
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol., 06 October 2022
Sec. Blood Cancer
Volume 1 - 2022 | https://doi.org/10.3389/frhem.2022.983424
This article is part of the Research TopicEditors' Showcase: Blood CancerView all 5 articles
Viral reactivation was previously reported after severe acute respiratory syndrome coronavirus‐2 (SARS-CoV-2) infection but was seldom documented after SARS-CoV-2 vaccination, except varicella-zoster virus and cytomegalovirus. Here, we present a case of reactive Epstein–Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis (HLH) and thrombosis with thrombocytopenia syndrome after receiving SARS-CoV-2 mRNA vaccination. Antiplatelet factor 4 antibody was detected, and the bone marrow study showed hemophagocytosis and was positive in the immunohistochemistry staining for EBV-encoded small nuclear RNAs and negative staining for CD3 and CD56 markers of small lymphocytes. The high percentage of CD38 high/HLA-DR+ cells among CD8+ T cells further confirmed HLH. After intravenous administration of immunoglobulin, the clinical symptoms, D-dimer level, fibrinogen, platelet count, EBV-DNA titer, and anti-PF4 level were all improved. Further investigation into the pathogenesis of vaccine-associated EBV reactivation, such as TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6), is warranted.
In the era of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), vaccination remains one of the essential prevention strategies to mitigate SARS‐CoV‐2 spread. Several kinds of vaccination are available worldwide, including nucleoside-modified messenger RNA (mRNA)-based vaccines, adenovirus-based DNA vector vaccines, and protein-based vaccines. Certain rare adverse events including thrombosis with thrombocytopenia syndrome (TTS), myocarditis, and Guillain–Barré syndrome were reported following SARS‐CoV‐2 vaccination (1, 2). Further, vaccine-associated viral reactivation was also reported. Varicella-zoster virus (VZV) reactivation was reported directly associated with the mRNA vaccine (3). Cytomegalovirus (CMV) reactivation was noted in a patient who received the adenovirus-based DNA vector vaccine ChAdOx1 nCoV-19 (4). Viral reactivation including VZV, CMV, and Epstein–Barr virus (EBV) was previously documented in patients with SARS‐CoV‐2 infection (5). Particularly, EBV reactivation in these patients correlates with higher mortality, and they suffer from long-lasting complications including fatigue, brain fog, and rashes (6). Nonetheless, whether EBV could be reactivated after SARS‐CoV‐2 vaccination remains unknown.
TTS is a rare but severe side effect of SARS‐CoV‐2 vaccination (1, 7). This complication is characterized by severe thrombosis with moderate-to-severe thrombocytopenia. The presence of antiplatelet factor 4 (PF4) antibodies is essential for both TTS diagnosis and intravenous immunoglobulin (IVIG) treatment.
Hemophagocytic lymphohistiocytosis (HLH) is a rare disease (8) (8). The pathogenesis of HLH mainly results from excessive immune activation, where activation of T cells, natural killer (NK) cells, and macrophages aberrantly induce tumor necrosis factor-α, interferon-γ, interleukin (IL)-1, IL-2, and IL-6. Consequently, a cytokine storm occurred, and immune homeostasis is disrupted. Limited cases regarding HLH triggered directly by influenza or SARS-CoV-2 vaccinations have been reported (9, 10). Since HLH is life threatening, immediate diagnosis and effective intervention are crucial. Herein, we present a case of reactive EBV-associated HLH and TTS following BNT162b2 mRNA SARS-CoV-2 vaccination and was successfully treated by IVIG treatment.
A 25-year-old Chinese man has a history of infectious mononucleosis with EBV at the age of 16 years based on the serology study. He had his second vaccination with the BNT162b2 mRNA COVID-19 vaccine on December 2, 2021. He remained well until 2 months later. He had a fever at 40°C with diffused headache and fatigue on February 17, 2022. The symptoms were aggravated at night, but there was no neurologic motor deficit of the head and limbs. He denied travel history and contact with wild animals and people showing similar symptoms. He also denied bowel habit change or urination abnormality. Physical examination did not reveal abnormality on the skin and joints. Laboratory data revealed elevated liver function (alanine aminotransferase, 323 U/L; reference, 10–50 U/L), thrombocytopenia (platelet, 75×109 cells/L; reference, 150–400 cells/L), elevated ferritin level (19,353 ng/mL; reference, 30–400 ng/mL), elevated D-dimer level (30.35 mg/L; reference, <0.55 mg/L), elevated lactate dehydrogenase (LDH) (283 U/L, reference value: ≦250 U/L) and low fibrinogen (63 mg/dL, reference value: 190–380 mg/dL). The abdominal echogram showed splenomegaly (75 × 54 mm), and computed tomography of the chest and abdomen revealed marked splenomegaly. He initially received a 2-week course of empirical antibiotics with ceftriaxone plus doxycycline. However, the fever fluctuated with episodic transient loss of consciousness. Ecchymosis and skin rash gradually developed all over the extremities. All cultures yielded no growth of pathogens.
We then conducted serological screening for viruses, atypical pathogens, and rheumatic illness (Supplementary Table 1). The EBV-DNA level was 25, 004 copies/mL (reference value: not detected). Otorhinolaryngologic examination and positron emission tomography of the whole body found neither active lesion over the field of ear, nose, and throat nor EBV-associated malignancies (lymphoma and nasopharyngeal carcinoma). There were positive IgG to the EBV early antigen and capsid antigen and negative IgM to the EBV capsid antigen, indicating chronic EBV reactivation. The anti-PF4 IgG test showed a positive result (optical density (OD), 0.6; reference, <0.4 OD), indicating probable TTS. The bone marrow study revealed the presence of hemophagocytosis (Figures 1A, B). Through immunohistochemistry (IHC) staining, EBV-encoded small nuclear RNAs were positive (Figure 1C) and small lymphocytes with no expression of CD3 and CD56 (Figures 1D, E). Modified flow cytometry for active T-cell phenotype was performed to distinguish the HLH or early sepsis (11). There were 44.2% CD8-positive T cells in the sample, and 19.2% of CD8+ T cells were composed of CD38 high/HLA-DR+ cells. For HLH diagnosis, the threshold was >7% CD38 high//HLA-DR+ among CD8+ T cells (Figure 1F). Therefore, the diagnosis of HLH was confirmed. We titrated the steroid dose and administered IVIG on March 4, 2022. The follow-up laboratory data revealed improvement in hepatic enzymes, platelet count, LDH, EBV-DNA titer, fibrinogen, D-dimer, and anti-PF4 antibody (Figure 2). The intravenous dose of dexamethasone was titrated every week and was shifted to oral administration before discharge. We arranged discharge on April 6, 2022. The written informed consent for publication was obtained from the patient. The institutional review board of the Chang Gung Memorial Hospital approved this study (IRB no. 202200809B0).
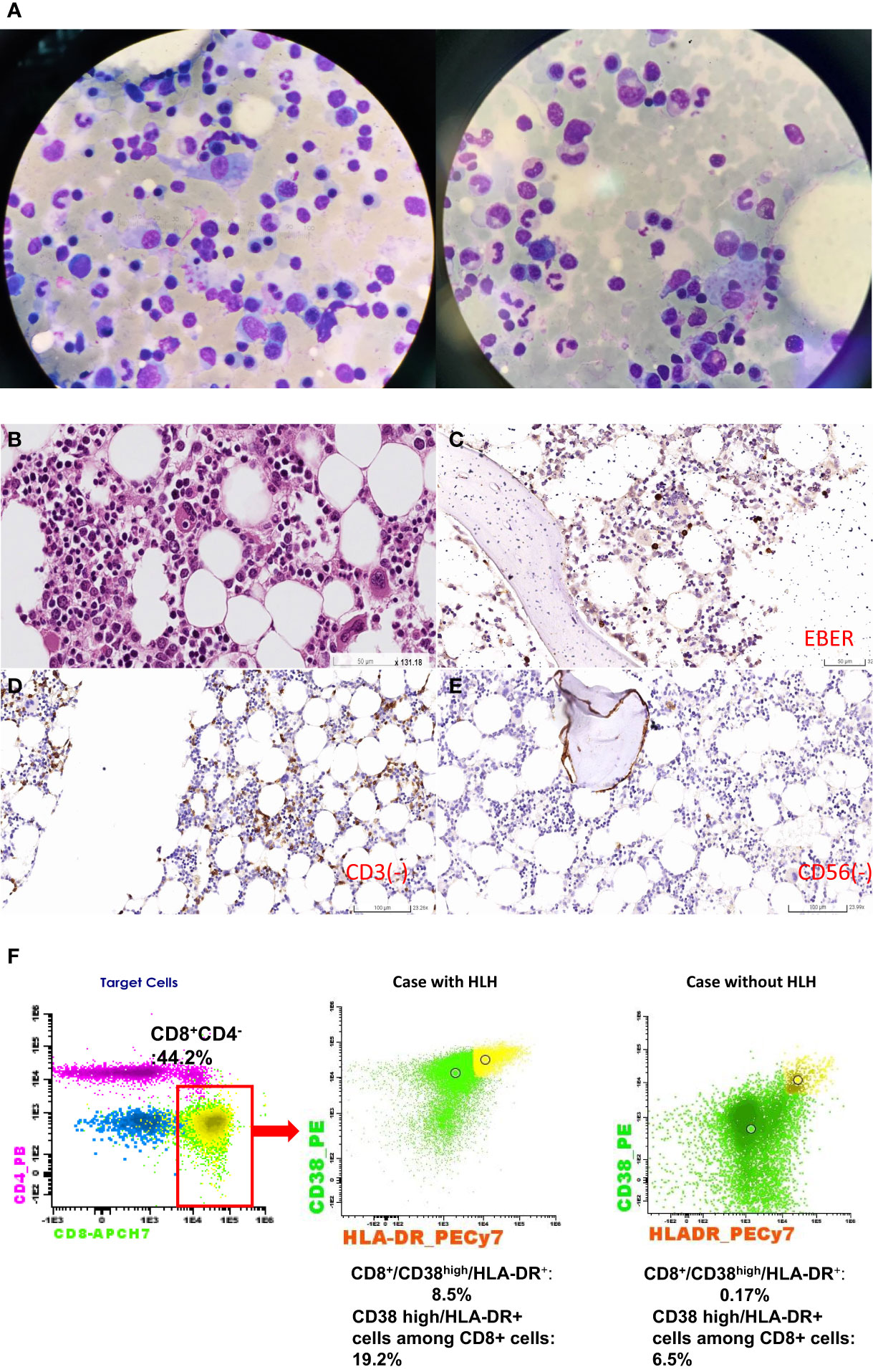
Figure 1 Bone marrow study. (A) Hemophagocytes were present. The red arrow indicated hemophagocytes. (B) The red arrow indicated foamy histocyte. Hemophagocyte was typically presented in hematoxylin and eosin staining (50× magnification). One histiocyte phagocytosed a nucleated red blood cell located in the middle of the figure (Red arrow). (C) Presence for EBV by IHC staining. The red arrow indicated that some lymphocytes were positive for EBV-encoded small RNA (EBER). (D) IHC staining for CD3-positive T cells. Unlike EBER-positive lymphocytes, CD3-positive cells (red arrow) were scarce with no aggregation, which excluded the possibility of T-cell lymphoma. (E) IHC staining for CD56-positive cells. There was no CD56-positive lymphocyte in the patient’s bone marrow. The purple part (B) was the bony structure of bone marrow. IHC, immunohistochemistry. (F) HLH was confirmed by flow cytometric analysis in peripheral blood T-cell phenotypes at diagnosis. In the left panel, the percentage of CD8+ T-cell was up to 44.2% in the red square, and 19.2% of CD8+ T-cells were composed of CD38 high/HLA-DR+ cells. We showed the difference in the percentage of CD38 high/HLA-DR+ cells in CD8+ T-cells between a case with HLH (the middle panel) and a case without HLH (the right panel) by using the same gating strategy. Only 0.17% CD8+/CD38 high/HLA-DR1+ cells is found in the patient without HLH but 8.5% CD8+/CD38 high/HLA-DR+ cells is found in our patient with HLH. The yellow dots represent the cells with CD8+/CD38 high/HLA-DR+ cells and the green dots represent the cell with CD8+/CD38 low or HLA-DR- cells.
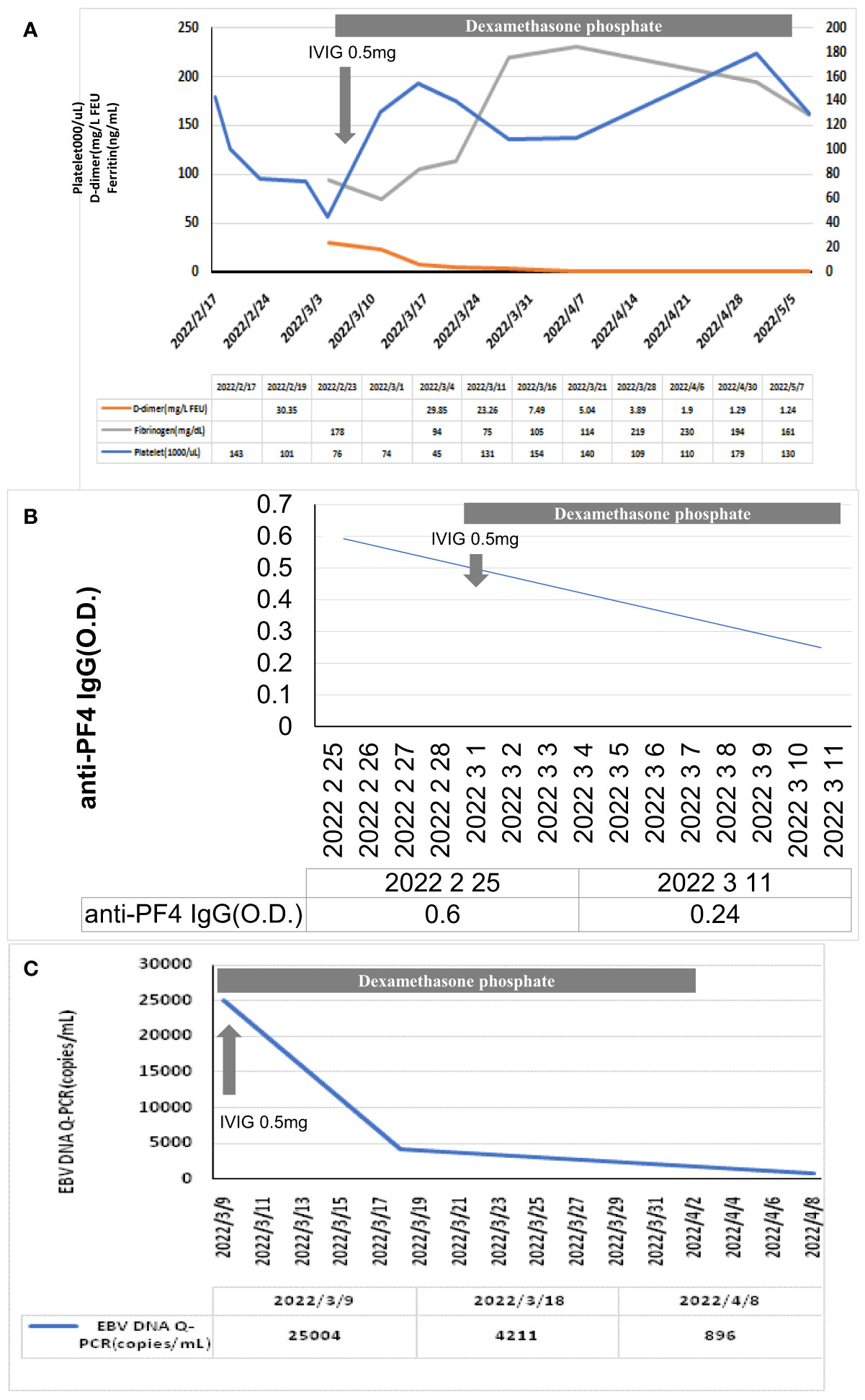
Figure 2 Clinical response of the levels of (A) D-dimer, platelet count, fibrinogen, (B) anti-PF4 antibody, and (C) EBV-DNA titers after steroid and IVIG use. IVIG, intravenous immunoglobulin. After IVIG treatment, the levels of D-dimer, platelet, and fibrinogen returned to the normal range gradually. Meanwhile, the EBV-DNA titer decreased after the anti-PF4 antibody decreased.
Certain viral reactivation following SARS‐CoV‐2 vaccination was previously reported including VZV and CMV (3, 4). We reported a rare case of reactivation of chronic EBV-associated HLH and TTS after the BNT162b2 mRNA SARS‐CoV‐2 vaccination. HLH is an aggressive syndrome of excessive immune activation that causes tissue destruction and multiple organ failure. The pathogenesis of HLH is associated with T cell activation with the excessive activation of immune cells and uncontrolled inflammation reaction (12). Infection is the common etiology (13, 14). Other possible causes for HLH development include malignancy and autoimmune disease (15). The EBV-associated HLH feature of the current case was shown at bone marrow biopsy using IHC staining and was further confirmed by the specific T-cell phenotype using flow cytometry (>7% CD38 high/HLA-DR+ among CD8+ T cells) in Supplementary Figure 1. Patient’s clinical presentations including high fever, cytopenia, and splenomegaly were also compatible with the clinical course of HLH. Moreover, the presence of anti-PF4 IgG and unusual coagulopathy (elevated D-dimer level and low fibrinogen level) were usually observed in patients with TTS presentation after SARS‐CoV‐2 vaccination (16). Immediate administration of high-dose steroid and IVIG alleviated the patient’s clinical symptoms and gradually improved the levels of D-dimer, fibrinogen, EBV-DNA titer, and anti-PF4 IgG and platelet count. To our knowledge, this is the first case clearly presenting reactivated EBV-associated HLH and TTS following SARS-CoV-2 mRNA vaccination.
The diagnosis of HLH needs to exclude multiple organ failure, malignancy, and sepsis. Timely and accurate diagnosis is critical for immediate curative treatment. The finding of hemophagocytosis in bone marrow is neither sensitive nor specific for HLH. There are two essential diagnostic criteria (Table 1). According to the HLH-2004 diagnostic guidelines, the clinical features should meet five of eight items for HLH diagnosis (17). A total score of HScore over 169 indicates at least 93% sensitivity and 86% specificity for adult HLH (18). In the present case with EBV reactivation following vaccination, the patient had cultures negative for pathogen growth, no presence of malignancies and no organ failure. His clinical presentation met five of the eight items of the HLH-2004 criteria and the total HScore was 206, indicating the presence of HLH. Recently, the distribution of unique T-cell subtype investigated by flow cytometry helps clinicians differentiate HLH from early sepsis and healthy individuals (11). Patients with >7% of CD38 high/HLA-DR+ cells among CD8+ T cells probably develop HLH (11). In our patient, 19.2% of CD8+ T cells were composed of CD38 high/HLA-DR+ cells in his T-cell distribution. Taken together, HLH is definitely present in this patient.
We compared our case with other previously reported vaccine-associated HLH cases following SARS-CoV-2 vaccination (Table 2). The salient clinical feature in the present case is the simultaneous presence of HLH and anti-PF4 antibody that was not reported in previous vaccine-associated HLH cases following SARS‐CoV‐2 vaccination (16). The presence of pathologic anti-PF4 antibody probably induced by the adenovirus vector or spike protein of SARS-CoV-2 leads to hyperinflammation syndrome mediated by macrophage-secreted IL-1β (19) (18). Currently, the presence of anti-PF4 antibody is considered one of the major criteria for vaccine-associated TTS (7). The positive finding of anti-PF4 IgG in our case also offers an indication for IVIG treatment that could neutralize this pathological antibody (16, 20).
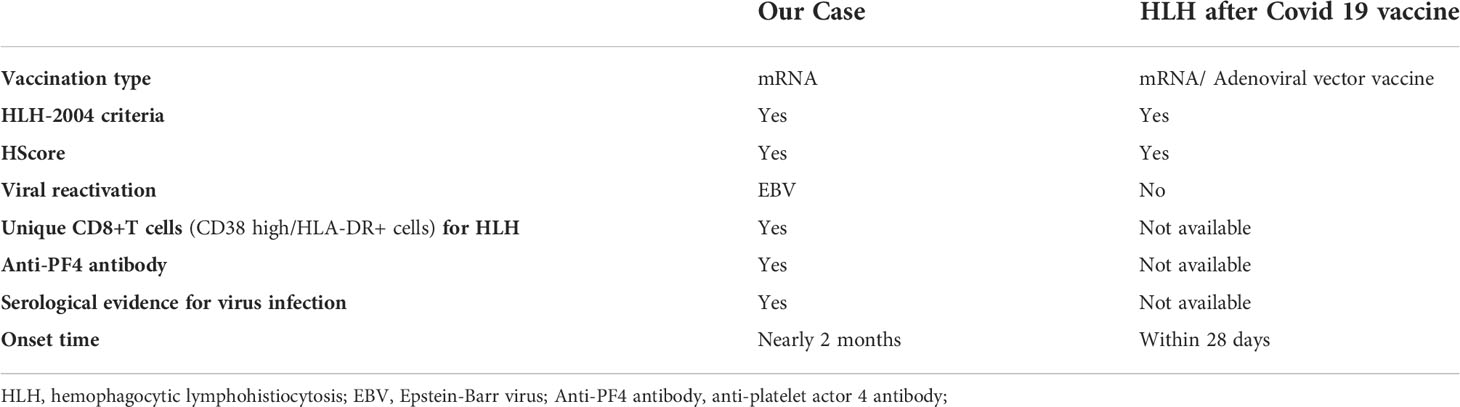
Table 2 The differences between our case with EBV-associated HLH and TTS after SARS‐CoV‐2 mRNA vaccination and other cases after Covid-19 vaccinations.
The goal of HLH management is to suppress a life-threatening hyperinflammation response. Several therapeutic strategies including chemotherapy, interferon-γ, alemtuzumab (anti-CD52), anakinra (anti-IL-1), anti-IL-6, and ruxolitinib (JAK2 inhibitor) are clinically applied to modulate immune hemostasis (21–24). IVIG treatment for HLH is only recommended for patients with less disease severity (14). The possible mechanism of IVIG for anti-inflammation ability is through suppressing the complement activation, neutralizing cytokines, and blocking macrophage Fc receptors (14, 25). In our case, IVIG was applied to neutralize the pathologic anti-PF4 antibody, not to target the EBV-infected lymphocytes. EBV-associated HLH is commonly seen in Asia, and early chemotherapy with the HLH-94 protocol improves the outcome (14, 26, 27). However, chemotherapy is seldom utilized for patients with vaccine-associated HLH. Targeting the underlying causes that induce HLH such as infection, vaccination, or rheumatic illness and using a less toxic therapeutic regimen could be an alternative for clinically stable patients with HLH (13). In our case, high-dose steroids and lower-dose IVIG (0.5 mg/kg) showed good clinical benefit in all clinical parameters including symptoms, laboratory data, and anti-PF4 antibody. Hence, early intervention using steroid and IVIG to patients with HLH could improve the outcomes.
The lack of data pertaining to essential inflammatory cytokine levels and NK cell activity are limitations to this case report. For example, the plasma levels of inflammatory cytokines, including TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6) can help to identify the inflammatory phenotype. Further, the investigation regarding the correlation between SARS-CoV-2 vaccination and EBV reactivation is needed as the vaccination program remains one of the globally recognized policies for SARS-CoV-2 prevention. Although steroids and IVIG are effective in the current case, close surveillance for clinical symptoms and laboratory data are required since the triggers to HLH could be EBV reactivation, anti-PF4 antibody, or both. Aggressive treatment including chemotherapy, anakinra, or ruxolitinib should be initiated immediately if the clinical presentation turns worse.
In conclusion, EBV-reactivated HLH and TTS could simultaneously occur following SARS-CoV2 vaccination. EBV has a malignant transformation potential and is associated with malignancies (28). The incidence of SARS-CoV2 vaccination-associated EBV reactivation should be closely monitored in the era of COVID-19.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the institutional review board of the Chang Gung Memorial Hospital (IRB no. 202200809B0). The written informed consent for publication was obtained from the patient.
Y-SC and J-JY treated the patient. Y-SC also wrote the manuscript. T-CC provide us the pathology result of bone marrow. Y-HW interpreted the result of the flow cytometry. C-YH and K-YY organized the group and revised the article. All authors read and approved the final manuscript.
This work was supported by the Ministry of Science and Technology, Taiwan (MOST111-2321-B-A49-007-) to C-YH.
We thank the patient and his family for letting us present his case in this report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2022.983424/full#supplementary-material
1. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis (2021) 21(7):939–49. doi: 10.1016/S1473-3099(21)00224-3
2. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following pfizer and moderna SARS-CoV-2 vaccination. Am J Hematol (2021) 96(5):534–7. doi: 10.1002/ajh.26132
3. Maldonado MD, Romero-Aibar J. The pfizer-BNT162b2 mRNA-based vaccine against SARS-CoV-2 may be responsible for awakening the latency of herpes varicella-zoster virus. Brain Behav Immun Health (2021) 18:100381. doi: 10.1016/j.bbih.2021.100381
4. Takahashi M, Makino S, Iizuka H, Noguchi M, Yoshida K. Chronic active Epstein-Barr virus-associated secondary hemophagocytic lymphohistiocytosis in pregnancy: a case report. BMC Pregnancy Childbirth (2021) 21(1):681. doi: 10.1186/s12884-021-04150-4
5. Simonnet A, Engelmann I, Moreau AS, Garcia B, Six S, El Kalioubie A, et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis No (2021) 51(3):296–9. doi: 10.1016/j.idnow.2021.01.005
6. Paolucci S, Cassaniti I, Novazzi F, Fiorina L, Piralla A, Comolli G, et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int J Infect Dis (2021) 104:315–9. doi: 10.1016/j.ijid.2020.12.051
7. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med (2021) 384(22):2092–101. doi: 10.1056/NEJMoa2104840
8. Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant (2010) 16(1 Suppl):S82–9. doi: 10.1016/j.bbmt.2009.11.014
9. Soliman S, Bakulina A. Hemophagocytic lymphohistiocytosis after inactivated influenza vaccination in a young man complicated by severe rhabdomyolysis. Cureus (2022) 14(3):e23334. doi: 10.7759/cureus.23334
10. Hieber ML, Sprute R, Eichenauer DA, Hallek M, Jachimowicz RD. Hemophagocytic lymphohistiocytosis after SARS-CoV-2 vaccination. Infection (2022) 26:1–6. doi: 10.1007/s15010-022-01786-y
11. Chaturvedi V, Marsh RA, Zoref-Lorenz A, Owsley E, Chaturvedi V, Nguyen TC, et al. T-Cell activation profiles distinguish hemophagocytic lymphohistiocytosis and early sepsis. Blood (2021) 137(17):2337–46. doi: 10.1182/blood.2020009499
12. Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood (2015) 125(19):2908–14. doi: 10.1182/blood-2015-01-551622
13. George MR. Hemophagocytic lymphohistiocytosis: review of etiologies and management. J Blood Med (2014) 5:69–86. doi: 10.2147/JBM.S46255
14. La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood (2019) 133(23):2465–77. doi: 10.1182/blood.2018894618
15. Lim SH, Park S, Jang JH, Kim K, Kim HJ, Kim SH, et al. Clinical significance of bone marrow hemophagocytosis in adult patients with malignancy and non-malignancy-induced hemophagocytic lymphohistiocytosis. Ann Hematol (2016) 95(2):325–35. doi: 10.1007/s00277-015-2523-8
16. Iba T, Levy JH. Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination. Trends Cardiovasc Med (2022) 32(5):249–56. doi: 10.1016/j.tcm.2022.02.008
17. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2007) 48(2):124–31. doi: 10.1002/pbc.21039
18. Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol (2014) 66(9):2613–20. doi: 10.1002/art.38690
19. Attwell L, Zaw T, McCormick J, Marks J, McCarthy H. Haemophagocytic lymphohistiocytosis after ChAdOx1 nCoV-19 vaccination. J Clin pathol (2022) 75(4):282–4. doi: 10.1136/jclinpath-2021-207760
20. Fiore MM, Kakkar VV. Platelet factor 4 neutralizes heparan sulfate-enhanced antithrombin inactivation of factor xa by preventing interaction(s) of enzyme with polysaccharide. Biochem Biophys Res Commun (2003) 311(1):71–6. doi: 10.1016/j.bbrc.2003.09.171
21. Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood (2011) 118(17):4577–84. doi: 10.1182/blood-2011-06-356261
22. Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol (2021) 12:614704. doi: 10.3389/fimmu.2021.614704
23. Marsh RA, Allen CE, McClain KL, Weinstein JL, Kanter J, Skiles J, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab. Pediatr Blood Cancer (2013) 60(1):101–9. doi: 10.1002/pbc.24188
24. Bami S, Vagrecha A, Soberman D, Badawi M, Cannone D, Lipton JM, et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer (2020) 67(11):e28581. doi: 10.1002/pbc.28581
25. Larroche C, Bruneel F, André MH, Bader-Meunier B, Baruchel A, Tribout B, et al. [Intravenously administered gamma-globulins in reactive hemaphagocytic syndrome Multicenter study to assess their importance, by the immunoglobulins group of experts of CEDIT of the AP-HP]. Ann Med Interne (Paris) (2000) 151(7):533–9.
26. Chellapandian D, Das R, Zelley K, Wiener SJ, Zhao H, Teachey DT, et al. Treatment of Epstein Barr virus-induced haemophagocytic lymphohistiocytosis with rituximab-containing chemo-immunotherapeutic regimens. Br J Haematol (2013) 162(3):376–82. doi: 10.1111/bjh.12386
27. Imashuku S, Kuriyama K, Teramura T, Ishii E, Kinugawa N, Kato M, et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol (2001) 19(10):2665–73. doi: 10.1111/bjh.15988
Keywords: hemophagocytic lymphohistiocytosis, Epstein Barr virus, thrombosis with thrombocytopenia syndrome, SARS-CoV-2 vaccination, intravenous immunoglobulin (IVIG)
Citation: Chang Y-S, Ye J-J, Cheng T-C, Wen Y-H, Huang C-YF and Yeh K-Y (2022) Case report: Reactive Epstein–Barr virus-associated hemophagocytic lymphohistiocytosis and thrombosis with thrombocytopenia syndrome following SARS-CoV-2 vaccination and treated with intravenous immunoglobulin. Front. Hematol. 1:983424. doi: 10.3389/frhem.2022.983424
Received: 01 July 2022; Accepted: 31 August 2022;
Published: 06 October 2022.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Prasenjit Guchhait, Regional Centre for Biotechnology (RCB), IndiaCopyright © 2022 Chang, Ye, Cheng, Wen, Huang and Yeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun-Yun Yeh, eWVodHluZ0BnbWFpbC5jb20=; Chi-Ying F. Huang, Y3lodWFuZzVAbnljdS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.