
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol. , 21 March 2024
Sec. Blood Cancer
Volume 3 - 2024 | https://doi.org/10.3389/frhem.2024.1345884
 Flavia Antonucci1*
Flavia Antonucci1* Serena Marotta1
Serena Marotta1 Maria Celentano1
Maria Celentano1 Mariangela Pedata1
Mariangela Pedata1 Cira Riccardi1
Cira Riccardi1 Cristina Luise1
Cristina Luise1 Angela Carobene1
Angela Carobene1 Simona Maria Muggianu1
Simona Maria Muggianu1 Assunta Viola2
Assunta Viola2 Mafalda Caputo1
Mafalda Caputo1 Stefania Leone1
Stefania Leone1 Ilaria Migliaccio1
Ilaria Migliaccio1 Barbara Pocali2
Barbara Pocali2 Mirella Alberti1
Mirella Alberti1 Claudio Falco1
Claudio Falco1 Mario Toriello3
Mario Toriello3 Felicetto Ferrara2
Felicetto Ferrara2 Alessandra Picardi1,4*
Alessandra Picardi1,4*To date, the effect of blinatumomab and donor lymphocyte infusion association as a salvage treatment for acute lymphoblastic leukemia (ALL) relapse after allogeneic transplant procedure is still unknown. Here, we report a case report of a patient with early relapse of ALL after allogeneic hematopoietic stem cell transplant successfully treated with a combination of blinatumomab and DLI.
Allogeneic hematopoietic stem cell transplantation (HSCT) represents the treatment of choice to achieve long-term survival in relapsed acute lymphoblastic leukemia (ALL). However, relapse after the allogeneic transplant procedure remains the main cause of treatment failure in this setting of patients. Blinatumomab, a bispecific T-cell engager (BiTE) against CD19 and CD3, has been approved for relapsed and/or refractory CD19-positive B-cell acute lymphoblastic leukemia (B-ALL) Ph+/− by the Food and Drug Administration, the European Medicines Agency, and Associazione Italiana del Farmaco (1). Although there are data regarding blinatumomab as salvage therapy before allogeneic HSCT, information on its use, alone or in combination with donor lymphocyte infusion (DLI), as treatment for early relapses after the allogeneic transplant procedure is scarce and is still a matter of debate (2, 3). However, due to the mechanism of action of blinatumomab, its association with DLI represents a powerful rationale in the case of CD19-positive ALL relapse after allogeneic HSCT (4, 5). Here, we present a case report of a patient affected by high-risk B-ALL who underwent allogeneic HSCT in third complete remission (CR), followed by early relapse successfully treated with a combination of blinatumomab and DLI.
In 2015, a 17-year-old male patient was diagnosed with pre-B Ph-ALL in a hospital in Central America and was treated according to local protocol based on a combination of vincristine, doxorubicin, asparaginase, and prednisone. The patient achieved the first CR, minimal residual disease (MRD) negative, on day +33. The relevant laboratory findings of the patient at diagnosis are described in Table 1.
In January 2018, the patient completed maintenance chemotherapy; however, after 1 year, hematological relapse occurred. He was treated with vincristine and prednisone, achieving second CR, MRD negative. In February 2019, the patient arrived at our department, where CR and MRD negativity were confirmed by bone marrow (BM) aspiration. Moreover, cerebrospinal fluid (CSF) assay and computed tomography (CT) documented the absence of extramedullary ALL. Furthermore, familial human leukocyte antigen (HLA) typing was performed, which showed the availability of an HLA-identical sister. In May 2019, he developed a second relapse, which was treated with a combination of high-dose cytosine arabinoside and mitoxantrone (HAM), achieving third hematological CR with MRD negativity using flow cytometry (FC) with a level of sensitivity up to 0.001%. In August 2019, the patient underwent HLA-identical allogeneic HSCT in third hematological CR. Peripheral blood stem cells (PBSCs) and a myeloablative thiotepa, Busilvex, and fludarabine schedule were used as the stem cell source and the conditioning regimen, respectively, while graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporin (CSA), short-course methotrexate (MTX), and low-dose (15 mg/kg) anti-thymocyte globulin (ATG) for the high relapse risk. Letermovir was used as the cytomegalovirus (CMV) prophylaxis. The characteristics of the recipient and the donor are described in Table 2.
The CD34-positive cell dose of the graft was 5 × 106/kg. Polymorphonuclear neutrophils and platelet engraftment were achieved on day +16 and day +20, respectively. The patient was discharged on day +30 after HSCT with a full donor chimerism, evaluated through the quantitative PCR method using insertion–deletion polymorphism (indl qRT-PCR). The BM aspiration, which was performed on day +60, showed pre-B ALL CD19-positive relapse with a mixed chimerism (76% donor). The CSF assay and CT documented the absence of extramedullary ALL relapse. CSA was promptly withdrawn, and eligibility for salvage therapy with chimeric antigen receptor T cells (CAR-T) was ruled out due to the too early relapse after allogeneic HSCT. Decision-making led to a salvage therapy based on the combination of blinatumomab and DLI after a single dose of vincristine that reduced the white blood cell (WBC) count from 10,000 to 900/μl and the BM blasts from 61% to 32%. Taking into account the very early timing of relapse and the lack of acute GVHD from HSCT to the relapse, the patient was administered blinatumomab and an intermediate dose of DLI (1 × 107/kg) on day +15 during blinatumomab infusion and in the absence of immunosuppressive prophylaxis. The patient achieved a fourth CR and MRD negativity and a full donor chimerism, documented by BM aspiration on day +30 after DLI. Grade III acute GVHD (aGVHD) with liver and skin involvement occurred 40 days after DLI. The treatment for GVHD consisted of high doses of steroids and, subsequently, for steroid refractoriness, infliximab (three doses) and extracorporeal photopheresis (ECP), thanks to which the patient achieved a full clinical response after 2 months. The development of GVHD, after the first dose of blinatumomab + DLI, did not allow further DLI. After 9 months of blinatumomab and DLI, extramedullary localization of the disease was suspected, with PET/CT positivity for multiple lymphadenopathies and a heteroplastic mass between the 11th thoracic and the 2nd lumbar vertebrae. Cytologic and FC analysis of the CSF and the mass revealed the extramedullary localization of pre-B ALL, with loss of CD22 and CD15 on both specimens. The FC characteristics of the patient’s disease are detailed in Table 3.
The patient received craniospinal radiotherapy and six intrathecal chemotherapies (methotrexate, 12 mg; cytosine arabinoside, 40 mg; and prednisone, 40 mg), obtaining fifth CR, MRD negative. At the last follow-up, in August 2022, the patient was in persistent CR, MRD negative after 36 and 32 months from allogeneic HSCT and the combination of blinatumomab and DLI, respectively. Subsequently, the patient returned to his country of origin and was lost to follow-up.
The timeline of the patient’s disease is shown in Figure 1.
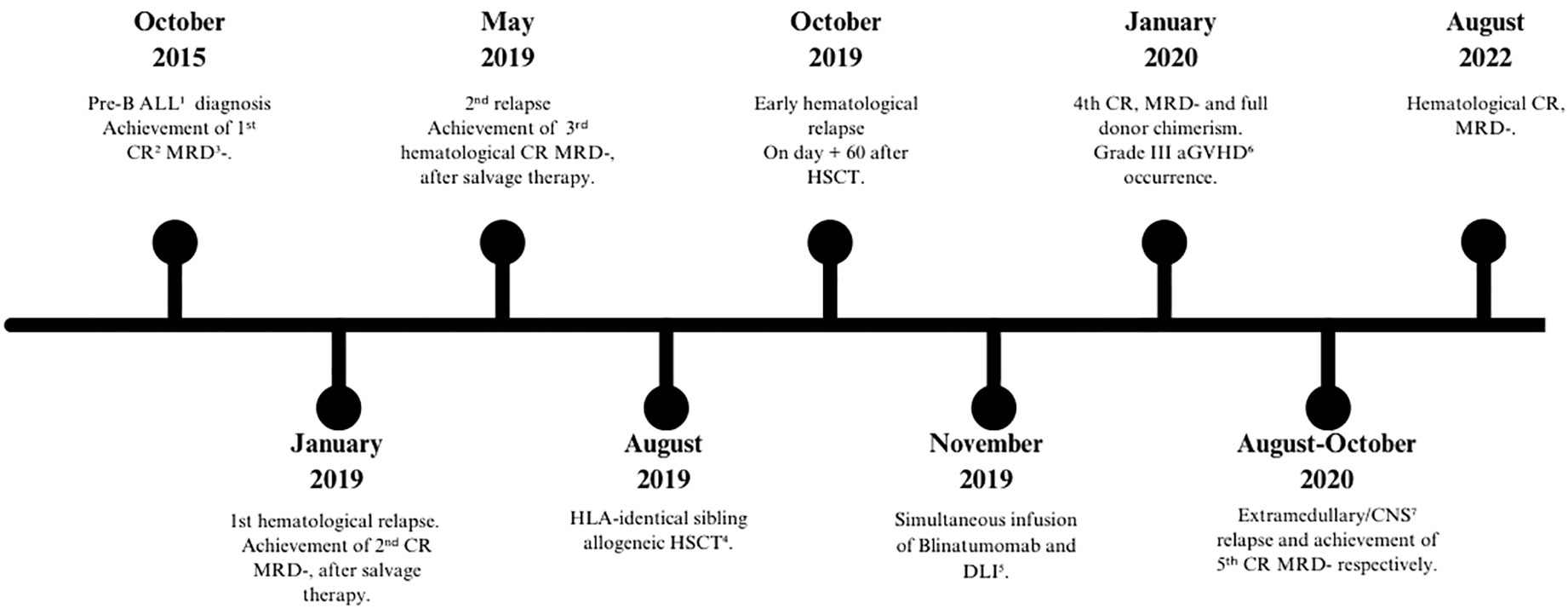
Figure 1 Timeline of patient’s disease. 1ALL, Acute Lymphoblastic Leukemia; 2CR, Complete Remission; 3MRD, Minimal Residual Disease; 4HSCT, Hematopoietic Stem Cell Transplantation; 5DLI, Donor Lymphocyte Infusion; 6aGVHD, acute Graft Versus Host Disease; 7CNS, Central Nervous System.
The use of DLI has shown poor efficacy in patients with early relapse of ALL after allogeneic HSCT (6, 7). Conversely, blinatumomab combined with DLI should be able to improve the graft versus leukemia effect of donor CD3-positive cells, exploiting the binding between CD19-positive blasts and CD3-positive donor lymphocytes with subsequent CD3-positive cell expansion, activation, and killing action against leukemic cells (8). Moreover, recent pharmacokinetic studies have shown that blinatumomab could lead to the suppression of regulatory B and T cells (9, 10). Furthermore, in a recent study, Chauvet et al. reported a multicenter experience on the use of blinatumomab and DLI in relapsed B-ALL after allogeneic HSCT (3). Overall, 72 patients were included in this clinical trial, of whom 50 received blinatumomab alone and 22 received blinatumomab in combination with DLI. In the subset of patients who received blinatumomab and DLI, the median time of DLI administration was 44 days (range, 35–74 days) after blinatumomab in 20 cases, whereas 2 patients received DLI before blinatumomab. The 2-year overall survival (OS) rates were 31% and 43% (p = ns) for those receiving blinatumomab alone and for blinatumomab in combination with DLI, respectively. However, the blinatumomab and DLI group versus the blinatumomab group achieved significantly better results in terms of CR (82% versus 50%, p = 0.018).
Based on the aforementioned rationale, confirmed by a recent clinical trial, our decision-making led to a salvage therapy for the treatment of relapsed B-ALL after allogeneic HSCT using the contemporary administration of DLI and blinatumomab (day +14 after blinatumomab start). Although a larger sample size is needed to confirm the efficacy of this therapeutic option, this case report supports its potential role in lengthening the OS and leukemia-free survival of patients with very poor prognosis. Furthermore, data regarding the efficacy of the simultaneous administration of DLI and blinatumomab are extremely scarce, and the onset of aGVHD associated with the 9-month leukemia-free survival of this case indicates that their contemporary infusion may increase their synergistic effect. However, the use of GVHD prophylaxis after simultaneous blinatumomab and DLI administration should be considered in order to avoid the occurrence of grade III–IV GVHD. Lastly, the current innovative CAR-T-cell strategy, even for adults with early relapse of B-ALL after allogeneic HSCT, represents a promising challenge, although the consolidation therapy with a second allogeneic transplant after CAR-T is still a matter of debate. There are no data on the comparison of CAR-T cells and the simultaneous administration of blinatumomab and DLI.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethical approval was not required for case report description. However, patient’s informed consent for data collection and use was collected, in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
FA: Writing – review & editing. SM: Writing – review & editing, Data curation. MCe: Writing – review & editing, Data curation. MP: Writing – review & editing, Data curation. CR: Writing – review & editing, Data curation. AC: Writing – review & editing, Data curation. SL: Writing – review & editing, Data curation. IM: Writing – review & editing, Data curation. SMM: Writing – review & editing, Data curation. AV: Writing – review & editing, Data curation. MCa: Writing – review & editing, Data curation. CL: Writing – review & editing, Data curation. BP: Writing – review & editing, Data curation. MA: Writing – review & editing, Data curation. CF: Data curation, Writing – review & editing. MT: Writing – review & editing, Data curation. FF: Writing – review & editing, Supervision. AP: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Associazione Italiana Contro le Leucemie – Naples Section, contributed to the publication fee.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Portell CA, Advani A. Novel targeted therapies in acute lymphoblastic leukemia. Leuk Lymphoma. (2014) 55:737–48. doi: 10.3109/10428194.2013.823493
2. Stein AS, Kantarjian H, Gökbuget N, Bargou R, Litzow MR, Rambaldi A. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25:1498–504. doi: 10.1016/j.bbmt.2019.04.010
3. Chauvet P, Paviglianiti A, Labopin M, Labussière H, Boissel N, Robin M, et al. Combining blinatumomab and donor lymphocyte infusion in B-ALL patients relapsing after allogeneic hematopoietic cell transplantation: a study of the SFGM-TC. Bone Marrow Transplant. (2023) 58:72–79. doi: 10.1038/s41409-022-01846-9
4. Ueda M, de Lima M, Caimi P, Tomlinson B, Little J, Creger R, et al. Concurrent blinatumomab and donor lymphocyte infusions for treatment of relapsed pre-B-cell ALL after allogeneic hematopoietic cell transplant. Bone Marrow Transplant. (2016) 51:1253–5. doi: 10.1038/bmt.2016.104
5. Gaballa MR, Banerjee P, Denai RM, Xianli J, Ganesh C, Khazal S, et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia. Blood. (2022) 139:1908–19. doi: 10.1182/blood.2021013
6. Collins RH Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. (2000) 26:511–6. doi: 10.1038/sj.bmt.1702555
7. Chang X, Zang X, Xia C-Q. New strategies of DLI in the management of relapse of hematological Malignancies after allogeneic hematopoietic. Bone Marrow Transplant. (2015) 51:324–32. doi: 10.1038/bmt.2015.28
8. Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation. When, if and for whom. Blood Rev. (2013) 27:55–62. doi: 10.1016/j.blre.2012.11.002
9. Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological Malignancies after stem cell transplantation revisited. Cancer Treat Rev. (2010) 36:528–38. doi: 10.1016/j.ctrv.2010.03.004
10. Michallet AS, Nicolini F, Furst S, Le QH, Dubois V, Hayette S, et al. Outcome and long-term follow-up of alloreactive donor lymphocyte infusions given for relapse after myeloablative allogeneic hematopoietic stem cell transplantations (HSCT). Bone Marrow Transplant. (2005) 35:60. doi: 10.1038/sj.bmt.1704807
Keywords: acute lymphoblastic leukemia, blinatumomab, donor lymphocyte infusion (DLI), hematopoietic stem cell transplantation, Graft Versus Host Disease
Citation: Antonucci F, Marotta S, Celentano M, Pedata M, Riccardi C, Luise C, Carobene A, Muggianu SM, Viola A, Caputo M, Leone S, Migliaccio I, Pocali B, Alberti M, Falco C, Toriello M, Ferrara F and Picardi A (2024) Case report: Donor lymphocyte infusion and blinatumomab as treatment for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Front. Hematol. 3:1345884. doi: 10.3389/frhem.2024.1345884
Received: 28 November 2023; Accepted: 19 February 2024;
Published: 21 March 2024.
Edited by:
Matteo Molica, S. Eugenio, ItalyReviewed by:
Georg-Nikolaus Franke, University Hospital Leipzig, GermanyCopyright © 2024 Antonucci, Marotta, Celentano, Pedata, Riccardi, Luise, Carobene, Muggianu, Viola, Caputo, Leone, Migliaccio, Pocali, Alberti, Falco, Toriello, Ferrara and Picardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flavia Antonucci, ZmxhdmlhYW50b251Y2NpMUBnbWFpbC5jb20=; Alessandra Picardi, YWxlc3NhbmRyYS5waWNhcmRpQGFvY2FyZGFyZWxsaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.