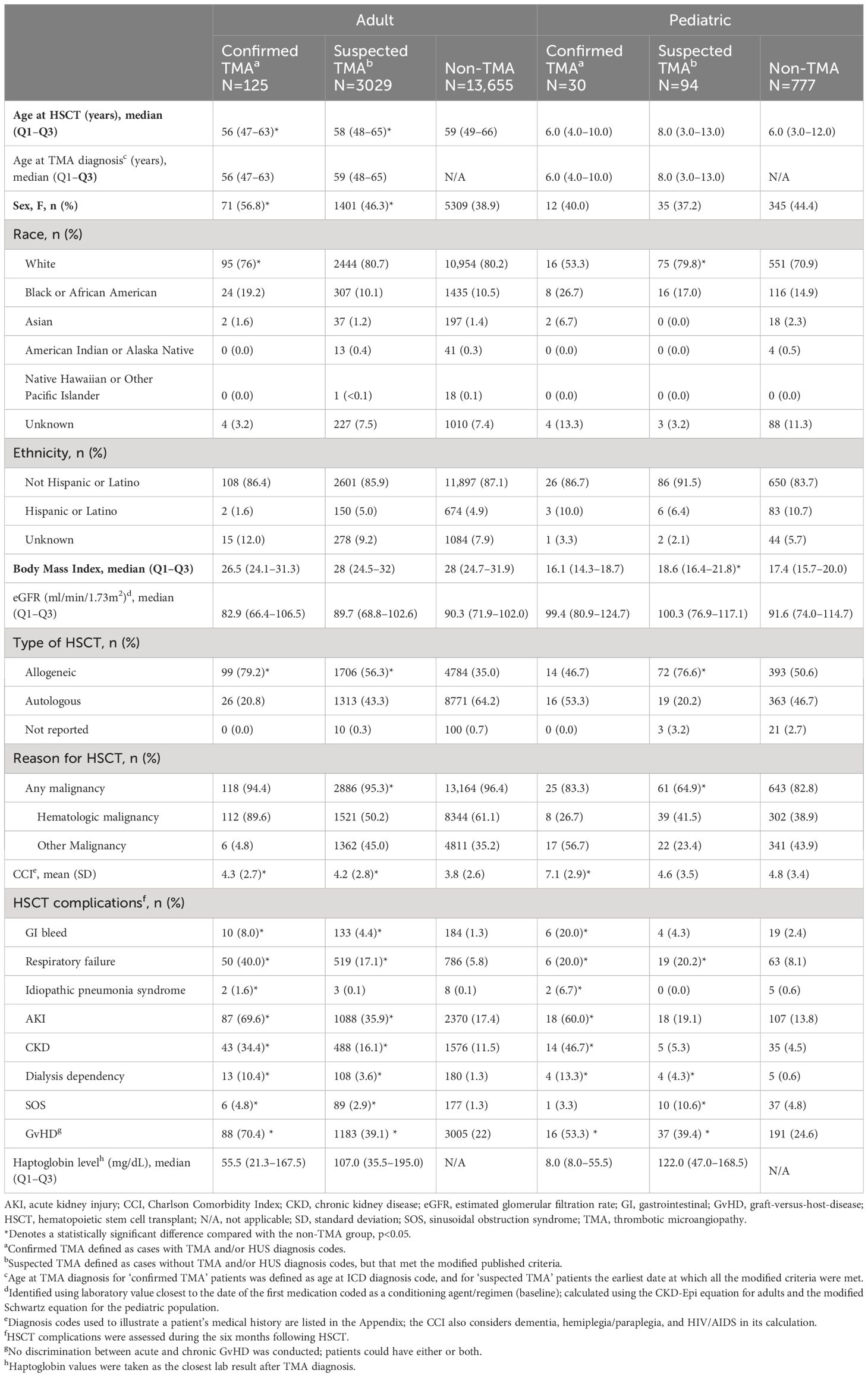
Table 3 Baseline demographics and characteristics in adult and pediatric patients within the confirmed, suspected or non-TMA cohorts following HSCT.
This study also assessed the clinical status of patients with HSCT-TMA after TMA diagnosis via measurements of hematologic and renal parameters. Hematologic improvement was defined as meeting platelet and lactate dehydrogenase (LDH) normalization criteria simultaneously within 40 days of each other; renal improvement was defined as any reduction in proteinuria from baseline, a 30% increase in eGFR, or discontinuation from dialysis. Full hematologic and renal improvement criteria are summarized in the footnotes of Table 4. Combined improvement was defined as meeting all hematologic and renal improvement criteria simultaneously, with initial normalized results within 40 days of each other, and time to resolution was measured from TMA diagnosis date (confirmed TMA) or the earliest date at which criteria were met (suspected TMA). The 40-day cut-off was selected based on initial exploratory analyses of the dataset.
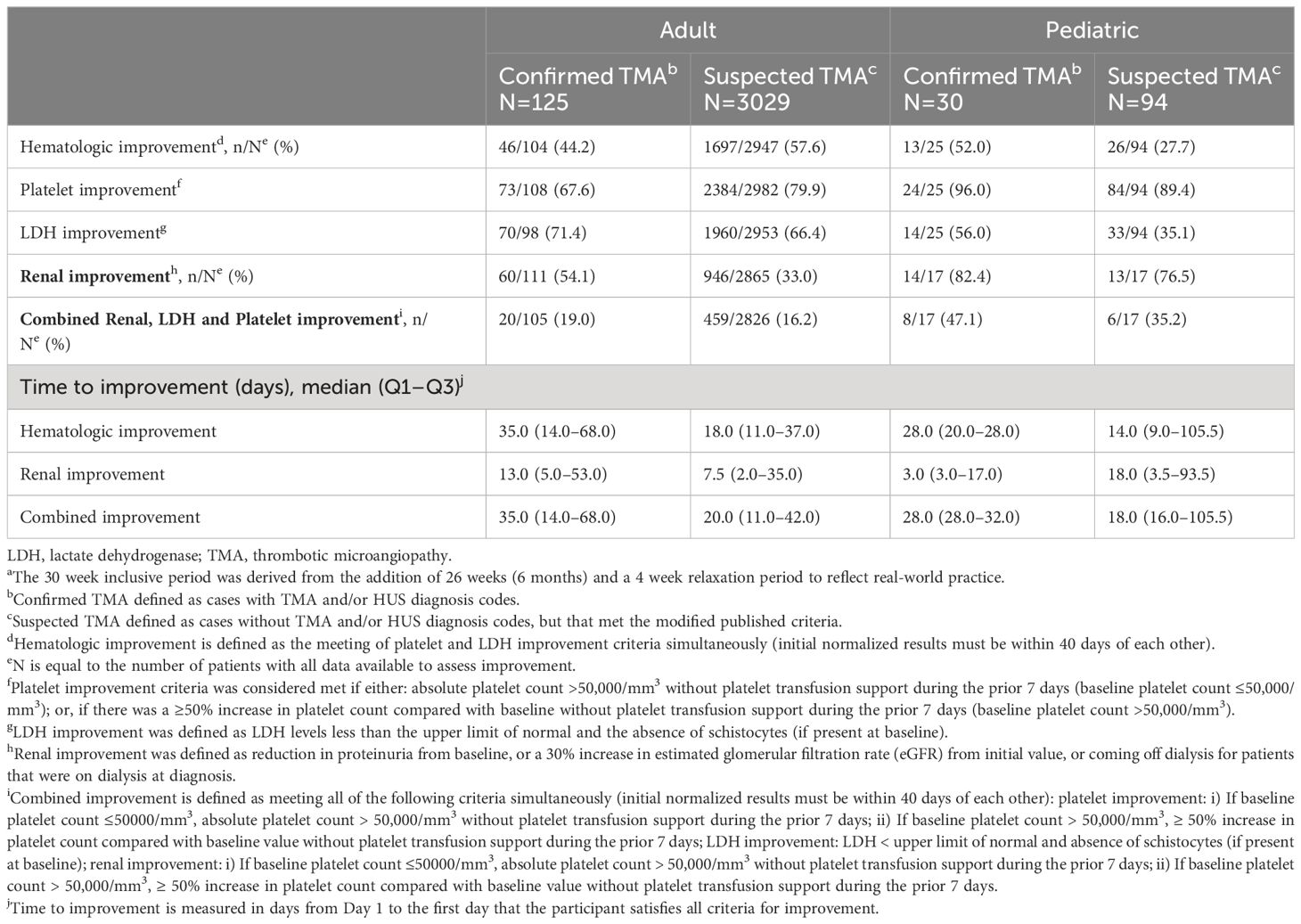
Table 4 TMA specific clinical status of adult and pediatric patients within 30 weeksa of TMA diagnosis.
Unadjusted all-cause healthcare resource utilization
Data pertaining to unadjusted HCRU during the 12 months post-HSCT were evaluated by total number of prescriptions; targeted therapies administered (including plasma exchange, complement inhibitors, rituximab, and defibrotide); and data on hospital visits (including inpatient hospitalizations, intensive care unit (ICU) stays, emergency room visits, outpatient visits, and readmission rates). HCRU was presented as unadjusted estimates.
Statistical analyses
All study objectives and patient metrics were characterized using means (standard deviations [SD]) and/or medians (interquartile ranges) for continuous variables, and absolute values and relative frequencies for categorical variables. Statistical comparisons were made using the student's t test for means, Mann-Whitney U test for medians, and Fisher's exact test for categorical variables; statistical significance was set at p<0.05, as commonly utilized in similar analyses. As the key goal of this analysis was to evaluate any underdiagnosis of HSCT-TMA, comparisons were only made between the confirmed TMA and non-TMA, and suspected TMA and non-TMA groups; no adjustments were made for multiple comparisons. No formal sample size/power calculations were performed, with all patients who met inclusion criteria included in the analysis.
Baseline eGFR values were set as the closest lab result to the date of administration of the first conditioning agent component; eGFR was calculated using the CKD-Epi equation (adults) and modified Schwartz equation (pediatrics) (18, 19). The following age-dependent upper limit of normal values for LDH measurements were used: ≤1 month old, 465 U/L; 2–17 months, 450 U/L; 18 months–10 years, 430 U/L, 11–17 years 287 U/L; ≥18 years, 281 U/L. Statistical comparisons were conducted separately for each mortality calculation (age at death or age at death +1).
Results
Patient population
A total of 24,565 HSCT procedures were identified from the TriNetX database within the study period; 23,014 (93.7%) were in adults (14,604 autologous and 8,254 allogeneic) and 1,551 (6.3%) were in pediatrics (652 autologous and 853 allogeneic). Concurrent conditioning agents were present in 72.1% of procedures, resulting in a final study population of 16,809 adult and 901 pediatric HSCT procedures (Figure 1). As noted in the Methods section, patients who met modified Cho et al. (adults) or Jodele et al. (pediatric) criteria for diagnosis of HSCT-TMA were deemed to be suspected cases. The proportion of patients meeting different numbers of components of these criteria is available in Supplementary Table 1.
Incidence of TMA in adult patients
Of the 16,809 adult HSCT procedures identified, only 125 (0.7%) had confirmed TMA based on billing diagnosis codes, while 3,029 (18.0%) had suspected TMA and 13,655 (81.2%) had no TMA (Figure 1). In adult patients with one HSCT procedure (n=14,708), 0.7% had confirmed TMA, 18.7% had suspected TMA and 80.5% did not have TMA; in patients with multiple HSCT procedures (n=1,026), 0.8% had confirmed TMA, 13.2% had suspected TMA and 86.1% did not have TMA. Confirmed and suspected TMA cases were more common after allogeneic (1.5% and 25.9%) compared with autologous (0.3% and 13%) transplantation, respectively (Table 3).
Incidence of TMA in pediatric patients
Of the 901 pediatric HSCT procedures identified, 30 (3.3%) had confirmed TMA based on billing diagnosis codes, 94 (10.4%) had suspected TMA and 777 (86.2%) had no TMA (Figure 1). Among patients with one HSCT procedure (n=566), 2.8% had confirmed TMA, 13.4% had suspected TMA and 83.7% did not have TMA; in patients with multiple HSCT procedures (n=144), 4.2% had confirmed TMA, 5.6% had suspected TMA and 90.3% did not have TMA. The incidence of confirmed TMA was similar between allogeneic and autologous HSCT patients; among those receiving allogeneic HSCT procedures, 2.9% had confirmed TMA and 15.0% had suspected TMA, whereas among those receiving autologous HSCT procedures, 4.0% had confirmed TMA and 4.8% had suspected TMA (Table 3).
Baseline demographics
The distribution of demographic and clinical characteristics within the confirmed, suspected, and non-TMA cohorts is presented in Table 3.
In the adult cohort, the proportion of females with confirmed (56.8%) and suspected TMA (46.3%) was significantly greater than in the non-TMA group (38.9%). Furthermore, incidence of confirmed and suspected TMA among White, Black and Asian patients was 0.7% and 18.1%, 1.4% and 17.4%, and 0.8% and 15.7%, respectively. Across all groups, the majority of patients required HSCT due to a hematologic malignancy.
In the pediatric cohort, the proportion of females with confirmed (40.0%) and suspected TMA (37.2%) was not significantly different than in the non-TMA group (44.4%). Furthermore, incidence of confirmed and suspected TMA among White, Black and Asian patients was 2.5% and 11.7%, 5.7% and 11.4%, and 10.0% and 0.0%, respectively. Fewer than half of all pediatric patients required HSCT due to hematologic malignancy; patients with suspected TMA most frequently underwent HSCT for hematologic malignancy (41.5% versus 23.4%).
Baseline laboratory parameters and medical history
Among adult patients, median baseline eGFR values were similar across all groups (Table 3). Mean pre-transplant Charlson Comorbidity Index (CCI) scores were significantly higher in patients with confirmed and suspected TMA, compared to patients without TMA. HSCT complications were significantly more common in adults within the confirmed and suspected TMA cohorts than the non-TMA cohort. Specifically, 70.4% of patients with confirmed TMA were also recorded as having acute or chronic GvHD, compared with 39.1% of patients with suspected TMA and 22.0% of patients without TMA. Adult patients with confirmed and suspected TMA also had a higher incidence of respiratory and renal dysfunction, as well as GI bleeding, compared to patients without TMA.
Among pediatric patients, median baseline eGFR values were similar across all groups. Mean pre-transplant CCI was significantly higher in patients with confirmed TMA versus patients without TMA but was not different between patients with suspected and no TMA. HSCT complications were significantly more common in pediatric patients within the confirmed and suspected TMA cohorts than the non-TMA cohort. Specifically, 53.3% of patients with confirmed TMA also had recorded acute or chronic GvHD, compared with 39.4% of patients with suspected TMA and 24.6% of patients without TMA. Pediatric patients with confirmed TMA also had a higher incidence of respiratory, renal and GI bleeding compared with patients without TMA, however patients with suspected TMA only had a significantly higher incidence of respiratory dysfunction.
Clinical status, outcomes and mortality after TMA diagnosis
Of adult patients with confirmed TMA with the results to meet the specified criteria, 46/104 (44.2%) met hematologic response criteria, 60/111 (54.1%) met renal response criteria, and 20/105 (19.0%) met combined improvement criteria (Table 4). Median times to hematologic, renal, and combined improvement in these patients were 35.0, 13.0 and 35.0 days, respectively. Of the pediatric patients with confirmed TMA and the results to meet the specified criteria, 13/25 (52.0%) met hematologic response criteria, 14/17 (82.4%) met renal response criteria, and 8/17 (47.1%) met combined improvement criteria. Median times to hematologic, renal and combined improvement were 28.0, 3.0 and 28.0 days, respectively). A similar trend was observed in times to hematologic, renal and combined improvement in adult and pediatric patients with suspected TMA.
Adult patients with confirmed and suspected TMA had significantly higher frequencies of oxygen/intubation and dialysis compared with non-TMA patients (Figure 2A). Pediatric patients with confirmed TMA had a higher frequency of oxygen/intubation and dialysis compared with non-TMA patients, while suspected TMA patients only had a higher frequency of oxygen/intubation versus non-TMA patients (Figure 2B).
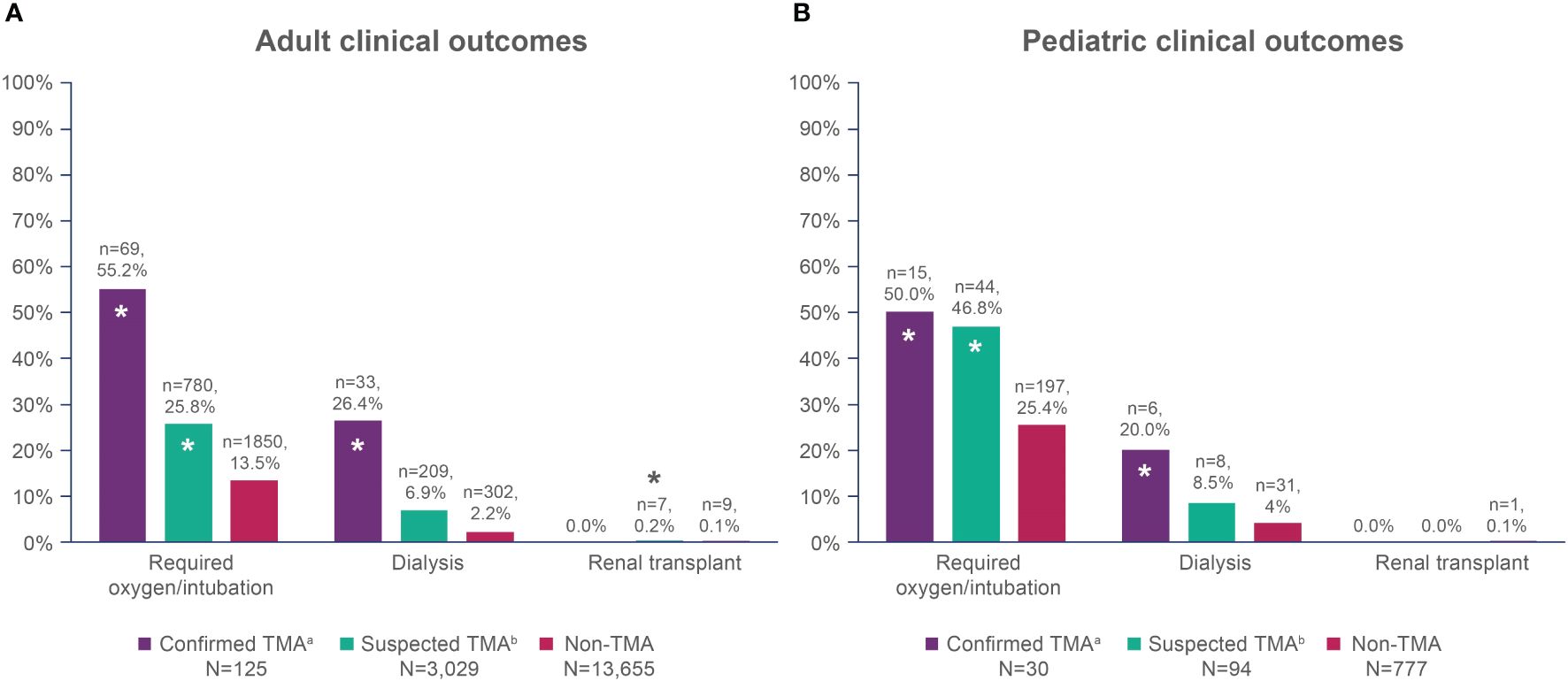
Figure 2 Overall clinical outcomes within one year of HSCT in (A) adult patients; and (B) pediatric patients. HSCT, hematopoietic stem cell transplant; TMA, thrombotic microangiopathy. *Denotes a statistically significant difference compared with the non-TMA group, p<0.05. aConfirmed TMA defined as cases with TMA and/or HUS diagnosis codes. bSuspected TMA defined as cases without TMA and/or HUS diagnosis codes, but that met the modified published criteria.
Mortality estimates in the first year after HSCT were significantly higher in adult patients with confirmed (27.2–40.0%) and suspected TMA (22.6–30.7%), compared with patients in the non-TMA cohort (8.2–13.6%). In pediatric patients, there was also a trend toward higher 1 year mortality for confirmed TMA (20.0–26.7%) and suspected TMA (16.0–23.4%) compared with patients without TMA (10.0–15.8%), although this did not reach the level of statistical significance (Figure 3).
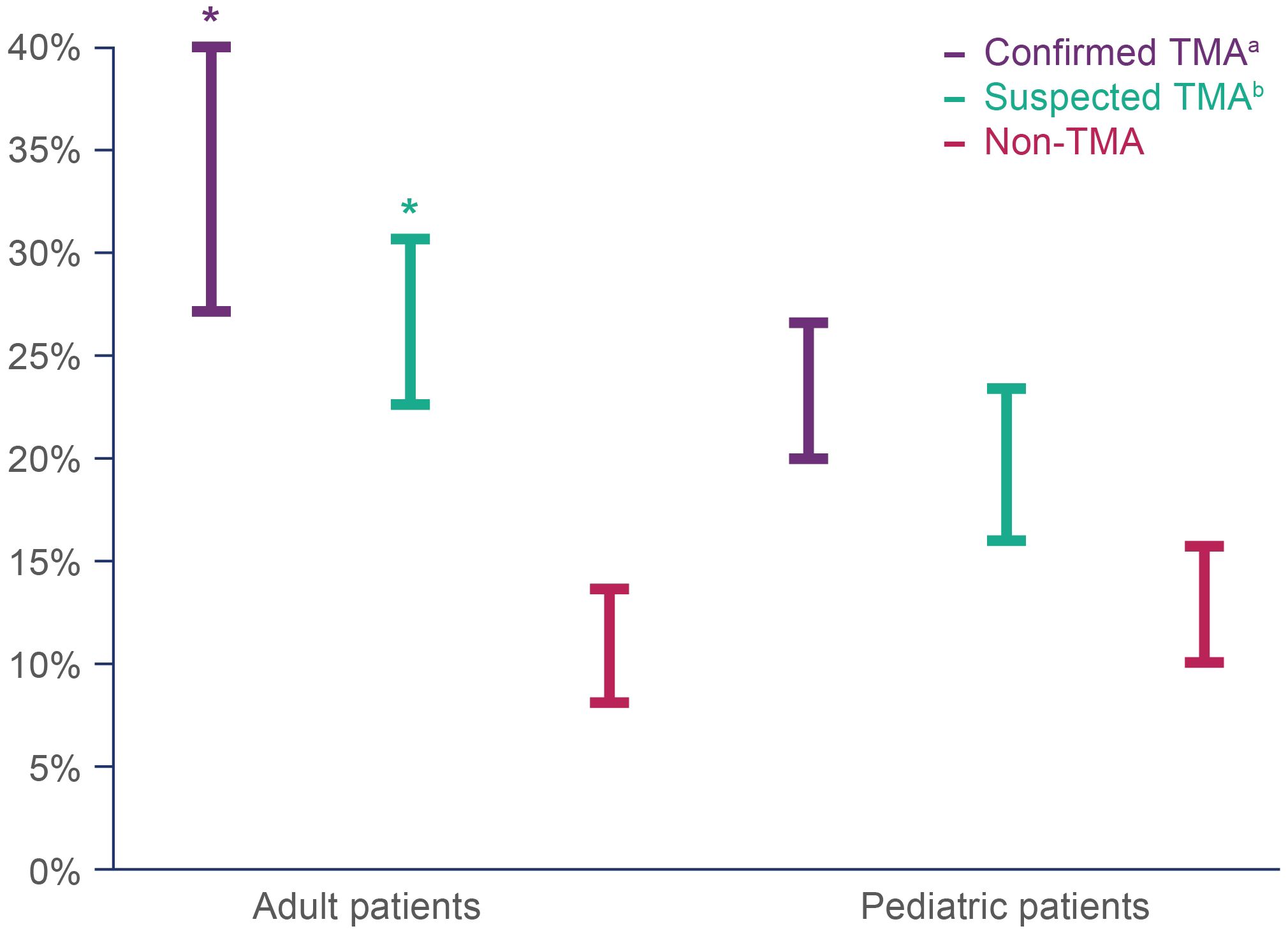
Figure 3 Estimated mortality range within one year of HSCT in adult and pediatric patients with confirmed, suspected and no TMA. Mortality was assessed as a dichotomous variable based on whether a patient had a recorded ‘age at death’. The lower end of the estimated mortality at one year was restricted to patients whose age at death was the same as their age at HSCT (index date), while the maximum estimated mortality included all patients whose age at death was equal to or one year older than their age at HSCT (index date). HSCT, hematopoietic stem cell transplant; TMA, thrombotic microangiopathy. *Denotes a statistically significant difference compared with the non-TMA group, p<0.05. aConfirmed TMA defined as cases with TMA and/or HUS diagnosis codes. bSuspected TMA defined as cases without TMA and/or HUS diagnosis codes, but that met the modified published criteria.
Healthcare resource utilization
All-cause healthcare resource utilization per patient within the first year after HSCT was higher in patients with confirmed or suspected TMA compared with non-TMA patients (Table 5). In adults, the median number of prescriptions per patient was highest in confirmed TMA cases, and 32 (25.6%) of this cohort received TMA-targeted therapy, including the complement inhibitors eculizumab and ravulizumab. Meanwhile, 33 (26.4%) and 24 (19.2%) patients received plasmapheresis and rituximab, respectively, and 1 (0.8%) patient received defibrotide. Suspected TMA patients also had significantly more prescriptions than non-TMA patients (median, 75.0 and 59.0, respectively), and very few patients received complement directed therapy; a small proportion of patients were given plasmapheresis (5.9%), rituximab (7.3%), or defibrotide (0.9%). Patients with confirmed and suspected TMA had a significantly higher frequency of total, inpatient, and outpatient visits per patient. Patients with confirmed and suspected TMA also had significantly higher readmission rates per patient and number of ICU admissions than non-TMA patients.
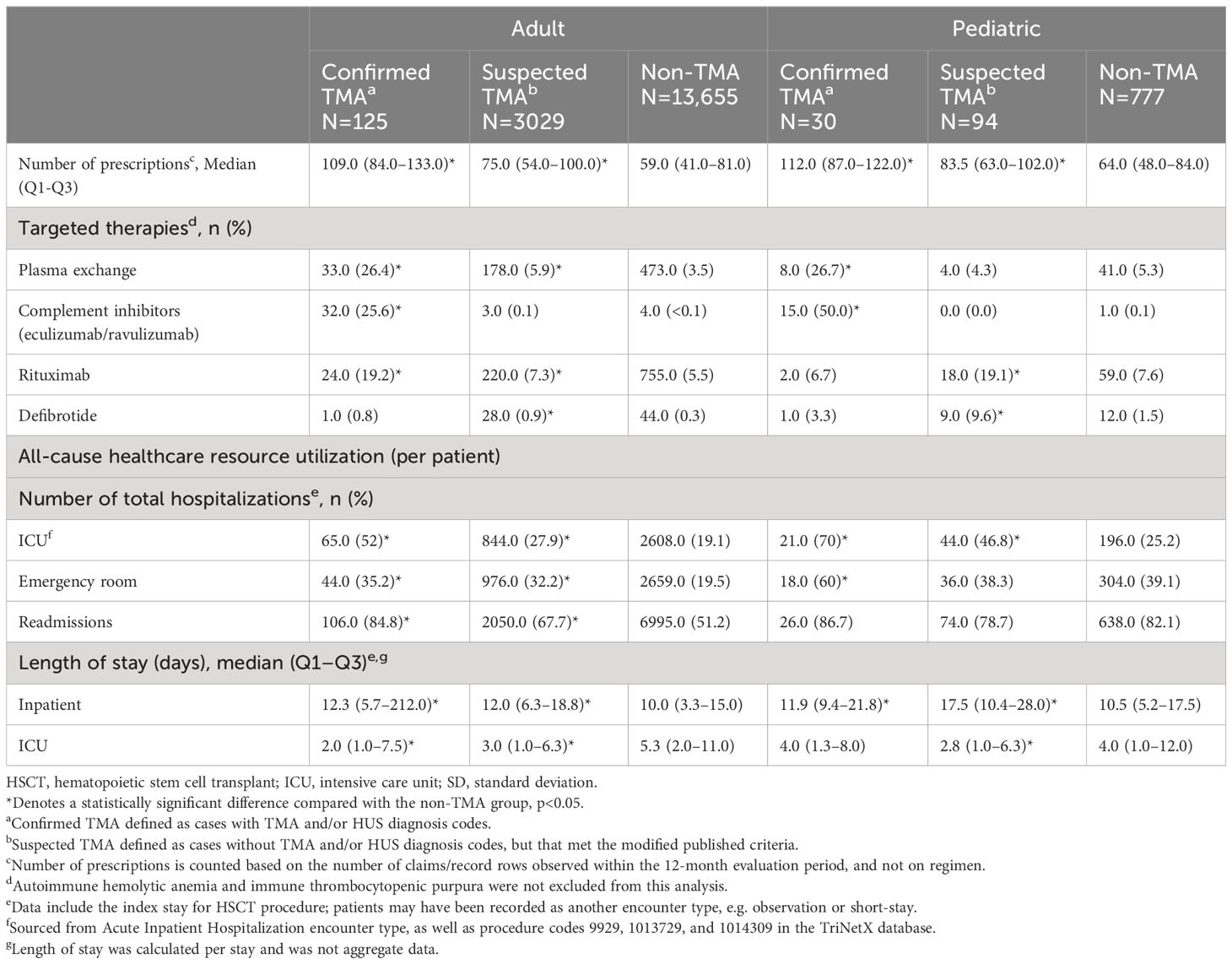
Table 5 Unadjusted all-cause healthcare resource utilization in adult and pediatric patients within 12 months of HSCT.
In pediatric patients, the median number of prescriptions per patient was significantly higher in confirmed TMA (112.0), and suspected TMA (83.5) patients than non-TMA patients (64.0). Among patients with confirmed TMA, 15 (50.0%) received TMA-targeted therapy, 8 (26.7%) were given plasmapheresis and only 2 (6.7%) and 1 (3.3%) patient received rituximab and defibrotide, respectively. Within the suspected TMA cohort, no patients received complement directed therapy, but a small proportion were given plasmapheresis (4.3%), rituximab (19.1%) or defibrotide (9.6%). The median number of total hospital visits per patient was not significantly different across confirmed, suspected, and non-TMA groups (33.0, 38.5 and 33.0, respectively), nor were median number of emergency visits, or readmission rates. However, length of inpatient stay was longer in patients with confirmed and suspected TMA than non-TMA cases, and they had more ICU admissions compared with non-TMA patients.
Discussion
Findings from this large EMR database study highlight an extremely low incidence of billing code confirmed HSCT-TMA diagnoses (0.7–3.3%), but a rate of suspected TMA (10.0–18.0%) aligning more closely to incidence estimates from the literature (6, 10). In addition to worse outcomes and higher burden of disease among patients with suspected TMA versus patients without TMA, this suggests HSCT-TMA is underdiagnosed, or at least, under-coded, by providers in current real-world practice. This study represents the first known effort to investigate HSCT-TMA systematically using a large real-world US-based EMR database for both adults and children.
The potential underdiagnosis identified in our study has also been reported elsewhere. For instance, a recent study in a large Japanese cohort observed a 5% incidence of TMA within 12 months of allogeneic HSCT (20). Furthermore, a large single center retrospective study of 1990 allogeneic HSCT patients identified 258 who met City of Hope criteria for “definite TMA”; of these, only 45 (17%) were diagnosed with TMA in real time (21). Importantly, this study further demonstrated long-term renal outcomes and overall survival were significantly worse in patients who met HSCT-TMA diagnostic criteria but did not have a recorded clinical diagnosis in real time, compared with patients without TMA (21). As the incidence of HSCT-TMA varies significantly, universal adoption of screening practices and diagnostic criteria is seemingly a necessity to support clinical diagnosis (8).
The ramifications of under-recognition and underdiagnosis of HSCT-TMA is of particular importance as high mortality and long-term morbidity burdens, including chronic kidney disease, associated with HSCT-TMA are potentially mitigatable with early intervention (16). Development of HSCT-TMA has been associated with substantial increases in risk of long-term kidney dysfunction and mortality (21), with more severe forms of the condition associated with long-term morbidity and mortality rates exceeding 80% (2, 6).
The absence of standardized diagnostic criteria for HSCT-TMA has rendered it difficult to consistently identify and perform comparative studies of this severe transplant complication (1). Original definitions of TMA following transplant from the Blood and Marrow Transplants Clinical Trials Network (CTN) and the International Working Group (IWG) were restrictive, mandating the presence of renal/neurologic dysfunction or schistocytes on smears. In an assessment of these criteria, Cho et al. identified a group of patients with “probable TMA”, who met most of the TMA criteria but did not have organ dysfunction (CTN) or sufficient schistocytes (IWG); these patients had poorer outcomes than patients without TMA. This led to their proposal of a more inclusive diagnostic definition of “overall TMA” in adult transplantation (1). However, widespread and consistent adoption of clinical criteria for HSCT-TMA has remained elusive, with other diagnostic criteria such as City of Hope and Jodele et al. criteria employed at various transplant centers in differing populations. The availability of such a variety of diagnostic criteria, alongside a lack of uniform application, has confounded the diagnosis and reporting of HSCT-TMA.
Our results identified sizable proportions of adult and pediatric patients with suspected TMA based on retrospectively applied clinical criteria; although many patients met one or two of the diagnostic criteria, in order to accurately estimate patients with TMA following HSCT, only those meeting four or more criteria within a specified time window were included in the suspected TMA cohort (Supplementary Table 1).
Our study also provides further evidence that these patients present similarly to those with confirmed TMA and have worse clinical outcomes than those without TMA. In particular, adult patients with confirmed and suspected TMA generally had higher CCI scores before transplantation, and a greater requirement for clinical intervention (e.g., dialysis, oxygen/intubation), a greater risk of mortality and higher healthcare resource utilization after HSCT than patients without TMA. Consistent with the literature, our results suggest that adult HSCT-TMA patients had worse clinical outcomes than pediatric HSCT-TMA patients (11, 22). However, some notable results should be highlighted. For example, in pediatric patients, despite numerical differences, no significant difference in mortality was observed between groups; this may be due to the small sample size of pediatric patients in our study, or more rapid identification and differential management of HSCT-TMA in pediatric compared with adult patients. The proportion of patients with acute or chronic GvHD was highest in adult (70.4%) and pediatric (53.5%) patients with confirmed TMA, although incidence remained significantly higher across all patients with suspected or confirmed TMA compared to patients without TMA. These findings are consistent with the literature that GvHD is commonly associated with HSCT-TMA (13). Interestingly, there was a relatively high proportion of pulmonary complications in patients with confirmed and suspected TMA; a similar higher proportion of lung-related complications has been reported in patients with aHUS, which as discussed shares many clinical similarities with HSCT-TMA (23). Overall, findings from this study reinforce the high clinical and healthcare utilization burden associated with both confirmed and suspected HSCT-TMA, and highlight potential areas of interest for future study.
More recently, refinements of the current clinical guidelines for the diagnosis of HSCT-TMA have been proposed. An expert opinion report – co-authored in collaboration with global HSCT societies – was recently published on the harmonization of definitions, diagnostic criteria and prognostic assessment of both adult and pediatric patients with HSCT-TMA (8). Although not published at the time our study was conducted, the modifications to the Jodele et al. criteria proposed in the harmonization consensus were akin to those utilized in our study; for example, the study highlighted that patients need to meet at least four of the seven applicable criteria at two time points within a specified time window. An initial sub-analysis of our data was completed to assess the differences in HSCT-TMA diagnosis during specific time periods (2009-2014 compared to 2015-2020); no differences between the two periods were observed. However, it would be of interest to explore how availability of these harmonized criteria impacts the diagnosis of HSCT-TMA in the future, as we believe a harmonized criteria may help to lessen the discrepancy of HSCT-TMA underdiagnosis in the real-world. It would also be interesting to explore whether the new harmonized diagnostic criteria could be applied to EMR systems in a future study, perhaps using artificial intelligence and machine learning approaches, to screen patients and alert clinicians in real time to the possibility of incipient HSCT-TMA.
Another notable finding from this study was that use of targeted therapies was low, even among patients with billing code confirmed TMA (25.6% in adults and 50.0% in pediatric patients), and very low in patients with suspected TMA (0.1% in adults and no pediatric patients), which may be a contributing factor to the associated poor clinical outcomes. Despite the growing evidence for complement involvement in the pathogenesis of HSCT-TMA, there are currently no FDA or EMA approved treatments, and the use of complement directed therapies such as eculizumab and ravulizumab, or endothelial protective agents such as defibrotide, in HSCT-TMA are “off label.” (12) As such, these costly therapies may be inaccessible within some healthcare systems, and likely contribute to the underutilization observed in our study and in real-world practice (10, 22, 24). Notably, a small number of adult and pediatric patients with suspected TMA in our study were given plasmapheresis, rituximab or defibrotide, possibly as these patients may have been treated for other endothelial syndromes such as liver VOD/SOS. A list of ongoing and recently completed clinical trials in the field of HSCT-TMA is provided in the Supplement (Supplementary Table 6).
Although our study is fortified by the very large numbers of patients in the TriNetX database, it has several limitations inherent both to its retrospective design and to the nature of the TriNetX database itself. The dataset contains entirely de-identified patient level data only, and there is the possibility of incomplete data entry for some variables in some patients. Further, consistency and completeness of data entry is not monitored. These data are also potentially subject to risk of mis-, under-, and/or delayed coding by busy clinical providers. The smaller percentages of patients meeting five or more of the modified diagnostic criteria may be explained by the lack of testing facilities and/or expertise for testing and interpretation of more specialized markers such as sC5b-9 and schistocytes at the local centers; for instance, no pediatric patients and only 202 adult patients had schistocytes data available in the database (Supplementary Table 1). This is an important consideration as diagnostic criteria could only be retrospectively applied to the available data, providing further support for the likely underdiagnosis of this condition in current clinical practice. As discussed in the Methods, during our study period there was no available diagnosis code(s) for HSCT-TMA, and we thus included patients with either a HUS or a TMA diagnosis code instead. We hope that the recent availability of the specific HSCT-TMA code will allow for more accurate and comprehensive diagnosis of patients. Further, results presented in this study are exclusively based on medical claims and EMR lab data, without access to patient notes, meaning our findings must be interpreted cautiously; for example, the suspected TMA group in our analysis is likely a heterogenous population of patients, some of whom may have alternative clinical explanations (such as other endothelial injury syndromes) for meeting TMA parameters. Also, as a database study, we did not have the ability to contact treating centers to obtain additional clinical information or case clarification. Despite these limitations, this study represents the first known effort to investigate HSCT-TMA systematically using a large real-world US EMR database for both adults and children. A previous smaller EMR study restricted to pediatric patients reported a HSCT-TMA incidence of 0.8%, and a mortality of 30.1%, which are in line with our study and reinforce the findings from our pediatric cohort (25).
Current and ongoing work in this field is necessary and may provide further diagnostic and pathophysiological insights. For instance, recent reports suggest that staining of tissues for C5b-9 deposition may be a useful tool to aid diagnosis of HSCT-TMA and differentiate the condition from others with similar clinical presentations, although further assessment is required before universal acceptance and adoption of this approach (26–28). Future research directions could also assess whether the number, or type, of diagnostic parameters met impact long-term prognosis.
In summary, this study showed confirmed and suspected HSCT-TMA are associated with a high mortality and morbidity burden, and HSCT-TMA is likely underdiagnosed in the real-world. Patients with suspected but undiagnosed TMA resemble confirmed TMA cases and have worse clinical outcomes, higher associated post-transplant complications, and higher HCRU burden compared to patients without TMA. Our study highlights the importance of initiatives such as the recently proposed harmonized HSCT-TMA diagnostic criteria in the facilitation of diagnosis uniformity and collaborative research in adults and children with HSCT-TMA. Finally, our study may also serve as a model for future retrospective real-world studies using large EMR based data in TMA and other diseases, or spur the future application of artificial learning to large EMRs that can prospectively alert clinicians to the potential presence of TMA in real time.
Data statement
Patient level data used in this study were obtained on a contractual basis from TriNetX. The analysis plan was limited to the results highlighted in this manuscript. The datasets generated are not publicly available because of their proprietary nature.
Data availability statement
Patient level data used in this study were obtained on a contractual basis from TriNetX. The analysis plan was limited to the results highlighted in this manuscript. The datasets presented in this article are not readily available because of their proprietary nature. Alexion will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development. Requests to access the datasets should be directed to https://alexion.com/contact-alexion/medical-information.
Ethics statement
As this study utilized retrospective electronic medical record data, ethical approval was not required in accordance with local legislation and institutional requirements.
Author contributions
YW: Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing. MS: Writing – original draft, Writing – review & editing. BS: Writing – original draft, Writing – review & editing. AT: Writing – original draft, Writing – review & editing. IA-D: Writing – original draft, Writing – review & editing. M-LO: Writing – original draft, Writing – review & editing. CD: Writing – original draft, Writing – review & editing. VH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This analysis and open access to the publication were funded by Alexion, AstraZeneca Rare Disease, Boston, MA. Medical writing support was provided by On Li Jasmine Lai, MSc and Alexander T. Hardy, PhD. of Bioscript Medical Communications, Macclesfield, UK and funded by Alexion AstraZeneca Rare Disease. Radha Narayan, PhD, of Alexion AstraZeneca Rare Disease provided critical review of the manuscript. Alexion, AstraZeneca Rare Disease, Boston, MA was responsible for the analysis of information contained in the TriNetX database and contributed to data analysis, interpretation, preparation, review, and approval of the manuscript for submission.
Conflict of interest
CD reports consulting for Alexion, Jazz Pharmaceuticals, and Allovir. VH has served as a consultant for Alexion, Omeros, Jazz Pharmaceuticals, and Allovir. YW, IA-D, and M-LO are employees of Alexion, AstraZeneca Rare Disease, and own stock/options in Alexion, AstraZeneca Rare Disease. AR and MS are employees of Genesis Research Group and received compensation from Alexion for design and conduct of this study. BS and AT were employees of Genesis Research Group at the time the study was conducted.
The authors declare that this study received funding from Alexion, AstraZeneca Rare Disease, Boston, MA. Authors Yan Wang, Imad Al-Dakkak and Moh-Lim Ong were employees of Alexion, AstraZeneca Rare Disease at the time of this study. The funder had the following involvement in the study: data analysis, interpretation, preparation, review and approval of the manuscript for submission.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2024.1405311/full#supplementary-material
References
1. Cho BS, Yahng SA, Lee SE, Eom K, Kim Y, Kim H, et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation. (2010) 90:918–26. doi: 10.1097/TP.0b013e3181f24e8d
2. Jodele S, Dandoy CE, Myers KC, El-Bietar J, Nelson A, Wallace G, et al. New approaches in the diagnosis, pathophysiology, and treatment of pediatric hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transfus Apher Sci. (2016) 54:181–90. doi: 10.1016/j.transci.2016.04.007
3. Brodsky RA. Complement in hemolytic anemia. Blood. (2015) 126:2459–65. doi: 10.1182/blood-2015-06-640995
4. Nester CM, Thomas CP. Atypical hemolytic uremic syndrome: what is it, how is it diagnosed, and how is it treated? Hematol Am Soc Hematol Educ Program. (2012) 2012:617–25. doi: 10.1182/asheducation-2012.1.617
5. Blasco M, Guillén-Olmos E, Diaz-Ricart M, Palomo M. Complement mediated endothelial damage in thrombotic microangiopathies. Front Med (Lausanne). (2022) 9:811504. doi: 10.3389/fmed.2022.811504
6. Choi CM, Schmaier AH, Snell MR, Lazarus HM. Thrombotic microangiopathy in haematopoietic stem cell transplantation. Drugs. (2009) 69:183–98. doi: 10.2165/00003495-200969020-00004
7. Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2005) 11:571–5. doi: 10.1016/j.bbmt.2005.06.001
8. Schoettler ML, Carreras E, Cho B, Dandoy CE, Ho VT, Jodele S, et al. Harmonizing definitions for diagnostic criteria and prognostic assessment of transplantation-associated thrombotic microangiopathy: A report on behalf of the European society for blood and marrow transplantation, American society for transplantation and cellular therapy, Asia-Pacific blood and marrow transplantation group, and center for international blood and marrow transplant research. Transplant Cell Ther. (2023) 29:151–63. doi: 10.1016/j.jtct.2022.11.015
9. Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, et al. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. (2015) 29:191–204. doi: 10.1016/j.blre.2014.11.001
10. Van Benschoten V, Roy C, Gupta R, Ouellette L, Hingorani S, Li A Incidence and risk factors of transplantation-associated thrombotic microangiopathy: A systematic review and meta-analysis. Transplant Cell Ther. (2022) 28:266.e261–266.e268. doi: 10.1016/j.jtct.2022.01.009
11. Higham CS, Collins G, Shimano KA, Melton A, Kharbanda S, Winestone LE, et al. Transplant-associated thrombotic microangiopathy in pediatric patients: pre-HSCT risk stratification and prophylaxis. Blood Adv. (2021) 5:2106–14. doi: 10.1182/bloodadvances.2020003988
12. Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. (2021) 56:1805–17. doi: 10.1038/s41409-021-01283-0
13. Meri S, Bunjes D, Cofiell R, Jodele S. The role of complement in HSCT-TMA: basic science to clinical practice. Adv Ther. (2022) 39:3896–915. doi: 10.1007/s12325-022-02184-4
14. Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. (2017) 390:681–96. doi: 10.1016/S0140-6736(17)30062-4
15. Ito S, Saito A, Sakurai A, Watanabe K, Karakawa S, Miyamura T, et al. Eculizumab treatment in paediatric patients diagnosed with aHUS after haematopoietic stem cell transplantation: a HSCT-TMA case series from Japanese aHUS post-marketing surveillance. Bone Marrow Transplant. (2024) 59:315–24. doi: 10.1038/s41409-023-02161-7
16. Dvorak CC, Higham C, Shimano KA. Transplant-associated thrombotic microangiopathy in pediatric hematopoietic cell transplant recipients: A practical approach to diagnosis and management. Front Pediatr. (2019) 7:133. doi: 10.3389/fped.2019.00133
17. Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood. (2014) 124:645–53. doi: 10.1182/blood-2014-03-564997
18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI 3rd, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
19. Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20:629–37. doi: 10.1681/ASN.2008030287
20. Matsui H, Arai Y, Imoto H, Mitsuyoshi T, Tamura N, Kondo T, et al. Risk factors and appropriate therapeutic strategies for thrombotic microangiopathy after allogeneic HSCT. Blood Adv. (2020) 4:3169–79. doi: 10.1182/bloodadvances.2020002007
21. Postalcioglu M, Kim HT, Obut F, Yilmam OA, Yang J, Byun BC, et al. Impact of thrombotic microangiopathy on renal outcomes and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24:2344–53. doi: 10.1016/j.bbmt.2018.05.010
22. Vasu S, Wu H, Satoskar A, Puto M, Roddy J, Blum W, et al. Eculizumab therapy in adults with allogeneic hematopoietic cell transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. (2016) 51:1241–4. doi: 10.1038/bmt.2016.87
23. Chapin J, Shore T, Forsberg P, Desman G, Besien KV, Laurence J. Hematopoietic transplant-associated thrombotic microangiopathy: case report and review of diagnosis and treatments. Clin Adv Hematol Oncol. (2014) 12:565–73.
24. Dandoy CE, Tsong WH, Sarikonda K, et al. Systematic review of signs and symptoms associated with hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transplant Cell Ther. (2023) 29:282.e281–282.e289. doi: 10.1016/j.jtct.2022.12.023
25. Ramgopal A, Sridar S, Dalal J, Kalpatthi R. Thrombotic microangiopathy: multi-institutional review of pediatric patients who underwent HSCT. J Pers Med. (2021) 11:20210525. doi: 10.3390/jpm11060467
26. Koopman JJE, van Essen MF, Rennke HG, de Vries APJ, van Kooten C. Deposition of the membrane attack complex in healthy and diseased human kidneys. Front Immunol. (2020) 11:599974. doi: 10.3389/fimmu.2020.599974
27. Alhomoud M, Magro C, Seshan S, Zhang T, Gomez-Arteaga A, Chokr N, et al. Deposition of complement components C5b-9 and MASP2 in tissues is not a feature of GVHD and may assist in discriminating GVHD from thrombotic microangiopathy following allogenic transplantation. Bone Marrow Transplant. (2023) 58:1270–4. doi: 10.1038/s41409-023-02089-y
28. Elhadad S, Chadburn A, Magro C, Besien KV, Roberson EDO, Atkinson JP, et al. C5b-9 and MASP2 deposition in skin and bone marrow microvasculature characterize hematopoietic stem cell transplant-associated thrombotic microangiopathy. Bone Marrow Transplant. (2022) 57:1445–7. doi: 10.1038/s41409-022-01723-5
Keywords: thrombotic microangiopathy, hematopoietic stem cell transplant, disease burden, incidence, diagnosis, clinical outcomes
Citation: Wang Y, Rava A, Smurzynski M, Shah B, Thanataveerat A, Al-Dakkak I, Ong M-L, Dvorak CC and Ho VT (2024) Real-world analysis of the underdiagnosis, clinical outcomes and associated burden of hematopoietic stem cell transplantation-associated thrombotic microangiopathy (HSCT-TMA) in the United States. Front. Hematol. 3:1405311. doi: 10.3389/frhem.2024.1405311
Received: 25 March 2024; Accepted: 17 June 2024;
Published: 15 July 2024.
Edited by:
Marcus O. Muench, Vitalant Research Institute, United StatesReviewed by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceSangeeta Hingorani, Seattle Children's Hospital, United States
Jeffrey Laurence, Cornell University, United States
Yue Han, The First Affiliated Hospital of Soochow University, China
Copyright © 2024 Wang, Rava, Smurzynski, Shah, Thanataveerat, Al-Dakkak, Ong, Dvorak and Ho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wang, eWFuLndhbmdAYWxleGlvbi5jb20=
†These authors share senior authorship
 Yan Wang
Yan Wang Andrew Rava
Andrew Rava Marlene Smurzynski2
Marlene Smurzynski2 Moh-Lim Ong
Moh-Lim Ong Christopher C. Dvorak
Christopher C. Dvorak