- 1Hematology Clinical Trial Unit, Hematology Clinic and Bone Marrow Transplantation Department, Laikon General Hospital, Athens, Greece
- 2Department of Allergy & Immunology, Cleveland Clinic, Abu Dhabi, United Arab Emirates
- 3Microbiology Department, College of Medicine, Kuwait University, Kuwait City, Kuwait
- 4Department of Hematology, Sultan Qaboos University Hospital, Muscat, Oman
- 5Department of Hematology, Kuwait Cancer Control Center, Shuwaikh, Kuwait
- 6Department of Paediatric, King Abdullah Specialized Children’s Hospital (KASCH), Ministry of the National Guard - Health Affairs, Riyadh, Saudi Arabia
- 7College of Medicine, King Saud Bin Abdulaziz University for Health Sciences (KSAU-HS), Riyadh, Saudi Arabia
- 8King Abdullah International Medical Research Centre (KAIMRC), Ministry of National Guard Health Affairs (MNGHA), Riyadh, Saudi Arabia
- 9Oncology Department, King Abdulaziz Medical City, Ministry of National Guard - Health Affairs, Jeddah, Saudi Arabia
- 10College of Medicine, King Saud Bin Abdulaziz University for Health Sciences (KSAU-HS), Jeddah, Saudi Arabia
- 11King Abdullah International Medical Research Centre (KAIMRC), Ministry of National Guard Health Affairs (MNGHA), Jeddah, Saudi Arabia
- 12Oncology Department, King Abdulaziz Medical City, Ministry of National Guard - Health Affairs, Riyadh, Saudi Arabia
- 13King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
- 14King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 15Department of Internal Medicine, American University of Beirut Faculty of Medicine, Beirut, Lebanon
- 16Department of Hematology, National Center for Cancer Care & Research, Hamad Medical Corporation, Doha, Qatar
- 17Department of Hematology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
- 18Department of Internal Medicine and Clinical Immunology, Hotel Dieu de France Hospital, Beirut, Lebanon
- 19Department of Hematology and Medical Oncology, American Hospital, Dubai, United Arab Emirates
- 20Immunology and allergy unit, Royal Hospital, Muscat, Oman
- 21Department of Hematology, Tawam Hospital, Abu Dhabi, United Arab Emirates
- 22Department of Hematology/Oncology, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates
Secondary immunodeficiency (SID), acquired hypogammaglobinemia, is an immunodeficiency caused by different factors like diseases, medications, and/or nutrition disorders. Most patients with hematological malignancies (HM), namely chronic lymphocytic leukemia (CLL) and multiple myeloma (MM), experience such SID. These patients have a consistently high risk of infection throughout the disease course. Traditional chemotherapy and novel agents used to treat HM may further increase infection susceptibility. Immunoglobulin replacement therapy (IgRT) is an effective management option for SID. The prevalence of SID in the Middle East needs better documentation. Healthcare providers should consider and evaluate SID in patients at risk, monitor for infection occurrence, and treat accordingly (including initiating IgRT when indicated). A Delphi initiative was conducted by a consensus panel of 15 experts from the Middle East who have over 20 years of experience in actively managing patients with SID. The modified Delphi process was used, and 16 questions reached a consensus on managing SID patients with IgRT. In addition, the consensus panel of Middle East experts recommended real-world practice recommendations regarding initiating, dosing, and discontinuing IgRT in managing SID. This consensus recommendation aims to assist healthcare practitioners in the Middle East in evidence-based clinical decision-making for better management of SID.
1 Introduction
Secondary immunodeficiency (SID), mainly in the form of acquired hypogammaglobulinemia in hematological malignancies (HMs), can develop due to either the underlying disease or as a consequence of therapy (1, 2). Patients with SID may experience increased susceptibility to infections, ranging from mild to severe, including opportunistic bacterial, viral, and fungal infections (3, 4). The spread of multidrug-resistant organisms such as multidrug-resistant gram-negative bacteria, vancomycin-resistant enterococcus, and methicillin-resistant Staphylococcus aureus further increases the incidence of severe infections and mortality (3).
Management of SID requires a thorough evaluation of the patient’s clinical and laboratory profiles to determine the most effective interventions. These interventions may include patient education, immediate access to antibiotics in emergencies, preventive antibiotic treatment, vaccination, and reduced immunosuppression or treatment of the underlying condition when feasible (5). The recently updated European Medicines Agency (EMA) guidelines have expanded the indication for the usage of immunoglobulin replacement therapy (IgRT) in SID to include a wide range of patients, such as those with various HMs, individuals undergoing B cell-depleting therapy and people experiencing hypogammaglobulinemia after bone marrow or solid organ transplantation. This is in addition to the previous EMA indication that included only chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) patients (6). Selected patients who suffer from severe or recurrent infections despite appropriate prophylactic antibiotics and vaccination and have low levels of quantitative serum immunoglobulin G (IgG) may be recommended for management with IgRT. Several studies have reported that IgRT effectively reduces severe infection rates in CLL or MM patients (7–9). However, other published research has not consistently replicated these results (10, 11). Data from a market research study analyzing secondary specialty pharmacies found that CLL and MM were the primary causes of secondary antibody deficiency leading to IgRT use, with approximately 39.2% to 54.9% of patients receiving IgRT for this condition (10). Acknowledging the complexity of the decision to use IgRT to manage SID is essential as it is multifactorial and involves physician-patient interaction and consensus. Additionally, more evidence that describes real-world practices around the initiation, dosing, and discontinuation of IgRT, specifically in managing patients with SID in the Middle East, is warranted. Therefore, developing evidence-based, region-specific recommendations from real-world experts for treating SID using IgRT is crucial. To achieve this goal, we present a set of consensus recommendations for managing SID with IgRT in the Middle East.
Wherever the term “HM patients at risk” is used, this implies CLL patients, MM patients, patients with B-lymphoproliferative neoplasms, patients on B-cell depleting therapies, hemopoietic stem cell transplant (HSCT) recipients, and patients on CAR T-cell therapies.
2 Materials and methods
The Middle East SID consensus recommendations were generated using a modified Delphi Method. A panel of 15 experts from the Middle East region, along with an international expert in IgRT and SID, was selected based on their expertise in managing patients with SID and their regional practice. The experts agreed upon the four most crucial areas of SID management that needed systematic literature review and detailed discussion to understand regional practices around a) Evaluation and diagnosis of SID, b) Prophylactic treatment of patients with SID, c) Monitoring Ig levels, d) Initiating, dosing, and discontinuation of IgRT. Eighteen (12) questions related to the four sections were generated through a modified Delphi process and distributed to all panel members via email in January 2023. Responses were collected within 30 days, and in March 2023, two virtual meetings were held to reach a consensus on managing SID in the Middle East. Experts voted anonymously on each recommendation statement presented in the multiple-choice format, and those with ≥70% agreement were included in the consensus. Statements not receiving agreement were discussed, revised if necessary, and re-voted.
The study was carried out in compliance with the standards outlined in the Declaration of Helsinki (11).
3 Results and discussion
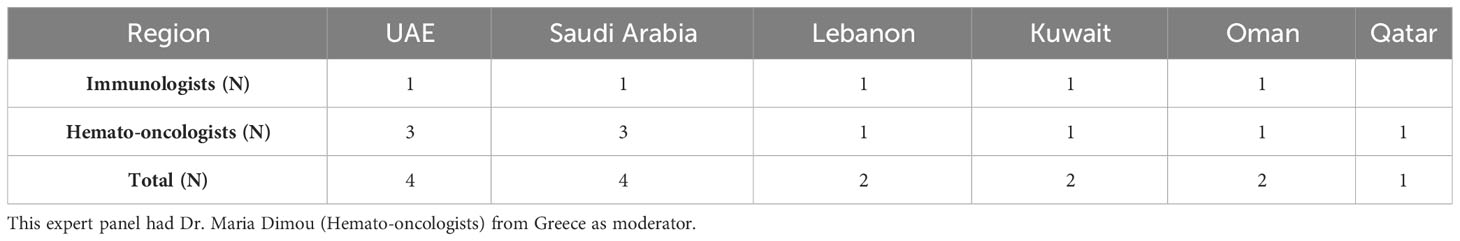
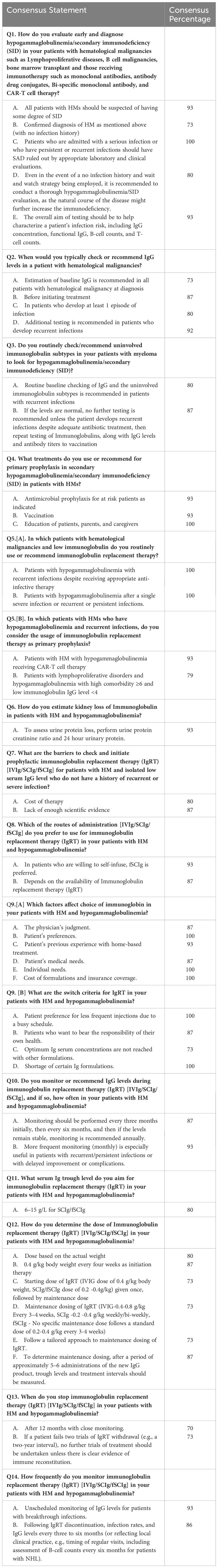
The Delphi panel from the Middle East consisted of Hematologists and Immunologists with extensive experience (average experience: 25 (35/15) years) in managing SID (Table 1). A total of 16 questions (12 + 2 questions written as two sub-questions each) with two or more statements are included after the agreement of the experts from the Middle East (Table 2).
3.1 Evaluation and diagnosis of SID
Lymphoid malignancies are frequently associated with SID, with reported incidences of some forms of hypogammaglobulinemia in newly diagnosed CLL, MM, and diffuse large B-cell lymphoma (DLBCL) reaching up to 25%, 80%, and 22%, respectively (13–15). The incidence of hypogammaglobulinemia increases during disease evolution in CLL patients. Therefore, infectious complications are common in CLL patients, with up to 80% of them experiencing such complications at some point during their disease (16). Severe or significant infections are experienced by 20% of CLL patients, accounting for an estimated 60% of deaths (16, 17).
Experts recommend that clinicians investigate SID in all HM at-risk patients through appropriate laboratory and clinical evaluations. In HM patients at risk without a history of infection, it would be prudent to obtain baseline quantitative serum IgG levels and then monitor the patient periodically during the natural course of the disease.
3.2 Primary and secondary prophylaxis in SID in patients with HMs
Experts from the Middle East typically recommend antimicrobial prophylaxis based on the patient’s underlying risk of infection (disease, type of therapy, etc.) as the primary prophylaxis for SID in these patients. Vaccination is also advised as a preventive measure to reduce the risk of infections in this population. To ensure early reporting of conditions in SID patients and timely initiation of treatment, patients, parents, and caregivers should receive education. Early recognition and management of infections are vital in reducing the morbidity and mortality associated with SID in patients with HMs. Therefore, appropriate prophylactic measures, vaccinations, and patient education are essential to optimize the management of SID in this patient population.
The role of IgRT as primary prophylaxis (before any infectious occurrence) for HM patients at risk with hypogammaglobulinemia is unclear. An international survey reported that the prescription practice for prophylactic IgRT in patients with SID varies among countries (12). The difference in IgRT usage between Italy, Germany, Spain, the United States, and the UK in patients with SID is more prevalent in the former four countries compared to the UK (12, 18). In 85% of cases, IgRT was prescribed after two or more severe infections, whereas in 65% of cases, it was prescribed after the first severe infection (secondary prophylaxis).In this study, IgRT was the primary prophylaxis given in 24% of the patients (18).
3.3 Monitoring IgG levels
Experts from the Middle East discussed in detail the practices carried out in everyday clinical practice regarding monitoring of IgG levels in patients with SID and unanimously recommended that regular evaluation and monitoring for hypogammaglobulinemia should be considered in CLL patients without a history of infection since they may develop SID during the natural course of the disease. Quantitative serum IgG levels should be determined at diagnosis and before initiation of treatment in all HM patients at risk. Additionally, IgG levels should be evaluated in patients who develop at least one episode of severe bacterial infection, and further testing is recommended in patients who develop recurrent or persistent infections. Routine baseline checking of IgG and the uninvolved immunoglobulin subtypes is recommended in MM patients with recurrent infections. If IgG levels are normal, no further testing is recommended unless the patient develops recurrent infections despite adequate antibiotic treatment. Repeating testing of uninvolved immunoglobulin and IgG levels is recommended in these patients. In addition, evaluation of the antibody titers to vaccination is advisable. An increased frequency of monitoring or monthly monitoring is recommended in patients with recurrent or persistent infections, delayed improvement, or complications.
The IgRT is recommended to be used in hypogammaglobulinemia patients with severe or recurrent infections, and monitoring of therapy through IgG trough levels is advisable: initially, every three months, followed by subsequent monitoring every six or more months if IgG trough levels have reached the preferable level. One possible approach is to check IgG levels monthly during treatment and every 3 to 6 months or annually. However, this approach is not recommended by the experts (Table 3).
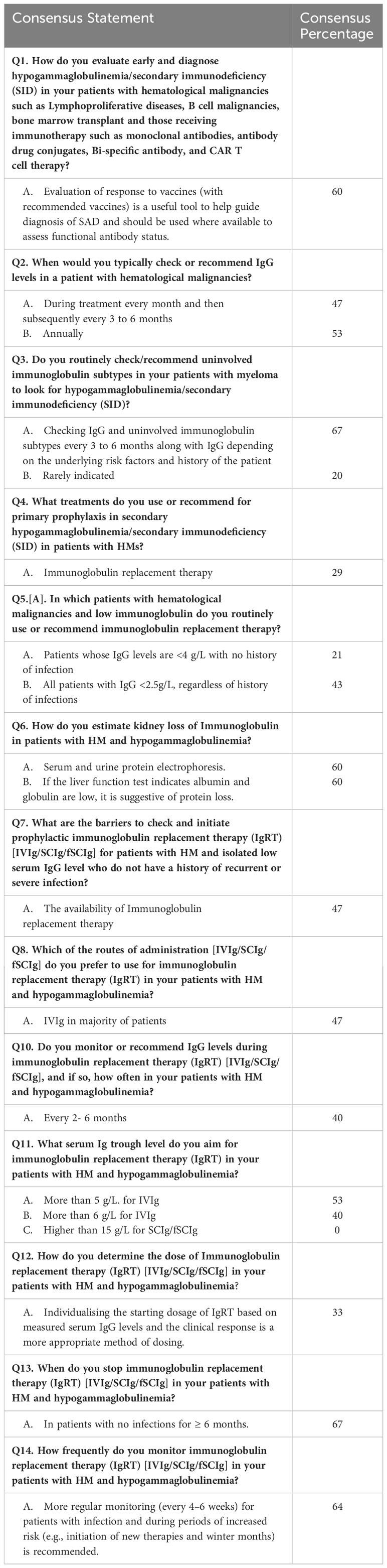
Table 3 Middle East recommendations for the management of SID that did not reach consensus (percentage agreement).
Early diagnosis and management of SID in high-risk HM patients are essential to minimize the risk of infectious complications and improve patient outcomes. These recommendations are in line with international guidelines.
Additionally, several methods were listed in the questionnaire to estimate kidney loss of immunoglobulin in patients with HM and hypogammaglobulinemia. To rule out organic causes of immunoglobulin loss, experts recommend evaluating urine protein loss, urine creatinine ratio, and 24-hour urine protein. Serum and urine protein electrophoresis can also be performed, detecting a loss of up to 60% of immunoglobulin. Liver function tests indicating low albumin and globulin levels are not recommended as suggestive of protein loss.
3.4 Initiating, dosing, and discontinuation of IgRT
Insufficient data are available on the occurrence of SID with new treatments. IgRT is recommended in patients with hematological malignancies and low immunoglobulin levels who have recurrent infections despite appropriate anti-infective treatment or who have had a single severe infection or recurrent or persistent infections. In addition, experts have reached a consensus that IgRT is also recommended as primary prophylaxis in specific subgroups of patients, including those with hypogammaglobulinemia receiving CAR (chimeric antigen receptor) T-cell therapy and those with lymphoproliferative disorders and hypogammaglobulinemia with comorbidities and IgG<4 g/l. However, there is no consensus on using IgRT in other HM patients whose IgG levels are <4 g/L or even <2.5 g/L with no history of infection (19).
The expert recommendation suggests a serum Ig trough level of 6-15 g/L when administering IgRT to patients with hematological malignancies and hypogammaglobulinemia via subcutaneous immunoglobulin (SCIg)/facilitated subcutaneous immunoglobulin (fSCIg).
In patients with hematological malignancies and isolated low serum IgG levels without a history of recurrent or severe infections, barriers to initiating prophylactic IgRT include cost and lack of adequate scientific evidence. The cost of IgRT is documented to be substantial and adds to the burden on healthcare systems (e.g., 100- 150,000 USD per year in the United States) (20). Although IgRT tends to decrease respiratory infections and hospitalizations, it is not universally effective across all treatment groups or disease stages. Expert recommendations emphasize the need for more robust randomized controlled trials to evaluate the efficacy and safety of IgRT in this patient population. Moreover, the availability of IgRT is considered a minor barrier. Nevertheless, the use of IgRT presents considerable obstacles, such as an annual 6-8% rise in worldwide demand and an uneven distribution of global supply (21). The experts acknowledge that the limited availability of IgRT during the COVID pandemic was a challenge in the Middle East, but not now.
According to expert recommendations, the preferred route of administration for IgRT in patients with hematological malignancies and hypogammaglobulinemia depends on certain factors. For example, if patients are willing to self-infuse, then SCIG or fSCIg is the preferred route of administration. The availability of IgRT is also a determining factor. In summary, the choice of immunoglobulins is based on the physician’s judgment, the patient’s preference, the patient’s previous experience with home-based treatment, the patient’s medical needs, the specific individual needs of the patients, and the cost of the formulations and availability of insurance coverage.
Furthermore, there is a need to change the definition of the dosing and discontinuation of IgRT (3). Expert recommendation for determining the dose of IgRT in patients with hematological malignancies and hypogammaglobulinemia includes initiating therapy with a dose of 0.4 g/kg body weight every 3-4 weeks, followed by maintenance dosing of 0.4-0.8 g/kg every 3-4 weeks for intravenous IgG (IVIG) and 0.2-0.4 g/kg weekly/bi-weekly for SCIg, while fSCIg does not follow a specific maintenance dose but a standard dose of 0.2-0.4 g/kg every 3-4 weeks. Alternatively, a tailored approach can be used to determine maintenance dosing based on trough levels and treatment intervals measured after approximately 5-6 administrations of the new IgG product. Breakthrough infections could also be another important reason for tailored IgRT doses and infusion intervals (e.g., increased doses and/or more frequent infusions). Dosing based on actual weight is recommended, while individualizing the starting dosage based on measured serum IgG levels and clinical response is not recommended.
Expert recommendation for the switch criteria for IgRT in patients with HMs and hypogammaglobulinemia consists of factors such as the patient’s preference for the frequency and number of infusions, their willingness to take charge of their health, the failure to achieve adequate IgG levels with other forms of treatment, and the unavailability of specific immunoglobulin formulations.
In the case of IgRT discontinuation, it is essential to monitor infection rates and IgG levels every three to six months. This can be based on local clinical practice and may include an assessment of B-cell counts every six months for patients with B-lymphoproliferative neoplasms to monitor B-cell reconstitution. However, per EMA recommendations, more regular IgG monitoring must be followed in the Middle East every 4-6 weeks for patients with infection and during periods of increased risk, such as initiation of new therapies and winter months.
Expert recommendations suggest that patients with hematological malignancies and hypogammaglobulinemia should be carefully monitored for when to stop IgRT using IVIg, SCIg, or fSCIg. Experts did not agree on IgRT discontinuation after 12 months of close monitoring or in patients who have not had any infections for at least six months. Experts agreed that if a patient fails two trials of IgRT withdrawal (e.g., a two-year interval), no further trials of treatment should be undertaken unless there is clear evidence of immune reconstitution. This approach is intended to avoid potential relapse or worsening of the condition and ensure that patients receive optimal long-term care.
4 Conclusion
This is the first consensus on guidance for managing SID in the Middle East. Adapting the global recommendations for the Middle East region can optimize the SID management approach and improve the overall standard of healthcare provided to these patients across the region.
Author contributions
MD: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. MAb: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. MAA: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. KA: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AAlh: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. FA: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AAls: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. MAl: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. AB: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HC: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. RE: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CI: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. FK: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. IN: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HO: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. MS: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Takeda Pharmaceutical Company Limited (Middle East) sponsored and provided funds for the development of the consensus. The sponsor had no input into the development of the voting statements, the analysis or interpretation of the results, or the decision to submit the manuscript for publication.
Acknowledgments
Authors thank Cogence Health DWC LLC for facilitating consensus and medical writing support.
Conflict of interest
All authors received honoraria from Cogence Health DWC LLC. Cogence Health DWC LLC received funding from Takeda Pharmaceutical Company Limited Middle East to facilitate this project. MAA declared she received speaker fees from AstraZeneca, Sanofi. AB declared he received research support from Novartis, Roche, Takeda, Jansen, Astellas, Celgene, Pfizer, and Amgen. He received advisory board and speaker fees from Novartis, Roche, Sanofi, Jazz, Adienne, Astellas, Takeda, Hikma, Celgene, Jansen, MSD, Abbvie, Pfizer and Amgen. RF declared he received consultancy, advisory board, and speakers-bureau honoraria from AZ, Roche, Janssen, Novartis, Biologix, Sobi, Newbridge, Gilead, BMS, MSD, Merck, Abbvie, Kyowa Kirin, Hikma, Pfizer, Sanofi, Servier, Amgen, Takeda. FK declared advisory board and speakers-bureau honoraria from Amgen, MSD, and Takeda.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAR, Chimeric Antigen Receptor; CLL, Chronic Lymphocytic Leukemia; DLBCL, Diffuse Large B-Cell Lymphoma; fSCIg, Facilitated Subcutaneous Immunoglobulin; HM, Hematological Malignancies; IgRT, Immunoglobulin Replacement Therapy; IVIG, Intravenous IgG; SCIg, Subcutaneous Immunoglobulin; SID, Secondary Immunodeficiency.
References
1. Sim B, Ng JY, Teh BW, Talaulikar D. Immunoglobulin replacement in hematological Malignancies: a focus on evidence, alternatives, dosing strategy, and cessation rule. Leuk Lymphoma. (2023) 64:18–29. doi: 10.1080/10428194.2022.2131424
2. Allegra A, Tonacci A, Musolino C, Pioggia G, Gangemi S. Secondary immunodeficiency in hematological Malignancies: focus on multiple myeloma and chronic lymphocytic leukemia. Front. Immunol. (2021) 12:738915. doi: 10.3389/fimmu.2021.738915
3. Ballo O, Tarazzit I, Stratmann J, Reinheimer C, Hogardt M, Wichelhaus TA, et al. Colonization with multidrug resistant organisms determines the clinical course of patients with acute myeloid leukemia undergoing intensive induction chemotherapy. PloS One. (2019) 14:1–12. doi: 10.1371/journal.pone.0210991
4. Carr RM, Oranu A, Khungar V. Chinen. Physiol. Behav. (2016) 176:139–48. doi: 10.1016/j.jaci.2009.08.040.Secondary
5. Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: Causes, diagnosis, and management. Front. Immunol. (2019) 10:33. doi: 10.3389/fimmu.2019.00033
6. European Medicines Agency. Old - Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) (2018). Available online at: http://www.ema.europa.eu/htms/human/qrd/docs/convention.pdf.
7. Günther G, Dreger B. Post-marketing observational study on 5% intravenous immunoglobulin therapy in patients with secondary immunodeficiency and recurrent serious bacterial infections. Microbiol. Immunol. (2013) 57:527–35. doi: 10.1111/1348-0421.12060
8. Compagno N, Cinetto F, Semenzato G, Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: A single-center experience in 61 patients. Haematologica. (2014) 99:1101–6. doi: 10.3324/haematol.2013.101261
9. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin. Lymphoma Myeloma Leuk. (2013) 13:106–11. doi: 10.1016/j.clml.2012.11.011
10. Gale PR, Chapel H, Bunch C, Rai L, Foon K, Courter S, et al. Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. N Engl. J. Med. (1988) 319:902–7. doi: 10.1056/NEJM198810063191403
11. Association WM. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
12. Na IK, Buckland M, Agostini C, Edgar JDM, Friman V, Michallet M, et al. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological Malignancies. Eur. J. Haematol. (2019) 102:447–56. doi: 10.1111/ejh.13223
13. Singh N, Mott SL, McCarthy AN, Syrbu S, Habermann TM, Feldman AL, et al. Prevalence and the impact of hypogammaglobulinemia in newly diagnosed, untreated diffuse large B cell lymphoma. Blood. (2019) 134:1604–4. doi: 10.1182/blood-2019-122737
14. Sørrig R, Klausen TW, Salomo M, Vangsted AJ, Frølund UC, Andersen KT, et al. Immunoparesis in newly diagnosed Multiple Myeloma patients: Effects on overall survival and progression free survival in the Danish population. PloS One. (2017) 12:1–15. doi: 10.1371/journal.pone.0188988
15. Parikh SA, Leis JF, Chaffee KG, Call TG, Hanson CA, Ding W, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer. (2015) 121:2883–91. doi: 10.1002/cncr.29438
16. Tadmor T, Welslau M, Hus I. A review of the infection pathogenesis and prophylaxis recommendations in patients with chronic lymphocytic leukemia. Expert Rev. Hematol. (2018) 11:57–70. doi: 10.1080/17474086.2018.1407645
17. Hensel M, Kornacker M, Yammeni S, Egerer G, Ho AD. Disease activity and pretreatment, rather than hypogammaglobulinaemia, are major risk factors for infectious complications in patients with chronic lymphocytic leukaemia. Br. J. Haematol. (2003) 122:600–6. doi: 10.1046/j.1365-2141.2003.04497.x
18. Edgar JDM, Richter AG, Huissoon AP, Kumararatne DS, Baxendale HE, Bethune CA, et al. Prescribing immunoglobulin replacement therapy for patients with non-classical and secondary antibody deficiency: an analysis of the practice of clinical immunologists in the UK and republic of Ireland. J. Clin. Immunol. (2018) 38:204–13. doi: 10.1007/s10875-017-0469-4
19. Link H, Kerkmann M, Holtmann L. Immunoglobulin substitution in patients with secondary antibody deficiency in chronic lymphocytic leukemia and multiple myeloma: a representative analysis of guideline adherence and infections. Support Care Cancer. (2022) 30:5187–200. doi: 10.1007/s00520-022-06920-y
20. Burt RK, Tappenden P, Balabanov R, Han X, Quigley K, Snowden JA, et al. The cost effectiveness of immunoglobulin vs. Hematopoietic stem cell transplantation for CIDP. Front. Neurol. (2021) 12:645263. doi: 10.3389/fneur.2021.645263
Keywords: secondary immunodeficiency, Middle East, Delphi consensus, immunoglobulin replacement therapy, acquired hypogammaglobinemia
Citation: Dimou M, Abuzakouk M, Al Ahmad M, Al Farsi K, Alhuraiji A, Al Roqi F, Alsaeed A, Alzahrani M, Bazarbachi A, Cherif H, El Fakih R, Irani C, Khan F, Nasr I, Osman HY and Siddiqui M (2024) Management of secondary immunodeficiency in hematological malignancies: a Delphi consensus from the Middle East. Front. Hematol. 3:1347708. doi: 10.3389/frhem.2024.1347708
Received: 01 December 2023; Accepted: 05 February 2024;
Published: 08 March 2024.
Edited by:
Emmanouil Nikolousis, European University Cyprus, CyprusReviewed by:
Senthilnathan Palaniyandi, University of Missouri, United StatesMatilde Scaldaferri, Matilde Scaldaferri, Italy
Copyright © 2024 Dimou, Abuzakouk, Al Ahmad, Al Farsi, Alhuraiji, Al Roqi, Alsaeed, Alzahrani, Bazarbachi, Cherif, El Fakih, Irani, Khan, Nasr, Osman and Siddiqui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Dimou, bXNkaW1vdUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Maria Dimou
Maria Dimou Mohamed Abuzakouk2†
Mohamed Abuzakouk2† Mona Al Ahmad
Mona Al Ahmad Fayhan Al Roqi
Fayhan Al Roqi Ali Bazarbachi
Ali Bazarbachi Honar Cherif
Honar Cherif Riad El Fakih
Riad El Fakih Iman Nasr
Iman Nasr