- 1School of Rehabilitation Science, McMaster University, Hamilton, ON, Canada
- 2Department of Physiology, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, Netherlands
- 3Centre for Heart, Lung, and Vascular Health, School of Health and Exercise Science, University of British Columbia, Kelowna, BC, Canada
- 4School of Physical Therapy, Western University, London, ON, Canada
- 5Memory and Motor Rehabilitation Laboratory (MEMORY-LAB), Feil and Oberfeld Research Centre, Jewish Rehabilitation Hospital, Montreal Center for Interdisciplinary Research in Rehabilitation, Laval, QC, Canada
- 6School of Physical / Occupational Therapy, McGill University, Montreal, QC, Canada
Introduction: Cognitive function is known to be associated with physical function, where greater walking capacity has been shown to have moderate to strong correlations with global cognitive function and other various domains of cognition in older adults with and without chronic conditions. Biological sex may moderate the relationship between cognitive and physical function, but whether sex differences exist in this association has not been examined in an aging population. The purpose of this study was to examine the associations between global cognitive function (Montreal Cognitive Assessment; MoCA), walking capacity (6-Minute Walk Test distance; 6 MWT) and sex in an aging population with broad ranges of cognitive and physical function.
Methods: Participants were assessed for global cognitive function (MoCA) and walking capacity (6 MWT). Multivariable regression analyses were performed to examine the interaction of sex in the association between MoCA and 6 MWT. First, we presented the unadjusted model (Model 1), then the model adjusted for age, history of stroke, and height (Model 2). To determine if there were sex-based differences in the association between global cognitive function and walking capacity, we included sex and an interaction term between sex*6 MWT distance in Models 3 and 4.
Results: Twenty-three females and 36 males were included in the multivariable regression analyses, respectively. Our sample represented broad ranges of cognitive and physical function levels, where MoCA scores ranged from 13 to 30, and 6 MWT distances from 203 to 750 m. 6 MWT distance was associated with MoCA in models unadjusted (R2 = 0.17; F(1,56) = 11.4; p < 0.01) and adjusted for age, stroke history, and height (R2 = 0.20; F(4,53) = 3.2; p = 0.02). No interaction with sex was found, but a main effect of sex was observed (R2 = 0.26; F(5,21) = 3.72; p = 0.03). When adjusting for age, height and history of stroke, males MoCA scores were 2.9 ± 1.3 less than the mean MoCA scores for females.
Discussion: Our findings confirm the positive relationship between cognitive and physical function in older adults. Notably, we also observed superior performance in global cognition among females that was consistent across a broad spectrum of walking capacity.
Introduction
Mild cognitive impairment is present in approximately 40% of older adults worldwide (1). It can impact performance of activities of daily living (2), and increase the risk of depression, apathy, irritability, lowered quality of life (3), and cardiovascular disease (4, 5). Cognitive function is known to be associated with physical function. Conversely, greater walking capacity [i.e., 6-Minute Walk Test (6 MWT) (6) distance] has been shown to have moderate to strong correlations with global cognitive function (7–14), memory (8, 9, 15), attention (9, 14, 16), verbal fluency (9, 14), and executive function (9, 14, 17) in older adults with (6–11, 15–17) and without (12–14) chronic conditions. With increasing age, declines in cognition (18) and walking capacity (19), as well as higher prevalence of chronic conditions such as stroke (20), often occur. Age-associated changes in physical function (21, 22) can compromise the ability for older adults to engage in physical activity (23), potentially contributing to further declines in cognitive and functional capacity.
Biological sex may moderate the relationship between cognitive and physical function, as it strongly influences aging-related processes, as well as the prevalence, diagnosis, severity, and outcomes of disease (24). It is unclear however whether the relationship between global cognitive function and walking capacity favours male or female sex. Levels of circulating brain derived neurotropic factor (BDNF), which plays an important role in promoting neuronal growth, brain plasticity and synaptic interactions (25), and regulates spinal density of mature neurons (26, 27), are higher in females (28, 29). Higher BDNF levels has also been attributed to the improvements observed in executive function in females following aerobic exercise interventions (30–32). Older females may also experience slower age-related declines in physical function such as aerobic fitness (33–38) where recent research has shown that females appear to be more efficient in oxygen extraction and uptake during walking compared to males (39).
Conversely, other studies have shown that fitness and physical function may be preserved to a greater extent in older males relative to females (40), attributed to declines in estrogen levels in females, reduced bone density and muscle mass, and greater central adiposity which contribute to reduced physical activity levels (41). However, when factors such as blood volume and oxygen carrying capacity are taken into account, aerobic fitness may not differ between males and females (42).
Given the evidence of sex differences in neurotrophic factors favouring older females and possible between-sex differences in physiology that contribute to physical function abilities, it may be that sex moderates the association between cognitive function and walking capacity. Thus, the objective of this study was to examine the moderating effect of sex on the associations between global cognitive function and walking capacity in an aging population with a range of cognitive and physical abilities.
Methodology
Study design
To include participants with a broad range of cognitive and physical function that is representative of an aging population, this secondary data analysis included community-dwelling middle aged and older adults with and without stroke. Data were pooled from an observational cohort study of individuals with and without stroke (n = 34 and 17, respectively) [Hamilton Integrated Research Ethics Board (HIREB) 13-348] and baseline data from a randomized controlled trial in middle aged and older adults with stroke (n = 11) [HIREB 4713; Centre de Recherche Interdisciplinaire en Réadaptation du Montréal Métropolitain (CRIR-1310-0218), clinical trials registry NCT03614585]. All participants provided written informed consent.
Participants
Participants without stroke were community dwelling older adults, 50–80 years old and able to walk at least 10 meters independently. Participants with stroke were 40–80 years old, ≥6 months following their first-ever stroke, living in the community, and able to walk at least 10-meters independently. We included the broader age range for individuals with stroke to account for the known mobility and cognitive impairment in this population. Individuals with stroke were excluded if they had a stroke of non-cardiogenic origin or tumor, scored <2 on the Modified Rankin Scale, had any contraindications to exercise testing (43), or class C or D American Heart Association Risk Criteria. Individuals were excluded if they possessed other neurological or musculoskeletal comorbidities, pain worsened with exercise, or cognitive, communication, or behavioural issues that could limit their ability to provide consent or follow instructions. In both studies, participants first completed their assessment of global cognitive function, which was subsequently followed by the assessment of walking capacity within the same visit to the lab. All participants were asked to abstain from food or drink for 4 h, smoking, caffeine and alcohol for 12 h and exercise for 24 h before each visit.
Assessments
Demographic information, including age, biological sex, and medical history were obtained in all participants. Stroke-related information were collected only for participants with stroke such as: time post-stroke, stroke type, location, and severity using the National Institutes of Health Stroke Scale (maximum score 42, higher scores indicate greater severity) (44).
Cognitive function
Global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) (45), which assesses attention, memory, visuospatial abilities, language, abstraction, concentration and orientation domains of cognition. The maximum possible score for the MoCA is 30, where higher scores indicate better function and scores <26 suggesting mild cognitive impairment (45). While the MoCA is generally used as a screening tool for cognitive impairment, previous studies have also used the MoCA and similar screening tools such as the Mini-Mental State Examination (MMSE) to explore the association between global cognitive function and walking capacity among older adults (7–14). The MoCA has excellent sensitivity and specificity in its ability to detect mild cognitive impairment, excellent internal consistency and concurrent validity with the MMSE (45), and excellent inter-rater reliability in older adults (46). The MoCA also has high sensitivity and specificity for detecting cognitive impairment in a cardiovascular population (47), and is feasible for cognitive screening in individuals with stroke (48), with excellent internal consistency and high concurrent validity with the MMSE (49, 50).
Walking capacity
The 6 MWT (6) was used to assess functional capacity. Standardized guidelines were followed (51). Participants were instructed to walk as far as possible over 6 min around a 65-meter indoor oval track, and total distance was measured using a measuring wheel. In a subset of 8 participants, the 6 MWT was conducted along a 20-meter straight indoor hallway due to space limitations. Total distance walked, resting and peak blood pressure, heart rate, and rate of perceived exertion were recorded. Participants were permitted to use gait aids and to take rest breaks if needed. Assessors refrained from providing encouragement throughout the test.
In older adults, the 6 MWT has high test-retest reliability and convergent validity with maximal treadmill testing (52). The 6 MWT has also demonstrated discriminant validity in its ability to distinguish between adults 60–69 years and 80–89 years of age, and between low- and high-active individuals (52). In individuals with stroke, the 6 MWT has excellent test-retest reliability (53), and concurrent validity with the Functional Independence Measure locomotion and motor subscales (54), V˙O2peak (53, 55), and preferred and fast walking speed (56).
Statistical analyses
Participants were included in the present analysis if they had data available on: (1) biological sex (2 levels: Male, Female); (2) walking capacity (6 MWT distance) and global cognitive function (MoCA); and (3) covariates of age, height and history of stroke (2 levels: Yes, No).
Participant demographics were described using means and standard deviations for normally distributed continuous variables, and medians and interquartile ranges for skewed data. Categorical variables were described using frequencies and percentages. All data was analyzed using StataIC Version 15 (StataCorp, College Station TX, USA) with an alpha set a priori at 0.05.
Multivariable regression analyses were performed to determine sex-based differences in the association between walking capacity (6 MWT distance, independent variable) and global cognitive function (MoCA score, dependent variable). We inspected variance inflation factors to ensure that multicollinearity was absent, and data were visually inspected for outliers and distribution. Assumptions for normality were tested using the Shapiro-Wilk test, while assumptions for homogeneity of the variances were tested using the Cook-Weisberg test. Residuals and leverage of the datapoints were examined for outliers. Any data points that influenced the beta-coefficients of the respective models by ≥10%, were deemed influential and thus removed (57).
Given our sample size of 59 participants, up to 6 variables were included in the regression models (58). We presented the unadjusted model (Model 1), then the model adjusted for age, history of stroke, and height (Model 2). Age and history of stroke were included due to their known associations with walking capacity (19, 23, 59–64) and global cognitive function (18, 65), and height was included to account for the known energy efficiency associated with walking with longer lower limb lengths (energy cost 2.6% lower for each 1 cm longer length) (66). To determine if there were sex-based differences in the association between global cognitive function and walking capacity, we included sex and an interaction term between sex*6 MWT distance in Models 3 and 4. The interaction term was subsequently removed, if deemed to be non-significant.
Results
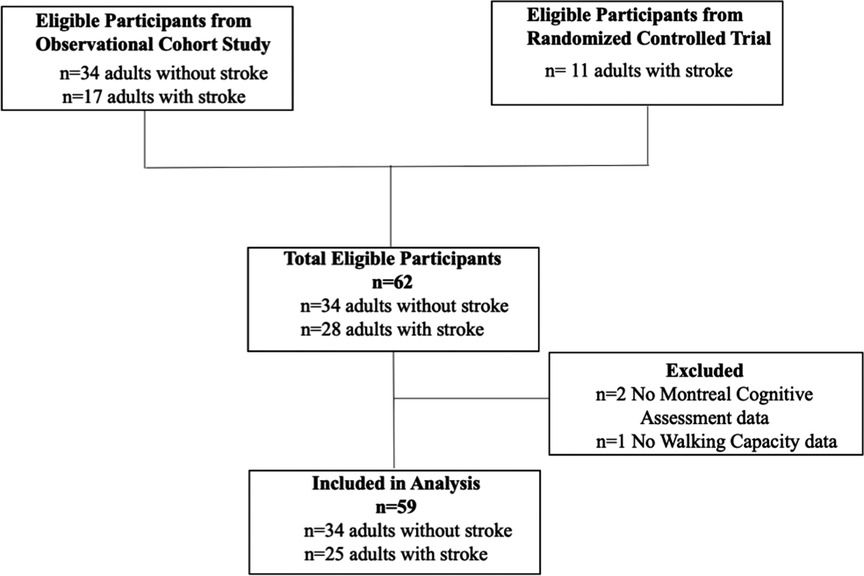
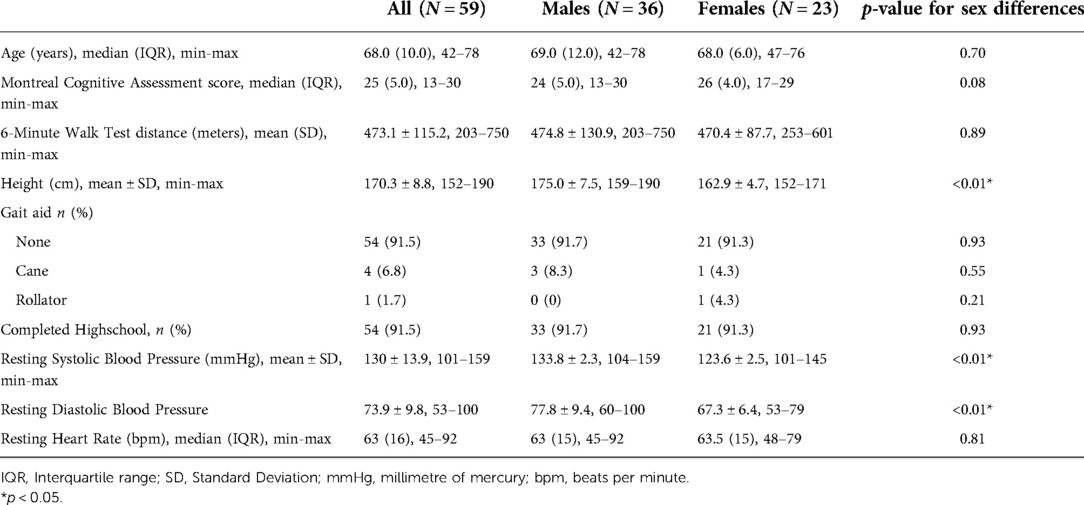
A flow chart of participants through the study is provided in Figure 1. Of 62 participants enrolled in both trials, the MoCA was not administered for two individuals with stroke due to aphasia and a language barrier, and 6 MWT was not performed for one individual with stroke due to significant knee pain. Therefore, 59 participants (23 females and 36 males) were included in the analyses. Participant characteristics are described in Table 1 for the entire sample, and disaggregated by sex.
For the 25 individuals with stroke, median time post-stroke was 3.3 years (IQR: 2.3) and were of mild to moderate severity (NIH-SS = 1.9 ± 1.2). Fifteen individuals (60%) experienced ischemic stroke, 5 (20%) experienced hemorrhagic stroke, and 5 (20%) were of unknown origin. Participants without stroke were older than those with stroke (70 (IQR: 6.0) vs. 62 (IQR: 14.0)). There were no sex differences in MoCA scores and 6 MWT distances among the individuals with stroke.
The whole sample represented a broad range of global cognitive impairment and walking capacities. MoCA scores ranged from 13 (severe cognitive impairment) to 30 (maximum score, no evidence of impairment) (45). The median MoCA scores for males was 24 (IQR: 5.0) and 26 (IQR: 4.0) for females, respectively. Distances walked on the 6 MWT were similarly broad, ranging from 203 to 750 meters, and representing 39–144% of age-matched reference values for males [mean (SD): 474.8 ± 130.9 meters], and 56–133% for females [mean (SD): 470.4 ± 87.7 meters] (67).
In the regression analysis, one outlier was removed due to its substantial influence (≥10%) on the beta-coefficient (n = 58 included in the final regression analysis). In the unadjusted model, 6 MWT distance was associated with global cognitive function, explaining 17% of the variance in MoCA scores (R2 = 0.17; F(1,56) = 11.44; p < 0.01) (Table 2; Model 1). In the model adjusted for age, history of stroke, and height, walking capacity was associated with global cognitive function (R2 = 0.20; R2 Change = 0.026; F(4,53) = 3.23; p = 0.02) (Table 2; Model 2). In Model 3, the interaction between sex and 6 MWT was deemed not significant and removed from the model. Sex alone was added to the final model, where a main effect of sex was observed (Model 4, R2 = 0.26; R2 Change = 0.068; F(5,21) = 3.72; p = 0.03). Figure 2 depicts the observed main effect of sex in the relationship between MoCA scores and 6 MWT distance when adjusting for age, height and history of stroke, whereby the males MoCA scores were 2.9 ± 1.3 less than the mean MoCA scores for females.
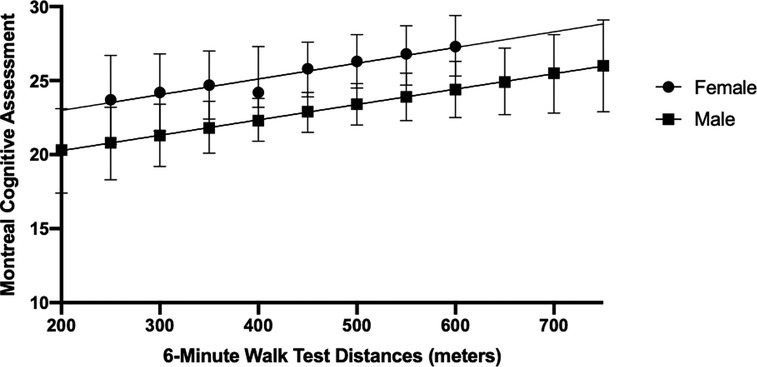
Figure 2. Marginsplot of sex differences in the association between global cognitive function (Montreal Cognitive Assessment scores) and Walking Capacity (6-Minute Walk Test distance) when adjusting for age, height and history of stroke. Main effect of sex (p < 0.05). Bars depict the 95% Confidence Intervals.
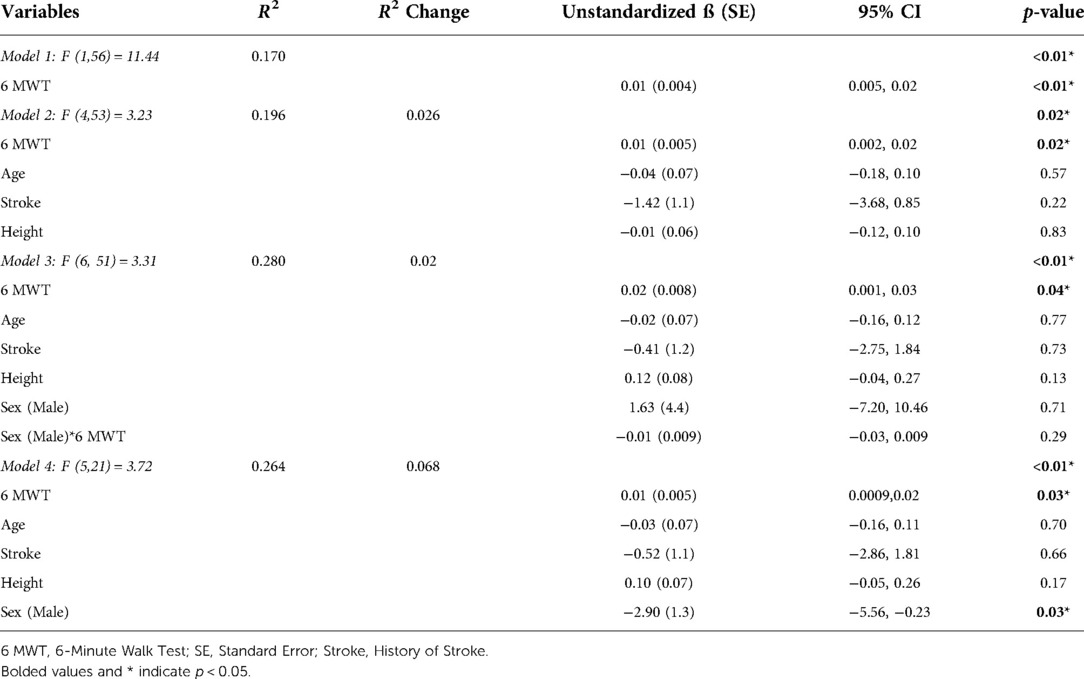
Table 2. Univariate and multivariable regression analyses examining the relationship between global cognitive function (Montreal Cognitive Assessment, dependent variable) and walking capacity (6-Minute Walk Test distance, independent variable) with models unadjusted (Model 1) and adjusted (Model 2), and sex (Model 3 for interaction, Model 4 for main effect).
Discussion
This study was the first to perform a sex-based analysis in the relationship between global cognitive function and walking capacity. The positive relationship between global cognitive function and walking capacity observed in the current study corroborate previous reports in older adults with less impairment in cognitive and physical function (12–14) and in populations with chronic conditions (6–11, 15–17). This body of evidence reinforces the importance of promoting exercise and physical activity behaviours to maintain cognitive and physical function among male and female older adults of all abilities. Indeed, exercise, in particular aerobic exercise and dual-task activities (cognitive tasks performed simultaneously with exercise), have the potential to improve physical fitness (68), and to induce structural and functional changes in the brain conducive for better cognitive functioning (69, 70). Older adults possessing high walking capacity have shown to have preserved grey matter (15) and reduced beta amyloid plaque accumulation (71) and are thus at lower risk for Alzheimer's disease (72).
While we did not observe an interaction between distance walked on the 6 MWT and sex in the association with cognitive function, there was a main effect of sex whereby males presented with lower MoCA scores than females across all distances of 6 MWT. Of note, the between-sex difference of 2.9 points in MoCA scores is clinically meaningful in older adult (73) and stroke (74) populations. Previous cohort studies have consistently reported that higher levels of physical activity participation are associated with reduced cognitive decline in older males (75). Thus, physical activity and exercise-based interventions may also be important for global cognitive function, particularly in older male adults.
The mechanisms underlying the apparent preservation of cognitive function in aging females relative to males are not entirely known, although neuroimaging studies may provide some insight. Older females present with greater gyrification (76) suggestive of attenuated brain atrophy, whereas older males have reduced white and grey matter parenchymal volume (77). Sex-specific morphological changes in the brain are consistent with observed functional differences as well, whereby females outperform males in areas of language, executive function, memory, attention and global cognition tasks from the third decade until the eighth decade of life (78–80).
Although beyond the scope of our current analysis, we acknowledge that hormonal influences may also be a contributing factor to the observed findings (81). Sex-based differences in cognitive function favouring younger adult females are also prominent in later adulthood in females with a history of estrogen supplementation or therapy use, suggesting a neuroprotective effect of estrogen even in older females (82–86). Estrogen aids in protecting against apoptosis and inflammatory processes such as beta amyloid accumulation, while also stimulating the synthesis of neurotransmitters, such as acetylcholine (87). Additionally, lower levels of circulating brain-derived neurotrophic factor, common in males (28, 29, 88), have been shown to be associated with poor cognitive function (89). Taken together, it may be that physiological and molecular mechanisms such as attenuated brain atrophy, the neuroprotective effect of estrogen in females with a history of estrogen supplementation or therapy, and higher levels of circulating neurotrophins critical to brain function may help explain the greater MoCA scores observed among older females across all levels of walking function in our adjusted model analyses.
Limitations
We acknowledge there are limitations to this study. For the current analysis, we prioritized the key variables shown to be most influential on the relationship between walking capacity and global cognitive function. Future studies may explore additional variables that may influence the relationship between sex, walking capacity, and global cognitive function such as estrogen supplementation or estrogen replacement history (83–86). Due to the exploratory nature of this study, we also opted to use a broad assessment of global cognitive function using the MoCA rather than domain-specific measures. Males are known to achieve greater outcomes on tasks of visuospatial abilities, while females outperform males in areas of language, executive function, memory, as well as global cognitive function (78–80). Future research may expand on these findings by examining sex-based differences in the associations between specific cognitive domains with walking capacity in an aging population.
Conclusion
Our findings confirm the positive relationship between cognitive and physical function in older adults. Notably, we also observed superior performance in global cognition among females that was consistent across a broad spectrum of walking capacity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Hamilton Integrated Research Ethics Board (HIREB) (13-348 and HIREB 4713); Centre de Recherche Interdisciplinaire en Réadaptation du Montréal Métropolitain (CRIR-1310-0218), clinical trials registry NCT03614585). The patients/participants provided their written informed consent to participate in this study.
Author contributions
EW and AT conceived the article. EW contributed to data collection and analysis, interpretation, and led the manuscript preparation. KSN, KM, ND, DAS, LR, HF, MR, and AT contributed to data collection and contributed to manuscript preparation. JR and JCM contributed to interpretation and manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
EW is supported by the McMaster Institute for Research on Aging and AGEWELL NCE Inc., a member of the Networks of Centres of Excellence program. EW is also supported by an Ontario Graduate Fellowship. KSN is supported by an Ontario Graduate Scholarship. KM is supported by an Ontario Graduate Scholarship. JCM holds a Canadian Institutes of Health Research Chair in Gender, Work and Health and the Dr James Roth Chair in Musculoskeletal Measurement and Knowledge Translation. LR holds a Doctoral Fellowship from Healthy Brains for Healthy Lives McGill and doctoral student bursary from the Quebec Rehabilitation Research Network (Réseau Provincial de Recherche en Adaptation-Réadaptation; REPAR). MR is supported by a Fonds Recherche Québec Santé (Junior I) Salary Award. AT is supported by a Clinician-Scientist Award (Phase II) from the Ontario Heart / Stroke Foundation (P-19-TA-1192).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. (2017) 29(10):1595–608. doi: 10.1017/S1041610217000473
2. Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. (2002) 58(5):758–64. doi: 10.1212/WNL.58.5.758
3. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. (2002) 288(12):1475–83. doi: 10.1001/jama.288.12.1475
4. Hussin NM, Shahar S, Yahya HM, Din NC, Singh DKA, Omar MA. Incidence and predictors of mild cognitive impairment (MCI) within a multi-ethnic Asian populace: a community-based longitudinal study. BMC Public Health. (2019) 19(1):1159. doi: 10.1186/s12889-019-7508-4
5. Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular disease risk factors and cognition in the elderly. Curr Cardiovasc Risk Rep. (2011) 5(5):407–12. doi: 10.1007/s12170-011-0189-x
6. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. (1985) 132(8):919–23. PMID: 3978515
7. Baldasseroni S, Mossello E, Romboli B, Orso F, Colombi C, Fumagalli S, et al. Relationship between cognitive function and 6-minute walking test in older outpatients with chronic heart failure. Aging Clin Exp Res. (2010) 22(4):308–13. doi: 10.1007/BF03324936
8. Cavalcante BR, Germano-Soares AH, Gerage AM, Leicht A, Tassitano RM, Bortolotti H, et al. Association between physical activity and walking capacity with cognitive function in peripheral artery disease patients. Eur J Vasc Endovasc Surg. (2018) 55(5):672–8. doi: 10.1016/j.ejvs.2018.02.010
9. Ferreira NV, Cunha PJ, da Costa DI, dos Santos F, Costa FO, Consolim-Colombo F, et al. Association between functional performance and executive cognitive functions in an elderly population including patients with low ankle–brachial index. Clin Interv Aging. (2015) 10:839–47. doi: 10.2147/CIA.S69270
10. Hayashi K, Oshima H, Shimizu M, Kobayashi K, Matsui S, Nishida Y, et al. Preoperative 6-minute walk distance is associated with postoperative cognitive dysfunction. Ann Thorac Surg. (2018) 106(2):505–12. doi: 10.1016/j.athoracsur.2018.03.010
11. Lee YH, Yoon ES, Park SH, Heffernan KS, Lee C, Jae SY. Associations of arterial stiffness and cognitive function with physical fitness in patients with chronic stroke. J Rehabil Med. (2014) 46(5):413–7. doi: 10.2340/16501977-1790
12. Matthé A, Roberson DN, Netz Y. The relationship between cognitive and physical function among residents of a Czech senior home. Acta Gymnica. (2015) 45(4):159–65. doi: 10.5507/ag.2015.019
13. Lord SR, Menz HB. Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil. (2002) 83(7):907–11. doi: 10.1053/apmr.2002.33227
14. Sherwood JJ, Inouye C, Webb SL, Zhou A, Anderson EA, Spink NS. Relationship between physical and cognitive performance in community dwelling, ethnically diverse older adults: a cross-sectional study. PeerJ. (2019) 7 [cited 2020 Jun 28]. p. 1–20. doi: 10.7717/peerj.6159. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6327882/30643695
15. Makizako H, Shimada H, Doi T, Park H, Yoshida D, Suzuki T. Six-minute walking distance correlated with memory and brain volume in older adults with mild cognitive impairment: a voxel-based morphometry study. Dement Geriatr Cogn Dis Extra. (2013) 3(1):223–32. doi: 10.1159/000354189
16. Schure MB, Borson S, Nguyen HQ, Trittschuh EH, Thielke SM, Pike KC, et al. Associations of cognition with physical functioning and health-related quality of life among COPD patients. Respir Med. (2016) 114:46–52. doi: 10.1016/j.rmed.2016.03.005
17. Loprinzi PD. Brief walking test and cognitive function among congestive heart failure patients: effect modification by duration of congestive heart failure. Int Cardiovasc Forum J. (2016) 6 [cited 2020 Jul 24]. p. 76–8. doi: 10.17987/icfj.v6i0.193. Available from: http://icfjournal.org/index.php/icfj/article/view/193
18. Murman DL. The impact of age on cognition. Semin Hear. (2015) 36(3):111–21. doi: 10.1055/s-0035-1555115
19. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. (1982) 37(5):560–4. doi: 10.1093/geronj/37.5.560
20. Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. (2010) 58(Suppl 2):S325–8. doi: 10.1111/j.1532-5415.2010.02915.x
21. Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. (2004) 7(4):405–10. doi: 10.1097/01.mco.0000134362.76653.b2
22. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. (2018) 47:123–32. doi: 10.1016/j.arr.2018.07.005
23. Törnbom K, Sunnerhagen KS, Danielsson A. Perceptions of physical activity and walking in an early stage after stroke or acquired brain injury. PLoS ONE. (2017) 12(3):e0173463. doi: 10.1371/journal.pone.0173463
24. Chiara M, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. (2010) 3(2):135–42. doi: 10.1161/CIRCOUTCOMES.110.868307
25. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. (2018) 38(3):579–93. doi: 10.1007/s10571-017-0510-4
26. Ji Y, Lu Y, Yang F, Shen W, Tang TTT, Feng L, et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. (2010) 13(3):302–9. doi: 10.1038/nn.2505
27. Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. (2014) 76(Pt C):628–38. doi: 10.1016/j.neuropharm.2013.05.029
28. Collins JM, Hill E, Bindoff A, King AE, Alty J, Summers MJ, et al. Association between components of cognitive reserve and serum BDNF in healthy older adults. Front Aging Neurosci. (2021) 13:489. doi: 10.3389/fnagi.2021.725914
29. Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, et al. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. (2012) 7(4):e35217. doi: 10.1371/journal.pone.0035217
30. Barha CK, Hsiung GYR, Best JR, Davis JC, Eng JJ, Jacova C, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. J Alzheimers Dis. (2017) 60(4):1397–410. doi: 10.3233/JAD-170221
31. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. (2003) 14(2):125–30. doi: 10.1111/1467-9280.t01-1-01430
32. Khattab S, Eng JJ, Liu-Ambrose T, Richardson J, MacDermid J, Tang A. Sex differences in the effects of exercise on cognition post-stroke: secondary analysis of a randomized controlled trial. J Rehabil Med. (2020) 52(1):jrm00002. doi: 10.2340/16501977-2615
33. Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc. (1987) 46(5):1824–9. PMID: 3493922
34. Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J Appl Physiol Respir Environ Exerc Physiol. (1982) 53(4):799–804. doi: 10.1152/jappl.1982.53.4.799
35. Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol. (2004) 97(2):781–9. doi: 10.1152/japplphysiol.00447.2003
36. Toth MJ, Gardner AW, Ades PA, Poehlman ET. Contribution of body composition and physical activity to age-related decline in peak VO2 in men and women. J Appl Physiol. (1994) 77(2):647–52. doi: 10.1152/jappl.1994.77.2.647
37. Weiss EP, Spina RJ, Holloszy JO, Ehsani AA. Gender differences in the decline in aerobic capacity and its physiological determinants during the later decades of life. J Appl Physiol. (2006) 101(3):938–44. doi: 10.1152/japplphysiol.01398.2005
38. Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. (2005) 112(5):674–82. doi: 10.1161/CIRCULATIONAHA.105.545459
39. Beltrame T, Villar R, Hughson RL. Sex differences in the oxygen delivery, extraction, and uptake during moderate-walking exercise transition. Appl Physiol Nutr Metab. (2017) 42(9):994–1000. doi: 10.1139/apnm-2017-0097
40. Liang J, Bennett JM, Shaw BA, Quiñones AR, Ye W, Xu X, et al. Gender differences in functional status in middle and older age: are there any age variations? J Gerontol B Psychol Sci Soc Sci. (2008) 63(5):S282–92. doi: 10.1093/geronb/63.5.S282
41. Kendall KL, Fairman CM. Women and exercise in aging. J Sport Health Sci. (2014) 3(3):170–8. doi: 10.1016/j.jshs.2014.02.001
42. Diaz-Canestro C, Pentz B, Sehgal A, Montero D. Sex differences in cardiorespiratory fitness are explained by blood volume and oxygen carrying capacity. Cardiovasc Res. (2021) 118(1):334–43. doi: 10.1093/cvr/cvab028
43. Riebe D, Franklin BA, Thompson PD, Garber CE, Whitfield GP, Magal M, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. (2015) 47(11):2473–9. doi: 10.1249/MSS.0000000000000664
44. Brott T, Adams H P, Olinger C P, Marler J R, Barsan W G, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20(7):864–70. doi: 10.1161/01.STR.20.7.864
45. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x
46. Feeney J, Savva GM, O’Regan C, King-Kallimanis B, Cronin H, Kenny RA. Measurement error, reliability, and minimum detectable change in the mini-mental state examination, montreal cognitive assessment, and color trails test among community living middle-aged and older adults. J Alzheimers Dis. (2016) 53(3):1107–14. doi: 10.3233/JAD-160248
47. McLennan SN, Mathias JL, Brennan LC, Stewart S. Validity of the montreal cognitive assessment (MoCA) as a screening test for mild cognitive impairment (MCI) in a cardiovascular population. J Geriatr Psychiatry Neurol. (2011) 24(1):33–8. doi: 10.1177/0891988710390813
48. Cumming TB, Marshall RS, Stroke LR, Deficits C. And rehabilitation: still an incomplete picture. Int J Stroke. (2013) 8(1):38–45. doi: 10.1111/j.1747-4949.2012.00972.x
49. Toglia J, Fitzgerald KA, O’Dell MW, Mastrogiovanni AR, Lin CD. The Mini-mental state examination and Montreal cognitive assessment in persons with mild subacute stroke: relationship to functional outcome. Arch Phys Med Rehabil. (2011) 92(5):792–8. doi: 10.1016/j.apmr.2010.12.034
50. Godefroy O, Fickl A, Roussel M, Auribault C, Bugnicourt JM, Lamy C, et al. Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. (2011) 42(6):1712–6. doi: 10.1161/STROKEAHA.110.606277
51. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (2002) 166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102
52. Rikli RE, Jones CJ. The reliability and validity of a 6-Minute walk test as a measure of physical endurance in older adults. J Aging Phys Act. (1998) 6(4):363–75. doi: 10.1123/japa.6.4.363
53. Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. (2004) 85(1):113–8. doi: 10.1016/S0003-9993(03)00436-2
54. Fulk GD, Echternach JL, Nof L, O’Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother Theory Pract. (2008) 24(3):195–204. doi: 10.1080/09593980701588284
55. Tseng BY, Kluding P. The relationship between fatigue, aerobic fitness, and motor control in people with chronic stroke: a pilot study. J Geriatr Phys Ther. (2009) 32(3):97–102. doi: 10.1519/00139143-200932030-00003
56. Tang A, Sibley KM, Bayley MT, McIlroy WE, Brooks D. Do functional walk tests reflect cardiorespiratory fitness in sub-acute stroke? J Neuroeng Rehabil. (2006) 3:23. doi: 10.1186/1743-0003-3-23
57. Tabachnick B, Fidell L. Using multivariate statistics. 6th ed. Vol. 28. Boston, MA, USA: Pearson (2012).
58. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. 2nd ed. New York: Springer-Verlag (2012) [cited 2020 May 7]. (Statistics for Biology and Health). Available from: https://www.springer.com/gp/book/9781461413523
59. Muren MA, Hütler M, Hooper J. Functional capacity and health-related quality of life in individuals post stroke. Top Stroke Rehabil. (2008) 15(1):51–8. doi: 10.1310/tsr1501-51
60. Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil. (2010) 91(10):1582–6. doi: 10.1016/j.apmr.2010.07.005
61. Patterson SL, Forrester LW, Rodgers MM, Ryan AS, Ivey FM, Sorkin JD, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. (2007) 88(1):115–9. doi: 10.1016/j.apmr.2006.10.025
62. Polese JC, Pinheiro MB, Faria CDCM, Britto RR, Parreira VF, Teixeira-Salmela LF. Strength of the respiratory and lower limb muscles and functional capacity in chronic stroke survivors with different physical activity levels. Braz J Phys Ther. (2013) 17(5):487–93. doi: 10.1590/S1413-35552012005000114
63. Kubo H, Nozoe M, Yamamoto M, Kamo A, Noguchi M, Kanai M, et al. Safety and feasibility of the 6-Minute walk test in patients with acute stroke. J Stroke Cerebrovasc Dis. (2018) 27(6):1632–8. doi: 10.1016/j.jstrokecerebrovasdis.2018.01.017
64. Carvalho C, Sunnerhagen KS, Willén C. Walking performance and muscle strength in the later stage poststroke: a nonlinear relationship. Arch Phys Med Rehabil. (2013) 94(5):845–50. doi: 10.1016/j.apmr.2012.11.034
65. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. (2014) 2(8):8. doi: 10.3978/j.issn.2305-5839.2014.08.05
66. Vannetti F, Pasquini G, Vitiello N, Molino-Lova R. Effects of lower limb length and body proportions on the energy cost of overground walking in older persons. ScientificWorldJournal. (2014) 2014 [cited 2020 Jun 4]. p. 1–6. doi: 10.1155/2014/318204. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4090425/25050389
67. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. (1998) 158(5 Pt 1):1384–7. doi: 10.1164/ajrccm.158.5.9710086
68. Paterson DH, Jones GR, Rice CL. Ageing and physical activity: evidence to develop exercise recommendations for older adults. Can J Public Health. (2007) 98(Suppl 2):S69–S108. doi: 10.1139/H07-111
69. Mandolesi L, Polverino A, Montuori S, Foti F, Ferraioli G, Sorrentino P, et al. Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front Psychol. (2018) 9 [cited 2021 Jun 15]. p. 1–11. doi: 10.3389/fpsyg.2018.00509. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5934999/
70. Jardim NYV, Bento-Torres NVO, Costa VO, Carvalho JPR, Pontes HTS, Tomás AM, et al. Dual-Task exercise to improve cognition and functional capacity of healthy older adults. Front Aging Neurosci. (2021) 13:33. doi: 10.3389/fnagi.2021.589299
71. Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. (2010) 67(1):71–9. doi: 10.1001/archneurol.2009.307
72. ten Kate M, Visser PJ, Bakardjian H, Barkhof F, Sikkes SAM, van der Flier WM, et al. Gray matter network disruptions and regional amyloid beta in cognitively normal adults. Front Aging Neurosci. (2018) 10 [cited 2021 Jan 16]. p. 1–11. doi: 10.3389/fnagi.2018.00067. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5863592/29599717
73. Krishnan K, Rossetti H, Hynan LS, Carter K, Falkowski J, Lacritz L, et al. Changes in Montreal cognitive assessment scores over time. Assessment. (2017) 24(6):772–7. doi: 10.1177/1073191116654217
74. Wu CY, Hung SJ, Lin KC, Chen KH, Chen P, Tsay PK. Responsiveness, minimal clinically important difference, and validity of the MoCA in stroke rehabilitation. Occup Ther Int. (2019) 2019 [cited 2021 May 19]. p. 1–7. doi: 10.1155/2019/2517658. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487084/
75. Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. (2013) 18(8):864–74. doi: 10.1038/mp.2012.162
76. Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Steinmetz H, et al. Gender differences in cortical complexity. Nat Neurosci. (2004) 7(8):799–800. doi: 10.1038/nn1277
77. Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. (1994) 14(8):4748–55. doi: 10.1523/JNEUROSCI.14-08-04748.1994
78. Caskie G, Schaie K, Willis S. Individual differences in the rate of change in cognitive abilities during adulthood. Gerontological society of America conference; San Francisco, USA (1999).
79. de Frias CM, Nilsson LG, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. (2006) 13(3–4):574–87. doi: 10.1080/13825580600678418
80. McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. (2016) 31(2):166–75. doi: 10.1037/pag0000070
81. Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The mayo clinic study of aging. Neurology. (2010) 75(10):889–97. doi: 10.1212/WNL.0b013e3181f11d85
82. Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Muñoz B, et al. Sex differences in cognition in healthy elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. (2012) 19(6):759–68. doi: 10.1080/13825585.2012.690366
83. Baldereschi M, Di Carlo A, Lepore V, Bracco L, Maggi S, Grigoletto F, et al. Estrogen-replacement therapy and Alzheimer’s disease in the Italian Longitudinal Study on Aging. Neurology. (1998) 50(4):996–1002. doi: 10.1212/WNL.50.4.996
84. Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. (2005) 76(1):103–5. doi: 10.1136/jnnp.2003.024927
85. Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. (1996) 156(19):2213–7. doi: 10.1001/archinte.1996.00440180075009
86. Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. (1996) 348(9025):429–32. doi: 10.1016/S0140-6736(96)03356-9
87. Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. (2008) 51(3):618–26. doi: 10.1097/GRF.0b013e318180ba10
88. Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore longitudinal study of aging. PLoS One. (2010) 5(4):e10099. doi: 10.1371/journal.pone.0010099
Keywords: global cognitive function, walking capacity, functional health, sex diferences, aging
Citation: Wiley E, Noguchi KS, Moncion K, D’Isabella N, Shkredova DA, Fang H, Richardson J, MacDermid JC, Rodrigues L, Roig M and Tang A (2022) The association between global cognitive function and walking capacity in individuals with broad ranges of cognitive and physical function: Are there sex differences?. Front. Rehabilit. Sci. 3:960437. doi: 10.3389/fresc.2022.960437
Received: 2 June 2022; Accepted: 26 August 2022;
Published: 12 September 2022.
Edited by:
Behdin Nowrouzi-Kia, University of Toronto, CanadaReviewed by:
Rodolfo Borges Parreira, Santa Casa of Sao Paulo, BrazilMasahiro Fujimoto, National Institute of Advanced Industrial Science and Technology (AIST), Japan
© 2022 Wiley, Noguchi, Moncion, D'isabella, Shkredova, Fang, Richardson, MacDermid, Rodrigues, Roig and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ada Tang YXRhbmdAbWNtYXN0ZXIuY2E=
Specialty Section: This article was submitted to Translational Research in Rehabilitation, a section of the journal Frontiers in Rehabilitation Sciences
 Elise Wiley
Elise Wiley Kenneth S. Noguchi
Kenneth S. Noguchi Kevin Moncion
Kevin Moncion Natalie D’Isabella1
Natalie D’Isabella1 Julie Richardson
Julie Richardson Joy C. MacDermid
Joy C. MacDermid Marc Roig
Marc Roig Ada Tang
Ada Tang