- 1Griffith Centre of Biomedical and Rehabilitation Engineering, Menzies Health Institute Queensland, Griffith University, Gold Coast, QLD, Australia
- 2BioLab³ - DIIEM - Roma Tre University, Roma, Italy
- 3Bionic Laboratory of Extremity Reconstruction and Department of Plastic & Reconstructive Surgery, Medical University of Vienna, Vienna, Austria
Editorial on the Research Topic
Bionic limb prostheses: advances in clinical and prosthetic care
by Frossard L, Conforto S and Aszmann OC. (2022). Front. Rehabilit. Sci. 3:950481. doi: 10.3389/fresc.2022.950481
Context
Importance of residuum health
The use of a prosthesis is essential to maintain function and wellbeing of individuals suffering from limb absence (1, 2). Consequently, providers of prosthetic care recommend bespoke interventions to sustain lenient interactions between individuals' residual limb and their prosthesis (3–7). The clinical management of this interface is critical because it greatly affects the residuum health (8).
Residuum health is influenced by intrinsic determinants inherent to personal demographics (e.g., gender, age, weight, and height) and surgical amputation (e.g., length of bone, muscle reassignments, muscle strength, and adipose tissue distribution) and extrinsic determinant-associated attachment (e.g., socket design and direct skeletal attachment) and prosthetic components (e.g., choice and alignment of components, control of the prosthetic joint movements, use of walking aids, and level of activity) (8). In all cases, interactions between intrinsic and extrinsic determinants are critical as residual tissues have limited physiological capacity to withstand direct loading applied by typical socket-suspended prostheses during daily activities (e.g., chafing and rubbing) (5, 9–12). In addition to general neurological residuum and phantom pain, individuals can experience a range of incapacitating neuromusculoskeletal dysfunctions compromising residuum health, such as acute and chronic skin issues, edema, neuroma, tendinitis, muscle contractures, stress fractures, osteopenia, and heterotopic bone growth, which altogether increases the risks of sound lower joints osteoarthrosis, and hyperlordosis (6, 13, 14).
Consequently, satisfactory residuum–prosthesis interface is difficult to achieve and sustain (15). Individuals with compromised residuum health are more at risk to experience unsuccessful prosthetic fitting arrangements (4, 16). Those with healthy residuum are more likely to maximize comfort, stability, and mobility when using a suitable prosthesis. Individuals tend to go up and down between low (e.g., bedridden, use of wheelchair, and two crutches without prosthesis), unsatisfactory (e.g., two crutches with prosthesis, one stick, and independent ambulation with pain), and satisfactory (independent ambulation without pain and participation in recreational and professional activities) levels of activity depending on their satisfaction with prosthetic fitting, functional abilities, and need for aids (17–20). Individuals are often trapped going back and forth between unsatisfactory and satisfactory health states depending on pain level with the prosthesis (19, 21). Pain leads to frequent, and too often permanent, prosthesis abandonment (22–24). Altogether, repeated episodes of care addressing prosthetic fitting generate great personal distress and heavy socioeconomic burdens (e.g., healthcare expenses and work absenteeism) (25–30).
Emergence of new bionic solutions
In the last few decades, we have witnessed promising developments in the production of bionic limb solutions that could possibly alleviate, separately or altogether, some of the residuum health and fitting issues (31–34). Some innovations provide better prosthetic attachment through osseointegrated implants that could either extend the residuum limb and facilitate socket fit (e.g., endoskeletal implant) or protrude the skin to allow the fitting of bone-anchored prostheses (e.g., endoskeletal-exoskeletal implant) (19, 35, 36). Other innovations aim predominantly to reduce pain and improve control of the prosthetic limbs, including regenerative peripheral nerve interfaces, targeted muscle reinnervation (TMR), agonist–antagonist myoneural interface, and sensory feedback (31, 37–40).
Altogether, these emerging bionic bone-anchored prostheses could dramatically alleviate socket-related issues and improve intuitive usage of artificial limbs (33, 41–43). Early evidence of the clinical outcomes of these new interventions has indicated that they have, altogether, the potential to engender life-changing benefits (e.g., body image, sitting comfort, osseoperception, pain reduction, prosthetic control, walking ability, and health-related quality of life) (44–47).
Need for more information about rehabilitation and prosthetic care bionic solutions
Reports of scientific advances of a particular solution tend to focus primarily on the design of interface between the body and the hardware (e.g., osseointegrated implants and electrodes), screening process (e.g., eligibility criteria), surgical techniques (e.g., number of stages and reinnervation matrices), fitting and design of prosthetic components (e.g., microprocessor-controlled joints and control algorithms) as well as short- to long-term outcomes (e.g., physical tasks and health-related quality of life) (48–52).
Although critical to successful clinical outcomes, rehabilitation procedures (e.g., training exercises) and prosthetic fitting recommendations (e.g., setting of components) for new solutions are often areas of continuous development and, therefore, are under-reported (53–59). The level of understanding and acceptance of pre- and postoperative clinical care may vary between interventions for lower or upper bionic limbs.
More information is needed to elucidate the relationships between surgical procedure, clinical care, prosthetic fitting, and outcomes of current and emerging interventions (e.g., efficacy and safety) that are critical for establishing an evidence-based reasonable, and eventually best, standard of care for current and future bionic solutions.
Contribution
Scope of the research topic
Initially, we identified a need for more information about:
• Preoperative interventions that could possibly maximize surgical and medical outcomes of bionic limb solutions (e.g., screening process, strength, and reconditioning, stretching program).
• Postoperative intervention following surgical insertion of osseointegrated implants (e.g., rehabilitation programs, prescription of loading progression, monitoring of loading exercises, design of static and dynamics load-bearing exercises, strength, and conditioning).
• Postoperative intervention after targeted muscle reinnervation (e.g., extraction of physiological signal, development of classifiers, design of fine and/or gross motor control training exercises, training for intuitive control).
• Fitting of bionic and/or bone-anchored prostheses (e.g., choice and alignment of prosthetic components, training with microprocessor-controlled joint units, fall prevention program).
• Short- and long-term outcome measures of efficacy and safety of bionic and/or bone-anchored prostheses extracted from standardized and non-standardized instruments (e.g., physical tasks and self-reported surveys).
• Quantitative evaluation of functional recovery with techniques based on kinematics and dynamics and on the processing of myoelectric signal of the non-amputee limb to study adaptation and recovery strategies also aimed at the optimal choice of prosthesis.
It will be unrealistic to expect that this Research Topic alone will outline the current “state-of-the-art” on these topics. Therefore, we decided to gather a series of highly focused articles presenting forthcoming ideas and concepts as well as preliminary data about current and emerging bionic solutions.
Outline of key contributions
This Research Topic features a total of 10 articles written by 54 authors (39% females and 61% males) from 23 institutions across 7 countries. It presents one Perspective, Review, Case Report, Brief Research Report, and six Original Research articles.
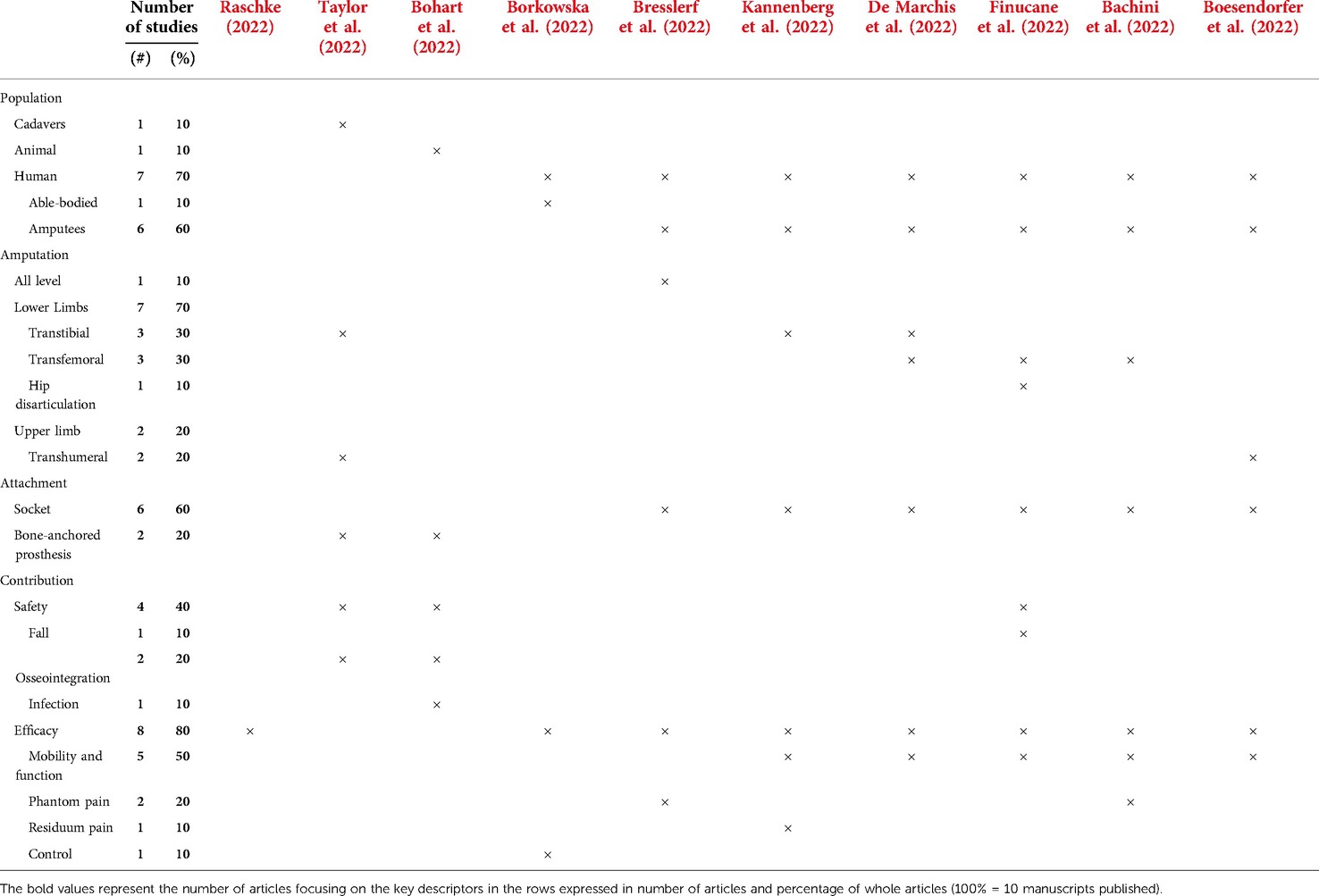
As detailed in Table 1, six manuscripts involved individuals with transtibial, transfemoral, hip disarticulation, and transhumeral amputations. Two other basic studies used cadavers and animal specimens. Six manuscripts focused on socket interface and three locked at the design of a percutaneous part, the osseointegration process, and the surgical procedure for direct skeletal attachment specific to bone-anchored prostheses. Four studies sought to improve safety of prosthetic care, more particularly reduction of fall, improvement of osseointegration, and reduction in the infection of future osseointegrated implants. Eight studies aimed at improving efficacy, particularly mobility and function, reduction of phantom and residuum pain, and control of prosthesis.
Overview of new bionic solutions
Raschke (2022) wrote an introductory review that provided critical insights into the historical developments of the prosthetic technology and practices within the greater context of successive industrial revolutions (Industry 1.0 to Industry 4.0). Raschke shared her astute perspective on the expected benefits of the current industry revolution. The unfolding Industry 4.0 is characterized by the convergence of physical, digital, and biological systems that support the creation of smart technology and cyber-physical systems enabling innovative bionic bone-anchored prostheses (e.g., advanced manufacturing, additive manufacturing, data analytics, augmented reality, simulation, horizontal/vertical integration, cybersecurity, cloud computing, and the industrial internet). Raschke also highlighted the importance of health economic assessments to determine the balance between the costs and the benefits of these innovations (25).
Taylor et al. (2022) used cadaveric mechanical testing, medical imaging, and finite-element analyses of humeri and tibia to improve the design of the percutaneous osseointegration docking system for direct skeletal prosthetic limb attachment. The translation of the exact system from the humerus to the tibia may not be suitable because of differences in impaction force and stress distribution. Each type of implant must be designed following a specific shape and mechanical constraints.
Bohart et al. (2022) used a porcine model to develop an infection-free integration between the skin and a percutaneous part of skin and bone integrated pylon for direct skeletal attachment of lower limb prostheses. Injections of botulinum toxin into the four thigh muscles of the distal thigh of the left hind leg were sufficient to provide noticeable immobilization the skin’s movement around the implant by the fourth week after the procedure. Injections of botulinum toxin might limit skin movements around a percutaneous part of an implant, thereby possibly reducing postoperative risks of infection.
Borkowska et al. (2022) performed a randomized cross-over study within able-bodied participants to assess the capacity of a new haptic sleeve to improve mechanotactile feedback. This study locked at changes in weak, normal, and strong grasp using visual, haptic, or combined feedback. The mechanotactile feedback provided by the haptic sleeve effectively improve grasping tasks and reduced energy expenditure.
Bresslerf et al. (2022) asked clinicians and end users to complete a System Usability Scale survey and semistructured interview to validate a new computer-assisted limb assessment (CALA) tool that can standardize documentation and visualization of phantom limb sensations and pain and quantify the patient’s body image. CALA allowed for an accurate description and quantitative documentation of phantom limb pain. This capacity to analyze, monitor, and report sensation and pain information can help to close the gap between the therapist's conception and the patient's perception of phantom limb sensation and pain.
Kannenberg et al. (2022) analyzed the outcomes of an online survey completed by 46 individuals with transtibial amputation to determine whether anecdotal reports on reduced musculoskeletal pain and improved patient-reported mobility were isolated occurrences or reflect a common experience in powered prosthetic ankle–foot users. Users reported improvements in mobility and reduction of sound knee and amputated side knee pain when using powered prosthetic ankle–foot compared with passive feet. However, a substantial proportion of powered prosthetic ankle–foot users also reverted to passive feet.
De Marchis et al. (2022) performed a multimodal prosthetic gait assessment using a series of kinematic, kinetic, and electrophysiological datasets collected on individuals with different types of amputations and prosthetic components for a project funded by the Italian Worker’s Compensation Authority. This study showed the importance of analyzing movement neural control and mechanical actuation of prosthetic limb as a whole rather than through segregated analyses focusing specific aspects. Multimodal prosthetic gait assessment can facilitate a more effective design of prostheses and therapies for patients fitted with conventional and new bionic limbs.
Finucane et al. (2022) asked individuals with a unilateral transfemoral or knee disarticulation amputation to follow new training (i.e., verbal, visual, tactile cueing, and patient education) to improve functional mobility (i.e., level-ground walking, stair climbing, incline walking, and sit-to-stand transitions) with a powered knee and ankle prostheses. This study provided new training techniques that can help individuals fitted with lower limb prostheses to take advantage of these powered devices and achieve their desired clinical outcomes.
Bachini et al. (2022) asked an individual with transfemoral amputation to wear four prosthetic interfaces stimulating specific areas of the residual limb (e.g., rigid and a semirigid socket with and without a focal pressure) to investigate if socket design can influence phantom sensations. Phantom sensations were different during distinct phases of the walking gait cycle depending on the four interfaces and led to changes in some gait spatiotemporal parameters. Phantom sensations were modulated by the prosthetic interface and could provide natural somatosensory information dynamically varying with gait phases.
Boesendorfer et al. (2022) reported the experience and outcomes of an individual who opted for an elective arm amputation to solve the lack of function due to obstetric brachial plexus injury. The participant showed a distinct improvement of function and high wearing times of the prosthesis at follow-up assessment. Selected patients who experience severe neurological deficit of biologic hand function might benefit from the elective amputation and subsequent restoration with the bionic hand.
Next steps
Sparking discussions
As highlighted by Raschke (2022), the successful development of bionic solutions integrating physical, digital, and biological systems will occur through a multitude of small increments. This Research Topic contributes to this global effort as it identifies knowledge gaps while, hopefully, sparking discussions about these new concepts capable of advancing clinical and prosthetic care of bionic limb prostheses.
From concept to standard of care
These articles should motivate more teams to engage in formalized research and publications further advancing these innovations. Accumulation of evidence through registered clinical trials will be required to facilitate clinical adoption and subsequent acceptance as standard of prosthetic care. Robust evidence will be required to overcome what Harris (2016) described as the “decline effect” (e.g., Initial strong results of new treatments tend to fade overtime with subsequent independent and stronger studies) (60). This will be critical to convince public and private healthcare funding bodies to support a particular innovation, particularly with the emergence of the fee-for-device business model (e.g., hospital, work cover, and insurance) (25–30).
Toward a global ecosystem
These clinical and prosthetic care innovations will contribute to the formation of a global ecosystem where a set of organizations and services will integrate the value chain of these bionic solutions through various commercial models. This emerging ecosystem will include providers of prosthetic solutions and administrators of healthcare organizations. More importantly, consumers will be at the heart of the ecosystem through involvement in the co-design of innovations and influence of consumers’ advocates. Involving all stakeholders will critical to warrant that these bionic innovations, indeed, improve safely the life of growing population of individuals suffering from limb loss worldwide.
Author contributions
LF contributed to conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, and funding acquisition. SC contributed to conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, and funding acquisition. OA contributed to conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing, visualization, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This contribution made by LF was partially supported by Bionics Queensland Challenge 2021 Major Prize–Mobility, the Australian National Member Society of the International Society for Prosthetics and Orthotics (ANMS ISPO) through the Research Grant awarded in 2021 and the FY19 Defense Medical Research and Development Program through the Joint Program Committee 8/Clinical and Rehabilitative Medicine Research Program Restoring Warfighters with Neuromusculoskeletal Injuries Research Award (RESTORE) under award no. W81XWH2110215-DM190659. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by these funding bodies (i.e., Bionics Queensland, ANMS ISPO, FY19 Defense Medical Research and Development Program. The contribution made by SC was partially supported by the program BRIC 2016-ID10 funded by INAIL.
Acknowledgments
The authors would like to express their gratitude to Agnes Sturma for her support during the preparation of this Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Demet K, Martinet N, Guillemin F, Paysant J, Andre JM. Health related quality of life and related factors in 539 persons with amputation of upper and lower limb. Disabil Rehabil. (2003) 25:480–6. doi: 10.1080/0963828031000090434
2. Shields C, Thorp H, Hendry G, Jayakaran P. Health-related quality of life in persons with dysvascular and traumatic lower limb amputation—a systematic review. Physiotherapy. (2015) 101(Suppl. 1):e673. doi: 10.1016/j.physio.2015.03.3514
3. Cavenett S. The effectiveness of total surface bearing compared to specific surface bearing prosthetic socket design on health outcomes of adults with a trans-tibial amputation: a systematic review. JBI Database of Systematic Reviews & Implementation Reports (2012).
4. McGrath MP, Gao J, Tang J, Laszczak P, Jiang L, Bader D, et al. Development of a residuum/socket interface simulator for lower limb prosthetics. Proc Inst Mech Eng H. (2017) 231:235–42. doi: 10.1177/0954411917690764
5. Dickinson A, Diment L, Morris R, Pearson E, Hannett D, Steer J. Characterising residual limb morphology and prosthetic socket design based on expert clinician practice. Prosthesis. (2021) 3:280–99. doi: 10.3390/prosthesis3040027
6. Pascale BA, Potter BK. Residual limb complications and management strategies. Curr Phys Med Rehabil Rep. (2014) 2:241–9. doi: 10.1007/s40141-014-0063-0
7. Fiedler G, Akins J, Cooper R, Munoz S, Cooper R. Rehabilitation of people with lower-limb amputations. Curr Phys Med Rehabil Rep. (2014) 2(4):263–72. doi: 10.1007/s40141-014-0068-8
8. Roy S, Mathew-Steiner S, Sen CK. Residual limb health and prosthetics. In: Vinjamuri R, editor. Prosthesis (2020). doi: 10.5772/intechopen.83819
9. Henao SC, Orozco C, Ramirez J. Influence of gait cycle loads on stress distribution at the residual limb/socket interface of transfemoral amputees: a finite element analysis. Sci Rep. (2020) 10:4985. doi: 10.1038/s41598-020-61915-1
10. Portnoy S, Yizhar Z, Shabshin N, Itzchak Y, Kristal A, Dotan-Marom Y, et al. Internal mechanical conditions in the soft tissues of a residual limb of a trans-tibial amputee. J Biomech. (2008) 41:1897–909. doi: 10.1016/j.jbiomech.2008.03.035
11. Bramley JL, Worsley PR, Bader DL, Everitt C, Darekar A, King L, et al. Changes in tissue composition and load response after transtibial amputation indicate biomechanical adaptation. Ann Biomed Eng. (2021) 49(12):3176–88. doi: 10.1007/s10439-021-02858-0
12. Frossard L, Beck J, Dillon M, Chappell M, Evans JH. Development and preliminary testing of a device for the direct measurement of forces and moments in the prosthetic limb of transfemoral amputees during activities of daily living. J Prosthet Orthot. (2003) 15:135–42. doi: 10.1097/00008526-200310000-00005
13. Paterno L, Ibrahimi M, Gruppioni E, Menciassi A, Ricotti L. Sockets for limb prostheses: a review of existing technologies and open challenges. IEEE Trans Biomed Eng. (2018) 65:1996–2010. doi: 10.1109/TBME.2017.2775100
14. Smith DG, Ehde DM, Legro MW, Reiber GE, Aguila M, Boone DA. Phantom limb, residual limb, and back pain after lower extremity amputations. Clin Orthop Relat Res. (1999) 361(361):29–38. doi: 10.1097/00003086-199904000-00005
15. Safari R. Lower limb prosthetic interfaces: clinical and technological advancement and potential future direction. Prosthet Orthot Int. (2020) 44(6):384–401. doi: 10.1177/0309364620969226
16. Steer JW, Worsley PR, Browne M, Dickinson A. Key considerations for finite element modelling of the residuum-prosthetic socket interface. Prosthet Orthot Int. (2021) 45:138–46. doi: 10.1177/0309364620967781
17. Pezzin LE, Dillingham TR, Mackenzie EJ, Ephraim P, Rossbach P. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil. (2004) 85:723–9. doi: 10.1016/j.apmr.2003.06.002
18. Kark L, Simmons A. Patient satisfaction following lower-limb amputation: the role of gait deviation. Prosthet Orthot Int. (2011) 35:225–33. doi: 10.1177/0309364611406169
19. Chadwell A, Diment L, Amigo EM, Morgado Ramirez DZ, Dickinson A, Granat MH, et al. Technology for monitoring everyday prosthesis use: a systematic review. engrXiv. (2020). doi: 10.31224/osf.io/ea2np
20. Frossard L, Hagberg K, Haggstrom E, Branemark R. Load-relief of walking aids on osseointegrated fixation: instrument for evidence-based practice. IEEE Trans Neural Syst Rehabil Eng. (2009) 17:9–14. doi: 10.1109/TNSRE.2008.2010478
21. Dillingham TR, Pezzin LE, MacKenzie EJ, Burgess AR. Use and satisfaction with prosthetic devices among persons with trauma-related amputations: a long-term outcome study. Am J Phys Med Rehabil. (2001) 80:563–71. doi: 10.1097/00002060-200108000-00003
22. Chatterjee S, Majumder S, RoyChowdhury A, Pal S. Review: problems with use of trans-tibial prosthesis. J Med Imaging Health Inform. (2016) 6:269–84. doi: 10.1166/jmihi.2016.1686
23. Smail LC, Neal C, Wilkins C, Packham TL. Comfort and function remain key factors in upper limb prosthetic abandonment: findings of a scoping review. Disabil Rehabil Assist Technol. (2021) 16:821–30. doi: 10.1080/17483107.2020.1738567
24. Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment: a survey of the last 25 years. Prosthet Orthot Int. (2007) 31:236–57. doi: 10.1080/03093640600994581
25. Raschke SU. Editorial opinion: value within the prosthetic and orthotic provision process. Can Prosthet Orthot J. (2022) 5(1). doi: 10.33137/cpoj.v5i1.38442.
26. Berg D, Frossard L. Health service delivery and economic evaluation of limb lower bone-anchored prostheses: a summary of the Queensland artificial limb service’s experience. Can Prosthet Orthot J. (2021) 4(2):1–22. doi: 10.33137/cpoj.v4i2.36210
27. Frossard L. Trends and opportunities in health economic evaluations of prosthetic care innovations. Can Prosthet Orthot J. (2021) 4(2):1–17. doi: 10.33137/cpoj.v4i2.36364
28. Guirao L, Samitier B, Frossard L. A preliminary cost-utility analysis of the prosthetic care innovations: case of the keep walking implant. Can Prosthet Orthot J. (2021) 4:1–16. doi: 10.33137/cpoj.v4i2.36366
29. Frossard L, Ferrada L, Quincey T, Berg D. Cost-effectiveness of transtibial bone-anchored prostheses using osseointegrated fixation: from challenges to preliminary data. J Prosthet Orthot. (2021) 33:184–95. doi: 10.1097/JPO.0000000000000372
30. Frossard L. A preliminary cost-utility analysis of the prosthetic care innovations: basic framework. Can Prosthet Orthot J. (2021) 4(2):1–0013. doi: 10.33137/cpoj.v4i2.36365
31. Marks LJ, Michael JW. Science, medicine, and the future: artificial limbs. Br Med J. (2001) 323:732–5. doi: 10.1136/bmj.323.7315.732
32. Farina D, Amsuss S. Reflections on the present and future of upper limb prostheses. Expert Rev Med Devices. (2016) 13:321–4. doi: 10.1586/17434440.2016.1159511
33. Aman M, Festin C, Sporer ME, Gstoettner C, Prahm C, Bergmeister KD, et al. Bionic reconstruction: restoration of extremity function with osseointegrated and mind-controlled prostheses. Wien Klin Wochenschr. (2019) 131:599–607. doi: 10.1007/s00508-019-1518-1
34. Pierrie SN, Gaston RG, Loeffler BJ. Current concepts in upper-extremity amputation. J Hand Surg Am. (2018) 43:657–67. doi: 10.1016/j.jhsa.2018.03.053
35. Guirao L, Samitier CB, Costea M, Camos JM, Majo M, Pleguezuelos E. Improvement in walking abilities in transfemoral amputees with a distal weight bearing implant. Prosthet Orthot Int. (2017) 41:26–32. doi: 10.1177/0309364616633920
36. Branemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. Bone Joint J. (2014) 96:106–13. doi: 10.1302/0301-620X.96B1.31905
37. Aman M, Sporer ME, Gstoettner C, Prahm C, Hofer C, Mayr W, et al. Bionic hand as artificial organ: current Status and future perspectives. Artif Organs. (2019) 43(2):109–18. doi: 10.1111/aor.13422
38. Schultz AE, Kuiken TA. Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PM R. (2011) 3:55–67. doi: 10.1016/j.pmrj.2010.06.016
39. Mastinu E, Branemark R, Aszmann O, Ortiz-Catalan M. Myoelectric signals and pattern recognition from implanted electrodes in two TMR subjects with an osseointegrated communication interface. Conf Proc IEEE Eng Med Biol Soc. (2018) 2018:5174–7. doi: 10.1109/EMBC.2018.8513466.
40. Ortiz-Catalan M, Mastinu E, Sassu P, Aszmann O, Branemark R. Self-contained neuromusculoskeletal arm prostheses. N Engl J Med. (2020) 382:1732–8. doi: 10.1056/NEJMoa1917537
41. Potter BK. From bench to bedside: a perfect fit? Osseointegration can improve function for patients with amputations. Clin Orthop Relat Res. (2016) 474:35–7. doi: 10.1007/s11999-015-4604-3
42. Aman M, Sporer ME, Gstoettner C, Prahm C, Hofer C, Mayr W, et al. Bionic hand as artificial organ: current status and future perspectives. Artif Organs. (2019) 43:109–18. doi: 10.1111/aor.13422
43. Aszmann OC, Vujaklija I, Roche AD, Salminger S, Herceg M, Sturma A, et al. Elective amputation and bionic substitution restore functional hand use after critical soft tissue injuries. Sci Rep. (2016) 6 :34960. doi: 10.1038/srep34960
44. Hagberg K, Ghassemi Jahani SA, Kulbacka-Ortiz K, Thomsen P, Malchau H, Reinholdt C. A 15-year follow-up of transfemoral amputees with bone-anchored transcutaneous prostheses. Bone Joint J. (2020) 102-B:55–63. doi: 10.1302/0301-620X.102B1.BJJ-2019-0611.R1
45. Atallah R, van de Meent H, Verhamme L, Frölke JP, Leijendekkers RA. Safety, prosthesis wearing time and health-related quality of life of lower extremity bone-anchored prostheses using a press-fit titanium osseointegration implant: a prospective one-year follow-up cohort study. PLoS ONE. (2020) 15:e0230027. doi: 10.1371/journal.pone.0230027
46. Lundberg M, Hagberg K, Bullington J. My prosthesis as a part of me: a qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. (2011) 35:207–14. doi: 10.1177/0309364611409795
47. Frossard L, Hagberg K, Häggström E, Gow DL, Brånemark R, Pearcy M. Functional outcome of transfemoral amputees fitted with an osseointegrated fixation: temporal gait characteristics. J Prosthet Orthot. (2010) 22:11–20. doi: 10.1097/JPO.0b013e3181ccc53d
48. Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. (2001) 38(2):175–81.11392650
49. Kunutsor SK, Gillatt D, Blom AW. Systematic review of the safety and efficacy of osseointegration prosthesis after limb amputation. Br J Surg. (2018) 105:1731–41. doi: 10.1002/bjs.11005
50. Yildiz KA, Shin AY, Kaufman KR. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: a review. J Neuroeng Rehabil. (2020) 17:43. doi: 10.1186/s12984-020-00667-5
51. Castiglia SF, Ranavolo A, Varrecchia T, De Marchis C, Tatarelli A, Magnifica F, et al. Pelvic obliquity as a compensatory mechanism leading to lower energy recovery: characterization among the types of prostheses in subjects with transfemoral amputation. Gait Posture. (2020) 80:280–4. doi: 10.1016/j.gaitpost.2020.06.013
52. Varrecchia T, Serrao M, Rinaldi M, Ranavolo A, Conforto S, De Marchis C, et al. Common and specific gait patterns in people with varying anatomical levels of lower limb amputation and different prosthetic components. Hum Mov Sci. (2019) 66:9–21. doi: 10.1016/j.humov.2019.03.008
53. Wood P, Small C, Mahoney P. Perioperative and early rehabilitation outcomes following osseointegration in UK military amputees. BMJ Mil Health. (2020) 166(5):294–301. doi: 10.1136/jramc-2019-001185
54. Vertriest S, Pather S, Sondergeld P, Frossard L. Rehabilitation programs after the implantation of transfemoral osseointegrated fixations for bone-anchored prostheses: a scoping review protocol. JBI Database System Rev Implement Rep. (2017) 15:1–13. doi: 10.11124/JBISRIR-2016-003023
55. Hagberg K, Branemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses: rehabilitation perspective. J Rehabil Res Dev. (2009) 46:331–44. doi: 10.1682/JRRD.2008.06.0080
56. Vertriest S, Coorevits P, Hagberg K, Branemark R, Haggstrom EE, Vanderstraeten G, et al. Static load bearing exercises of individuals with transfemoral amputation fitted with an osseointegrated implant: loading compliance. Prosthet Orthot Int. (2017) 41:393–401. doi: 10.1177/0309364616640949
57. Vertriest S, Coorevits P, Hagberg K, Branemark R, Haggstrom E, Vanderstraeten G, et al. Static load bearing exercises of individuals with transfemoral amputation fitted with an osseointegrated implant: reliability of kinetic data. IEEE Trans Neural Syst Rehabil Eng. (2015) 23:423–30. doi: 10.1109/TNSRE.2014.2337956
58. Frossard L, Gow DL, Hagberg K, Cairns N, Contoyannis B, Gray S, et al. Apparatus for monitoring load bearing rehabilitation exercises of a transfemoral amputee fitted with an osseointegrated fixation: a proof-of-concept study. Gait Posture. (2010) 31:223–8. doi: 10.1016/j.gaitpost.2009.10.010
Citation: Frossard L, Conforto S and Aszmann OC (2022) Editorial: Bionics limb prostheses: Advances in clinical and prosthetic care. Front. Rehabilit. Sci. 3:950481. doi: 10.3389/fresc.2022.950481
Received: 22 May 2022; Accepted: 18 July 2022;
Published: 18 August 2022.
Edited by:
Areerat Suputtitada, Chulalongkorn University, ThailandReviewed by:
Mohammad Sami Al-Wardat, Aqaba University of Technology, Jordan© 2022 Frossard, Conforto and Aszmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laurent Frossard bGF1cmVudGZyb3NzYXJkQG91dGxvb2suY29t
Specialty Section: This article was submitted to Medical and Surgical Rehabilitation, a section of the journal Frontiers in Rehabilitation Sciences
 Laurent Frossard
Laurent Frossard Silvia Conforto
Silvia Conforto Oskar C. Aszmann
Oskar C. Aszmann