- 1Department of Internal Medicine, Trinity Health Oakland, Pontiac, MI, United States
- 2Wayne State University, Detroit, MI, United States
- 3Department of Internal Medicine, University of Connecticut, Farmington, CT, United States
- 4Ross University School of Medicine, Bridgetown, Barbados
- 5Department of Internal Medicine, Detroit Wayne County Authority Health, Detroit, MI, United States
- 6Department of Hematology and Oncology, Trinity Health Oakland, Pontiac, MI, United States
Background and aims: The Omicron variant, one of the variants causing the coronavirus disease of 2019 (COVID-19), was first identified in November 2021 and became the predominant variant in 2022. Although causing less severe disease, this variant and its subvariants have been associated with increased transmissibility and limited protection despite vaccination and prior infection. Individuals with sickle cell disease (SCD) are particularly at greater risk of severe illness and death, and studies regarding the effectiveness of COVID-19 vaccination have been limited in this population. The study aims to determine the effectiveness of COVID-19 vaccination during this period among individuals with SCD and to examine various factors that can influence the likelihood of COVID-19 infection and severity among SCD individuals.
Methods: This is a retrospective analysis of adult patients (≥18 years) with SCD who had emergency and inpatient encounters between January 1 and December 31, 2022. Multivariable regression analysis was performed to determine the effectiveness of the COVID-19 vaccine among this population.
Results: The study found that COVID-19 vaccination lowered the infection risk among SCD individuals by over 70% if they have received at least one dose of the vaccine. The study also found that individuals with SCD and a history of acute chest syndrome were over 3 times more likely to have a COVID-19 infection diagnosis than those without a history of acute chest syndrome.
Conclusion: The study confirms the effectiveness of the COVID-19 vaccine among individuals with SCD during the Omicron period of the COVID-19 pandemic.
1 Introduction
Sickle cell disease (SCD) afflicts approximately 100,000 individuals in the United States, with a prevalence of 1 in 365 among African Americans and 1 in 16,300 among Hispanic Americans. Its prevalence is even more pronounced in various global regions, including Africa, South and Central America, the Caribbean, Saudi Arabia, India, and select Mediterranean nations (1). Notably, individuals affected by sickle cell disease exhibit an elevated mortality rate in contrast to the general population, which was particularly emphasized during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (2, 3).
This is primarily related to the fact that individuals with SCD are considered to have a weakened immune system. Several factors contribute to a compromised immune system in SCD patients. One crucial factor is functional asplenia, which reduces the body’s ability to filter blood and increases the risk of infections from encapsulated organisms. Additionally, innate immunity through the complement system and adaptive immunity involving regulatory T-cell function and IgM-secreting memory B cells are impaired in SCD patients (4, 5). This impairment of cellular immunity encompasses a decrease in the percentage of CD4+ and CD8+ T cells circulating in the bloodstream, a decrease in the ratio of CD4+ helper T cells to CD8+ suppressor T cells, and the loss of memory B cells (6). These latter impairments in immunity are a major factor that impacts vaccines’ effectiveness and protective capacity to prevent infections in individuals with SCD. At the time of this paper’s writing, there is limited data on how well patients with SCD respond to SARS-CoV-2 vaccination. Recognizing these challenges is critical for developing tailored interventions to enhance vaccination strategies for this vulnerable population (4, 7).
The Omicron variant of SARS-CoV-2, also known as B.1.1.529, exhibited an unprecedented global spread, contributing to record-breaking surges in new infections (8, 9). According to the Centers for Disease Control and Prevention (CDC), it has emerged as the predominant variant since January 2022 (10). Although causing less severe disease, the Omicron variant and its subvariants have been associated with increased transmissibility and limited protection despite vaccination and prior infection (11). Its rapid surge to dominance can be attributed to critical mutations in the virus structure that significantly heightened its ability to spread, its virulence, and its capacity to evade the immune system, thereby impacting available treatment options (9, 11).
One remarkable characteristic of the Omicron variant is the substantial number of mutations found in its spike protein, which poses a substantial threat to the effectiveness of current COVID-19 vaccines and antibody therapies (12). Within the spike protein, there are a concerning number of mutations to the receptor binding proteins which is the primary target of neutralizing antibodies (8). Consequently, the Omicron variant has become notably resistant to neutralization not only by serum from individuals who have recovered from COVID-19 but also from those who have been vaccinated (8). These extensive mutations in the spike protein raise serious concerns about the potential compromise of current vaccines and therapeutic antibody treatments.
As COVID-19 vaccination rates rise and restrictions are eased, the impact on individuals with sickle cell disease (SCD), particularly during the Omicron period of the pandemic, remains an area that requires thorough investigation and study. The study aims to determine the effectiveness of COVID-19 vaccination during this period among individuals with SCD. Additionally, the study aims to examine various factors that can influence the likelihood of COVID-19 infection and severity among SCD individuals.
2 Methods
Adult individuals with SCD (age ≥ 18 years old) who had emergency and inpatient encounters at three large hospital centers in Michigan during the prevalence of the Omicron variant from January 1, 2022, to December 31, 2022 (10, 11) were included in the study. Patients were identified using the International Statistical Classification of Diseases and Related Health Problems, Tenth Version (ICD-10) codes. Patients with a conflicting diagnosis of Sickle cell trait upon chart review were excluded in the analysis. The study protocol was approved by the Institutional Review Board (IRB) of the Trinity Health System in Michigan (IRB 2023-010), which waived the requirement for informed consent given the retrospective nature of the study.
Electronic medical records (EMR) were retrospectively reviewed to gather patient data including demographics, details of COVID-19 infection, underlying comorbidities, and other clinical information, while the immunization registry in Michigan was used to obtain COVID-19 vaccination status. The categorization of COVID-19 vaccination status as fully, partially, or non-vaccinated was established based on the definitions provided by the Centers for Disease Control and Prevention (CDC) (13). The severity of the disease was determined by referring to the COVID-19 treatment guidelines outlined on the official website of the National Institutes of Health (14). Multivariable regression analysis was conducted to determine the clinical correlation between COVID-19 vaccination status and infection and to examine various factors influencing COVID-19 severity. Statistical analyses were performed using IBM SPSS version 28.0 statistical software, and a p-value of <0.05 was considered statistically significant.
3 Results
Among the 101 adult patients with SCD (age ≥ 18 years old) included in the study, 97% were Black, with a mean age of 40 ± 15 years. The majority had the hemoglobin SC (42.6%) or SS (27.7%) genotype. A total of 47.5% of the individuals with SCD were vaccinated by December 2022. Among these, 12.5% had received partial vaccination, 50% were fully vaccinated, and 37.5% had received a booster. The distribution of SCD genotypes did not significantly differ based on vaccination status (% vaccinated: Hb SC-51.4%, SS-31.4%, S beta-thalassemia-17.1%, p=0.18). Table 1 summarizes the baseline demographics of individuals with SCD included in the study.

Table 1 Baseline characteristics of individuals with sickle cell disease (SCD) included in the study.
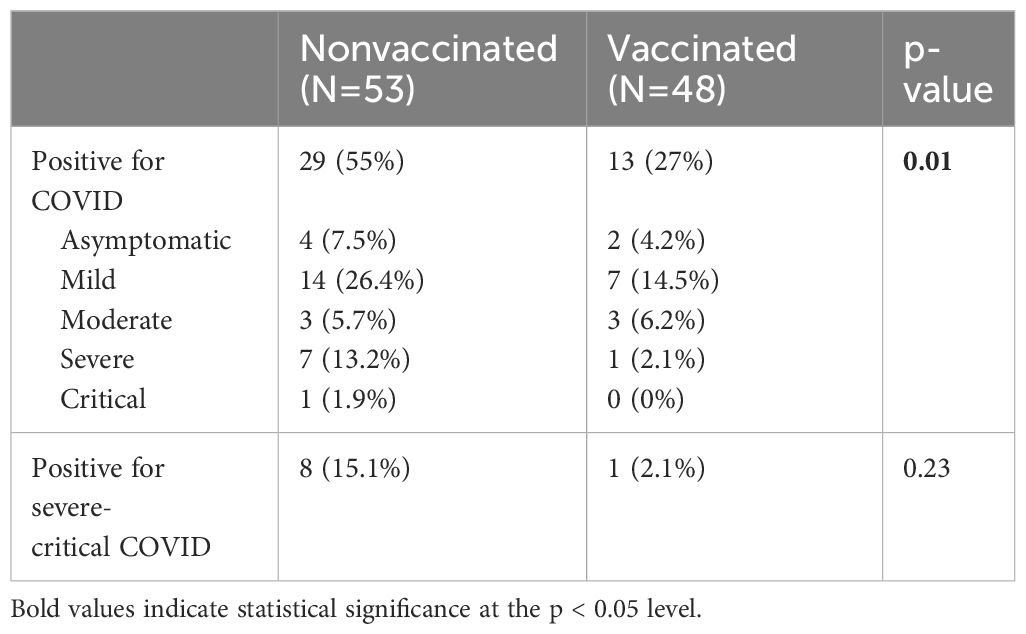
A total of 41.6% (n=42) of individuals with SCD were confirmed to have COVID-19 disease. The majority of cases were categorized as mild (20.8%, n=21), followed by severe (7.9%, n=8), asymptomatic (5.9%, n=6), moderate (5.9%, n=6) and critical 1% (n=1). No significant association was observed between the hemoglobin genotypes and COVID-19 infection (SC-54.5%, SS-36.4%, S beta-thalasemmia-9.1%, p=0.96). Notably, there was no reported mortality due to COVID-19 in our sample population. COVID-19 infection rates were notably higher in non-vaccinated individuals compared to vaccinated ones (55% vs. 27%, p=0.01, Table 2). Although the non-vaccinated group had a greater percentage of severe to critical illness, this difference was not statistically significant (15.1% vs. 2.1%, p=0.2, Table 2).
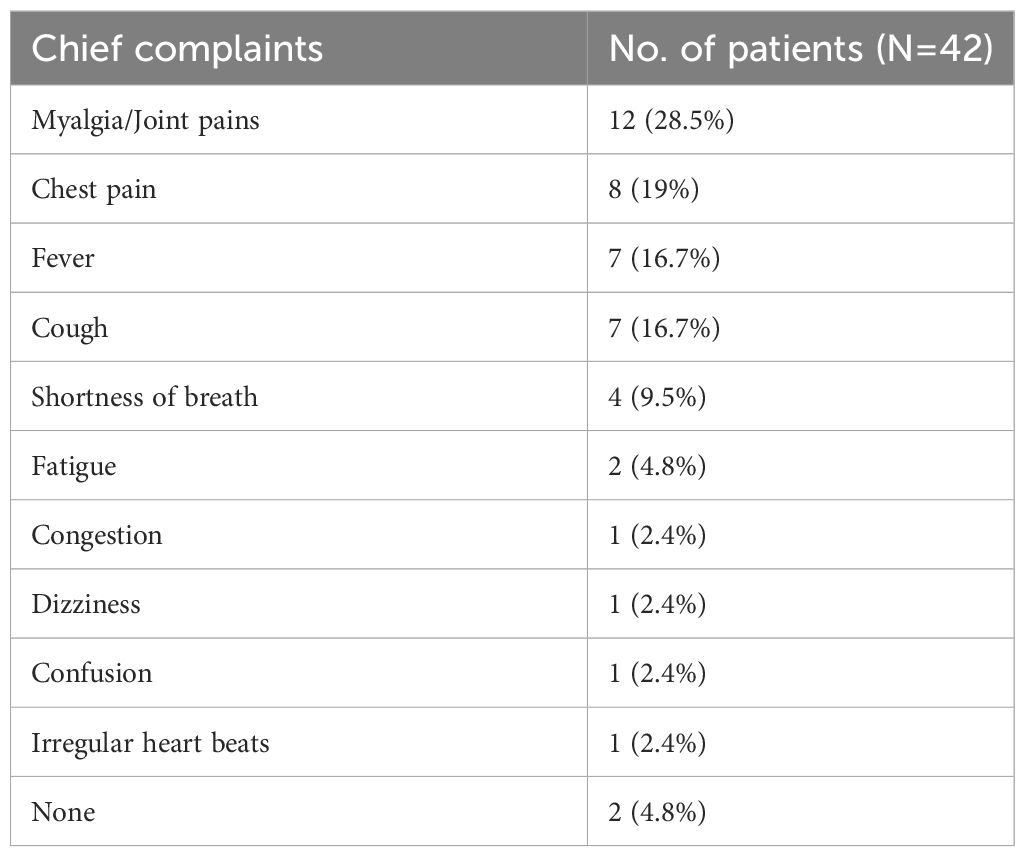
The most common presenting symptoms at the time of diagnosis were myalgia (28.5%) and chest pain (19%), as shown in Table 3. Interestingly, individuals with a prior history of acute chest syndrome were over three times more likely to be diagnosed with COVID-19 infection than those without (OR=3.68, 95% CI=1.47-9.17). This was likely due to a significant overlap between the symptoms of acute chest syndrome and COVID-19 (15), warranting more prompt emergency visits from patients.
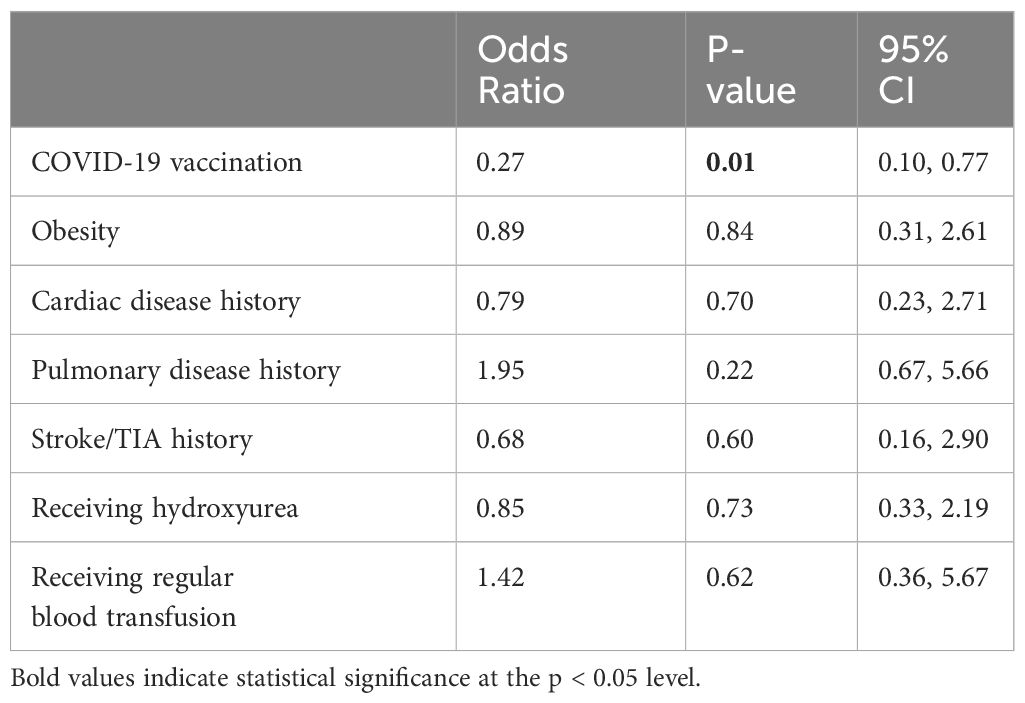
The mean number of days between the latest dose of COVID-19 vaccination and COVID-19 infection was 297.9 ± 160.5 days. The study revealed that receiving at least one dose of COVID-19 vaccination reduced the risk of infection among individuals with SCD by more than 70% (OR=0.27, 95% CI=0.10-0.77, Table 4). Notably, there was a significant difference in preventing COVID-19 infection among the various vaccination subgroups of partial, full, and booster (33% vs. 25% vs. 28%, respectively, p=0.045). However, due to a limited number of individuals with COVID-19 infection who were vaccinated, it was not possible to determine whether complete or booster vaccinations are more effective. Additionally, the results of the multivariable logistic regression analysis indicated that factors such as obesity, pre-existing cardiac history, pulmonary history, renal disease, stroke, or transient ischemic attack, were not significantly associated with COVID-19 severity in this sample population (p>0.05, Table 4).
4 Discussion
The current study contributes valuable real-world data to the limited body of existing literature concerning the efficacy of COVID-19 vaccination and the outcomes of COVID-19 infection in individuals with sickle cell disease, particularly during the period when the Omicron variant was predominant.
Previous studies have reported a lower effectiveness of the COVID-19 vaccine against the Omicron variant compared to other variants (11, 16). However, our study has demonstrated vaccine effectiveness among individuals with sickle cell disease despite the relatively lower vaccination rate in our cohort which was 47.5% compared to the general population’s rate of 70% as of December 2022 (17). This observation parallels the findings observed by Han et al. in 2021, when the Delta variant was predominant (11). Their retrospective study found that at least one dose of the COVID-19 vaccine was associated with a 70% reduction in the risk of infection, but no significant difference in the prevention of severe COVID-19 infection was observed (18), consistent with the results of our study. In our study, the mean interval of days from the latest dose of COVID-19 vaccination and COVID-19 infection was 297.9 ± 160.5 days. This is in contrast to studies in the general population that have shown the vaccine offers immediate protection and remains effective for at least 112 days after the primary vaccine series or at least 84 days after a booster dose (11). According to a recent study conducted by the American Society of Hematology Research Collaborative, the COVID-19 vaccines were found to be as effective in people with sickle cell disease (SCD) as they are in the general population. The study indicates that the immune response generated in people with SCD persisted for at least six months after vaccination (19). Another study by Nakahara et al. also found that patients with SCD produced a strong IgG antibody response to the COVID-19 vaccine, which had comparable neutralizing activity to non-SCD matched controls (7). However, it is important to note that neither study specifically focused on patients during the prevalence of the Omicron variant. Our study focused on the effectiveness of the COVID-19 vaccine during the predominance of the Omicron variant. The results are reassuring and similar to those of previous studies conducted under different circumstances. The analysis shows that participants in our study demonstrated a robust immune response to the vaccine, indicating the resilience of the immune response in the context of the Omicron variant. These findings offer encouraging insights into the efficacy of the vaccine among individuals with sickle cell disease.
In this cohort, the majority of SCD individuals experienced mild disease, with only 8.9% experiencing severe-critical illness, this is consistent with a comprehensive literature review of 71 studies performed by Hoogenboom et al. which found that a majority of SCD individuals had mild-moderate COVID-19 disease course (20). However, in the same study, SCD patients had 2 to 7 fold increased risk of hospitalization and 1.2 fold increased risk of COVID-19 related death compared to adults without SCD (20). It should be noted, however, that these studies were done before the Omicron period, when Delta was the most prevalent variant. One recent prospective study in France showed less severe COVID-19 disease observed among SCD individuals during Omicron period regardless of their protection status against COVID-19 disease (21). One proposed mechanism given by Hui et al. in an ex vivo study showed that Omicron has lower replication competence in human lungs, thus, causing less severe disease (22). However, Omicron variant has been shown to have increased transmissibility than other variants as it replicates faster in the bronchi compared to other variants (22).
The study found that the most common presenting symptom among patients were myalgia (28.5%), chest pain (19%), cough (16.7%) and fever (16.7%). This is in comparison with a recent study in France, which was also conducted during the Omicron period, where cough, fever and asthenia were the three most common complaints (21). Interestingly, we found that individuals with SCD and a history of acute chest syndrome were over three times more likely to receive a COVID-19 infection diagnosis than those without such history. This is consistent with other studies that reported a higher prevalence of recent vaso-occlusive crises among SCD patients with COVID-19 infection (2, 23, 24). One possible explanation for this is that COVID-19 is postulated to trigger vaso-occlusive crises and acute chest syndrome. Alternatively, both conditions may present with a significant overlap in symptoms warranting more prompt emergency visits from patients (2, 3, 13).
The impact of COVID-19 infection on individuals with SCD varies based on the hemoglobin genotype, potentially resulting in more severe outcomes for those with the SC genotype compared to the SS/S beta thalassemia genotype (25). A subgroup analysis was conducted in our study to assess the influence of hemoglobin genotypes on COVID-19 infection, but no significant association was identified. The prevalence of COVID-19 vaccination was similar across different hemoglobin genotypes. However, evaluating the difference in effectiveness of the COVID-19 vaccine across hemoglobin genotypes was not possible due to limited sample size when stratified by vaccination status. There is currently a paucity of literature addressing the variation in the effectiveness of COVID-19 vaccines based on SCD genotype, and this can serve as a direction for future studies.
The analysis had certain limitations. Firstly, the retrospective design of the study restricted the scope of our findings. Furthermore, our data only encompassed emergency and inpatient encounters that occurred from January 1 to December 31, 2022, for individuals with sickle cell disease (SCD). This may have excluded asymptomatic or undiagnosed cases of COVID-19 among SCD individuals who did not require emergency or inpatient care. While Omicron is the predominant variant during the study period (10, 11), confirmation of the Omicron variant was unavailable and reliance was placed on the period of predominance as determined from surveillance data. In addition, due to limited sample size when divided by vaccination status, subgroup analysis to determine the influence of hemoglobin genotypes on the effectiveness of COVID-19 vaccine was not feasible. Lastly, our study predominantly focused on less severe cases of COVID-19 among SCD individuals, making it imperative for future research to explore the factors contributing to more severe COVID-19 cases and unfavorable outcomes within this specific population.
5 Conclusion
Receiving at least one dose of the COVID-19 vaccine offers protection among individuals with SCD, even with the emergence of Omicron and its subvariants during the COVID-19 pandemic. Additionally, it is crucial that individuals with SCD remain vigilant and seek medical care if they experience chest pain, as this could be the only symptom of COVID-19 among this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statement
Ethical review and approval was provided by the Institutional Review Board (IRB) of the Trinity Health System in Michigan (IRB 2023-010). Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
KA: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. CLM: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CA: Formal analysis, Supervision, Writing – original draft, Writing – review & editing. GK: Supervision, Writing – review & editing. TC: Data curation, Writing – review & editing. GC: Data curation, Writing – review & editing. NC: Data curation, Writing – review & editing. RC: Data curation, Writing – review & editing. DD: Data curation, Writing – review & editing. LD: Data curation, Writing – review & editing. GD: Data curation, Writing – review & editing. EE: Data curation, Writing – review & editing. VG: Data curation, Writing – review & editing. LL: Data curation, Writing – review & editing. CM: Data curation, Writing – review & editing. OO: Data curation, Writing – review & editing. JO: Data curation, Writing – review & editing. TQ: Data curation, Writing – review & editing. SR: Data curation, Writing – review & editing. JG: Investigation, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our gratitude to Dr. Fabian Fregoli, Chief Medical Officer of Trinity Health Oakland, Dr. Heidi Kromrei, Director of Medical Education at Trinity Health Oakland, and Melody Dallo, IRB/Research Administrator of Trinity Health Oakland for their unwavering support toward resident’s scholarly activities. Additionally, we extend our thanks to Karen Hagglund for providing us with statistical assistance and invaluable advice on data analysis. Preliminary abstract results from this study were presented at the American Society of Hematology Conference on December 8, 2023, in San Diego, California, USA (26).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. CentersforDiseaseControlandPrevention. Data and statistics of sickle cell disease (2023). Available at: https://www.cdc.gov/ncbddd/sicklecell/data.html#print.
2. Panepinto JA, Brandow A, Mucalo L, Yusuf F, Singh A, Taylor B, et al. Coronavirus disease among persons with sickle cell disease, United States, march 20-may 21, 2020. Emerg Infect Dis. (2020) 26:2473–6. doi: 10.3201/eid2610.202792
3. Payne AB, Schieve LA, Abe K, Hulihan M, Hooper WC, Hsu LL. COVID-19 and sickle cell disease-related deaths reported in the United States. Public Health Rep. (2022) 137:234–8. doi: 10.1177/00333549211063518
4. Han J, Saraf SL, Gordeuk VR. Vaccination in sickle cell disease: Immunocompromised or immunocompetent? Br J Haematol. (2023) 202:916–8. doi: 10.1111/bjh.18942
5. Barrett DJ, Ammann AJ. Pneumococcal vaccine in sickle cell disease: IgG and IgM antibody response. Rev Infect Dis. (1981) 3 Suppl:S179–82. doi: 10.1093/clinids/3.Supplement_1.S179
6. Balandya E, Reynolds T, Obaro S, Makani J. Alteration of lymphocyte phenotype and function in sickle cell anemia: Implications for vaccine responses. Am J Hematol. (2016) 91:938–46. doi: 10.1002/ajh.24438
7. Nakahara H, Cheedarla N, Verkerke HP, Cheedarla S, Wu SC, Hendrickson JE, et al. Enhanced IgG immune response to COVID-19 vaccination in patients with sickle cell disease. Br J Haematol. (2023) 202:937–41. doi: 10.1111/bjh.18899
8. Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. (2022) 602:676–81. doi: 10.1038/s41586-021-04388-0
9. Hoffmann M, Arora P, Pöhlmann S. Understanding Omicron: Transmissibility, immune evasion and antiviral intervention. Clin Transl Med. (2022) 12:e839. doi: 10.1002/ctm2.839
10. Ma K, Shirk P, Lambrou A, Hassell N, Zheng X-Y, Payne A, et al. Genomic surveillance for SARS-coV-2 variants: circulation of omicron lineages - United States, january 2022-may 2023. MMWR Morbidity mortality weekly Rep. (2023) 72:651–6. doi: 10.15585/mmwr.mm7224a2
11. Del Rio C, Malani PN. COVID-19 in 2022-the beginning of the end or the end of the beginning? Jama. (2022) 327:2389–90. doi: 10.1001/jama.2022.9655
12. Scott L, Hsiao NY, Moyo S, Singh L, Tegally H, Dor G, et al. Track Omicron’s spread with molecular data. Science. (2021) 374:1454–5. doi: 10.1126/science.abn4543
13. CentersforDiseaseControlandPrevention. Vaccination data definitions (2023). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/reporting-vaccinations.html#data-definitions.
14. COVID-19TreatmentGuidelinesPanel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National institutes of health (Accessed 10-04-23).
15. Arun Shet M, Ataga K, Wun T, Hseih M, King A, Naik R, et al. COVID-19 and sickle cell disease: frequently asked questions: american society of hematology (2021). Available at: https://www.hematology.org/covid-19/covid-19-and-sickle-cell-disease#:~:text=There%20is%20significant%20overlap%20in,a%20low%20threshold%20for%20imaging.
16. Wu N, Joyal-Desmarais K, Ribeiro PAB, Vieira AM, Stojanovic J, Sanuade C, et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. (2023) 11:439–52. doi: 10.1016/S2213-2600(23)00015-2
17. Prevention CfDCa. COVID data tracker. Atlanta, GA: U.S. Department of health and human services, CDC2023, september 26 . Available at: https://covid.cdc.gov/covid-data-tracker.
18. Han J, Zhang X, Molokie RE, Njoku F, Hussain FA, Farooqui M, et al. COVID-19 vaccination status and disease burden in patients with sickle cell disease. Blood. (2022) 140:5027–8. doi: 10.1182/blood-2022-166597
19. Hematology ASo. Study suggests COVID-19 vaccine safe and effective for individuals with sickle cell disease2023 (2023). Available at: https://www.hematology.org/newsroom/press-releases/2023/study-suggests-covid-19-vaccine-safe-and-effective-for-individuals-with-sickle-cell-disease.
20. Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, et al. Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: A critical appraisal of the literature. Blood Rev. (2022) 53:100911. doi: 10.1016/j.blre.2021.100911
21. Derdevet J, Ranque B, Khimoud D, Joseph L, Michon A, Flamarion E, et al. Efficacy of COVID-19 vaccination in adult patients with sickle cell disease during the Omicron wave in France. Eur J Haematol. (2023) 111:509–12. doi: 10.1111/ejh.14034
22. Hui KPY, Ho JCW, M-c C, K-c Ng, Ching RHH, K-l L, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature. (2022) 603:715–20. doi: 10.1038/s41586-022-04479-6
23. Alkindi S, Elsadek RA, Al-Madhani A, Al-Musalhi M, AlKindi SY, Al-Khadouri G, et al. Impact of COVID-19 on vasooclusive crisis in patients with sickle cell anaemia. Int J Infect Dis. (2021) 106:128–33. doi: 10.1016/j.ijid.2021.03.044
24. Minniti CP, Zaidi AU, Nouraie M, Manwani D, Crouch GD, Crouch AS, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood Adv. (2021) 5:207–15. doi: 10.1182/bloodadvances.2020003456
25. Arlet J-B, de Luna G, Khimoud D, Odièvre M-H, de Montalembert M, Joseph L, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. Lancet Haematol. (2020) 7:e632–e4. doi: 10.1016/S2352-3026(20)30204-0
Keywords: sickle cell disease, COVID-19, SARS-CoV-2, Omicron, COVID-19 vaccine, Pandemic, sickle cell, SCD
Citation: Aldecoa KAT, Macaraeg CSL, Arsene C, Krishnamoorthy G, Chng T, Cherry G, Chowdhury N, Clark R, Deeb D, Deptula L, Dietz G, Eto E, Golston V, Lawson L, Mbionwu C, Okponyia O, Orejuela J, Qipo T, Raut S and Goodman J (2024) Evaluating the effectiveness of COVID-19 vaccines in adults with sickle cell disease during the Omicron period of COVID-19 pandemic. Front. Hematol. 3:1365268. doi: 10.3389/frhem.2024.1365268
Received: 04 January 2024; Accepted: 13 March 2024;
Published: 09 April 2024.
Edited by:
Immacolata Tartaglione, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Richard Oscar Francis, Columbia University, United StatesVijendra Singh, Wayne State University, United States
Copyright © 2024 Aldecoa, Macaraeg, Arsene, Krishnamoorthy, Chng, Cherry, Chowdhury, Clark, Deeb, Deptula, Dietz, Eto, Golston, Lawson, Mbionwu, Okponyia, Orejuela, Qipo, Raut and Goodman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kim Abbegail Tan Aldecoa, YWxkZWNvYS5raW1AZ21haWwuY29t
†These authors have contributed equally to this work
‡ORCID: Kim Abbegail Tan Aldecoa, orcid.org/0000-0002-5914-0118
 Kim Abbegail Tan Aldecoa
Kim Abbegail Tan Aldecoa Chef Stan L. Macaraeg
Chef Stan L. Macaraeg Camelia Arsene1,2,4
Camelia Arsene1,2,4 Nabila Chowdhury
Nabila Chowdhury