- Cohen Center for Recovery from Complex Chronic Illnesses, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Introduction: Long COVID involves debilitating symptoms, many of which mirror those observed with dysautonomia, and care must be taken with rehabilitation for autonomic dysfunction to avoid post-exertional malaise/post-exertional symptom exacerbation. Resonant breathing (breathing slowly at a defined rate of breaths per minute) requires less exertion and can potentially improve autonomic function. The objective of this work was to report on the impact of a resonant breathing program on self-reported symptoms and wellbeing in people with Long COVID.
Methods: A retrospective analysis of de-identified data was completed in a convenience sample of people with Long COVID, who participated in the Meo Health (formerly known as Stasis HP) resonant breathing program. Participants completed baseline and follow up surveys.
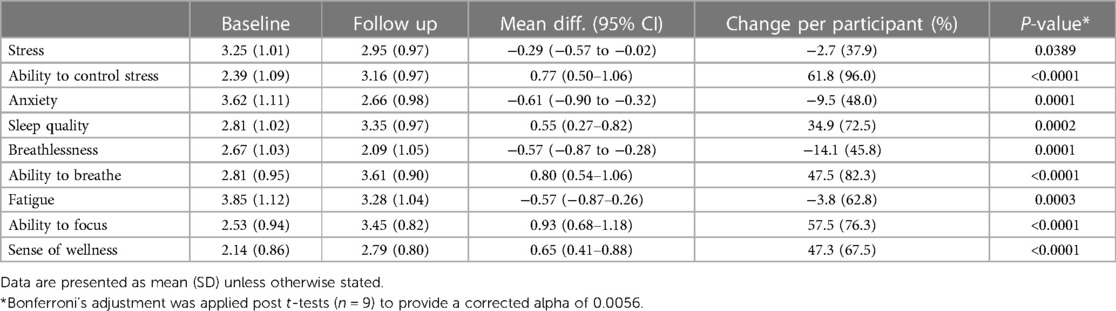
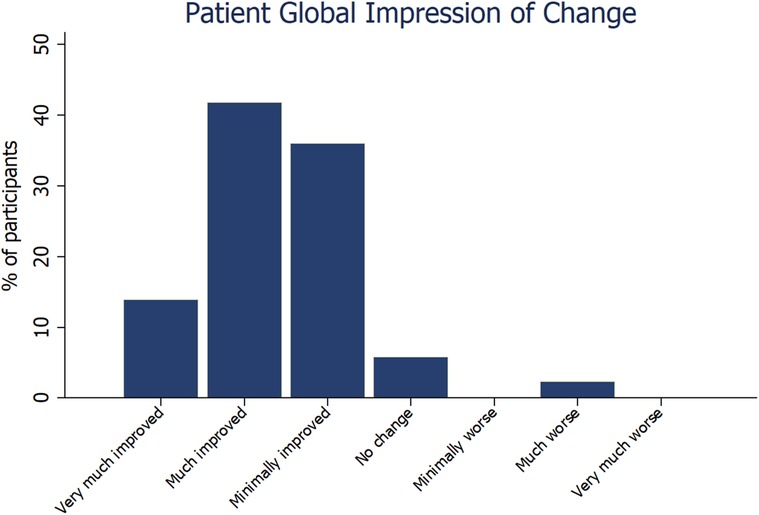
Results: Data were available for 99 participants. Most measures of symptoms and wellbeing improved at follow up, with the largest differences per participant seen in sense of wellness (47.3%, p < 0.0001), ability to focus (57.5%, p < 0.0001), ability to breathe (47.5%, p < 0.0001), ability to control stress (61.8%, p < 0.0001) and sleep quality (34.9%, p = 0.0002). Most (92%) participants reported improvement at follow up on the Patient Global Impression of Change Scale.
Conclusion: Self-reported symptoms and wellbeing improved in people with Long COVID completing resonant breathing. Resonant breathing can be considered as an option within the broader treatment plan of people with Long COVID.
Introduction
Post-acute sequelae of SARS-CoV-2 infection (PASC), also known as Long COVID, is a post-acute infection syndrome that involves debilitating symptoms and affects at least 6% (almost 20 million) of the adult US population (1). These persistent symptoms include post-exertional malaise, fatigue, weakness, pain, shortness of breath, cognitive dysfunction, sleep disturbances, fevers, and gastrointestinal issues, and affect every organ system (2–4). Further, many symptoms of Long COVID mirror those observed with dysautonomia, including orthostatic intolerance, palpitations, tachycardia, syncope, labile blood pressure, dizziness, and exercise intolerance (5).
Orthostatic intolerance and postural orthostatic tachycardia syndrome (POTS) can commonly follow acute viral infections, and are similarly observed in up to 80% of people with Long COVID (5–8). Prior to the COVID-19 pandemic, approximately 50% of individuals with POTS reported a history of viral infection prior to symptom onset, suggesting this as a potential trigger (9). The presence of autonomic dysfunction in Long COVID is possibly due to mechanisms such as viral- or immune-mediated disruption of the autonomic nervous system (10), brainstem signaling abnormalities (11), and/or small fiber neuropathy (12). In addition, sympathetic mediated hypocapnia has been reported in people with autonomic dysfunction (13), and in Long COVID in the absence of hyperventilation (14), with an overlap observed in the symptoms of hypocapnia and Long COVID.
While autonomic rehabilitation can be of benefit to some people with Long COVID, care must be taken to avoid post-exertional malaise/post-exertional symptom exacerbation (PEM/PESE) (6). Encouraging people with Long COVID to engage in physical activity that exceeds their exertional tolerance has been a commonly identified cause of worsening symptoms in self-report surveys (2, 3). Furthermore, there is evidence to suggest that overexertion of people with Long COVID who have PEM/PESE can lead to local and global physiological damage (15). As such, strategies that may regulate autonomic nervous system activity in Long COVID with minimal exertion should be investigated for feasibility and tolerability. Resonant breathing (breathing slowly at a defined rate of breaths per minute) requires less exertion than current approaches for autonomic rehabilitation (most commonly graded exercise therapy (16), and have previously been demonstrated to alleviate hypocapnia and balance sympathetic and parasympathetic activation (17). The physiological benefit of resonant breathing is underpinned by increased responsiveness of the baroreflex (18, 19), leading to improved homeostatic control of blood pressure and heart rate, alongside increased parasympathetic activation (17). Heart rate variability (HRV), an indirect measure of autonomic regulation, is decreased (i.e., worse) in people with Long COVID and is associated with increased self-reported fatigue (20). Resonant breathing could theoretically serve to counterbalance this abnormally increased sympathetic nervous system activity and autonomic dysfunction occurring in Long COVID (21).
Consensus guidelines for the treatment of autonomic dysfunction in Long COVID encourage clinicians to identify and address the most disabling symptoms (6, 22). Given the potential ability of resonant breathing exercises to improve the function of the autonomic nervous system, including this as part of the broader approach to autonomic rehabilitation has merit. The primary objective of this work was to report on the impact of an online resonant breathing program on self-reported symptoms and wellbeing in people with Long COVID.
Methods
Study design
This was a retrospective analysis of de-identified data from a convenience sample of people with Long COVID, who participated in the Meo Health (formerly known as Stasis HP) resonant breathing program between September 2020 and November 2021. This study was determined to be exempt from the need for ethics review by the Mount Sinai Program for the Protection of Human Subjects due to the de-identified nature of the data (STUDY-24-00246).
Participants
People aged 18 years or older with Long COVID were either referred to the Meo Health program via their care provider, or self-referred to the program. Participants were included in the analysis if they completed at least 4 weeks of the Meo Health program and completed both baseline and follow up surveys. All participants had confirmed (by polymerase chain reaction (PCR) and/or antibody test) or probable (diagnosed by a medical doctor in accordance with World Health Organization recommendations (23) previous COVID-19 infection and diagnosis of Long COVID (defined as experiencing symptoms >12 weeks since initial symptom onset). There were no specific exclusion criteria.
Resonant breathing protocol
Participants underwent an initial 4-week progressive resonant breathing program using a 4:6 (inspiratory:expiratory) second cadence. Participants were instructed via recorded video content, and advised to complete the resonant breathing exercises twice per day for five days per week (upon waking and in the evening prior to sleep). Instructions for nasal unblocking were also provided. The time of the sessions were initially set at 10 min in length, increasing up to 30 min by the end of week four as tolerated. Participants were advised to avoid caffeine or other stimulants prior to the sessions. All participants had the option to request an online supervised session to check technique, but this was not mandatory. At onboarding, participants were encouraged to continue the program beyond the initial 4 weeks (for 12 weeks and beyond), as a mainstay part of their broader Long COVID treatment plan. No other treatment was provided as part of the Meo Health program.
Outcome survey
A survey designed for the program by Meo Health was administered prior to commencement and at a follow up date according to the length of time the participant used the program. The survey collected basic demographic data (gender and age), and self-reported symptoms and wellbeing on a Likert scale (1–5, 1 = Very low, 5 = Very high), including stress, ability to control stress, anxiety, sleep quality, breathlessness, ability to breathe, fatigue, ability to focus, and sense of wellness. The first survey also included self-reported results from the max exhale test (MET) (amount of time it takes the participant to exhale as slowly as possible after full nasal inspiration) and breath hold test (BHT) (time able to hold breath after normal inhalation), while the follow up survey included the Patient Global Impression of Change (PGIC), and how many times per week and minutes per session the resonant breathing was performed.
Statistical analysis
Statistical analyses were undertaken with Stata (StataCorp, Stata Statistical Software Release: V.14). Data were analyzed using descriptive statistics, t-tests pairwise correlation and linear regression. Categorical data are presented as frequencies and proportions. Continuous data are presented as mean and standard deviation (SD), median and interquartile range (IQR) or median and range. Bonferroni's adjustment was applied post t-tests (n = 9) to provide a corrected alpha of 0.0056. Level of significance was set to 0.05 for all other tests.
Results
Data were available for 99 participants [78 (78%) female, aged median (IQR) 49 (41–57) years] who were enrolled in the program for at least 4 weeks and completed both baseline and follow up surveys. The median time at follow up was 95 (29–378) days. Baseline MET was 28 (16–47) s, and BHT was 41 (28–50) s. Participants reported completing the resonant breathing program 7 (5–8) times per week, for 8 (8–13) min per session.
Nearly all (89%) measures of symptoms and wellbeing improved at follow up, with the largest mean (SD) percentage improvement per participant observed in ability to control stress [61.8 (96.0)%, p < 0.0001], ability to focus [57.5 (76.3)%, p < 0.0001], ability to breathe [47.5 (82.3)%, p < 0.0001], sense of wellness [47.3 (67.5)%, p < 0.0001], and sleep quality [34.9 (72.5)%, p = 0.0002] (Table 1). Most (92%) participants reported some level of improvement at follow up on the Patient Global Impression of Change Scale (Figure 1). The median time at follow up was not a predictor of changes in any outcomes. Improvement in ability to control stress was positively correlated with the number of times the Meo Health program was used each week (r = 0.38; p < 0.001). Improvement in ability to control stress was also associated with reduced self-reported anxiety (r = −0.29; p = 0.003) and improved sleep (r = 0.30; p = 0.003).
Discussion
The emergence of Long COVID has presented significant challenges to healthcare and the scientific community, with no approved treatments currently available (pharmacological or non-pharmacological). These data provide insight into the potential role of resonant breathing in the alleviation of some of the symptoms of Long COVID, alongside an ability to improve self-reported measures of wellbeing in a small cohort.
The positive outcomes observed in people with Long COVID performing at least 4 weeks of resonant breathing support the hypothesis of improved autonomic function as the possible mechanism, aligning with previous findings highlighting the potential role of breathwork as a therapy to address autonomic dysfunction in Long COVID (24) and other conditions (25–27). Controlled breathing may improve autonomic function via improving the baroreflex (18, 19), enhancing parasympathetic activity and mitigating stress responses (28). Symptoms of autonomic dysfunction feature heavily in many presentations of Long COVID (2, 3, 10), therefore the reported data are encouraging. Resonant breathing is a relatively simple technique that can be easily integrated into the treatment programs of people with Long COVID.
In addition, using HRV biofeedback may further optimize the benefits of resonant breathing (29), warranting further investigation. HRV biofeedback can be integrated into smartphone applications and is therefore accessible for use by people in the home setting. The feasibility of using HRV biofeedback has been demonstrated in a small number (13) of people with Long COVID, with observed improvements in measures of symptoms and wellbeing (21). Home-based rehabilitation programs are feasible in people with Long COVID (30, 31), and resonant breathing could be implemented remotely to reduce the burden on the patient. The broader rehabilitation program for people with Long COVID should address all potential sources of symptomatology, including autonomic (6), cardiovascular (32), pulmonary (33), neurological (34), cognitive (35) and musculoskeletal (36). As resonant breathing may be useful to assist with symptom stabilization, potential synergistic effects with other treatment approaches should be investigated.
The improvements in outcomes including stress, focus, breathing, wellness and sleep mirror what has been reported in other groups performing resonant breathing, with evidence suggesting that this technique has a range of positive effects on stress reduction, mood regulation, awareness, and interoceptive sensitivity across various populations (37–40). In addition to benefits in autonomic function, resonant breathing may help attenuate some of the feelings of anxiety associated with living with a severe complex chronic illness of proven immunological origin (4). The association between frequency of weekly use of the Meo Health program and improvements in ability to control stress, with a subsequent association with improvements in self-reported anxiety, supports the need for consistency in performing a resonant breathing program to achieve the desired results within this domain.
The majority of participants in this cohort were female (78%) which aligns with other studies reporting a bias towards Long COVID affecting women disproportionately (1–3). The age of participants in the present study was also similar to these cohorts. Long COVID affects more females possibly due to differences in hormones (41) as well as the ability of women to mount a faster and larger immune response (42), which may result in the longer-term symptoms experienced.
There are several limitations to be considered with this report. The data were analyzed retrospectively from a small cohort of people with Long COVID, with no prospective study design or control group. Information regarding how long each participant had been experiencing symptoms of Long COVID was not obtained, making it difficult to determine any level of benefit relating to how soon resonant breathing was commenced after initial infection. Further, while it is presumed that the participants were completing the program consistently up until the time at follow up, the data are self-reported and there may be discrepancies between responses and actual rates of adherence to the program. Other therapies being used were not considered and therefore could not be controlled for in the analysis, and there is a possibility that some participants experienced improvements in their outcomes spontaneously over time; however, recovery from Long COVID can be relatively uncommon during this timeframe, with 85% still reporting symptoms at one year (43). Obtaining objective measures of autonomic function was also beyond the scope of this study, and therefore any suspected improvements could not be quantified and reported.
Conclusion
This study provides some insight into the potential role and benefit of resonant breathing, and its feasibility as a simple and easy to use non-pharmacological treatment option for Long COVID. Long COVID impacts multiple body systems and requires a multi-targeted treatment approach (44), and a low-exertion therapy for improving self-reported symptoms and wellbeing has the potential to be of benefit for many people with Long COVID. These results now need to be reproduced in larger prospective studies with adequate controls, appropriate testing of autonomic function, and with sufficient power to explore the relationships between age, sex and other variables on outcomes.
Data availability statement
The datasets presented in this article are not readily available because data will only be made available via reasonable request to the corresponding author. Requests to access the datasets should be directed to David Putrino,ZGF2aWQucHV0cmlub0Btb3VudHNpbmFpLm9yZw==.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JP: Writing – original draft, Writing – review & editing. JT-M: Methodology, Writing – original draft, Writing – review & editing. LT: Data curation, Methodology, Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. DP: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
JW previously held a non-financial advisory role with Stasis HP.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adjaye-Gbewonyo D, Vahratian A, Perrine CG, Bertolli J. Long COVID in Adults: United States, 2022. NCHS Data Brief, no 480. Hyattsville, MD: National Center for Health Statistics (2023). doi: 10.15620/cdc:132417
2. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
3. Tabacof L, Tosto-Mancuso J, Wood J, Cortes M, Kontorovich A, McCarthy D, et al. Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation. Am J Phys Med Rehabil. (2022) 101(1):48–52. doi: 10.1097/PHM.0000000000001910
4. Klein J, Wood J, Jaycox JR, Dhodapkar RM, Lu P, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. (2023) 623(7985):139–48. doi: 10.1038/s41586-023-06651-y
5. Larsen NW, Stiles LE, Miglis MG. Preparing for the long-haul: autonomic complications of COVID-19. Auton Neurosci. (2021) 235:102841. doi: 10.1016/j.autneu.2021.102841
6. Blitshteyn S, Whiteson JH, Abramoff B, Azola A, Bartels MN, Bhavaraju-Sanka R, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2022) 14(10):1270–91. doi: 10.1002/pmrj.12894
7. Eldokla AM, Mohamed-Hussein AA, Fouad AM, Abdelnaser MG, Ali ST, Makhlouf NA, et al. Prevalence and patterns of symptoms of dysautonomia in patients with long-COVID syndrome: a cross-sectional study. Ann Clin Transl Neurol. (2022) 9(6):778–85. doi: 10.1002/acn3.51557
8. Haloot J, Bhavaraju-Sanka R, Pillarisetti J, Verduzco-Gutierrez M. Autonomic dysfunction related to postacute SARS-CoV-2 syndrome. Phys Med Rehabil Clin N Am. (2023) 34(3):563–72. doi: 10.1016/j.pmr.2023.04.003
9. Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. (1999) 74(11):1106–10. doi: 10.4065/74.11.1106
10. Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in “long COVID”: rationale, physiology and management strategies. Clin Med (Lond). (2021) 21(1):e63–7. doi: 10.7861/clinmed.2020-0896
11. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. doi: 10.3389/fmicb.2021.698169
12. Novak P, Mukerji SS, Alabsi HS, Systrom D, Marciano SP, Felsenstein D, et al. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann Neurol. (2022) 91(3):367–79. doi: 10.1002/ana.26286
13. Stewart JM, Pianosi P, Shaban MA, Terilli C, Svistunova M, Visintainer P, et al. Postural hyperventilation as a cause of postural tachycardia syndrome: increased systemic vascular resistance and decreased cardiac output when upright in all postural tachycardia syndrome variants. J Am Heart Assoc. (2018) 7(13):e008854. doi: 10.1161/JAHA.118.008854
14. Wood J, Tabacof L, Tosto-Mancuso J, McCarthy D, Kontorovich A, Putrino D. Levels of end-tidal carbon dioxide are low despite normal respiratory rate in individuals with long COVID. J Breath Res. (2021) 16(1):017101. doi: 10.1088/1752-7163/ac3c18
15. Appelman B, Charlton BT, Goulding RP, Kerkhoff TJ, Breedveld EA, Noort W, et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun. (2024) 15(17):17. doi: 10.1038/s41467-023-44432-3
16. Fu Q, Levine BD. Exercise and non-pharmacological treatment of POTS. Auton Neurosci. (2018) 215:20–7. doi: 10.1016/j.autneu.2018.07.001
17. Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. (2006) 67(3):566–71. doi: 10.1016/j.mehy.2006.02.042
18. Sakakibara M, Kaneda M, Oikawa LO. Efficacy of paced breathing at the low-frequency peak on heart rate variability and baroreflex sensitivity. Appl Psychophysiol Biofeedback. (2020) 45(1):31–7. doi: 10.1007/s10484-019-09453-z
19. Lin G, Xiang Q, Fu X, Wang S, Wang S, Chen S, et al. Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. J Altern Complement Med. (2012) 18(2):143–52. doi: 10.1089/acm.2010.0607
20. Haischer MH, Opielinski LE, Mirkes LM, Uhrich TD, Bollaert RE, Danduran M, et al. Heart rate variability is reduced in COVID-19 survivors and associated with physical activity and fatigue. Physiol Rep. (2024) 12(2):e15912. doi: 10.14814/phy2.15912
21. Astin R, Banerjee A, Baker MR, Dani M, Ford E, Hull JH, et al. Long COVID: mechanisms, risk factors and recovery. Exp Physiol. (2023) 108(1):12–27. doi: 10.1113/EP090802
22. Herrera JE, Niehaus WN, Whiteson J, Azola A, Baratta JM, Fleming TK, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients [published correction appears in PM R. 2022 jan;14(1):164]. PM R. (2021) 13(9):1027–43. doi: 10.1002/pmrj.12684
23. World Health Organization. WHO COVID-19 Case Definition. Available online at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Surveillance_Case_Definition-2020.2 (Accessed February 18, 2021)
24. Corrado J, Iftekhar N, Halpin S, Li M, Tarrant R, Grimaldi J, et al. HEART Rate variability biofeedback for long COVID dysautonomia (HEARTLOC): results of a feasibility study. Adv Rehabil Sci Pract. (2024) 13:27536351241227261. doi: 10.1177/27536351241227261
25. Mourya M, Mahajan AS, Singh NP, Jain AK. Effect of slow- and fast-breathing exercises on autonomic functions in patients with essential hypertension. J Altern Complement Med. (2009) 15(7):711–7. doi: 10.1089/acm.2008.0609
26. Garg P, Mendiratta A, Banga A, Bucharles A, Victoria P, Kamaraj B, et al. Effect of breathing exercises on blood pressure and heart rate: a systematic review and meta-analysis. Int J Cardiol Cardiovasc Risk Prev. (2023) 20:200232. doi: 10.1016/j.ijcrp.2023.200232
27. Herawati I, Mat Ludin AF, Mutalazimah M, Ishak I, Farah NMF. Breathing exercise for hypertensive patients: a scoping review. Front Physiol. (2023) 14:1048338. doi: 10.3389/fphys.2023.1048338
28. Russo MA, Santarelli DM, O'Rourke D. The physiological effects of slow breathing in the healthy human. Breathe (Sheff). (2017) 13(4):298–309. doi: 10.1183/20734735.009817
29. Lehrer PM, Gevirtz R. Heart rate variability biofeedback: how and why does it work? Front Psychol. (2014) 5:756. doi: 10.3389/fpsyg.2014.00756
30. Palacios-Ceña D, Bautista-Villaécija O, Güeita-Rodríguez J, García-Bravo C, Pérez-Corrales J, Del Corral T, et al. Supervised telerehabilitation and home-based respiratory muscle training for post–COVID-19 condition symptoms: a nested qualitative study exploring the perspectives of participants in a randomized controlled trial. Phys Ther. (2024) 104(5):pzae043. doi: 10.1093/ptj/pzae043
31. Philip KEJ, Owles H, McVey S, Pagnuco T, Bruce K, Brunjes H, et al. An online breathing and wellbeing programme (ENO breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial. Lancet Respir Med. (2022) 10(9):851–62. doi: 10.1016/S2213-2600(22)00125-4
32. Whiteson JH, Azola A, Barry JT, Bartels MN, Blitshteyn S, Fleming TK, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cardiovascular complications in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2022) 14(7):855–78. doi: 10.1002/pmrj.12859
33. Maley JH, Alba GA, Barry JT, Bartels MN, Fleming TK, Oleson CV, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of breathing discomfort and respiratory sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2022) 14(1):77–95. doi: 10.1002/pmrj.12744
34. Melamed E, Rydberg L, Ambrose AF, Bhavaraju-Sanka R, Fine JS, Fleming TK, et al. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of neurologic sequelae in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2023) 15(5):640–62. doi: 10.1002/pmrj.12976
35. Fine JS, Ambrose AF, Didehbani N, Fleming TK, Glashan L, Longo M, et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of cognitive symptoms in patients with post-acute sequelae of SARS-CoV-2 infection (PASC). PM R. (2022) 14(1):96–111. doi: 10.1002/pmrj.12745
36. Maccarone MC, Coraci D, Regazzo G, Sarandria N, Scanu A, Masiero S. Evolution of musculoskeletal symptoms in long COVID syndrome: a lexical analysis to approach requirements for an interdisciplinary management. Joint Bone Spine. (2024) 91(1):105623. doi: 10.1016/j.jbspin.2023.105623
37. Zaccaro A, Piarulli A, Laurino M, Garbella E, Menicucci D, Neri B, et al. How breath-control can change your life: a systematic review on psycho-physiological correlates of slow breathing. Front Hum Neurosci. (2018) 12. doi: 10.3389/fnhum.2018.00353
38. Yeh G, Horwitz R. Integrative medicine for respiratory conditions: asthma and chronic obstructive pulmonary disease. Med Clin N Am. (2017) 101(5):925–41. doi: 10.1016/j.mcna.2017.04.008
39. Farb NAS, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci. (2013) 8(1):15–26. doi: 10.1093/scan/nss066
40. Van Diest I, Verstappen K, Aubert AE, Widjaja D, Vansteenwegen D, Vlemincx E. Inhalation/exhalation ratio modulates the effect of slow breathing on heart rate variability and relaxation. Appl Psychophysiol Biofeedback. (2014) 39(3):171–80. doi: 10.1007/s10484-014-9253-x
41. Silva J, Takahashi T, Wood J, Lu P, Tabachnikova A, Gehlhausen JR, et al. Sex differences in symptomatology and immune profiles of long COVID. medRxiv. (2024):2024.02.29.24303568. doi: 10.1101/2024.02.29.24303568
42. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
43. Tran V-T, Porcher R, Pane I, Ravaud P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat Commun. (2022) 13:1812. doi: 10.1038/s41467-022-29513-z
Keywords: Long COVID, resonant breathing, autonomic nervous system, autonomic dysfunction, rehabilitation
Citation: Polizzi J, Tosto-Mancuso J, Tabacof L, Wood J and Putrino D (2024) Resonant breathing improves self-reported symptoms and wellbeing in people with Long COVID. Front. Rehabil. Sci. 5:1411344. doi: 10.3389/fresc.2024.1411344
Received: 2 April 2024; Accepted: 25 June 2024;
Published: 12 July 2024.
Edited by:
Stefano Masiero, University of Padua, ItalyReviewed by:
Maria Chiara Maccarone, University of Padua, ItalyJared M. Gollie, Washington DC VA Medical Center, United States
© 2024 Polizzi, Tosto-Mancuso, Tabacof, Wood and Putrino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Putrino, ZGF2aWQucHV0cmlub0Btb3VudHNpbmFpLm9yZw==
 Jessica Polizzi
Jessica Polizzi Jenna Tosto-Mancuso
Jenna Tosto-Mancuso Laura Tabacof
Laura Tabacof Jamie Wood
Jamie Wood David Putrino
David Putrino