
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Hematol. , 26 April 2024
Sec. Hematopoiesis and Stem Cells
Volume 3 - 2024 | https://doi.org/10.3389/frhem.2024.1386973
This article is part of the Research Topic Hematopoietic Stress in Stem Cell Homeostasis and Disease Pathogenesis View all 5 articles
 Flavia Bigi1,2
Flavia Bigi1,2 Paola Tacchetti1
Paola Tacchetti1 Alessandro Giorgi2
Alessandro Giorgi2 Gaia Mazzocchetti1,2
Gaia Mazzocchetti1,2 Vincenza Solli1,2
Vincenza Solli1,2 Simona Barbato1,2
Simona Barbato1,2 Barbara Sinigaglia1,2
Barbara Sinigaglia1,2 Elena Campanini3
Elena Campanini3 Emanuele Favero1,2
Emanuele Favero1,2 Marco Talarico1,2
Marco Talarico1,2 Michele Puppi1,2
Michele Puppi1,2 Ilaria Rizzello1,2
Ilaria Rizzello1,2 Serena Rocchi1,2
Serena Rocchi1,2 Katia Mancuso1,2
Katia Mancuso1,2 Lucia Pantani1
Lucia Pantani1 Michele Cavo1,2*
Michele Cavo1,2* Elena Zamagni1,2
Elena Zamagni1,2The impact of daratumumab on CD34+ hematopoietic stem cell (HSC) mobilization has recently been a matter of concern. To address this issue, we compared CD34+ HSC-related outcomes in patients with multiple myeloma treated with daratumumab-based quadruplets (N = 44) and bortezomib/thalidomide/dexamethasone (N = 50) before cyclophosphamide-based mobilization. Plerixafor was more often required in the daratumumab group (52% vs. 20%, p = 0.002) and, despite a lower total yield, retained its efficacy in boosting HSC harvesting (+90% vs. +79%, p = 0.463). As a result, the same proportion of patients reached their planned collection goal in the two groups, suggesting its potential to overcome the interference of daratumumab on HSC mobilization. No clinically significant differences were observed in the immediate post-autologous HSC transplant interval in the two groups.
Daratumumab (D)-based combination therapies have become the new standard of care in newly diagnosed multiple myeloma (NDMM), with autologous stem cell transplantation (ASCT) remaining an essential phase in the treatment paradigm for eligible (TE) patients (pts). Concerns have been recently raised about the adverse impact of D on CD34+ hematopoietic stem cell (HSC) mobilization: sub-analyses of clinical trials (1, 2) and retrospective studies (3–10) have shown lower peaks circulating CD34+ cells, increased use of plerixafor (PLX), higher number of days of leukapheresis, lower yields of collected HSCs, and more mobilization failures in D-treated compared with non-D-treated pts. A delayed engraftment after ASCT in pts in the D-groups has also been discussed (1, 3, 7).
We ran a retrospective analysis of 44 TE-NDMM pts who received D-based induction therapies before ASCT between 2019 and 2022 at our institution. Twenty-six pts (59%) were treated with D-bortezomib/thalidomide/dexamethasone (DVTd) outside clinical studies, as per our institutional practice after its approval in Italy in December 2021; the remaining 18 pts (41%) were randomly assigned to the D-bortezomib/cyclophosphamide/dexamethasone (DVCd) arm of the phase 2 EMN18 trial (NCT03896737). Pts exposed to lenalidomide as part of induction were excluded, to specifically assess the impact of D on HSCs. Fifty TE-NDMM pts matched for age and sex who had received VTd induction between 2017 and 2021 served as the control group.
At the end of induction, all pts received mobilization therapy on an outpatient basis, consisting of cyclophosphamide (CY) at 2 g/m2, as per our policy, or 3 g/m2, according to the EMN18 trial design, followed by 10 mcg/kg/day granulocyte-colony stimulating factor (G-CSF) from day +6. Circulating CD34+ cells were assessed starting on day +11 after CY with the BD™ stem cell kit and cytofluorimetric assay. Pts with >40 CD34+ cells/µL underwent daily leukapheresis with the Spectra Optia® Apheresis System for up to three consecutive days. Poor mobilizers (<20 CD34+ cells/µL) and pts with 20–40 CD34+ cells/µL after an additional day of G-CSF therapy received PLX at the dose of 0.24 mg/kg, followed by leukapheresis on the next day. PLX was also administered after the first or second day of HSC collection in case of suboptimal harvesting.
The minimum target of CD34+ cells/kg to safely perform a single or double ASCT was set at 3 × 106 and 6 × 106, respectively. The indication for single or double ASCT has changed over time based on the results of the EMN02 trial (11) and the most recent EHA/ESMO guidelines (12); accordingly, the EMN18 trial design required tandem ASCT only in pts with high-risk cytogenetics. CD34+ cells were cryopreserved in liquid nitrogen with homologous plasma and dimethyl sulfoxide; before each ASCT, post-thaw CD34+ cell vitality was assessed by trypan blue staining. Neutrophil recovery was defined as >500/µL and platelet recovery was defined as >20,000/µL without transfusions.
The primary endpoints of our analysis were the number of total harvested CD34+ cells and the achievement of the collection goal; secondary endpoints included other collection outcomes, PLX use and efficacy, and post-ASCT engraftment and complications. Univariate analyses were performed with the Kruskal–Wallis test for numerical variables and Fisher’s exact test for categorical variables.
Baseline characteristics were comparable between the two groups (Supplementary Table 1). The majority of pts in both groups received four cycles of induction therapy; in the remaining pts, two additional cycles were administered to maximize the depth of response, or as a bridge in case of delayed HSC collection (Supplementary Table 2).
Regarding our primary endpoints, a trend toward lower total median CD34+ collected cells was observed in D pts over controls (6.74 vs. 8.03 × 106 CD34+ cells/kg, p = 0.06) (Supplementary Figure 1); overall, 84% of pts met their planned collection goal and more than 90% of pts harvested enough CD34+ cells to perform at least one ASCT in both groups.
The median number of days of leukapheresis was similar (1.94 vs. 1.68, p = 0.08), but D pts had a significantly lower HSC yield on the first day of collection (3.48 vs. 5.92 × 106 CD34+ cells/kg, p = 0.004), which resulted in a significantly higher proportion of D pts needing more than a single apheresis (78% vs. 56%, p = 0.047) to reach the collection goal. Mobilization failure was observed in three D pts vs. no patient in the control group, but the difference was not significant. One D-treated patient and 4 VTd pts successfully underwent a second mobilization attempt to reach their planned collection target; this difference was influenced by the higher collection goal in VTd pts and was not statistically significant (Table 1).
The three D pts who failed mobilization were likely to have lower hemoglobin concentration at diagnosis (6.2, 6.4, and 10.8 g/dL, respectively) than the other pts in the same group (median, 11.6 g/dL). No other baseline characteristics and neither the number of induction cycles (hence of D doses), D-free interval before CY, use of CY as part of induction therapy, nor response to induction or mobilizing CY dose showed a significant impact on harvest outcomes.
PLX was used in 23 D and 10 VTd pts (52% vs. 20%, p = 0.002), of whom 8 and 5 received two doses, respectively (not significant). Timing and indications for PLX were similar: the first dose (PLX1) was mostly given to poor mobilizers, while the second dose (PLX2) was mostly administered after the first day of leukapheresis, aimed at reaching the 6 × 106 CD34+ cells/kg goal (Table 1). No patient-, disease-, or therapy-related variables were associated with the use of PLX in D pts.
The median peak of circulating CD34+ cells/µL on the first planned collection day was significantly lower in the D group than in the control group (21 vs. 81, p < 0.001). We analyzed the kinetics of circulating CD34+ cells in the D subgroup upon PLX use: the median CD34+ cell concentration was significantly higher after PLX1 and PLX2 compared to pre-PLX (42 and 30 vs. 9 CD34+ cells/µL with padj < 0.001 and 0.034, respectively; the decrease after PLX2 was not significant) (Figure 1). Notably, these data were not available for all D pts.
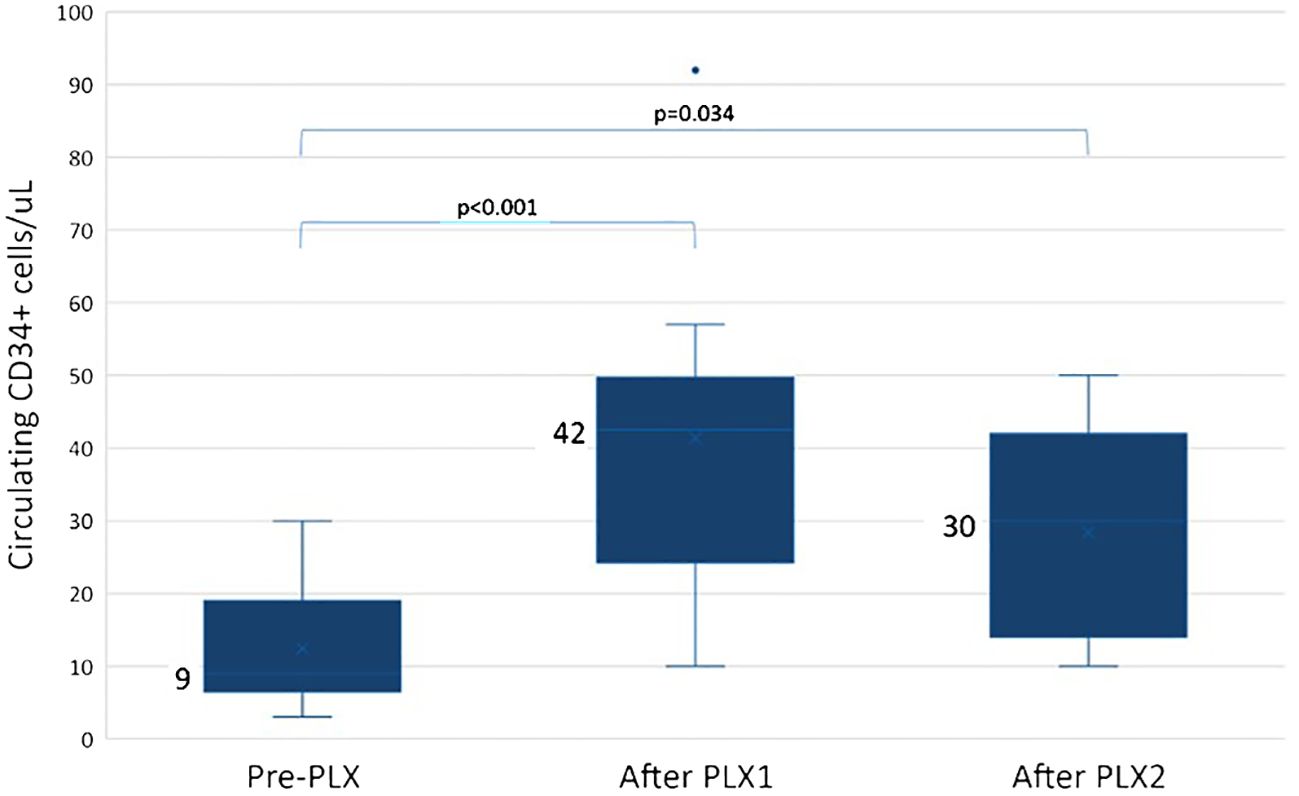
Figure 1 Circulating CD34+ cells in daratumumab-treated patients who received plerixafor (PLX). In daratumumab-treated pts, circulating CD34+ cells increased from 9 CD34+ cells/µL pre-PLX (range 3–30 CD34+ cells/µL) to 42 CD34+ cells/µL after the first dose of PLX (PLX1, range 10–92 CD34+ cells/µL, padj < 0.001) and to 30 CD34+ cells/µL after the second dose (PLX2, range 10–50 CD34+ cells/µL, padj = 0.034); the decrease after PLX2 was not statistically significant. Pre-PLX, N = 14; after PLX1, N = 14; after PLX2, N = 5.
Among D pts who received PLX, the median yield of CD34+ collected cells was null, 3.48 × 106 cells/kg, and 1.94 × 106 cells/kg before PLX, after PLX1, and after PLX2, respectively. The benefit was similar in controls: the median increase in collected CD34+ cells after PLX1 was of +90% and +79% in D and VTd pts, respectively, and of +10 and +16% after PLX2, compared with pre-PLX (Figure 2; Supplementary Figure 2). PLX enabled 83% of D pts and 40% of controls to reach their collection goal and ensured the harvesting of enough cells for at least one ASCT in all but two pts in the D group.
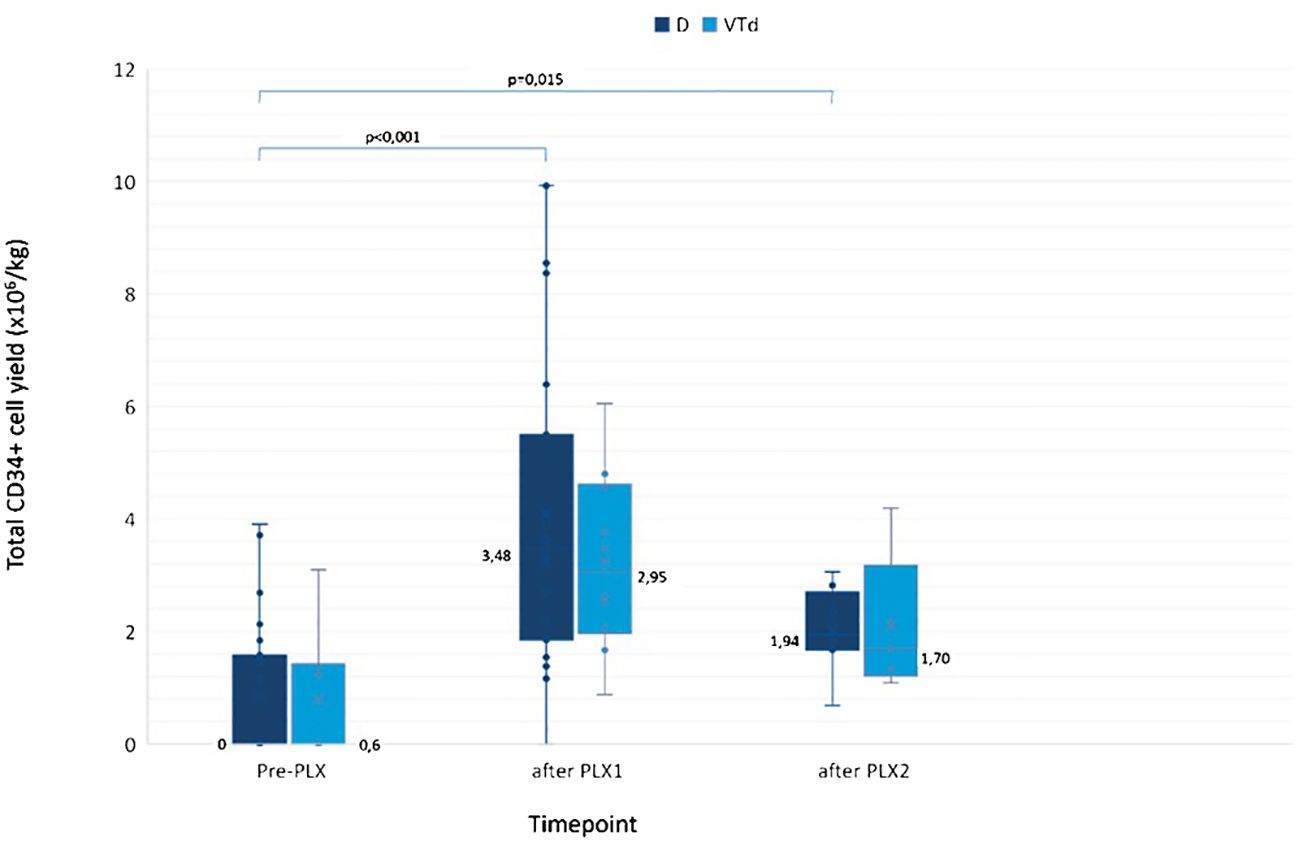
Figure 2 CD34+ cells collected in patients who received PLX. The median CD34+ cell yield in D vs. VTd pts was 0 (range 0–1.52) vs. 0.60 (range 0–3.19) CD34+ cells/kg pre-PLX; 3.48 (range 0–9.93) vs. 2.95 (range 0.88–6.05) CD34+ cells/kg after PLX1; 1.94 (range 0.69–3.06) vs. 1.70 (range 1.09–4.19) CD34+ cells/kg after PLX2. The median increase in CD34+ cell yield was similar in the two groups: by 1.9 times in D pts vs. 1.8 times in VTd pts (p = 0.463) after PLX1 and by 1.1 vs. 1.2 times (p = 1) after PLX2, compared to pre-PLX. Pre-PLX D, N = 22; VTd, N = 10; after PLX1 D, N = 22; VTd, N = 10; after PLX2 D, N = 8; VTd, N = 5. D, daratumumab; PLX, plerixafor; PLX1, first dose of plerixafor; PLX2, second dose of plerixafor; VTD, bortezomib/thalidomide/dexamethasone.
To date, all VTd pts and 93% of D pts have undergone one ASCT (ASCT1). The rate of a suboptimal stem cell yield hampering a second planned ASCT (ASCT2) was 16% in both groups. The proportion of pts who actually received a double ASCT in the D group (11%) was lower than that in the control group (44%), reflecting the aforementioned data from the literature (11, 12) and the EMN18 trial design; five D pts were waiting to undergo the procedure at the time of analysis.
Post-thaw CD34+ cell vitality was higher in the D vs. VTd group prior to ASCT1 (75% vs. 67%, p = 0.024), and the median number of reinfused CD34+ cells/kg was similar in the two groups. Nevertheless, hematopoietic recovery was slower in D pts after ASCT1, for both neutrophils (12 vs. 11 days, p < 0.001) and platelets (14 vs. 12 days, p < 001), and more erythrocyte transfusions (36% vs. 14% of pts requiring ≥2 units, p = 0.031) were needed in D pts. However, no significant difference was found in terms of platelet transfusions, incidence of febrile neutropenia, life-threatening infectious complications, antibiotic therapy duration, or length of hospitalization among the two groups (Table 2). The comparison of the same variables for ASCT2 showed no statistical difference between the two groups.
Collectively, our analysis confirmed the interference of D-based induction therapies on the subsequent HSC mobilization, which was not affected by the cumulative D dose, or by the D-free interval before mobilization or CY dose in the range between 2 and 3 g/m2. This is in line with the results of two other monocentric studies showing no impact of D-free interval before leukapheresis (6), number of D doses, or both (9). A Swedish multicentric study found an impact of age and radiation on the total stem cell yield (13), while a recently published case report from the Mayo Clinic group presented a case of a successful mobilization after a 3-month washout period from prolonged D administration (20 doses) following two previous mobilization failures closer to D (14). CY at the dose of 4 g/m2 combined with on-demand PLX has recently been reported to be effective in most pts receiving DVTd induction therapy, though the lack of a control group hampered researchers to draw any firm conclusions (13).
Since numerous studies have demonstrated a higher requirement for PLX in D-treated pts, our analysis was aimed at evaluating its efficacy in this population compared with a non-D-treated group. PLX has shown to be effective in improving circulating CD34+ cell levels and mitigating the effects of D on HSC collection outcomes, demonstrating efficacy in boosting the CD34+ cell yield similar to that of the control group. These data are consistent with recent findings showing upregulation of cell adhesion genes on CD34+ cells after D-based therapy, suggesting this mechanistic effect as potentially crucial in impairing the mobilization of HSCs (15).
Overall, the addition of D to the induction triplets did not significantly compromise the feasibility of planned ASCT(s). Engraftment was slightly delayed after ASCT1, but this did not translate into higher rates of complications.
The main limitations of our study include the small sample size reflecting the monocentric design and the retrospective nature, which may have limited the statistical power of several analyses. On the other hand, the case–match comparison somewhat increases the reliability of the study. Published experiences comprise a variety of D-based combinations, mobilization strategies, and collection targets, making a proper meta-analysis impractical. Prospective randomized studies are required to formally assess the optimal mobilization strategy for D-treated pts.
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request, without undue reservation.
The studies involving humans were approved by Comitato Etico Area Vasta Emilia Romagna. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FB: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PT: Conceptualization, Investigation, Writing – review & editing. AG: Investigation, Writing – review & editing. GM: Data curation, Formal analysis, Writing – review & editing. VS: Data curation, Formal analysis, Writing – review & editing. SB: Writing – review & editing, Writing – original draft. BS: Investigation, Writing – review & editing. EC: Investigation, Writing – review & editing. EF: Investigation, Writing – review & editing. MT: Investigation, Writing – review & editing. MP: Investigation, Writing – review & editing. IR: Investigation, Writing – review & editing. SR: Investigation, Writing – review & editing. KM: Investigation, Writing – review & editing. LP: Investigation, Writing – review & editing. MC: Conceptualization, Formal analysis, Supervision, Writing – review & editing. EZ: Conceptualization, Formal analysis, Investigation, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work reported in this publication was funded by the Italian Ministry of Health, RC-2023-2778909.
PT has received Honoraria from Amgen, Bristol-Myers Squibb/Celgene, Janssen, Takeda, AbbVie, Sanofi, GlaxoSmithKline and Oncopeptides; SR receives Honoraria from Amgen, GlaxoSmithKline and Janssen; IR has received Honoraria from Amgen, GlaxoSmithKline and Sanofi; SR has received Honoraria from Amgen, GlaxoSmithKline and Janssen; KM has received Honoraria from Celgene, Takeda, Amgen, Sanofi and Janssen; LP has received Honoraria from Janssen and Amgen; MC has served in a consulting/advisory role for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Menarini Stemline, Sanofi, and Karyopharm Therapeutics; and has received honoraria from Amgen, AbbVie, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm Therapeutics, Menarini Stemline, Sanofi, and Karyopharm Therapeutics; EZ has received honoraria from Janssen, Bristol-Myers Squibb, Amgen, Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2024.1386973/full#supplementary-material
1. Hulin C, Offner F, Moreau P, Roussel M, Belhadj K, Benboubker L, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab + bortezomib/thalidomide/dexamethasone in the phase 3 CASSIOPEIA study. Haematologica. (2021) 106:2257–60. doi: 10.3324/haematol.2020.261842
2. Chhabra S, Callander N, Watts NL, Costa LJ, Thapa B, Kaufman JL, et al. Stem cell mobilization yields with daratumumab and lenalidomide–containing quadruplet induction therapy in newly diagnosed multiple myeloma: findings from the MASTER and GRIFFIN trials. Transplant Cell Ther. (2023) 29(3):174.e1–174.e10. doi: 10.1016/j.jtct.2022.11.029
3. Al Saleh AS, Sidiqi MH, Gertz MA, Muchtar E, Lacy MQ, Warsame RM, et al. Delayed neutrophil engraftment in patients receiving Daratumumab as part of their first induction regimen for multiple myeloma. Am J Hematol. (2020) 95:E8–E10. doi: 10.1002/ajh.25654
4. Edmisson J. Despite use of upfront plerixafor and G-CSF, daratumumab exposure reduces stem cell mobilization in patients with multiple myeloma | Elsevier enhanced reader. Blood. (2022) 140:4295–6. doi: 10.1182/blood-2022-169367
5. Oza S, Slotky R, Vissa P, Phull P, Kaur S, Suh HC, et al. Effect of daratumumab on stem cell mobilization and engraftment kinetics post autologous stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. (2022) 140:10441–2. doi: 10.1182/blood-2022-171098
6. Manjappa S, Fox R, Reese J, Firoozamand A, Schmikla H, Nall S, et al. Impact of daratumumab on stem cell collection, graft composition and engraftment among multiple myeloma patients undergoing autologous stem cell transplant. Blood. (2020) 136:35–7. doi: 10.1182/blood-2020-142115
7. Papaiakovou EE, Terpos E, Kanellias N, Migkou M, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. Impact of daratumumab-containing induction on stem cell mobilization and collection, engraftment and hospitalization parameters among multiple myeloma patients undergoing autologous stem cell transplantation. Blood. (2021) 138:3886. doi: 10.1182/blood-2021-149499
8. Luan D, Christos PJ, Ancharski M, Guarneri D, Pearse R, Rossi AC, et al. Timing of daratumumab administered pre-mobilization in multiple myeloma impacts pre-harvest peripheral blood CD34+ Cell counts and plerixafor use. Blood. (2020) 136:15–6. doi: 10.1182/blood-2020-140811
9. Bhutani M. Stem cell mobilization characteristics for transplant eligible patients with newly diagnosed multiple myeloma (NDMM) treated with carfilzomib, lenalidomide, dexamethasone, and daratumumab (KRd-dara) | Elsevier enhanced reader. Blood. (2022) 140:4393–5. doi: 10.1182/blood-2022-160071
10. Lemonakis K, Tatting L, Lisak M, Carlson K, Crafoord J, Blimark CH, et al. Impact of daratumumab based induction on stem cell collection parameters in Swedish myeloma patients. Haematologica. (2023) 108(2):610–4. doi: 10.3324/haematol.2022.281610
11. Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. (2020) 7:e456–68. doi: 10.1016/S2352-3026(20)30099-5
12. Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up y behalf of the EHA Guidelines Committee * and ESMO Guidelines Committee. (2021) 17. doi: 10.1016/j.annonc.2020.11.014
13. Liberatore C, Perini T, Passeri C, Ferla V, Fioritoni F, Girlando V, et al. Higher cyclophosphamide dose grants optimal stem-cell collection after daratumumab-based induction in multiple myeloma. Haematologica (2023) 108(12):3502–5. doi: 10.3324/haematol.2023.283452
14. Seth A, Murray D, Buadi FK, Gertz MA, Yadav U, Kumar SK, et al. Failure of mobilization of hematopoietic stem cells associated with elevated serum levels of anti-CD38 monoclonal antibody. Eur J Haematol. (2023) 111:318–21. doi: 10.1111/ejh.14008
Keywords: multiple myeloma, mobilization, hematopoietic stem cell (HSC), daratumumab, plerixafor
Citation: Bigi F, Tacchetti P, Giorgi A, Mazzocchetti G, Solli V, Barbato S, Sinigaglia B, Campanini E, Favero E, Talarico M, Puppi M, Rizzello I, Rocchi S, Mancuso K, Pantani L, Cavo M and Zamagni E (2024) Interference of daratumumab and efficacy of plerixafor on haematopoietic stem cell collection in Multiple Myeloma. Front. Hematol. 3:1386973. doi: 10.3389/frhem.2024.1386973
Received: 16 February 2024; Accepted: 08 April 2024;
Published: 26 April 2024.
Edited by:
Pontus Lundberg, Roche, SwitzerlandReviewed by:
Daniele Derudas, Ospedale Oncologico Armando Businco, ItalyCopyright © 2024 Bigi, Tacchetti, Giorgi, Mazzocchetti, Solli, Barbato, Sinigaglia, Campanini, Favero, Talarico, Puppi, Rizzello, Rocchi, Mancuso, Pantani, Cavo and Zamagni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Cavo, bWljaGVsZS5jYXZvQHVuaWJvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.