
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Hematol. , 13 June 2024
Sec. Blood Cancer
Volume 3 - 2024 | https://doi.org/10.3389/frhem.2024.1335161
 Theo Leitner1*
Theo Leitner1* Cyrus Khandanpour1,2*
Cyrus Khandanpour1,2* Knut Wendelin3
Knut Wendelin3 Fuat Oduncu4
Fuat Oduncu4 Christoph Kimmich5
Christoph Kimmich5 Ralph Naumann6
Ralph Naumann6 Miriam Kull7
Miriam Kull7 Hartmut Goldschmidt8
Hartmut Goldschmidt8 Martin Ehmer9
Martin Ehmer9 Claudia Kiewitz9
Claudia Kiewitz9 Hans Salwender10*
Hans Salwender10*Therapy for relapsed and refractory multiple myeloma (RRMM) remains challenging. While monoclonal antibodies against CD38 combined with pomalidomide have demonstrated efficacy in clinical trials, real-world data remain sparse. We present real-world data from a compassionate use program (CUP) of isatuximab given in combination with pomalidomide and dexamethasone according to the German Compassionate Use Directive ahead of commercial availability for adult patients with RRMM. Patients had received at least two prior lines of therapy, including lenalidomide and a proteasome inhibitor (PI), and had demonstrated disease progression on the last therapy. Isatuximab was administered as part of the clinical routine. In total, 18 patients were included in the CUP before the official market availability of isatuximab. The data reflect a heterogeneous population in terms of age, risk factors, previous diseases, and treatments. Most of the patients had received two full isatuximab cycles. The analysis showed no new safety signals, supporting the manageable toxicity profile of isatuximab and highlighting its potential in real-world settings.
Despite substantial improvements in the treatment of multiple myeloma (MM) during the past decade, the management of relapsed and refractory multiple myeloma (RRMM) remains challenging. While patients respond well to initial therapy, often leading to long disease-free periods, most patients will relapse eventually (1–3). Monoclonal antibodies targeting CD38 have shown good efficacy in the treatment of MM (4, 5). CD38, a transmembrane glycoprotein with ectoenzymatic activity, expressed in hematological malignancies, is highly and uniformly expressed on MM cells, with >98% of MM patients testing positive for CD38 (6). Preclinical studies demonstrated enhanced antitumor effects of isatuximab when combined with immunomodulatory agents (IMiDs) and proteasome inhibitors (PIs) (4, 7). Isatuximab in combination with pomalidomide has been investigated in various anti-myeloma trials. Prospective phase 1 and phase 3 studies provide the highest level of evidence for the safety and efficacy of Isa-Pd in patients with RRMM (8, 9). The phase 3 ICARIA-MM study demonstrated remarkable responses and improved outcomes of Isa-Pd in pomalidomide-naive RRMM patients compared to Pd alone (8). In addition, the pivotal phase 3 IKEMA trial evidenced significant improvements in progression-free survival and depth of response in patients with RRMM receiving isatuximab in combination with carfilzomib and dexamethasone (7). However, real-world data on Isa-Pd in RRMM patients are limited. Here, we report the first German real-world safety data of isatuximab administration in combination with pomalidomide and dexamethasone in clinical routines, within a compassionate use program before market availability, in RRMM patients who have received at least two prior lines of anti-myeloma therapy, including lenalidomide and a PI, and who have demonstrated disease progression on the therapy.
The program was open for enrollment from July 2020 to January 2021 07–2020 to 01–2021, prior to regular market access in February 2021. All patients diagnosed with relapsed and refractory multiple myeloma must have demonstrated disease progression on their last therapy (having undergone at least two prior therapy lines), according to the anticipated label, as well as no other satisfactory treatment option with an alternative drug approved in Germany. Treatment consisted of isatuximab (given in a dose of 10 mg/kg intravenously; weekly (once a week) (QW) of the first cycle and Q2W in subsequent cycles) and a background medication of pomalidomide (4 mg orally administered, every day from day 1–21), combined with dexamethasone (40 mg and 20 mg if age ≥75 years, oral or intravenous application; QW of each cycle). The length of each cycle was 28 days. Treatment was repeated until disease progression or unacceptable toxicity. The CUP was terminated as soon as isatuximab was made commercially available, as per local regulations. Patients continued treatment with commercial drugs, if applicable.
All data were collected within the CUP for isatuximab prior to regular market access in February 2021. Statistical analyses were provided as descriptive summaries. The baseline characteristics collected for each patient were age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, vital signs, number of prior therapy lines, time from initial diagnosis, subtype MM disease, pretreatment, MM state examined (yes/no), method of examination, concomitant medication, and concomitant disease. The CUP was conducted in accordance with the principles laid down by the 18th World Medical Assembly (Helsinki, 1964) and all applicable amendments laid down by the World Medical Assembly.
In total, 18 patients from 12 sites in Germany participated in the CUP. All patients were eligible for treatment with isatuximab as defined by the inclusion and exclusion criteria. Ten patients (55.6%) were women, and eight (44.4%) were men. The median age was 67 years (range: 47–78, Table 1). Most of the patients (10 patients; 55.6%) had an ECOG performance status of 1, followed by five patients (27.78%) and three patients (16.67%) with an ECOG performance status of 0 and 2, respectively. The median time from the initial diagnosis of MM to enrollment in the CUP was 7 years (range: 1–17). Table 1 summarizes the baseline characteristics of the CUP population.
All patients had received lenalidomide and a proteasome inhibitor (bortezomib, carfilzomib, or ixazomib) and had demonstrated progress on their last MM therapy. Prior to enrollment in the CUP, the median number of prior lines of therapy amounted to three (range: 2–11). Half of the patients (nine patients; 50.0%) were pretreated with pomalidomide, and eight patients (44.4%) were pretreated with anti-CD38. Of all patients, eight (44.4%) received concomitant medication, and nine (50.0%) were registered with concomitant disease at baseline (Table 1).
In total, 11 patients experienced 29 adverse events (AEs) (Table 2). One serious AE (SAE) occurred in one patient before the patient had received the first dose of isatuximab, and therefore, this event was not classified as treatment-emergent AE (TEAE) (Table 2). Of all 28 TEAEs, 19 (67.9%) were documented within the first cycle of therapy, of which 10 occurred during the first visit of the first cycle or maximum 4 days afterward. Nine TEAEs (32.1%) were documented during subsequent visits. Most of the patients had recovered from the TEAEs at the end of the program (8 of 11 patients; 72.7%), one patient (9.1%) did not recover (hip fracture grade 3, not related to therapy), and two patients died (18.2%) following disease progression, which was not related to therapy. TEAEs related to isatuximab, dexamethasone, and pomalidomide were documented in six patients (33.3%), one patient (5.6%), and two patients (11.1%), respectively. Ten of the 28 TEAEs (35.7% of all TEAEs) of any grade possibly related to isatuximab were monitored. Thereof, five grade 3 or higher TEAEs related to isatuximab were documented in four patients (22.2%): febrile neutropenia, urinary tract infection, decreased neutrophil count, decreased platelet count, and hypertension (Table 2). Of all related TEAEs, angina pectoris, infusion-related reactions, dyspnea, laryngeal obstruction, and hypertension were further classified into the category of adverse events of special interest (AESIs) according to the investigator’s decision. Serious TEAEs related to isatuximab, according to the investigator’s assessment, were a decreased platelet count (grade 4), a urinary tract infection (grade 3), and angina pectoris (grade 2; Table 2). The drug was withdrawn consequent to the last two SAEs; no action was taken in the case of the event ‘platelet count decreased’. All serious TEAEs related to isatuximab were recovered/resolved. In general, TEAEs leading to permanent premature discontinuation of isatuximab were reported for six patients (33.3%; Table 3; Figure 1). Those were febrile neutropenia (grade 3), angina pectoris (grade 2), urinary tract infection (grade 3), infusion-related reaction (grade 2), dyspnea (grade 1), laryngeal obstruction (grade 2), and hypertension (grade 3). TEAEs leading to death were not related to isatuximab treatment (plasma cell myeloma and disease progression). In summary, in these heavily pretreated RRMM patients, the CUP did not reveal any new safety concerns.
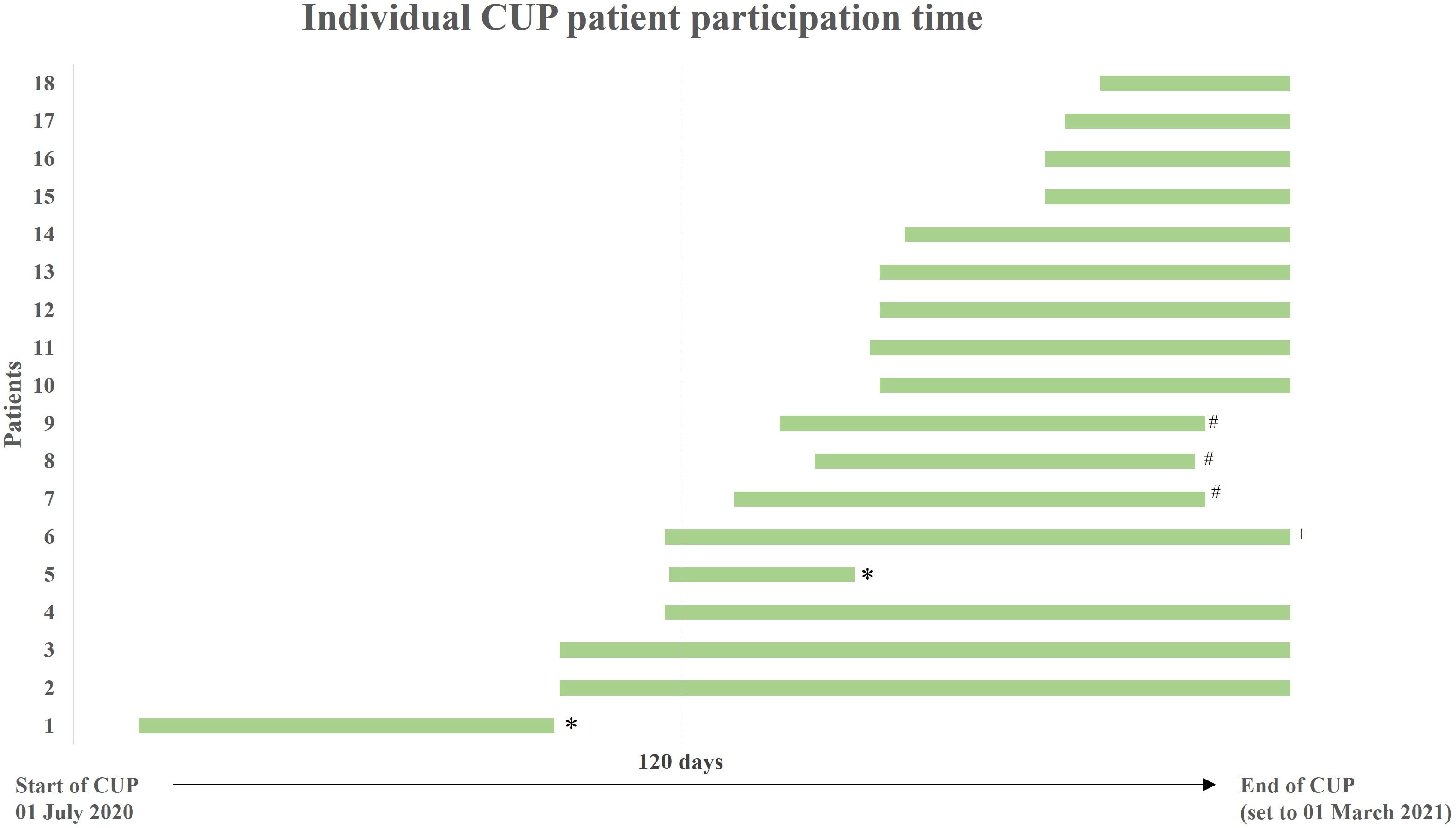
Figure 1 Individual patient participation time in the Compassionate Use Program (CUP). Green bars indicate the individual duration of CUP participation for each patient (isatuximab therapy start-end of therapy/CUP). The time point for all patients that were treated until the regular end of CUP was set to 01 March 2021. The length of bars does not precisely correlate with the number of administered cycles. *discontinuation due to death; #discontinuation due to progression; +discontinuation due to other reasons.
Patients who are refractory to approved therapy regimens have limited treatment options. Revised guidelines recommend the combination of novel agents with the existing standard of care regimen. In particular, the administration of triple and quadruplet therapy combinations creates complex but promising therapy scenarios (10). Patients enrolled in the CUP had limited alternative treatment options, and the CUP provided access to isatuximab, which was not finally approved at the time but had been shown to be effective and safe in relapsed and refractory MM. Isatuximab was recommended based on the results of a phase 1 study and the randomized, multinational phase III ICARIA-MM study, which evaluated the efficacy and safety of a triple therapy consisting of isatuximab with pomalidomide and dexamethasone in 307 adult patients with relapsed and refractory MM who had received at least two previous lines of therapy, including lenalidomide and PI (8, 9). The ICARIA-MM study was the only comparable phase 3 study at the time of CUP conduct. The inclusion criteria closely aligned with the present CUP. In contrast to the ICARIA-MM trial, the CUP included patients refractory to previous treatment with an anti-CD38 mAb and who had received prior therapy with pomalidomide. Therefore, our data reflect a heterogeneous and real-world study population encompassing variations in age, risk factors, previous diseases, and treatments. The treatment scheme of isatuximab, pomalidomide, and dexamethasone in the ICARIA-MM trial was equal to this CUP. While TEAEs were reported for 99.3% of ICARIA-MM patients, 61.1% of CUP patients experienced TEAEs. Serious adverse events were documented for 61.8% and 33.3% of ICARIA-MM and CUP patients, respectively. TEAEs leading to discontinuation were higher in the real world, as demonstrated by 33.3% of CUP patients vs. 7.2% of ICARIA-MM patients. This discrepancy might result from the great difference in patient numbers (152 vs. 18 patients) and observation periods (maximum exposure 76.7 vs. 16 weeks plus 30 days after the last dose) due to the termination of the CUP as well as from the high number of prior therapy lines received by CUP patients (8). In addition to the small number of patients and short follow-up, the lack of cytogenetic data and risk stratification limits the comparability of the study results. After the CUP ended, three real-world studies on isatuximab combination therapy were initiated: one retrospective study on Isa-Pd in RRMM patients across 24 UK cancer centers (11), a non-interventional study (NIS) of patients enrolled in the French early-access program (IMAGE) (12), and a German NIS on the safety and efficacy of Isa-Pd and Isa-Kd (IONA-MM) (13). In the study from the UK, for safety data, 107 patients were followed for a median of 3.7 months (IQR: 0.5–12.4 months). The median number of prior therapies was 3 (IQR: 2–5). Similar to the CUP, prior therapies included PIs (99.1%), IMiDs (100%), antiCD38 (4.7%), and others. The median number of Isa-Pd cycles administered was 4 (IQR: 2–8). The median of any AEs per patient in the total cohort was 2 (range: 1–9) for those who experienced them. Any-grade AEs were experienced by 87.9%, compared to 99.3% of ICARIA-MM patients and 61.1% in this CUP (see above) (11). Both IMAGE and IONA-MM include a substantial fraction of lenalidomide-refractory patients and report an expected safety profile (12, 13). The data enable a comparison of Isa-Pd patients (and Isa-Kd in IONA-MM) in the real world to the registration studies.
The above real-world evidence supports isatuximab as a viable additional treatment option and a potential alternative to the first approved CD38 monoclonal antibody, daratumumab. However, a direct comparison between isatuximab and daratumumab is not possible due to the lack of published head-to-head clinical trials and their use in different treatment regimens. A published matched, adjusted indirect comparison evaluated the safety and efficacy of Isa-Kd versus Dara-Rd in relapsed multiple myeloma and showed a significant improvement in progression-free survival but no significant overall survival benefit with Isa-Kd compared to Dara-Rd. In terms of safety, reduced rates of some, but not all, adverse events were documented in the Isa-Kd cohort (14). Isatuximab has been shown to be effective in newly diagnosed high-risk multiple myeloma. In the GMMG-CONCEPT trial, the combination of isatuximab with carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) showed high rates of sustained minimal residual disease (MRD) negativity with a tolerable safety profile in patients with high-risk disease. The data suggest a potential indication for isatuximab in these patients (15). Due to the lack of comparability between differently designed clinical trials, the choice of CD38 antibodies will continue to depend on the availability of approved combination therapies, considering individual patient factors such as previous treatment history, risk stratification, and potentially informative biomarkers such as copy number alterations of chromosome 1q (16, 17).
In conclusion, the data set of 18 RRMM patients enrolled in this CUP represents the first German data recorded outside of clinical trials analyzing the safety of isatuximab in previously treated MM patients in routine clinical practice. The CUP data confirm the results of the ICARIA-MM study in a real-world setting: There were no new safety concerns with isatuximab combination therapy, even in patients treated with multiple prior lines of therapy.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was obtained from participants or their legal guardians/next of kin, in accordance with national legislation and institutional requirements.
TL: Investigation, Writing – original draft, Writing – review & editing. CyK: Conceptualization, Data curation, Funding acquisition, Investigation, Validation, Writing – original draft, Writing – review & editing. KW: Investigation, Writing – original draft, Writing – review & editing. FO: Investigation, Writing – original draft, Writing – review & editing. ChK: Investigation, Writing – original draft, Writing – review & editing. RN: Investigation, Writing – original draft, Writing – review & editing. MK: Investigation, Writing – original draft, Writing – review & editing. HG: Investigation, Writing – original draft, Writing – review & editing. ME: Investigation, Resources, Writing – original draft, Writing – review & editing. ClK: Investigation, Resources, Writing – original draft, Writing – review & editing. HS: Conceptualization, Data curation, Funding acquisition, Investigation, Resources, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Study funding was provided by Sanofi, Germany.
Sanofi and the authors thank the patients and their families, the investigators, study coordinators, and support staff. Medical writing and/or editorial assistance was provided by Sandra Baumgart, PhD, an employee of Alcedis GmbH (Gießen, Germany). This assistance was funded by Sanofi, Germany.
TL: Advisory board: Janssen. HS: Honoraria: AbbVie, Amgen, BMS/Celgene, Chugai, Genzyme, GSK, Janssen, Oncopeptides, Pfizer, Roche, Sanofi, Sebia, TAD Pharma, Takeda. RN: Advisory board: Amgen, BMS/Celgene, Gilead, GlaxoSmithKline GSK, Janssen, Oncopeptides, Sanofi, Takeda. ChK: Advisory Board: Sanofi, Amgen, GSK, Janssen; Honoraria: AstraZeneca, Takeda. MK: Advisory board: Oncopeptides, Takeda, GSK. HG: Research funding: Amgen, BMS, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp and Dohme MSD, Sanofi, Mundipharma GmbH, Takeda, Novartis; Honoraria: Amgen, BMS, Celgene, Chugai, GlaxoSmithKline GSK, Janssen, Novartis, Sanofi; Consultancy: Adaptive Biotechnology, Amgen, BMS, Celgene, Janssen, Sanofi, Takeda; Advisory Board: Adaptive Biotechnology, Amgen, BMS, Celgene, Janssen, Sanofi, Takeda. ME, and ClK: Employees of Sanofi and may hold shares and/or stock options in the company. CyK.: Research funding: AstraZeneca, Sanofi; Consultancy: AstraZeneca, BMS/Celgene, Glaxo Smith Kline, Janssen, Pfizer, Sanofi, Takeda; Advisory Board: AstraZeneca, BMS/Celgene, Glaxo Smith Kline, Janssen, Pfizer, Sanofi, Takeda.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ravi P, Kumar SK, Cerhan JR, Maurer MJ, Dingli D, Ansell SM, et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic Malignancies. Blood Cancer J. (2018) 8:26. doi: 10.1038/s41408-018-0065-8
2. Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia. (2017) 31:2443–8. doi: 10.1038/leu.2017.138
3. Yong K, Delforge M, Driessen C, Fink L, Flinois A, Gonzalez-McQuire S, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. (2016) 175:252–64. doi: 10.1111/bjh.14213
4. Frampton JE. Isatuximab: A review of its use in multiple myeloma. Target Oncol. (2021) 16:675–86. doi: 10.1007/s11523-021-00827-0
5. van de Donk NWCJ, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. (2018) 9:2134. doi: 10.3389/fimmu.2018.02134
6. van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. (2018) 131:13–29. doi: 10.1182/blood-2017-06-740944
7. Moreau P, Dimopoulos MA, Yong K, Mikhael J, Risse ML, Asset G, et al. Isatuximab plus carfilzomib/dexamethasone versus carfilzomib/dexamethasone in patients with relapsed/refractory multiple myeloma: IKEMA Phase III study design. Future Oncol Lond Engl. (2020) 16:4347–58. doi: 10.2217/fon-2019-0431
8. Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet Lond Engl. (2019) 394:2096–107. doi: 10.1097/01.HS9.0000561576.58696.ae
9. Mikhael J, Richardson P, Usmani SZ, Raje N, Bensinger W, Karanes C, et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood. (2019) 134:123–33. doi: 10.1182/blood-2019-02-895193
10. Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J. (2020) 10:94. doi: 10.1038/s41408-020-00359-2
11. Djebbari F, Rampotas A, Vallance G, Panitsas F, Basker N, Sangha G, et al. Efficacy of isatuximab with pomalidomide and dexamethasone in relapsed myeloma: results of a UK-wide real-world dataset. HemaSphere. (2022) 6:e738. doi: 10.1097/HS9.0000000000000738
12. Decaux O, Lafore R, Iaquinta D, Tekle C, Leleu X. Isatuximab plus pomalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma in real-life context in France: IMAGE subgroup analysis based on prior lines of therapy and refractory status. Blood. (2022) 140:10969–70. doi: 10.1182/blood-2022-162602
13. Manasanch EE, Beksac M, Cavo M, Knauf W, Tsukada N, Tekle C, et al. MM-086 real-world experience with isatuximab (Isa) in patients with relapsed and/or refractory multiple myeloma: IONA-MM first interim analysis. Clin Lymphoma Myeloma Leuk. (2022) 22:S405–6. doi: 10.1016/S2152-2650(22)01590-7
14. Richter J, Lin PL, Garcia-Horton V, Guyot P, Singh E, Zhou ZY, et al. Matching-adjusted indirect comparison of isatuximab plus carfilzomib and dexamethasone with daratumumab plus lenalidomide and dexamethasone in relapsed multiple myeloma. Cancer Med. (2023) 12:8005–17. doi: 10.1002/cam4.5584
15. Leypoldt LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, et al. Isatuximab, carfilzomib, lenalidomide, and dexamethasone for the treatment of high-risk newly diagnosed multiple myeloma. J Clin Oncol. (2024) 42:26–37. doi: 10.1200/JCO.23.01696
16. D’Agostino M, Ruggeri M, Aquino S, Giuliani N, Arigoni M, Gentile M, et al. Impact of gain and amplification of 1q in newly diagnosed multiple myeloma patients receiving carfilzomib-based treatment in the forte trial. Blood. (2020) 136:38–40. doi: 10.1182/blood-2020-137060
Keywords: isatuximab, multiple myeloma, relapsed/refractory, real-world, compassionate use
Citation: Leitner T, Khandanpour C, Wendelin K, Oduncu F, Kimmich C, Naumann R, Kull M, Goldschmidt H, Ehmer M, Kiewitz C and Salwender H (2024) First clinical experience of isatuximab safety and tolerability in relapsed and refractory multiple myeloma: real-world data from a compassionate use program in Germany. Front. Hematol. 3:1335161. doi: 10.3389/frhem.2024.1335161
Received: 08 November 2023; Accepted: 30 April 2024;
Published: 13 June 2024.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Samo Zver, University Medical Centre Ljubljana, SloveniaCopyright © 2024 Leitner, Khandanpour, Wendelin, Oduncu, Kimmich, Naumann, Kull, Goldschmidt, Ehmer, Kiewitz and Salwender. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theo Leitner, dGhlby5sZWl0bmVyQHVrc2guZGU=; Cyrus Khandanpour, Y3lydXMua2hhbmRhbnBvdXJAdWtzaC5kZQ==; Hans Salwender, aC5zYWx3ZW5kZXJAYXNrbGVwaW9zLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.