
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Rehabil. Sci. , 19 September 2022
Sec. Rehabilitation in Neurological Conditions
Volume 3 - 2022 | https://doi.org/10.3389/fresc.2022.987601
This article is part of the Research Topic Neuromuscular Adaptations to Sensorimotor Stimulation Protocols: Potential Rehabilitative Interventions for Individuals with Central or Peripheral Neuromuscular Injuries. View all 6 articles
Background: Research on stroke rehabilitation often addresses common difficulties such as gait, balance or physical activity separately, a fragmentation contrasting the complexity in clinical practice. Interventions aiming for recovery are needed. The purpose of this study was to investigate effects of a comprehensive low-cost physical therapy intervention, I-CoreDIST, vs. usual care on postural control, balance, physical activity, gait and health related quality of life during the first 12 weeks post-stroke.
Methods: This prospective, assessor-masked randomized controlled trial included 60 participants from two stroke units in Norway. Participants, who were randomized to I-CoreDIST (n = 29) or usual care physical therapy (n = 31), received 5 sessions/week when in-patients or 3 sessions/week as out-patients. Primary outcomes were the Trunk Impairment Scale-modified Norwegian version (TISmodNV) and activity monitoring (ActiGraphsWgt3X-BT). Secondary outcomes were the Postural Assessment Scale for Stroke, MiniBesTEST, 10-meter walk test, 2-minute walk test, force-platform measurements and EQ5D-3L. Stroke specific quality of life scale was administered at 12 weeks. Linear regression and non-parametric tests were used for statistical analysis.
Results: Five participants were excluded and seven lost to follow-up, leaving 48 participants in the intention-to-treat analysis. There were no significant between-group effects for primary outcomes: TIS-modNV (p = 0,857); daily average minutes of sedative (p = 0.662), light (p = 0.544) or moderate activity (p = 0.239) and steps (p = 0.288), or secondary outcomes at 12 weeks except for significant improvements on EQ5D-3L in the usual care group. Within-group changes were significant for all outcomes in both groups except for activity levels that were low, EQ5D-3L favoring the usual care group, and force-platform data favoring the intervention group.
Conclusions: Physical therapy treatment with I-CoreDIST improved postural control, balance, physical activity and gait during the first 12 weeks after a stroke but is not superior to usual care.
Stroke is a common cause of physical and cognitive disabilities. It is associated with lower levels of health-related quality of life (HRQOL) (1) and low levels of physical activity both during in-patient rehabilitation (2, 3) and in the long term (4, 5). Physical therapy is integral to the rehabilitation chain after a stroke, and is effective in reducing the burden of disability (6, 7). Strong evidence exists to support that increased dose and intensity of physical therapy increase functional gains (6). Recommendations, however, are often not achieved.
Research on stroke rehabilitation often addresses either gait, balance or upper limb function or specific treatments targeting single impairments (8). This fragmentation in research is in contrast to the complexity encountered by physical therapists in clinical stroke rehabilitation (9, 10), where the patients' movement problem often constitutes a combination of impairments and their mutual influence on each other. The main aims of physical therapy after a stroke are to improve walking, balance and functional movement (6), for which trunk control is a prerequisite (11–13). Reduced trunk control is common after a stroke and often persists into the sub-acute and chronic phases (12, 13). Such dysfunction is associated with poor functional mobility, reduced independence in activities of daily living and increased risk of falls (13–15). Recent reviews have concluded that there is evidence to support that trunk control, sitting and standing balance and mobility may significantly improve following trunk training after a stroke (13, 16–18). Findings support intensive rehabilitation treatment targeting trunk control to regain mobility and gait early after a stroke (14). The examined effect of trunk training is often in addition to usual care, thus separating the training of trunk control from the training of functional tasks, balance and gait. In daily activities these are inextricably linked, for example through the fine adjusted timing of anticipatory postural adjustments, that occur prior to the center of mass displacements associated with movements (19). The timing and symmetry of anticipatory postural adjustments are often affected after a stroke (20). There is a need to investigate if integrating trunk training and usual care could lead to greater functional gains.
New interventions in stroke rehabilitation should comprise clearly defined evidence (Langhorne 2009) and science-based methods (Nielsen 2015), and should aim to enhance recovery as opposed to compensatory strategies (21, 22). I-CoreDIST1 (Table 1) is a comprehensive, innovative rehabilitation method where activation of core muscles is enhanced and integral to all exercises without compromising focus on functional tasks or intensity. We support Kibler's (23) definition of core stability as “the ability to control the position and motion of the trunk over the pelvis and leg to allow optimum production, transfer and control of force and motion to the terminal segment in integrated kinetic chain activities”, (p. 190). This view incorporates an extended perspective of core muscles as all muscles on the trunk and those attached to the trunk, thus including muscles on the shoulder and hip girdle. The novelty of this approach lies within its integration of core muscle activation into exercises that incorporate functional activities, muscle strength, active muscle lengthening, upper limb function, gait and endurance. The structured assessment, clinical reasoning aids and the variation of exercises ensures individual tailoring and specificity. I-CoreDIST is designed to follow the patient through the course of rehabilitation, thus addressing fragmentation of care delivery and lack of continuity between care centers, a recognized barrier to recovery in stroke rehabilitation (9, 24, 25). The implementation of I-CoreDIST in the sub-acute stage after a stroke has successfully been explored in a non-controlled pilot study that demonstrated significant improvements in balance, postural control, walking-speed and -distance from baseline to 4 and 12 weeks (26).
The purpose of this study was to investigate the effect of I-CoreDIST when implemented in sub-acute, post-stroke physical therapy by addressing the following research question: Is physical therapy with I-CoreDIST better at improving postural control, levels of physical activity, balance, gait and HRQOL than usual care physical therapy when implemented during the first 12 weeks after a stroke.
This assessor-blinded, two arm parallel group, randomized controlled trial (RCT) was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT04069767) prior to inclusion of participants. The study adhered to the CONSORT guidelines and to guidelines for data protection set by the involved institutions.
The study was approved by the Regional Committee of Medical and Health Research Ethics (REK North: 2017/1961) and complies with the Declaration of Helsinki. All participants provided written informed consent prior to inclusion. The funders played no role in the design, conduct or reporting of this study.
The study was conducted in collaboration with two hospitals in two regions of Norway, two rehabilitation units and six surrounding municipalities. Participants were recruited at the hospitals stroke units where they underwent baseline testing prior to discharge and a follow-up assessment after 12 weeks. Inclusion started in September 2019 and ended in December 2021. Due to lockdown and subsequent restrictions related to the Covid-19 pandemic, inclusion and physiotherapy treatment for already included participants were stopped between March and June 2020.
Eligible participants, aged 18–85, had to be admitted to one of the two stroke units with a confirmed new stroke, have a premorbid modified Ranking Scale (mRS) of 0–3, be able to sit for 10 s at baseline testing, and to have a Trunk Impairment Scale-modified Norwegian version (TIS-modNv) score of <15. Exclusion criteria were inability to cooperate in physical therapy, ongoing substance abuse, severe disease, known dementia or other mental or cognitive disability preventing participation in physical therapy. After inclusion a baseline-assessment, evaluating trunk control, balance and gait along with self-administered questionnaire on health-related quality of life, was administered.
After baseline assessment, the participants were randomly assigned to one of two trial arms, A and B, in a 1:1 ratio. Randomization was stratified into two groups based on functional disability at baseline defined by mRS < 4 or ≥4 to minimize selection bias and to preserve homogeneity between arms. A digital solution, RedCap (Research Electronic Data Capture) tools hosted at the Northern Norway Regional Health Authority was used for randomization and data collection. Randomization was performed by an investigator, not connected to assessment or treatment of the patients, who informed the relevant physical therapist at rehabilitation units and/or municipalities of group allocation. The participants and the outcome assessors were blinded to group allocation.
The flow of patients through the study is summarized in Figure 1. The intervention period commenced after discharge from the stroke unit and lasted through the patient's individual rehabilitation course for 12 weeks. Time of and destination at discharge were not affected by participation in the study. Each physical therapy session lasted 60 min and was performed 5–6 days/per week if in a rehabilitation unit, and 3 sessions/week if in home based or outpatient treatment. Both groups received equal doses of physical therapy. Written reports followed the patient throughout the rehabilitation chain along with medical and multidisciplinary care as usual. Registrations of frequency and content of I-CoreDIST and usual care sessions were recorded for 12 weeks by the physical therapists.
The principles behind the I-CoreDIST intervention is outlined in Table 1. In I-CoreDIST structured core muscle activation is actively incorporated into exercises that simultaneously demand muscle strength, active muscle lengthening and endurance. These exercises specifically aim to improve, balance, gait, transfers upper limb function and functional activities, thus enhancing the training of the specific aspects of trunk function needed in everyday activities. The intervention started with an assessment to identify the patient's movement problems, supported by clinical reasoning charts, and contains 44 exercises, each with five levels of difficulty to allow for specificity and individualization. All physical therapists who treated participants in the I-CoreDIST group received 45 h of training prior to commencement of the study, one follow-up day during inclusion, and an educational package containing (1) the theoretical rationale behind the approach, (2) assessment and clinical reasoning charts and (3) images and descriptions of all exercises (Figures 2–4).
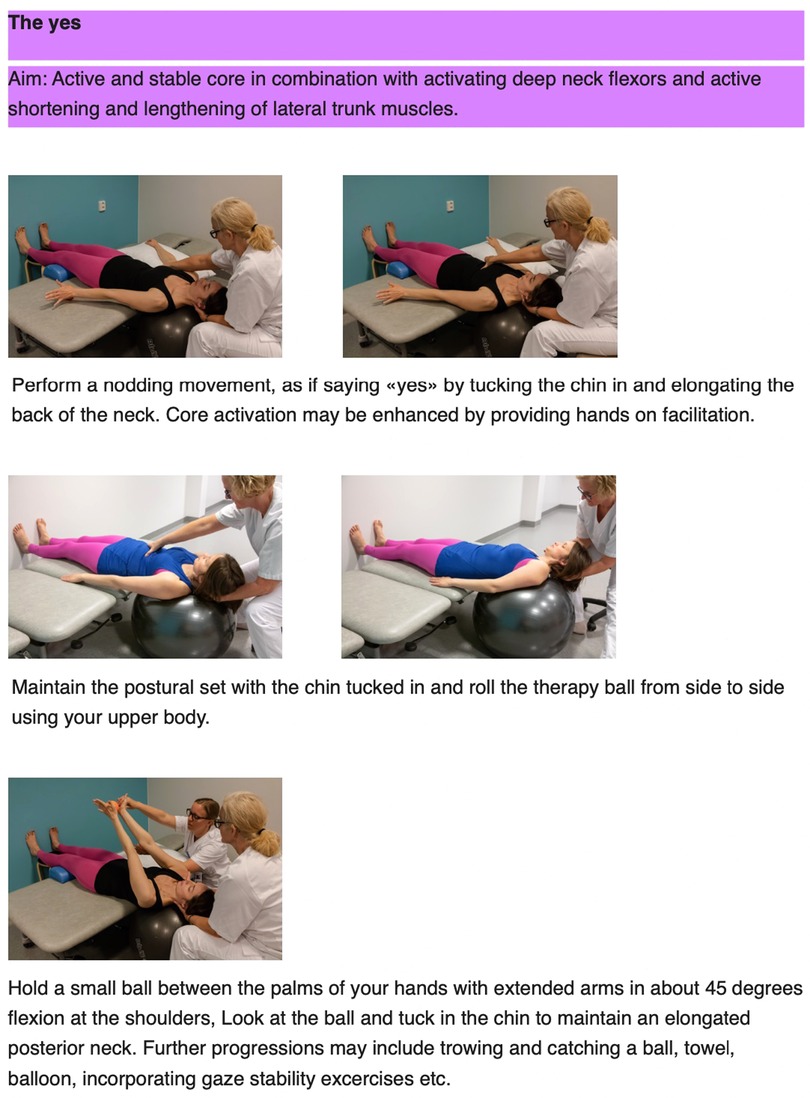
Figure 2. Example of exercise aiming for optimal adaptation to the base of support, an active core as well as enhancement of concentric and eccentric mucle activity in the neck.

Figure 3. Example of exercise aiming for optimal adaptation to the base of support, an active core, activity in large muscle groups in a standing position while challenging postural control and balance.

Figure 4. Example of exercise that aim for optimal adaptation to the base of support, an active core while practicing transferring the centre of gravity forward as in a sit to stand transfer.
There were no guidelines regarding the content of physical therapy, each individual therapist made treatment choices according to existing guidelines and what was usually offered to this patient group in that particular institution or municipality. The content of usual care in clinical practice in Norway is highly variable and poorly documented. Approaches towards stroke rehabilitation vary between the different schools of physiotherapy and traditions within institutions and municipalities. The Norwegian guidelines for treatment and rehabilitation after a stroke provide only general advice on including; intensive task related training containing a strength component for patients with paresis, training of transfers, gait and cardiovascular fitness, bilateral or constraint induced arm training (27). Specific training of trunk control is not a part of the recommendation for rehabilitation of sensorimotor disturbances after a stroke (27), but is part of the treatment tradition in some institutions.
The primary outcomes were trunk control, evaluated by TIS-modNV and physical activity, measured by an accelerometer and quantified into sedentary time, time in light, moderate and vigorous activity and number of steps. TIS-modNV is a 0–16-point scale, for which the ability to sit without support for 10 s is a prerequisite. It is considered a valuable tool for evaluation of trunk control and The scale has been proven reliable (ICC = 0.85) and valid for the stroke population (28), is sensitive, and do not have a ceiling effect.The minimal detectable change (MDC) is 2.9 points (28). ActiGraph Wgt3X-BT (ActiGraph, LCC, Pensacola, United States) is a 3-axis accelerometer used to record physical activity. It has been proven reliable in an adult population (29) and valid (ICC = 0.70) for use in the stroke population (30). Levels of physical activities are reflective of recovery of the activity limitation often experienced by stroke patients (31). The participants were instructed to wear the activity monitor in a waistband 24 h/day for seven consecutive days, after both baseline testing and the 12-week follow-up assessment. The participants were instructed to remove the device during showers/baths only. The devices were initialized and data were downloaded using ActiLife Software (ActiGraph, LCC, Pensacola, United States). Data were collected at a frequency of 100 Hz.
Secondary outcomes were postural control, balance, gait speed and distance, and HRQOL. We used the Swedish Postural Assessment Scale for stroke -Norwegian Version (SwePASS-NV) to measure postural control and the ability to maintain equilibrium during positional changes. It is sensitive for assessment of postural control after a stroke, and has excellent validity (α = 0.99, p < 0.001) (32), and reliability (ICC ≥ 0.99) (33). The scale ranges from 0 to 36 and has a ceiling, but no floor effect. The MDC in subacute stroke is 2.2 points (34). MiniBESTest was used to measure pro-and reactive balance in standing and walking on a scale from 0 to 28. It has a floor effect, as participants must be able to stand without support. The Norwegian version has shown good reliability (ICC = 0.95) and validity (35). The MDC for MiniBESTest is 3.2 points. In addition, the minimal clinically important difference (MCID) for detecting small changes is 4 points and five points for detecting substantial changes (36). Stability during quiet stance was assessed by calculating sway amplitude using AMTI AccuGait Optimized™ (Advanced Mechanical technology, Inc., Watertown, United States) multi-axis force plate system. Data on center of pressure (COP) displacements in cm were collected for 30 s with a frequency of 50 Hz (37) in the domains of eyes open and eyes closed and root mean square (RMS) values of the COP displacements were calculated. Reliability has been established for measuring COP displacements during quiet stance in the anteroposterior (AP) (ICC = 0.77) and mediolateral (ML) (ICC = 0.74) directions in a stroke population (38). Participants who were able to walk with or without an aid performed: (1) 10-Meter Walk Test (10 MWT), measuring walking speed (meters/s) at preferred and fast paces, reliable (ICC = 0.76) and valid for use in the stroke population (39, 40). MCID for 10 MWT preferred pace is 0.16 m/s (41) and 0.13 m/s for the 10 MWT fast pace (42) and (2) The 2-Minute Walk Test (2 MWT), measuring the total distance walked in two minutes, conducted on a 20 m walkway, also reliable (ICC = 0.85) for the stroke population (43). For non-ambulant participants, 0 meter/s was recorded at baseline or 12 weeks. HRQOL was reported using EQ-5D-3L and the stroke specific quality of life scale (SSQOL). EQ-5D-3L is a questionnaire used to assess self-perceived HRQOL, comprising five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, each with three levels of response, and a VAS scale (0–100) recording perceived health (44). EQ5D-3L has been proven reliable and valid for use in a stroke population (45, 46). SSQOL assesses health-related quality of life specific for stroke survivors. It is a 49-item questionnaire, addressing 12 domains: self-care, vision, language, mobility, work/productivity, upper extremity function, thinking, personality, mood, family roles, social roles and energy (47). The Norwegian translation has shown excellent reliability (ICC = 0.97) and validity (48). SSQOL was administered only at 12 weeks retest as it was not considered appropriate in the acute stage.
Sample size was calculated based on the mean and standard deviation of TIS-modNV-scores from a preceding pilot study (26). A difference of 0.67 standard deviation (SD) (1.93 points) between the intervention and the control group was considered clinically relevant. Thirty-seven individuals in each group were required to obtain an 80% chance to detect a difference of 1.93 points on TIS-modNV between the groups with a significance level of 0.05 (alpha) on two-sided tests.
Prior to statistical analysis the COP data were filtered using a fourth order Butterworth filter applied at 10 Hz (49) using BalanceClinic software (AMTI). The raw COP-data were imported to MatLab (Mathworks, Natick, MA, United States) where average RMS-values of COP-displacements in the AP (COPy) and ML (COPx) planes were calculated using the formula , and . Raw activity data were converted into daily average minutes of sedative time, light, moderate and vigorous activity using the ActiLife Software (ActiGraph, LCC, Pensacola, United States). Data were downloaded for all days, but day 1 and 8 were excluded due to differences in starting time. EQ5D profiles were summarized by calculating index values for each respondent (50). We utilized the value set from Denmark (51) as there is no available sets for Norway. This value set has previously been utilized in a Norwegian stroke population (52). Index values were also calculated for the SSQOL-data, converting scores from the 49 individual items into average scores for the 12 domains. Missing data were handled using person mean imputation and replaced by the domain average if one missing in a three-question domain or two missing in a five/six question domain. Forms were discarded if more than five missing items.
We performed an intention-to-treat analysis. Continuous variables are presented as means and standard deviations (SD) or median and interquartile range (IQR) depending on normality distribution. Categorical variables are presented as numbers and percentages. A multiple linear regression model was used to test if group allocation significantly predicted 12-week retest score when adjusting for baseline scores. If the data violated the assumptions for linear regression analysis, we performed a natural log transformation or used a Mann-Whitney U test for between-group differences. Within-group differences were calculated using paired samples t-test given a normal distribution of data and Wilcoxon signed rank test if not. Significance level was set at p < 0.05. All analyses were carried out using IBM SPSS (Statistics version 27 SPSS INC., Chicago IL).
A total of 60 participants were recruited between September 2019 and September 2021. Baseline characteristics are outlined in Table 2. Twentynine participants were randomized to the intervention group (I-CoreDIST) and 31 to the usual care group (Figure 1). The groups did not significantly differ in baseline characteristics, but there was a trend towards higher mean age (p = 0.17), lower premorbid levels of function (mRS) (p = 0.12) and higher scores for stroke severity on the NIH Stroke Scale (NIHSS) (p = 0.22) in the intervention group. The intervention group also had a higher rate of bilateral strokes, while the control group had a higher rate of hemorrhagic strokes. In the intervention group, six participants were lost to follow-up and another four were excluded from analysis. In the usual care group, one was lost to follow-up and one was excluded from analysis (Figure 1).
We used a multiple linear regression model for TIS-modNV, SwePASS-NV, MiniBesTEST, 10 MWT preferred and fast paces, activity data and 2 MWT and EQ5D-3L-scores (Table 3). There were some missing activity data at baseline as four monitors were not returned. In addition, three were excluded from analysis due to faulty monitors or a lack of registered activity in bouts exceeding that presumed to be inactivity. At retest, 15 participants did not attend or did not return the monitor, one was excluded due to little wear-time. Data in the categories of average minutes of moderate activity and average number of steps per day were skewed, thus natural log transformation were performed. The fitted regression model was a poor fit for the force platform data even after log transformation and as a result non-parametric tests were used to determine between-group differences.
Group allocation was not a significant predictor of 12-week retest score when adjusted for baseline differences for the primary outcomes TIS-modNV (p = 0.857), or for the activity data across all categories: Sedative minutes/day (p = 0.228), minutes of light activity/day (p = 0.155), minutes of moderate activity/day (p = 0.127), average number of steps/day (p = 0.887) (Table 3). Paired samples t-tests revealed significant within-group changes for TIS-modNV (p < 0.001) in both groups (Table 4) and Wilcoxons signed rank test showed significant within group changes in favor of the usual care group in the categories “minutes of moderate activity” per day (p = 0.005) and “average number of steps/day” (p = 0.042) for the activity data. There was a trend towards lower p-values for the intervention group regarding reduction in sedative minutes/day and increase in minutes of light activity/day (Table 4).
For the secondary outcome measures, the regression model and Mann-Whitney U test showed no significant differences between groups at 12-week retest (Tables 3, 5), except for EQ5D-3L-scores where group allocation significantly predicted 12-week retest scores in favor of the usual care group (p = 0.003) (Table 3). There were significant within-group changes in both groups on MiniBesTest (p < 0.001), 10 MWT at preferred pace (intervention group: p = 0.007, usual care group p < 0.001), SwePASS-NV (Intervention group: p = 0.001, usual care group p < 0.001) and 2 MWT (intervention group: p = 0.01, usual care group p ≤ 0.001). Only the usual care group showed significant improvements in 10 MWT fast pace (p < 0.001) and EQ5D (p < 0.001) at 12-week retest when compared to baseline (Table 4). Within-group changes for the force-platform data were significant in favor of the intervention group in the domain of COPx with eyes open (p = 0.05) and COPy with eyes open (p = 0.01) and eyes closed (p = 0.03) (Table 5).
Regarding the SSQOL, 43 forms were returned and 17 of these had missing data. Two were discarded due to two missing items in a three-question domain. Both groups shared similar trends with regards to which domains had the highest (“vision” and “self-care”) or lowest (“energy”) scores. The usual care group had higher median scores at 12 weeks in all domains, but “vision” where scores were equal (Table 6) and had a higher total index score at 12 weeks post stroke. Differences between groups were more pronounced in the cognitive-social-mental components than in the physical health components of the SSQOL (Figure 5). Mann-Whitney U test showed significant group differences in index scores, all favoring of the usual care group in the domains of “language” (p = 0.005), “mobility” (p = 0.036), “upper extremity function” (p = 0.011), thinking (p = 0.011), personality (p = 0.019) and mood (p = 0.006) domains.
The calculation of average number of weeks in physical therapy was based on the returned forms from the physical therapists (Supplementary Material). Participants in the intervention group: completed on average 7.94 (SD 3.45) weeks of physiotherapy. In the usual care group, the participants completed an average of 10.36 (SD 2.31) weeks of physiotherapy. Differences in how the forms were filled out made it difficult to determine the number of sessions completed by each participant.
Results show that there were no significant differences between groups following 12 weeks of intensive physiotherapy training with either I-CoreDIST or usual care when adjusted for baseline differences, suggesting that there were no added benefits from the implementation of I-CoreDIST during the sub-acute stage after a stroke. Our results are in line with previous research in stroke rehabilitation where results of clinical trials often are neutral (53, 54), meaning there is no statistical significant difference between groups at endpoint (55). We did encounter some well-known challenges in stroke rehabilitation RCT's, such as issues with recruitment rates, group heterogeneity and implementation fidelity that that are likely to have impacted upon results (53). In addition, the I-CoreDIST intervention is complex, defined as “containing several interacting components, targeting more than one organizational level of health care and offering considerable flexibility/tailoring” (56, 57). The intervention is low-cost and designed for implementation in clinical practice. While its flexibility allows for broad use and individualization, it is in opposition to the often highly standardized delivery of interventions in an RCT and would require increased power to yield statistically significant results. The registrations of content in treatment also suggest a degree of similarities between interventions as in the returned forms 71.4% of the usual care group reported having included postural control (Supplementary Material). However, interviews with a subgroup of participants (n = 19) revealed that experiences with participation in the study differed predominantly with regards to the content of therapy (58). Interviews confirm a greater focus on postural and movement control in the intervention group while participants in the usual care group describe an approach of structured training of strength and endurance measured through increased resistance or number of repetition (58).
Following 12 weeks of 3–5 weekly physiotherapy sessions, both groups showed both statistically and clinically significant improvements in measures of postural control and balance, sustained low levels of physical activity, and variable improvements in gait speed and distance.
For the primary outcomes, participants in both groups had a mean change near the previously reported MDC for TIS-modNV (29), indicating a true measure of improved trunk control exceeding that is associated with error. Only the usual care group showed statistically significant changes in activity levels for the categories of moderate activity and steps, equaling 56 moderate active mins/week and a daily average of 3,327 steps. Despite improvements in balance and that all participants had regained ambulation at 12-week retest, activity levels in both groups are well under the 150–300 min of moderate activity recommended for the general population in Norway (59) and the 20–60 min of aerobic activity 2–3 times/week recommended for the stroke population (60). There was a non-significant reduction in sedative minutes/day (Intervention: −27, Usual care: −7) and an increase in minutes of light activity/day (Intervention: 36, Usual care: 7) in favor of the intervention group. The high levels of sedative time, complete lack of vigorous physical activity and low average number of steps across groups is a cause for concern, both with regards to recovery and secondary prevention (60). Our results are in line with previous research on the stroke population (4), and may suggest suboptimal intensity in or duration of physical therapy sessions at baseline and little uptake of physical activity after the 12-week treatment period and retest. Apart from physical barriers, social factors, support and cognitive impairments have been suggested to influence levels of physical activity after a stroke (61, 62). These issues need further investigation.
With regards to secondary outcomes, improvements in PASS were statistically significant in both groups, though only the usual care group reached the MDC of 2.2 points. Both groups were within the category “good postural control” (31–36 points) at baseline and the previously reported ceiling effect in this measure (34, 63, 64). Both groups exceeded the required change of 5 points constituting substantial clinically important changes on the MiniBESTest (36), that together with improvements in TIS-modNV and PASS suggest overall improved postural control and balance in both groups. Force plate assessments of standing balance with eyes open and eyes closed showed statistically significant reduction in sway amplitudes in both AP and ML directions for the intervention group only implying improved balance control (49). This indicates that the focus on core activation and trunk control as recommended in the literature (13, 15, 16) and implemented in the I-CoreDIST intervention has resulted in reduced postural sway, that generally indicates improved postural stability (49, 65).
In measures of gait speed and distance, both groups exceeded the MCID on 10 MWT preferred pace (41) and fast pace (42), and displayed gait speeds well beyond the <0.8 m/s required for efficient community ambulation (66) at 12-week retest. Only the usual care group reached statistically significant within-group changes in 10 MWT fast pace. This suggests that the I-CoreDIST intervention did not target high walking speeds sufficiently.
Improvements in EQ5D were significant for the usual care group only and SSQOL-scores were generally lower in the intervention group. Group differences in SSQOL were more pronounced in the domains of thinking, personality, mood, social roles and energy than in the domains of self-care, vision, language, mobility, work/productivity and upper extremity function. The SSQOL and EQ5D indicate a lower HRQOL in the intervention group that seems more related to cognitive/mental than physical components. This may suggest a larger proportion of cognitive/mental problems in this group, which may have been caused by the stroke, result from the lower premorbid function, a higher age and stroke severity, or a combination of these. Exercise interventions are known to have small to moderate beneficial effects on HRQOL in physical and mental health domains that diminish at longer-term follow up, and no significant effects on societal or participatory domains, (67). The limited uptake of physical activity after the intervention, as indicated by the activity monitoring at 12-weeks along with lower HRQOL-scores on cognitive/mental components, supports these notions.
The major limitation of this study is that it is underpowered (n = 60) with regards to the sample size calculations (n = 74). In addition, ten participants were lost to follow-up in the intervention group. Four were excluded, and six discontinued physiotherapy or did not attend retest. The reasons given were mainly related to travel time to the physiotherapist/hospital and fear of Covid-19 on public transportation/in the physiotherapy clinic/hospital. With regards to implementation fidelity, further investigations into issues of recruitment and retention, such as barriers and effects of participation for both participants and physiotherapists and the quality of I-CoreDIST training and materials would have been beneficial. Participants in the usual care group, on average received physiotherapy for 2.4 weeks more than those in the intervention group. Registration forms revealed a vulnerability regarding absence, sick leave etc., particularly for the physiotherapists treating the intervention group. Only 1–2 physiotherapists had I-CoreDIST training on most sites, resulting in limited ability for another therapist to cover in case of absence. No additional training was required to treat the usual care group. These issues were further reinforced by Covid regulations and reallocation of staff related to the handling of pandemic. The 12-week follow up period is relatively short and a long-term follow up would have been beneficial.
A 12-week physiotherapy program with either I-CoreDIST or usual care implemented during the first 12 weeks showed no differences between groups, except for significant gains in HRQOL in favor of the usual care group. Both groups showed significant improvements on measures of postural control, balance and gait.
The datasets presented in this article are not readily available as we do not have permission from the ethical committee to share data. Requests to access the datasets should be directed tobWFyaWFubmUuc2l2ZXJ0c2VuMkBub3JkbGFuZHNzeWtlaHVzZXQubm8=.
The study involved human participants and was reviewed and approved by the Regional Committee for Medical Research Ethics North Norway (REK North: 2017/1961). All patients/participants provided written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
BN and ECA is responsible for the idea and development of the CoreDIST-intervention, and the adaptation to stroke patients together with MS. All authors have contributed to the design of the project and formulation of research goals and aims. BN and MS have been responsible for project administration and MS has been the site principal investigator. MS has been responsible for the investigation and data curation. All authors have participated in creating a statistical analysis plan. MS conducted the formal analysis of data, supervised by BN, KBA and ECA. MS drafted the manuscript. All authors contributed to the article and approved the submitted version.
The study was funded by the Northern Norway Regional Health Authority (Grant Number: HNF1459-19).
We would like to thank the individuals who participated in this study, Nordland and Nord-Trøndelag Hospital Trust for their support, the four physical therapists that conducted the testing and the physical therapists who treated the patients, the participating municipalities and the user representative. We extend our gratitude to Tonje Braathen for help with statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2022.987601/full#supplementary-material.
1I-CoreDIST: I = individualised, Core = trunk, D = dual task, I = intensive, S = specific, stability, somatosensory stimulation, T = teaching, training. Individualised Core activation combined with DISTal functional movement
1. Haley WE, Roth DL, Kissela B, Perkins M, Howard G. Quality of life after stroke: a prospective longitudinal study. Qual Life Res. (2011) 20(6):799–806. doi: 10.1007/s11136-010-9810-6
2. Sjoholm A, Skarin M, Churilov L, Nilsson M, Bernhardt J, Linden T. Sedentary behaviour and physical activity of people with stroke in rehabilitation hospitals. Stroke Res Treat. (2014) 2014:591897. doi: 10.1155/2014/591897
3. Barrett M, Snow JC, Kirkland MC, Kelly LP, Gehue M, Downer MB, et al. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil. (2018) 25(5):366–74. doi: 10.1080/10749357.2018.1458461
4. Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys Ther. (2017) 97(7):707–17. doi: 10.1093/ptj/pzx038
5. Saunders DH, Mead GE, Fitzsimons C, Kelly P, van Wijck F, Verschuren O, et al. Interventions for reducing sedentary behaviour in people with stroke. Cochrane Database Syst Rev. (2021) (6). doi: 10.1002/14651858.CD012996.pub2
6. Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst Rev. (2014) (4). doi: 10.1002/14651858.CD001920.pub3
7. Verbeek JM, van Wegen E, van Peppen R, van der Wees PJ, Heniks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS One. (2014) 9(2):e87987. doi: 10.1371/journal.pone.0087987
8. Dee M, Lennon O, O'Sullivan C. A systematic review of physical rehabilitation interventions for stroke in low and lower-middle income countries. Disabil Rehabil. (2020) 42(4):473–501. doi: 10.1080/09638288.2018.1501617
9. DiCarlo JA, Gheihman G, Lin DJ. Reimagining stroke rehabilitation and recovery across the care continuum: results from a design-thinking workshop to identify challenges and propose solutions. Arch Phys Med Rehabil. (2021) 102(8):1645–57. doi: 10.1016/j.apmr.2021.01.074
10. Langhorne P, Bernhardt J, Kwakkel G. Stroke care 2: stroke rehabilitation. Lancet. (2011) 377(9778):1693–702. doi: 10.1016/S0140-6736(11)60325-5
11. Likhi M, Jidesh VV, Kanagaraj R, George JK. Does trunk, arm, or leg control correlate best with overall function in stroke subjects? Top Stroke Rehabil. (2013) 20(1):62–7. doi: 10.1310/tsr2001-62
12. Verheyden G, Vereeck L, Truijen S, Troch M, Herregodts I, Lafosse C, et al. Trunk performance after stroke and the relationship with balance, gait and functional ability. Clin Rehabil. (2006) 20(5):451–8. doi: 10.1191/0269215505cr955oa
13. Van Criekinge T, Truijen S, Schröder J, Maebe Z, Blanckaert K, van der Waal C, et al. The effectiveness of trunk training on trunk control, sitting and standing balance and mobility post-stroke: a systematic review and meta-analysis. Clin Rehabil. (2019) 33(6):992–1002. doi: 10.1177/0269215519830159
14. Isho T, Usuda S. Association of trunk control with mobility performance and accelerometry-based gait characteristics in hemiparetic patients with subacute stroke. Gait Posture. (2015) 44:89–93. doi: 10.1016/j.gaitpost.2015.11.011
15. Cabrera-Martos I, Ortiz-Rubio A, Torres-Sánchez I, López-López L, Jarrar M, Valenza MC. The effectiveness of core exercising for postural control in patients with stroke: a systematic review and meta-analysis. PM/R. (2020) 12(11):1157–68. doi: 10.1002/pmrj.12330
16. Gamble K, Chiu A, Peiris C. Core stability exercises in addition to usual care physiotherapy improve stability and balance after stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2021) 102(4):762–75. doi: 10.1016/j.apmr.2020.09.388
17. Borges Souza DC, Santos M, da Silva Ribeiro NM, Maldonado IL. Inpatient trunk exercises after recent stroke: an update meta-analysis of randomized controlled trials. J Alzheimers Dis. (2019) 44(3):369–77. doi: 10.3233/NRE-182585
18. Ravichandran H, Sharma HR, Haile TG, Gelaw AY, Gebremeskel BF, Janakiraman B. Effects of trunk exercise with physioball to improve trunk balance among subjects with stroke: a systematic review and meta-analysis. J Exerc Rehabil. (2020) 16(4):313–24. doi: 10.12965/jer.2040292.146
19. Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett. (2012) 506(2):256–60. doi: 10.1016/j.neulet.2011.11.018
20. Curuk E, Lee Y, Aruin AS. Individuals with stroke use asymmetrical anticipatory postural adjustments when counteracting external perturbations. Motor Control. (2019) 23(4):461–71. doi: 10.1123/mc.2018-0083
22. Frykberg GE, Vasa R. Neuroplasticity in action post-stroke: challenges for physiotherapists. Eur J Physiother. (2015) 17:56–65. doi: 10.3109/21679169.2015.1039575
23. Kibler WB, Press J, Sciascia A. The role of core stability in athletic function. Sports Med. (2006) 36(3):189–98. doi: 10.2165/00007256-200636030-00001
24. Norrving B, Barrick J, Davalos A, Dichgans M, Cordonnier C, Guekht A, et al. Action plan for stroke in Europe 2018–2030. Eur Stroke J. (2018) 3(4):309–36. doi: 10.1177/2396987318808719
25. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2016) 47(6):e98–169. doi: 10.1161/STR.0000000000000098
26. Normann B, Arntzen EC, Sivertsen M. Comprehensive core stability intervention and coordination of care in acute and subacute stroke rehabilitation-a pilot study. Eur J Physiother. (2019) 21(4):187–96. doi: 10.1080/21679169.2018.1508497
27. Norwegian Directorate of Health. Nasjonal faglig retningslinje for behandling og rehabilitering ved hjerneslag. (National guidelines for treatment and rehabilitation in stroke). Oslo: Norwegian Directorate of Health (2017).
28. Gjelsvik B, Breivik K, Verheyden G, Smedal T, Hofstad H, Strand LI. The trunk impairment scale - modified to ordinal scales in the Norwegian version. Disabil Rehabil. (2012) 34(16):1385–95. doi: 10.3109/09638288.2011.645113
29. Aadland E, Ylvisaker E. Reliability of the actigraph GT3X + accelerometer in adults under free-living conditions. PLoS One. (2015) 10(8):e0134606. doi: 10.1371/journal.pone.0134606
30. Campos C, DePaul VG, Knorr S, Wong JS, Mansfield A, Patterson KK. Validity of the ActiGraph activity monitor for individuals who walk slowly post-stroke. Top Stroke Rehabil. (2018) 25(4):295–304. doi: 10.1080/10749357.2018.1446487
31. Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. (2010) 91(2):288–97. doi: 10.1016/j.apmr.2009.10.025
32. Benaim C, Perennou DA, Villy J, Rousseaux M, Pelissier JY. Validation of a standardized assessment of postural control in stroke patients - the postural assessment scale for stroke patients (PASS). Stroke. (1999) 30(9):1862–8. doi: 10.1161/01.STR.30.9.1862
33. Breistein K, Gjelsvik BEB, Jørgensen L. The postural assessment scale for stroke patients: translation into Norwegian, cultural adaptation, and examination of reliability. Eur J Physiother. (2017) 19(4):207–14. doi: 10.1080/21679169.2017.1334817
34. Chien C-WBS, Hu M-HP, Tang P-FP, Sheu C-FP, Hsieh C-LP. A comparison of psychometric properties of the smart balance master system and the postural assessment scale for stroke in people who have had mild stroke. Arch Phys Med Rehabil. (2007) 88(3):374–80. doi: 10.1016/j.apmr.2006.11.019
35. Hamre C, Botolfsen P, Tangen GG, Helbostad JL. Interrater and test-retest reliability and validity of the Norwegian version of the BESTest and mini-BESTest in people with increased risk of falling. BMC Geriatr. (2017) 17(1):92. doi: 10.1186/s12877-017-0480-x
36. Beauchamp MK, Niebuhr R, Roche P, Kirkwood R, Sibley KM. A prospective study to establish the minimal clinically important difference of the Mini-BESTest in individuals with stroke. Clin Rehabil. (2021) 35(8):1207–15. doi: 10.1177/02692155211025131
38. Gray VL, Ivanova TD, Garland SJ. Reliability of center of pressure measures within and between sessions in individuals post-stroke and healthy controls. Gait Posture. (2014) 40(1):198–203. doi: 10.1016/j.gaitpost.2014.03.191
39. Busk H, Holm P, Skou S, Seitner S, Siemsen T, Wienecke T. Inter-rater reliability and agreement of 6 minute walk test and 10 meter walk test at comfortable walk speed in patients with acute stroke. Physiother Theory Pract. (2022):1–9. doi: 10.1080/09593985.2022.2030830. [Epub Ahead of Print]35109744
40. Cheng DK-Y, Dagenais M, Alsbury-Nealy K, Legasto JM, Scodras S, Aravind G, et al. Distance-limited walk tests post-stroke: a systematic review of measurement properties. NeuroRehabilitation. (2021) 48(4):413–39. doi: 10.3233/NRE-210026
41. Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. (2010) 90(2):196–208. doi: 10.2522/ptj.20090079
42. Hiengkaew VP, Jitaree KM, Chaiyawat PP. Minimal detectable changes of the berg balance scale, fugl-Meyer assessment scale, timed “up / go” test, gait speeds, and 2-Minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. (2012) 93(7):1201–8. doi: 10.1016/j.apmr.2012.01.014
43. Kosak M, Smith T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. (2005) 42(1):103–7. doi: 10.1682/JRRD.2003.11.0171
44. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med. (2001) 33(5):337–43. doi: 10.3109/07853890109002087
45. Cameron LJ, Wales K, Casey A, Pike S, Jolliffe L, Schneider EJ, et al. Self-reported quality of life following stroke: a systematic review of instruments with a focus on their psychometric properties. Qual Life Res. (2022) 31(2):329–42. doi: 10.1007/s11136-021-02944-9
46. Hunger M, Sabariego C, Stollenwerk B, Cieza A, Leidl R. Validity, reliability and responsiveness of the EQ-5D in German stroke patients undergoing rehabilitation. Qual Life Res. (2012) 21(7):1205–16. doi: 10.1007/s11136-011-0024-3
47. Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. (1999) 30(7):1362–9. doi: 10.1161/01.STR.30.7.1362
48. Pedersen SG, Heiberg GA, Nielsen JF, Friborg O, Stabel HH, Anke A, et al. Validity, reliability and Norwegian adaptation of the stroke-specific quality of life (SS-QOL) scale. SAGE Open Med. (2018) 6:2050312117752031. -. doi: 10.1177/2050312117752031
49. Mansfield A, Inness EL. Force plate assessment of quiet standing balance control: perspectives on clinical application within stroke rehabilitation. Rehabil Process Outcome. (2015) 2015. doi: 10.4137/RPO.S20363
50. Szende A, Oppe M, Devlin N, EuroQol G. EQ-5D Value sets: inventory, comparative review and user guide. Dordrecht: Springer Netherlands: Imprint: Springer (2007).
51. Wittrup-Jensen KU, Lauridsen J, Gudex C, Pedersen KM. Generation of a danish TTO value set for EQ-5D health states. Scand J Public Health. (2009) 37(5):459–66. doi: 10.1177/1403494809105287
52. Waehler IS, Saltvedt I, Lydersen S, Fure B, Askim T, Einstad MS, et al. Association between in-hospital frailty and health-related quality of life after stroke: the nor-COAST study. BMC Neurol. (2021) 21(1):100. doi: 10.1186/s12883-021-02128-5
53. Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. (2020) 19(4):348–60. doi: 10.1016/S1474-4422(19)30415-6
54. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. (2009) 8(8):741–54. doi: 10.1016/S1474-4422(09)70150-4
55. Bresee L. The importance of negative and neutral studies for advancing clinical practice. Can J Hosp Pharm. (2017) 70(6):403–4. doi: 10.4212/cjhp.v70i6.1706
56. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new medical research council guidance. Br Med J (Clin Res Ed). (2008) 337:a1655. doi: 10.1136/bmj.a1655
57. Richards DA, Hallberg IR. Complex interventions in health: An overview of research methods. Beaverton: Ringgold Inc. (2015).
58. Sivertsen M, De Jaegher H, Arntzen EC, Alstadhaug KB, Normann B. Embodiment, tailoring, and trust are important for co-construction of meaning in physiotherapy after stroke: a qualitative study. Physiother Res Int. (2022) 27(3):e1948. doi: 10.1002/pri.1948
59. The Norwegian Directorate of Health. In: Health TNDo, editors. Physical activity in prevention and treatment. Oslo: The Norwegian Directorate of Health (2022). Avaiable at: https://www.helsedirektoratet.no/faglige-rad/fysisk-aktivitet-i-forebygging-og-behandling
60. Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45(8):2532–53. doi: 10.1161/STR.0000000000000022
61. Viktorisson A, Andersson EM, Lundstroem E, Sunnerhagen KS. Levels of physical activity before and after stroke in relation to early cognitive function. Sci Rep. (2021) 11(1):9078. doi: 10.1038/s41598-021-88606-9
62. Espernberger KR, Fini NA, Peiris CL. Personal and social factors that influence physical activity levels in community-dwelling stroke survivors: a systematic review of qualitative literature. Clin Rehabil. (2021) 35(7):1044–55. doi: 10.1177/0269215521993690
63. Huang Y-J, Lin G-H, Lee S-C, Hsieh C-L. A comparison of the responsiveness of the postural assessment scale for stroke and the berg balance scale in patients with severe balance deficits after stroke. J Geriatr Phys Ther. (2020) 43(4):194–8. doi: 10.1519/JPT.0000000000000247
64. Persson CU, Kjellberg S, Lernfelt B, Westerlind E, Cruce M, Hansson P-O. Risk of falling in a stroke unit after acute stroke: the fall study of gothenburg (FallsGOT). Clin Rehabil. (2018) 32(3):398–409. doi: 10.1177/0269215517728325
65. Quijoux F, Nicolaï A, Chairi I, Bargiotas I, Ricard D, Yelnik A, et al. A review of center of pressure (COP) variables to quantify standing balance in elderly people: algorithms and open-access code. Physiol Rep. (2021) 9(22):e15067. doi: 10.14814/phy2.15067
66. Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair. (2008) 22(6):672–5. doi: 10.1177/1545968308318837
Keywords: physical therapy, stroke, rehabilitation, trunk control, balance, gait, physical activity, health related quality of life
Citation: Sivertsen M, Arntzen EC, Alstadhaug KB and Normann B (2022) Effect of innovative vs. usual care physical therapy in subacute rehabilitation after stroke. A multicenter randomized controlled trial. Front. Rehabilit. Sci. 3:987601. doi: 10.3389/fresc.2022.987601
Received: 6 July 2022; Accepted: 30 August 2022;
Published: 19 September 2022.
Edited by:
Shih-Wei Huang, Taipei Medical University, TaiwanReviewed by:
Akhila Veerubhotla, New York University, United States© 2022 Sivertsen, Arntzen, Alstadhaug and Normann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marianne Sivertsen bWFyaWFubmUuc2l2ZXJ0c2VuMkBub3JkbGFuZHNzeWtlaHVzZXQubm8=
Specialty Section: This article was submitted to Rehabilitation in Neurological Conditions, a section of the journal Frontiers in Rehabilitation Sciences
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.