
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Rehabil. Sci., 16 October 2023
Sec. Rehabilitation in Neurological Conditions
Volume 4 - 2023 | https://doi.org/10.3389/fresc.2023.1205154
 Dirk Arnold1,2
Dirk Arnold1,2 Jovanna Thielker1,2
Jovanna Thielker1,2 Carsten M. Klingner2,3,4
Carsten M. Klingner2,3,4 Orlando Guntinas-Lichius1,2,4
Orlando Guntinas-Lichius1,2,4 Gerd Fabian Volk1,2,4*
Gerd Fabian Volk1,2,4*
Introduction: Although many different treatments were developed for facial palsy, only a few therapeutic options are available for facial synkinesis. Electrical stimulation of specific muscles via implants could be useful in restoring facial symmetry in synkinetic patients. A challenge in developing stimulation devices is finding the right stimulation location, type, and amplitude. This work assesses the ability to selectively stimulate the zygomaticus muscle (ZYG) in patients with oral-ocular synkinesis to elicit a visually detectable response of the ipsilateral corner of the mouth (COM), without causing a reaction of the orbicularis oculi muscle (OOM). We aimed to assess how close to the COM the stimulation should be delivered in order to be selective.
Methods: A total of 10 patients (eight females, two males) were enrolled. Facial function was graded according to the Sunnybrook facial grading system. Needle EMG was used to test the activities of the muscles, during volitional and “unintended” movements, and the degree of synkinesis of the ZYG and OOM. Two ball electrodes connected to an external stimulator were placed on the paretic ZYG, as close as possible to the COM.
Results: Independent of the waveform with which the stimulation was presented, a selective ZYG response was observed within 4.5 cm of the horizontal plane and 3 cm of the vertical plane of the COM. When the distance between the electrodes was kept to ≤2 cm, the amplitude necessary to trigger a response ranged between 3 and 6 mA when the stimulation was delivered with triangular pulses and between 2.5 and 3.5 mA for rectangular pulses. The required amplitude did not seem to be dependent on the applied phase duration (PD), as long as the PD was ≥5 ms.
Conclusion: Our results show that selective stimulation of the ZYG presenting synkinetic ZYG–OOM reinnervation can be achieved using a broad PD range (25–1,000 ms) and an average amplitude ≤6 mA, which may be further decreased to 3.5 mA if the stimulation is delivered via rectangular rather than triangular waves. The most comfortable and effective results were observed with PDs between 50 and 250 ms, suggesting that this range should be selected in future studies.
Clinical Trial Registration: [https://drks.de/search/de/trial/DRKS00019992], identifier (DRKS00019992).
Facial palsy, which is an umbrella term for incomplete facial paresis and complete facial paralysis, results from temporary or permanent dysfunction of the peripheral facial nerve (FN). Ordinarily, the FN splits into the temporal, zygomatic, buccal, marginal mandibular, and cervical branches (1), which innervate the main facial muscles responsible for mimic and expression. Despite the frequently observed simplification in anatomical literature, the anatomical position of the FN and the path of its various branches have high inter- and intraindividual variability (2–4). This can result in a higher number of distal branches than those commonly described and an increased occurrence of nerve anastomoses and plexus. For example, the innervation of the zygomaticus muscle (ZYG) and the orbicularis oculi muscle (OOM) has been traced back to either the deep buccal or the zygomatic branches (5, 6). Apparently, the distribution of innervating nerve branches is not consistently determined developmentally in these areas. The distribution is influenced to a greater extent by molecules within the extracellular matrix, which causes erratic reinnervation patterns. This finally leads to the development of facial synkinesis (7–9). Facial synkinesis is the most common long-term sequelae of facial paralysis with axonal damage. It is defined as the “unintended” movement of a muscle during the volitional movement of another muscle, through misdirected regrowth of axons during reinnervation after a lesion on the FN (10, 11). The severity of synkinetic reinnervation mainly depends on the size and location of the damage. Since axons have been found to regenerate with an average rate of 1 mm per day (7, 12), clinical signs of synkinesis are usually only detected between 3 and 4 months and 2 years after the nerve injury (10). Facial muscle synkinesis usually includes oral-ocular synkinesis, i.e., unintended eye closure during volitional mouth movement, and ocular-oral synkinesis, i.e., unintended mouth movement during volitional eye closure (13). These can appear alone or in combination with each other. The main problem for patients suffering from facial synkinesis beyond visual disturbances is the inability to properly convey emotions. This in turn can lead to psychological issues, such as depression, social isolation, and anxiety (14–18). Consequently, facial synkinesis often has a strong negative impact on the quality of life of a patient (19–21).
The repertoire of treatment options for facial palsy in general is much more extensive than that for patients with facial synkinesis. Thus, in addition to various training methods (22–27) and surgical interventions to reconstruct the innervation (28, 29), electrical stimulation is deemed as a safe and effective tool for the treatment of facial palsy (30) and in decelerating the atrophy of the affected muscles without negatively affecting muscle reinnervation (31–33).
In contrast, the most common treatment of facial synkinesis is the injection of botulinum toxin into the affected muscle(s) every 3–6 months (34, 35). However, many studies recommend training therapy in addition to or as an alternative to botulinum toxin treatment (36, 37) and in combination with EMG biofeedback (38–40).
Electrical stimulation to overcome the synkinetic activity of individual muscles has lately emerged as a promising approach (41). Based on different studies (42–44), the effectiveness of electrical stimulation is primarily dependent on the ability to activate a specific muscle with appropriate parameters.
Active implants could be a new approach to restoring smile symmetry or eye closure for reinnervated yet facially paralyzed patients. In most synkinetically reinnervated patients, volitional maneuvers such as smiling or blinking are possible to a certain extent, but when an individual tension threshold is exceeded, the synkinetically reinnervated muscles are coactivated. With an implant that detects the activity levels of the target muscles (e.g., left and right ZYG), electrical stimulation could be used to enhance the contraction of the concerned muscle before that threshold, without the patient having to actively increase the activity. Thus, it would be possible to reduce or even avoid the synkinetic effects with selective activation. To date, such implants have only been described in theory (45, 46), and have not been used in clinical practice. Rapid developments in chip-, electrode-, and transmission technology and deep learning approaches provide the perspective that such implants will become possible in the future. However, several problems must first be addressed; for instance, the electrodes should be as thin as a sheet of paper. Recent developments are already moving in the right direction [e.g., tattoo electrodes (47–49)]. In addition, they should be capable of sending electromyography (EMG) signals wirelessly from the healthy side as a trigger, to the control unit on the paralyzed side. A determination of the position at which the stimulation should be placed is therefore imperative (4, 42) because selective stimulation can only be successful if the innervating nerve of the target muscle is stimulated distal to the branching axons that are responsible for the unintended activity. In our opinion, the implantation of a stimulation device would be useful especially when improvements can no longer be achieved with non-invasive methods. However, non-invasive training methods using surface electrodes could also benefit from our results.
The work presented herein assessed the ability to selectively stimulate the ZYG in patients with oral-ocular synkinesis, as diagnosed via needle EMG, to elicit a visually detectable response of the ipsilateral corner of the mouth (COM), without causing a reaction of the ipsilateral eye. In particular, we aimed to assess how close to the respective COM the stimulation should be delivered on the ipsilateral ZYG, to be selective without causing OOM activation. The clear definition of the optimal stimulation region is a critical element of the subsequent development of implantable solutions for the treatment of facial synkinesis.
Data were collected from June 2018 to February 2021 at the Facial Nerve Center of the ENT Department, Jena University Hospital, as a longitudinal, open-label, prospective, case series-based, proof-of-principle clinical investigation. This study was approved by the Ethics Committee of Jena University Hospital in 2018 (application number 5403–02/18) and has been registered in the German Clinical Trials Register (Deutsches Register Klinischer Studien, DRKS 00019992). All participants gave written informed consent prior to their inclusion in this study.
The inclusion criteria were as follows: age ≥18 years old; onset of the palsy ≥6 months ago; diagnosed facial palsy with aberrant reinnervation of the ZYG and/or the OOM; anatomic, physiological, and mental conditions compatible with participation; and high motivation with realistic expectations regarding the participation.
The exclusion criteria were as follows: pregnant or breast-feeding women; any head (e.g., facial and/or neck) surgery (except patient 8), pharmacological treatment (e.g., botulinum toxin injection, wrinkle treatment), or physiotherapeutic program within the last 3 months before enrollment; other clinical diseases that might result in alteration of the outcomes (e.g., muscular and/or skin diseases, epilepsy); use of an active medical implant; known allergies or intolerance to the material used for this clinical investigation; ongoing participation in other drug and/or medical device clinical investigations that could affect the results of the present clinical investigation; and in the opinion of the principal investigator, anything that would place the subject at increased risk or preclude the subject's full compliance with the general requirements.
A total of 10 patients (eight females, two males) were enrolled in the study. The patients were referred to the ENT Department at Jena University Hospital after already being diagnosed with facial palsy by MRI and clinical examinations in other specialized clinics. The mean age at enrollment was 52.4 ± 11.5 years (range 35–69; median = 51). The interval from the onset of unilateral facial paralysis to the time of enrollment ranged from 6 months to 36 years (Table 1). One patient underwent a reconstructive surgical treatment, 20% of the patients were treated with botulinum toxin (more than 3 months earlier than enrollment), 10% required artificial tears, and 80% underwent speech therapy and/or other physiotherapeutic treatment.
Before starting the stimulation procedure, two independent examiners assessed the symptoms of the patients according to the Sunnybrook facial grading system (50). Here, the examiners visually recorded the symmetry at rest, the symmetry during defined movements (raising eyebrows, gently closing the eyelid, smiling with open mouth, showing teeth, pursing lips), and the synkinesis during the same movements. Afterward, they weighted them (symmetry at rest ×5, symmetry at movement ×4, synkinesis ×1) to combine into a total score.
In addition, using needle EMG (Synergy T5, Viasys, CareFusion, San Diego, CA, USA), the examiners evaluated pathological spontaneous activity (PSA) and rated it 0 = none or 1 = present in multiple areas, muscle reactions during the volitional maneuvers of smiling (ZYG) and blinking (OOM), and unintended activity of these muscles during maneuvers in which they would not normally be expected to be activated [blinking (ZYG) and smiling (OOM)] (51). The activity was graded subjectively by the examiner as “none,” “single fiber pattern,” “strongly decreased,” “moderately decreased,” “mildly decreased,” or “normal.” A classification of synkinesis into “none,” “mild,” “moderate,” or “strong” was based on the detected unintended activities of both muscles. Synkinesis was graded as “mild” if the unintended activity of the ZYG or the OOM or both muscles was assessed as “strongly decreased.” In contrast, synkinesis was “strong” if the activity was “mildly decreased” and “moderate” if a moderate attenuation was detected.
With the patient in a sitting position, two ball electrodes (8 mm Ø; Physiomed Elektromedizin AG, Schnaittach, Germany) connected to an external stimulator (STMIsola, BIOPAC Systems, Inc., Germany) were placed on the paretic ZYG [the skin was prepared using conducting ultrasound gel (not shown in figure) (Sonosid/Asid Bonz GmbH], as close as possible to the respective COM with an interelectrode distance of 1.5 cm (Figure 1). In addition to the research stimulation device used in this study (STMIsola, BIOPAC Systems, Inc. Germany), we have good experience with commercially available stimulation devices for patients that output pulse durations and pulse forms such as those used in this study, e.g., MED-EL's STIWELL, Krauth + Timmermann’s Paresestim, and Schuhfried's Stimulette.
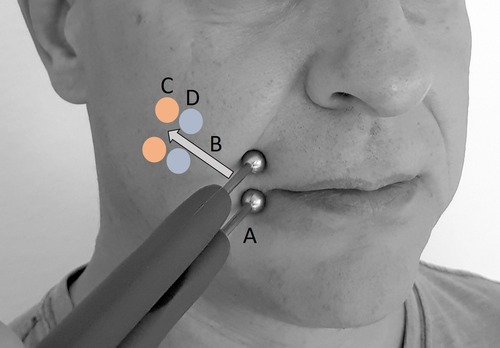
Figure 1. Stimulation procedure with two ball electrodes (conducting ultrasound gel not shown). (A) Starting position for test stimulation. (B) Both electrodes were moved retrograde along the assumed facial nerve path, without detaching either electrode. (C) Position of first unspecific muscle reactions. (D) Last position of specific zygomaticus muscle (ZYG) response and position for all PD tests.
Using biphasic electrical stimulation, delivered with triangular or rectangular waveforms, a phase duration (PD) of 100 ms, and a frequency of 1 Hz, the examiner increased the amplitude from 0.5 to a maximum of 25 mA, in 0.5 mA increments, until a selective ZYG response was achieved without patient discomfort. The stimulation was aimed at the (re)innervating nerve branches. A current-controlled stimulation was used to ensure the consistency of the physiological responses between sessions. The voltage is dependent on the impedance between the electrodes. A selective ZYG response was determined visually as the respective COM lifting. At this point, the ball electrodes were moved retrograde along the assumed facial nerve path, in the direction of the ear, without detaching either electrode from the skin of the patient until a selective ZYG response was no longer observed within the aforementioned amplitude range. The last position where a selective ZYG response was possible without the unintended activity of the OOM or unspecific cocontractions of other muscles and without discomfort being experienced by the patient was used for the analysis. At this point of stimulation, the response of the ZYG to the use of a PD of 1,000, 500, 250, 100, 50, 25, 15, 10, 5, and 1 ms was assessed in this sequence. The whole stimulation procedure lasted up to 25 min per patient.
Data were analyzed using IBM SPSS statistics software (Version 25, IBM, NY, USA) for medical statistics. The distribution of continuous data was described using mean values ± standard deviation. Qualitative data were presented in absolute and relative frequencies. Due to the small sample size, as a proof of principle clinical investigation, no statistical analyses could be carried out and the data were evaluated descriptively.
Table 2 shows the results of the EMG examinations for each patient. PSA at multiple areas of the affected ZYG could be detected in only one patient (2). No PSA was observed in any patients in the OOM. All patients were capable of intentional movement of the ZYG to varying degrees. “Normal” activity (09), “strongly decreased” activity (07), and “single fiber pattern” activity (06) were observed once each. The volitional activity was “mildly decreased” in three patients (1, 4, and 5) and “moderately decreased” in four patients (2, 3, 8, and 10). In contrast, no volitional activity of the OOM was observed in two patients (7 and 8). A “strongly decreased” volitional activity was observed in one patient (4) and “mildly decreased” volitional activity also in one patient (1). Four of the patients (2, 3, 6, and 10) had a “moderately decreased” volitional OOM activity, and two patients (5 and 9) had “normal” OOM activity.
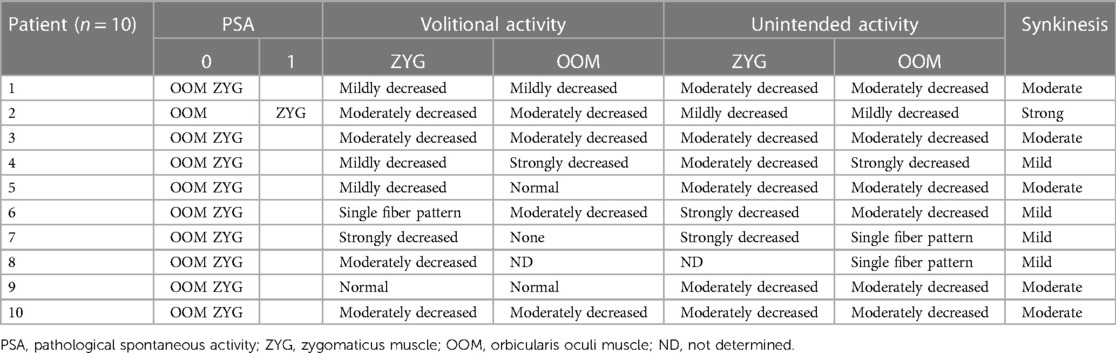
Table 2. Needle electromyography results of all patients during volitional maneuvers such as smiling (ZYG) or blinking (OOM) or unintended maneuvers such as blinking (ZYG) or smiling (OOM).
Finally, the following synkinesis classifications resulted from the measured unintended muscle movements: The ZYG–OOM synkinesis was “strong” in only one patient (2), “moderate” in five patients (1, 3, 5, 9, and 10), and “mild” in four patients (4, 6, 7, and 8).
The mean composite score was 45.9 ± 22.4 (range 5–80; median = 47.5), representing a “moderate” post-paralytic facial syndrome. A moderate loss of resting symmetry was confirmed by a mean resting symmetry score of 10 ± 4.1 (range 5–15; median = 10). The average voluntary movement score was 61.2 ± 22.3 (range 20–88; median = 64). This corresponds to a “moderate” impairment of volitional movement. There was a “mild”-to-“moderate” synkinesis between the ZYG and the OOM with a mean synkinesis score of 5.3 ± 3.4 (range 0–10; median = 6).
No adverse events were observed for the entire duration of the study.
Independent of the waveform of the stimulation, a selective ZYG response was only observed with stimulation within approximately 4.5 cm of the horizontal plane and 3 cm of the vertical plane from the respective COM. It was not possible to determine a discomfort threshold that was valid for all the patients, because the subjective sensation varied greatly between the patients. The patients who had already undergone electrical stimulation tended to show greater sensitivity thresholds than patients for whom it was the first electrical stimulation.
When wave stimulation with triangular pulses was used, a selective ZYG response was observed in this area in nine patients (Figure 2). In patient 8, the threshold of discomfort was reached before the ZYG could be activated selectively with PDs between 15 and 1,000 ms. Shorter PDs were not tested for that reason. Among the responders, in patient 5 a specific ZYG response could only be observed with 100 ms, while discomfort threshold was reached with the other PDs tested (250, 500 and 1000). Only 250 ms was effective in patient 1. The other PDs were not completely tested because they caused discomfort and/or unspecific responses to the patient. A mean amplitude of 4 ± 2.5 mA (range 1.4–9; median = 3.3 mA) was required to elicit a selective ZYG response with a PD of 100 ms.

Figure 2. Results of zygomaticus muscle (ZYG) stimulation presented as triangular waves at various phase durations (PDs in ms). No stimulation response (Stim response) at any amplitude (mA) as selective ZYG activation was possible in patient 8 for any tested PD, because the threshold of discomfort was always reached before. For patients 1 and 5, a selective ZYG response could be elicited only with 100 and 250 ms, respectively. Interruptions in the lines within the line chart occur when the ZYG of a patient does not respond to each PD with a selective activity.
Stimulations with rectangular pulses were performed in nine patients because one patient (1) declined to perform the test. Stimulation successfully triggered a selective ZYG response in eight of the nine patients. In one patient (7), only unspecific reactions without discomfort were observed with a PD range of between 50 and 1,000 ms (data not shown), and other PDs were not tested. The remaining eight patients showed selective stimulations with different PDs, but patient 8 only with 250 ms and patient 4 with 250 and 1,000 ms. Other PDs caused either discomfort or unspecific facial muscle response. A mean amplitude of 2.6 ± 2.3 mA (range 1–7; median = 1.8 mA) was required to observe a selective ZYG response with a PD of 100 ms. The results of all patients (n = 10) and all PDs are shown in Figure 3.
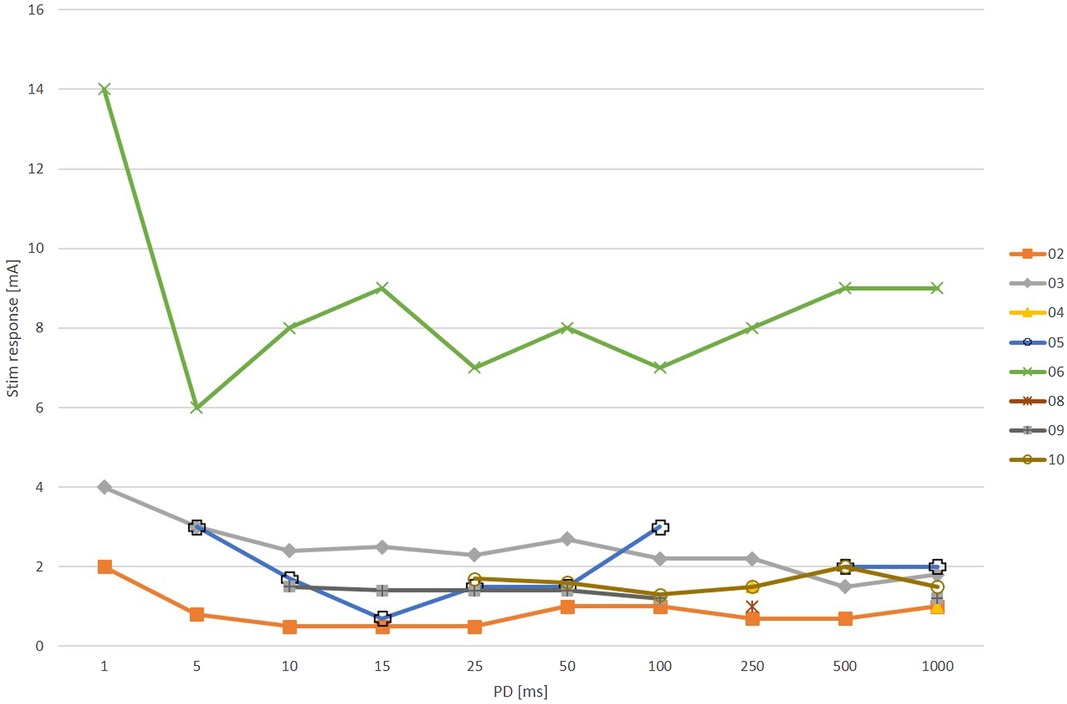
Figure 3. Results of zygomaticus muscle (ZYG) stimulation presented as rectangular waves at various phase durations (PDs in ms). Patient 1 declined to perform the test and only unspecific facial muscle reactions were detectable in patient 7 (data not shown). A selective ZYG activation was possible in patient 8 only with a PD of 250 ms and PDs of with 250 and 1,000 ms in patient 4. As mentioned before, interruptions in the lines occur when the ZYG of a patient does not respond to each PD with a selective activity (Stim response = stimulation response amplitude in mA).
The numbers of successful selective ZYG responses for all PDs of triangular and rectangular waveforms are presented in Table 3. Again, patient 1 declined the test. The PDs of 100 ms and 250 ms were most effective for triangular pulses and 1,000 ms for rectangular pulses.
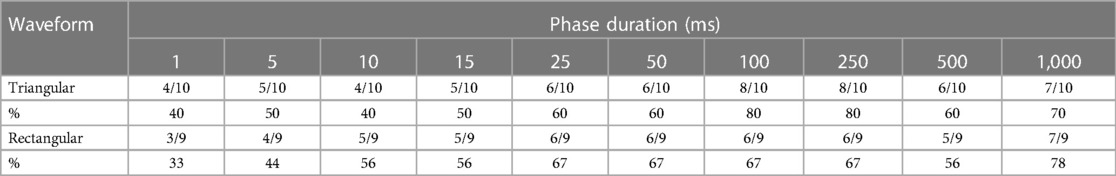
Table 3. Number and percentage of successful stimulations of the zygomaticus muscle with different phase durations and waveforms.
The mean threshold amplitudes of the rectangular pulses were slightly lower and did not vary as much as those of the triangular pulses (Figure 4). The mean amplitudes for rectangular pulses were all around 3 mA except for a PD of 1 ms, which required an amplitude of approximately 7 mA. Stimulations with triangular pulses required amplitudes between 3 and 5 mA except for the PDs of 1 ms and 500 ms, which required amplitudes of 10 mA and 6 mA, respectively.
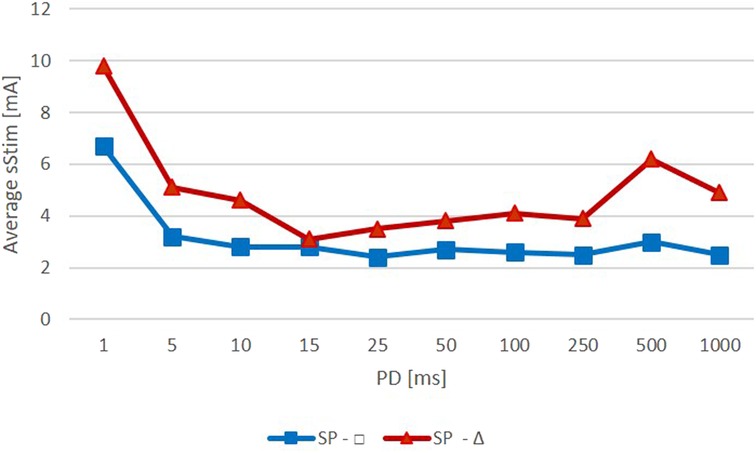
Figure 4. Average amplitude required to observe a specific zygomaticus muscle (ZYG) response with stimulation delivered as triangular (red) or rectangular (blue) waveform (Average Stim, average surface stimulation in mA; SP, stimulation pulse; PD, phase duration in ms).
The EMG assessment showed that, prior to the testing session, four patients (40%) had a “mild,” five patients (50%) had a “moderate,” and one patient (10%) had a “strong” ZYG–OOM synkinesis. The Sunnybrook assessment confirmed these results and showed that all the enrolled patients had a “moderate” impairment of volitional mouth and eye movements and had a “moderate” loss of facial symmetry (52). The composite score results confirmed “moderate” facial paresis. While both assessments provided comparable results concerning facial paresis symptoms and the presence of synkinetic reinnervation, EMG was found to be more sensitive in detecting initial, subclinical signs of reinnervation (e.g., single fiber pattern) before its effect became clinically relevant and thus assessable through visual outcome measures, such as the Sunnybrook assessment.
The wide range of inter- and intraindividual variation of the innervation path of the FN is well-known, (1–6, 53–57). Similarly, the pattern of synkinetic reinnervation can also be highly variable as a consequence (58, 59). The EMG data presented supports this. In all patients studied, the ZYG and OOM were activated to some extent when smiling or blinking was performed and their activation should not have occurred.
The irregularity of the distribution of the sprouting nerve endings of different motor units during reinnervation and the extent of their re-connection to an undetermined number of muscle fibers in one or more muscles determine the degree of synkinesis (60). The axons may be concentrated in a particular region or may be more widely distributed throughout the muscle. Therefore, when considering the best position for the placement of subcutaneous implantable electrodes for selective stimulation of the ZYG, to restore at least partial facial symmetry, it is important to understand which area of the cheek should be targeted. We were able to observe a selective ZYG response to electrical stimulation delivered, with either triangular or rectangular waveforms, as long as it was delivered on the relevant branch of the facial nerve within an area of approximately 4.5 cm × 3.0 cm above the respective COM. This area was not that different between patients, which indicates that at least in the 10 patients included in this study, the synkinetic axons branch proximal to this area.
In patients with synkinetic reinnervated facial muscles, the stimulation parameter selection is crucial to avoid synkinetic responses or discomfort. PD and amplitude determine the size and strength of the electrical field and, thus, which nerve branches, or even denervated muscle fibers, were activated by the pulses. The distance between the electrodes is also important to create a selective electrical field. If the distance was kept at ≤2 cm, as in the present study, the amplitude necessary to trigger a selective ZYG response would be in a range of between 3 and 6 mA or between 2 and 3 mA, depending on whether the stimulation was delivered with a triangular or a rectangular waveform, respectively. The amplitude required did not seem to depend on the PD applied as long as the latter was ≥5 ms. For lower PDs (e.g., 1 ms), an amplitude equal to twice that value was required to see the same effects with longer PDs. This observation was expected, because short PDs require higher amplitudes to reach the same effect as longer ones (≥5 ms), due to the very short delivery time and the reduced electrical field that is generated, which causes poorer nerve and muscle fiber recruitment and activation (61).
A relevant difference between stimulation of paralyzed vs. post-paralyzed ZYG is that in the former a selective response is achieved with a combination of PD and amplitude that are inversely proportional (61); a shorter PD requires a higher amplitude. In contrast, in the case of post paralyzed ZYG, the amplitude did not change with increasing PDs ≥5 ms. This difference is the result of the presence of reinnervated fibers in the post-paralyzed muscles that are ready to respond to stimulations with short PDs and low amplitudes, which are absent in the paralyzed muscles. Accordingly, in paretic muscles, even a low-magnitude electric field would be able to activate a sufficient number of intact nerve fibers/motor units to elicit a visually detectable response. Furthermore, the plateau beyond which no further intensity increase would improve the ZYG response is more easily reached in post-paralyzed than in completely denervated paralyzed muscles. Still, simultaneous activation of denervated muscle fibers cannot be ruled out since, as recently shown by Arnold et al. (42), they are particularly reactive to longer PDs and lower amplitudes. However, the article cited 6 cm × 4 cm hydrogel electrodes were used, which are much bigger than the ball electrodes used in the present study and produce a larger electrical field with each PD (43).
The PD of 500 ms stimulated with triangular pulses was an exception that required higher amplitudes (in addition to the 1 ms PD). A slightly greater amplitude, compared with the amplitudes at the other PDs, was required to elicit a selective ZYG response (Figure 4). However, this was due to the amplitude value of patient 6 (500 ms, 19 mA) (Figure 2). In this patient, higher amplitudes for all PDs were required to activate the ZYG. That is the only patient in which “single fiber patterns” were detected when testing the volitional activity of the ZYG, i.e., a very weak reinnervation of the muscle was present. Also, the unintended activity of the muscle was “strongly decreased.” This means that in this patient, only a few muscle fibers could be reached via intact nerve branches by electrical stimulation and consequently a greater current was required to elicit a visible response from the ZYG.
Similar EMG results were observed in patient 7. Again, the volitional and unintended activity of the ZYG was “strongly decreased,” and the muscle could only be visibly activated in response to stimulation with longer PDs (100–1,000 ms) and slightly increased amplitudes (Figure 2). Using rectangular pulses, selective activation of the ZYG was not possible at all. There were always unspecific muscle responses in the face before the threshold was reached, which could be explained by the increased electrical field.
As the present study was primarily aimed at the selective response of the ZYG, the strength of its volitional and unintentional activation certainly plays a decisive role in the search for the required amplitude. Similar to the optimal stimulation parameters, a suitable electrode position is highly patient-specific, and their determination is important for adequate performance of electrical stimulation in patients with synkinesis after facial nerve palsy. At present, it is relevant for electrical stimulation with surface electrodes, especially for practicing the denervated or reinnervated muscles, and we could show that selective activation is also possible in synkinetically reinnervated muscles. However, in the future, when the implantation of electrodes is established, it might be also important for implants. This will also depend on the kind of electrode systems, which will be used (intraneural electrodes, extraneural cuff electrodes, or electrodes embedded into muscle tissue). As already mentioned, stimulations delivered as triangular waves released less energy than those delivered as rectangular waves. This is an important observation for the future development of implantable electrical stimulation devices that could help to optimize the lifetime of an implant. Further considerations are that since the implanted electrodes would deliver the stimulation directly on the target nerve/muscles, thus bypassing the connective tissue and the skin, they would most likely be compatible with lower amplitudes than those used in this study. Finally, it is important to note that none of the patients enrolled in this study suffered any adverse events during or after the conclusion of the stimulation session, which confirms the high safety profile of surface electrical stimulation, even when used on very sensitive body regions, such as the face.
The main limitation of our study was the subjective assessment of volitional and unintended activities by the examiners. The evaluation of the degree of synkinesis was performed by facial palsy experts supported by needle EMG to objectify the assessments. Demeco et al. (62) developed a grading system that can be used in future studies to further enhance the objectification of the findings. Büchern et al. (63) also developed another approach to objectify and standardize facial analysis using 3D sensors.
Moreover, our sample size was small. The intention was a proof of principle clinical investigation. This has been successfully achieved. Next step, the results have to be confirmed in a larger sample also allowing detailed statistical analyses. Because we did not permanently treat the patients with the described parameters and we have not investigated the stimulation effects on nerves, musculature, or connective tissue, it is not possible to recommend treatments for rehabilitation or training with specific stimulation parameters based on this publication.
Surface electrical stimulation can be used to activate specific facial muscles safely and selectively without a synkinetically reinnervated patient's discomfort and/or unselective activation of other ipsi- and/or contralateral muscles. In particular, the selective stimulation of the ZYG showing synkinetic ZYG–OOM reinnervation can be achieved using a broad range of PD (25–1,000 ms) and an average amplitude ≤6 mA that may be decreased further to 3.5 mA if the stimulation is delivered via rectangular rather than triangular waves. The most comfortable and effective results were observed with PDs between 50 and 250 ms, suggesting that this range should be preferred in future studies. We observed that the electrode position on the face and the distance and configuration are critical to observe a specific response within the observed range.
In summary, our results showed that specific stimulation of at least some facial muscles, such as the ZYG, would be compatible with an implantable solution that would represent an interesting alternative for patients suffering from facial synkinesis and not profiting from the currently available standard treatments.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Jena University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
GV and OG contributed to the conception and design of the study. DA, GV, JT, and CK were responsible for collecting the data. DA and GV analyzed the data. DA wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
We would like to thank Silvia Rosellini for the data analysis, Una Doyle for proofreading and editing the manuscript, and colleagues from our department for helping with data collection for this study.
The study was sponsored by MED-EL Elektromedizinische Geräte GmbH, Innsbruck, Austria. The funder had the following involvement with the study: study design and data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kochhar A, Larian B, Azizzadeh B. Facial nerve and parotid gland anatomy. Otolaryngol Clin North Am. (2016) 49:273–84. doi: 10.1016/j.otc.2015.10.002
2. Roostaeian J, Rohrich RJ, Stuzin JM. Anatomical considerations to prevent facial nerve injury. Plast Reconstr Surg. (2015) 135:1318–27. doi: 10.1097/prs.0000000000001244
3. Adidharma L, Bly RA, Theeuwen HA, Holdefer RN, Slimp J, Kinney GA, et al. Facial nerve branching patterns vary with vascular anomalies. Laryngoscope. (2020) 130:2708–13. doi: 10.1002/lary.28500
4. Raslan A, Volk GF, Möller M, Stark V, Eckhardt N, Guntinas-Lichius O. High variability of facial muscle innervation by facial nerve branches: a prospective electrostimulation study. Laryngoscope. (2017) 127:1288–95. doi: 10.1002/lary.26349
5. Kehrer A, Engelmann S, Bauer R, Taeger C, Grechenig S, Kehrer M, et al. The nerve supply of zygomaticus major: variability and distinguishing zygomatic from buccal facial nerve branches. Clin Anat. (2018) 31:560–5. doi: 10.1002/ca.23044
6. Kehrer A, Engelmann S, Ruewe M, Geis S, Taeger C, Kehrer M, et al. Anatomical study of the zygomatic and buccal branches of the facial nerve: application to facial reanimation procedures. Clin Anat. (2019) 32:480–8. doi: 10.1002/ca.23332
7. Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg Clin N Am. (1991) 2:31–42. doi: 10.1016/S1042-3680(18)30755-1
8. Benjamin B. Vocal cord paralysis, synkinesis and vocal fold motion impairment. ANZ J Surg. (2003) 73:784–6. doi: 10.1046/j.1445-2197.2003.02799.x
9. Salles AG, da Costa EF, Ferreira MC, do Nascimento Remigio AF, Moraes LB, Gemperli R. Epidemiologic overview of synkinesis in 353 patients with longstanding facial paralysis under treatment with botulinum toxin for 11 years. Plast Reconstr Surg. (2015) 136:1289–98. doi: 10.1097/prs.0000000000001802
10. Celik M, Forta H, Vural C. The development of synkinesis after facial nerve paralysis. Eur Neurol. (2000) 43:147–51. doi: 10.1159/000008154
11. Crumley RL. Mechanisms of synkinesis. Laryngoscope. (1979) 89:1847–54. doi: 10.1288/00005537-197911000-00020
12. Cancalon PF. Survival and subsequent regeneration of olfactory neurons after a distal axonal lesion. J Neurocytol. (1987) 16:829–41. doi: 10.1007/bf01611989
13. Nakamura K, Toda N, Sakamaki K, Kashima K, Takeda N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngol Head Neck Surg. (2003) 128:539–43. doi: 10.1016/s0194-59980223254-4
14. Paolucci T, Cardarola A, Colonnelli P, Ferracuti G, Gonnella R, Murgia M, et al. Give me a kiss! An integrative rehabilitative training program with motor imagery and mirror therapy for recovery of facial palsy. Eur J Phys Rehabil Med. (2020) 56:58–67. doi: 10.23736/s1973-9087.19.05757-5
15. Saadi R, Shokri T, Schaefer E, Hollenbeak C, Lighthall JG. Depression rates after facial paralysis. Ann Plast Surg. (2019) 83:190–4. doi: 10.1097/sap.0000000000001908
16. De Stefani E, Ardizzi M, Nicolini Y, Belluardo M, Barbot A, Bertolini C, et al. Children with facial paralysis due to Moebius syndrome exhibit reduced autonomic modulation during emotion processing. J Neurodev Disord. (2019) 11:12. doi: 10.1186/s11689-019-9272-2
17. Dobel C, Miltner WH, Witte OW, Volk GF, Guntinas-Lichius O. Emotional impact of facial palsy. Laryngorhinootologie. (2013) 92:9–23. doi: 10.1055/s-0032-1327624
18. Volk GF, Hesse S, Geißler K, Kuttenreich AM, Thielker J, Dobel C, et al. Role of body dysmorphic disorder in patients with postparalytic facial synkinesis. Laryngoscope. (2021) 131:E2518–24. doi: 10.1002/lary.29526
19. Bernd E, Kukuk M, Holtmann L, Stettner M, Mattheis S, Lang S, et al. Newly developed biofeedback program for facial muscle training in patients with facial paralysis. Hno. (2018) 66:686–92. doi: 10.1007/s00106-018-0542-1
20. Chang YS, Choi JE, Kim SW, Baek SY, Cho YS. Prevalence and associated factors of facial palsy and lifestyle characteristics: data from the Korean National Health and Nutrition Examination Survey 2010–2012. BMJ Open. (2016) 6:e012628. doi: 10.1136/bmjopen-2016-012628
21. Volk GF, Granitzka T, Kreysa H, Klingner CM, Guntinas-Lichius O. Nonmotor disabilities in patients with facial palsy measured by patient-reported outcome measures. Laryngoscope. (2016) 126:1516–23. doi: 10.1002/lary.25695
22. Morishima N, Kamiya T, Naito Y, Morisaka A, Ishikawa T, Tachibana K, et al. Effect of muscle strengthening on peripheral facial palsy: a randomized controlled trial. Phys Ther Res. (2020) 23:59–65. doi: 10.1298/ptr.E10000
23. Martineau S, Rahal A, Piette É, Chouinard AM, Marcotte K. The mirror effect plus protocol for acute Bell’s palsy: a randomised and longitudinal study on facial rehabilitation. Acta Otolaryngol. (2021) 141:203–8. doi: 10.1080/00016489.2020.1842905
24. Beurskens CH, Heymans PG, Oostendorp RA. Stability of benefits of mime therapy in sequelae of facial nerve paresis during a 1-year period. Otol Neurotol. (2006) 27:1037–42. doi: 10.1097/01.mao.0000217350.09796.07
25. Boschetti CE, Lo Giudice G, Spuntarelli C, Apice C, Rauso R, Santagata M, et al. Kabat rehabilitation in facial nerve palsy after parotid gland tumor surgery: a case-control study. Diagnostics (Basel). (2022) 12(3):1–11. PMID: 35328118; PMCID: 8947506. doi: 10.3390/diagnostics12030565
26. Pereira LM, Obara K, Dias JM, Menacho MO, Lavado EL, Cardoso JR. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clin Rehabil. (2011) 25:649–58. doi: 10.1177/0269215510395634
27. Wamkpah NS, Jeanpierre L, Lieu JEC, Del Toro D, Simon LE, Chi JJ. Physical therapy for iatrogenic facial paralysis: a systematic review. JAMA Otolaryngol Head Neck Surg. (2020) 146:1065–72. doi: 10.1001/jamaoto.2020.3049
28. Volk GF, Pantel M, Guntinas-Lichius O. Modern concepts in facial nerve reconstruction. Head Face Med. (2010) 6:25. doi: 10.1186/1746-160x-6-25
29. Pinkiewicz M, Dorobisz K, Zatoński T. A comprehensive approach to facial reanimation: a systematic review. J Clin Med. (2022) 11(10):1–25. PMID: 35629016; PMCID: 9143601. doi: 10.3390/jcm11102890
30. Marotta N, Demeco A, Inzitari MT, Caruso MG, Ammendolia A. Neuromuscular electrical stimulation and shortwave diathermy in unrecovered bell palsy: a randomized controlled study. Medicine (Baltimore). (2020) 99:e19152. doi: 10.1097/md.0000000000019152
31. Puls WC, Jarvis JC, Ruck A, Lehmann T, Guntinas-Lichius O, Volk GF. Surface electrical stimulation for facial paralysis is not harmful. Muscle Nerve. (2020) 61:347–53. doi: 10.1002/mus.26784
32. Cercone M, Jarvis JC, Ducharme NG, Perkins J, Piercy RJ, Willand MP, et al. Functional electrical stimulation following nerve injury in a large animal model. Muscle Nerve. (2019) 59:717–25. doi: 10.1002/mus.26460
33. Cheetham J, Perkins JD, Jarvis JC, Cercone M, Maw M, Hermanson JW, et al. Effects of functional electrical stimulation on denervated laryngeal muscle in a large animal model. Artif Organs. (2015) 39:876–85. doi: 10.1111/aor.12624
34. Guntinas-Lichius O, Prengel J, Cohen O, Mäkitie AA, Vander Poorten V, Ronen O, et al. Pathogenesis, diagnosis and therapy of facial synkinesis: a systematic review and clinical practice recommendations by the international head and neck scientific group. Front Neurol. (2022) 13:1–19. PMID: 36438936; PMCID: 9682287. doi: 10.3389/fneur.2022.1019554
35. Cooper L, Lui M, Nduka C. Botulinum toxin treatment for facial palsy: a systematic review. J Plast Reconstr Aesthet Surg. (2017) 70:833–41. doi: 10.1016/j.bjps.2017.01.009
36. Lee JM, Choi KH, Lim BW, Kim MW, Kim J. Half-mirror biofeedback exercise in combination with three botulinum toxin A injections for long-lasting treatment of facial sequelae after facial paralysis. J Plast Reconstr Aesthet Surg. (2015) 68:71–8. doi: 10.1016/j.bjps.2014.08.067
37. Mandrini S, Comelli M, Dall'angelo A, Togni R, Cecini M, Pavese C, et al. Long-term facial improvement after repeated BoNT-A injections and mirror biofeedback exercises for chronic facial synkinesis: a case-series study. Eur J Phys Rehabil Med. (2016) 52:810–8.27164539
38. Brach JS, VanSwearingen JM, Lenert J, Johnson PC. Facial neuromuscular retraining for oral synkinesis. Plast Reconstr Surg. (1997) 99:1922–31. doi: 10.1097/00006534-199706000-00017
39. VanSwearingen JM, Brach JS. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast Reconstr Surg. (2003) 111:2370–5. doi: 10.1097/01.Prs.0000061007.36637.88
40. Volk GF, Roediger B, Geißler K, Kuttenreich AM, Klingner CM, Dobel C, et al. Effect of an intensified combined electromyography and visual feedback training on facial grading in patients with post-paralytic facial synkinesis. Front Rehabil Sci. (2021) 2:746188. doi: 10.3389/fresc.2021.746188
41. Volk GF, Thielker J, Möller MC, Majcher D, Mastryukova V, Altmann CS, et al. Tolerability of facial electrostimulation in healthy adults and patients with facial synkinesis. Eur Arch Otorhinolaryngol. (2020) 277:1247–53. doi: 10.1007/s00405-020-05818-x
42. Arnold D, Thielker J, Klingner CM, Puls WC, Misikire W, Guntinas-Lichius O, et al. Selective surface electrostimulation of the denervated zygomaticus muscle. Diagnostics (Basel). (2021) 11(2):1–13. PMID: 33525522; PMCID: 7912406. doi: 10.3390/diagnostics11020188
43. Gomez-Tames JD, Gonzalez J, Yu W. A simulation study: effect of the inter-electrode distance, electrode size and shape in transcutaneous electrical stimulation. Annu Int Conf IEEE Eng Med Biol Soc. (2012) 2012:3576–9. doi: 10.1109/embc.2012.6346739
44. Mäkelä E, Venesvirta H, Ilves M, Lylykangas J, Rantanen V, Ylä-Kotola T, et al. Facial muscle reanimation by transcutaneous electrical stimulation for peripheral facial nerve palsy. J Med Eng Technol. (2019) 43:155–64. doi: 10.1080/03091902.2019.1637470
45. Tobey DN, Sutton D. Contralaterally elicited electrical stimulation of paralyzed facial muscles. Otolaryngology. (1978) 86:ORL-812–8. doi: 10.1177/019459987808600528
46. Nicolaidis SC, Williams HB. Muscle preservation using an implantable electrical system after nerve injury and repair. Microsurgery. (2001) 21:241–7. doi: 10.1002/micr.1047
47. Ferrari LM, Ismailov U, Badier J-M, Greco F, Ismailova E. Conducting polymer tattoo electrodes in clinical electro- and magneto-encephalography. NPJ Flexible Electronics. (2020) 4:4. doi: 10.1038/s41528-020-0067-z
48. Ferrari LM, Sudha S, Tarantino S, Esposti R, Bolzoni F, Cavallari P, et al. Ultraconformable temporary tattoo electrodes for electrophysiology. Adv Sci (Weinh). (2018) 5:1700771. doi: 10.1002/advs.201700771
49. Chandra S, Li J, Afsharipour B, Cardona AF, Suresh NL, Tian L, et al. Performance evaluation of a wearable tattoo electrode suitable for high-resolution surface electromyogram recording. IEEE Trans Biomed Eng. (2021) 68:1389–98. doi: 10.1109/tbme.2020.3032354
50. Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngol Head Neck Surg. (1996) 114:380–6. doi: 10.1016/s0194-59989670206-1
51. Guntinas-Lichius O, Volk GF, Olsen KD, Mäkitie AA, Silver CE, Zafereo ME, et al. Facial nerve electrodiagnostics for patients with facial palsy: a clinical practice guideline. Eur Arch Otorhinolaryngol. (2020) 277:1855–74. doi: 10.1007/s00405-020-05949-1
52. Hu WL, Ross B, Nedzelski J. Reliability of the Sunnybrook facial grading system by novice users. J Otolaryngol. (2001) 30:208–11. doi: 10.2310/7070.2001.20148
53. Marur T, Tuna Y, Demirci S. Facial anatomy. Clin Dermatol. (2014) 32:14–23. doi: 10.1016/j.clindermatol.2013.05.022
54. Gosain AK. Surgical anatomy of the facial nerve. Clin Plast Surg. (1995) 22:241–51. doi: 10.1016/S0094-1298(20)30965-2
55. Monkhouse WS. The anatomy of the facial nerve. Ear Nose Throat J. (1990) 69(10):677–83, 686–7. PMID: 2286163.2286163
56. Diamond M, Wartmann CT, Tubbs RS, Shoja MM, Cohen-Gadol AA, Loukas M. Peripheral facial nerve communications and their clinical implications. Clin Anat. (2011) 24:10–8. doi: 10.1002/ca.21072
57. Proctor B. The anatomy of the facial nerve. Otolaryngol Clin North Am. (1991) 24:479–504. doi: 10.1016/S0030-6665(20)31112-9
58. Grosheva M, Nohroudi K, Schwarz A, Rink S, Bendella H, Sarikcioglu L, et al. Comparison of trophic factors’ expression between paralyzed and recovering muscles after facial nerve injury. A quantitative analysis in time course. Exp Neurol. (2016) 279:137–48. doi: 10.1016/j.expneurol.2016.02.020
59. Grosheva M, Wittekindt C, Guntinas-Lichius O. Prognostic value of electroneurography and electromyography in facial palsy. Laryngoscope. (2008) 118:394–7. doi: 10.1097/MLG.0b013e31815d8e68
60. Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, et al. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. (2005) 21:391–402. doi: 10.1111/j.1460-9568.2005.03877.x
61. Geddes LA, Bourland JD. The strength-duration curve. IEEE Trans Biomed Eng. (1985) 32:458–9. doi: 10.1109/tbme.1985.325456
62. Demeco A, Marotta N, Moggio L, Pino I, Marinaro C, Barletta M, et al. Quantitative analysis of movements in facial nerve palsy with surface electromyography and kinematic analysis. J Electromyogr Kinesiol. (2021) 56:102485. doi: 10.1016/j.jelekin.2020.102485
Keywords: electrical stimulation, surface electrodes, facial paralysis, facial palsy, zygomaticus muscle, muscle atrophy, reinnervation
Citation: Arnold D, Thielker J, Klingner CM, Guntinas-Lichius O and Volk GF (2023) Selective zygomaticus muscle activation by ball electrodes in synkinetically reinnervated patients after facial paralysis. Front. Rehabil. Sci. 4:1205154. doi: 10.3389/fresc.2023.1205154
Received: 13 April 2023; Accepted: 25 September 2023;
Published: 16 October 2023.
Edited by:
Agnes Sturma, University of Applied Sciences Wien, AustriaReviewed by:
Kenji Kondo, The University of Tokyo, Japan© 2023 Arnold, Thielker, Klingner, Guntinas-Lichius and Volk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Fabian Volk ZmFiaWFuLnZvbGtAbWVkLnVuaS1qZW5hLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.