
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Rehabil. Sci., 05 April 2022
Sec. Interventions for Rehabilitation
Volume 3 - 2022 | https://doi.org/10.3389/fresc.2022.857955
Introduction: At hospital stroke units, the time available to assess the patient's limitations in activities and participation is limited, although being essential for discharge planning. Till date, there is no quick-to-perform instrument available that captures the patient's actual performance during daily activities from a motor, cognitive, and communication perspective within the International Classification of Functioning, Disability and Health (ICF) framework. Therefore, the aim was to develop and validate a shortened version of the Lucerne ICF-Based Multidisciplinary Observation Scale (Short-LIMOS) that observes the patient's performance across ICF-domains and is applicable in the context of an acute stroke unit.
Methods: The Short-LIMOS was developed by reducing the original 45-item LIMOS to the ten most important items using a multivariable linear regression ANOVA with data of 836 stroke patients collected during inpatient neurorehabilitation. The Short-LIMOS's reliability, validity, and responsiveness were evaluated with data of 416 stroke patients in the acute stroke unit.
Results: A significant equation [F(10,825) = 232.083] with R2 of 0.738 was found for the following ten items for the Short-LIMOS: maintaining a body position (d415), changing basic body position (d410), climbing stairs (d4551), eating (d550), dressing (d540), communicating with—receiving—written messages (reading) (d325), applying knowledge, remembering facts (d179), solving complex problems (d1751), making simple decisions (d177), and undertaking a simple task (d2100). Principal component analysis revealed a Short-LIMOS motor and a Short-LIMOS cognition/communication component. The Short-LIMOS had a high internal consistency and good test-retest reliability. A moderate construct validity was shown by the significant correlation with the Barthel Index. The Short-LIMOS had neither floor nor ceiling effects.
Discussion and Conclusion: The developed Short-LIMOS was found to be reliable and valid within a population of (hyper)acute and subacute stroke patients. The added value of this multidisciplinary assessment is its comprehensiveness by capturing the patient's actual performance on the motor, cognitive, and communication domain embedded in an ICF-framework in <10 mins.
In an acute stroke unit setting, impairments due to stroke need to be detected within a noticeably short time in order to make appropriate decisions on acute medical treatment. In addition to acute medical treatment, it is crucial to assess which activities of daily living (ADLs) the patient can still perform and which not, as the ability to perform ADLs has been shown to be an important factor to consider in discharge planning (1–3). This requires an assessment tool that can capture and measure the patient's actual ADL performance. An acute stroke unit setting is not optimally suited for capturing the patients' actual ADL performance level: the premises are usually oriented toward acute medical care, and monitoring facilities often prevent patients to perform certain ADLs by themselves, even though they might theoretically be able to do so. Therefore, the observation and reliable assessment of ADL performance on acute stroke units represents a challenge. In this context, the development and validation of a specific ADL assessment tool for the acute stroke unit seems to be of crucial importance.
The Lucerne ICF-based Multidisciplinary Observation Scale (LIMOS) was developed to assess stroke patients' performance in the ADLs across the domains of the International Classification of Functioning, Disability and Health (ICF) (4). The LIMOS has an adequate reliability (4), validity (4, 5), and responsiveness (6), and is highly predictive of the patients' discharge destination after inpatient stroke rehabilitation (3). A central strength of the LIMOS is its comprehensiveness, i.e., it does not only include physical function during the ADLs, but also items regarding communication, learning and applying knowledge, interpersonal relationships, general tasks, and domestic life. Another advantage of the LIMOS is that—in contrast to, e.g., the Functional Independence Measure (FIM) (7) or the Barthel Index (BI) (8)—it does not present floor or ceiling effects (9, 10). Therefore, the LIMOS is particularly suited for the use in a subacute, multidisciplinary neurorehabilitation setting. However, with its 45 items, the scale is too extensive for the use in an acute stroke unit setting, in which decisions, amongst others regarding discharge destination, must be made in a noticeably short amount of time. Thus, for the acute stroke unit setting, a shortened version of the LIMOS is required. Apart from a short administration time, adequate measurement properties of the scale are needed to be useful in the environment of an acute stroke unit. Measurement properties reflect the quality of an outcome measure and can be assigned to one of the following three domains: reliability, validity, and responsiveness (11). Reliability is defined as “the degree to which the measurement is free from measurement error” (11). Validity reflects “the degree to which a health-related patient-reported outcome instrument [or: outcome measure] measures the construct(s) it purports to measure” (11). Finally, responsiveness refers to “the ability of a health-related patient-reported outcome instrument [or: outcome measure] to detect change over time in the construct to be measured” (11).
The aim of the present study was to develop a short version of the LIMOS (so-called Short-LIMOS), and to investigate its reliability, validity, and responsiveness. The goal of the Short-LIMOS was to be suitable for the use in an acute stroke unit setting, and to reflect not only the patients' physical functioning, but also their cognitive and communication performance.
The development and validation of the Short-LIMOS was achieved in two phases. In the first phase, the Short-LIMOS was developed by reducing the original 45-item LIMOS to the ten most important items. In the second phase, the Short-LIMOS's reliability, validity, responsiveness, and floor and ceiling effects were assessed.
A retrospective analysis of data routinely collected during inpatient stroke rehabilitation at the Neurocenter of the Luzerner Kantonsspital, Switzerland, between January 2014 and February 2019, was conducted.
Data from 836 stroke patients were analyzed. Patients with a cerebral stroke of any type, as confirmed by brain computed tomography or magnetic resonance imaging, were included. Patients were not included in the study if they were admitted for re-rehabilitation beyond the early subacute phase post-stroke (12). Patients received multidisciplinary rehabilitation according to national guidelines and local protocols (13), in which the focus lays on repetitive, task-oriented training.
Medical and demographic data such as age, sex, diagnosis, time since stroke, and length of stay in the neurorehabilitation center were collected from patient records.
With the LIMOS, the stroke patients' performance during the ADL is assessed in a reliable and valid manner (4, 6). The LIMOS was administered as part of the clinical routine by trained members of the multidisciplinary team (i.e., nurses, occupational therapists, physical therapists, speech therapists, and neurologists). Administration occurred within the first 72 h after admission to the neurorehabilitation center, and again within the last 72 h before discharge. The LIMOS is based on the ICF framework (14), and consist of 45 items that are categorized in four components: interpersonal activities, motor and self-care; communication; knowledge and general tasks; and domestic life. The patients are observed during everyday hospital life and not in a test situation; this is important since the patients' behavior can be influenced by the test situation itself (i.e., induce the patients to overachieve and not to behave at their typical level of performance). For each item, the level of assistance needed by the patient is assessed by the respective health professional by means of a 5-point Likert scale, with higher scores reflecting a higher independence degree, i.e., 1 = the patient is not able to fulfill a task at all or needs more than 75% of assistance (i.e., complete assistance); 2 = the patient is able to fulfill a task with an assistance of 25–75% (i.e., severe assistance); 3 = the patient is able to fulfill a task with an assistance of <25%, or under supervision (i.e., moderate assistance); 4 = the patient is able to fulfill a task independently, but needs increased time and/ or auxiliary materials/ aids (i.e., slight assistance); and, 5 = the patient is able to fulfill a task independently (i.e., no assistance needed). Correspondingly, the LIMOS total score (calculated as the sum of all item scores) ranges from 45 to 225, with higher scores representing a higher degree of independence (4, 6).
For all analyses, a two-tailed p-value with a critical threshold of <0.05 was used for accepting statistical significance. Descriptive statistics for nominal data were expressed as number of patients and percentages. Descriptive statistics for non-normally distributed quantitative data included median as well as first and third quartiles (Q1–Q3). Descriptive statistics for normally distributed data included mean and standard deviation.
In order to develop the Short-LIMOS, we aimed to define the LIMOS items that, at admission to the neurorehabilitation center (independent variables, see Supplementary Material 1), could explain most of the ADL performance at discharge, as assessed by the LIMOS total score (dependent variable). For this purpose, we first performed a multivariable, stepwise, forward linear regression analysis ANOVA, followed by an enter method linear regression ANOVA.
A prospective analysis of data collected between April 2019 and March 2021 as part of the clinical routine at the acute stroke unit at the Neurocenter of the Luzerner Kantonsspital, Switzerland was conducted.
Acute stroke patients were tested in the acute stroke unit. Patients received multidisciplinary rehabilitation according to national guidelines and local protocols (13), in which the focus lays on repetitive, task-oriented training in the acute stroke unit.
Medical and demographic data such as age, gender, diagnosis, time since stroke, National Institutes of Health Stroke Scale (NIHSS) (15, 16) score, and length of stay in the acute stroke unit were collected from patient records.
Each stroke patient was assessed with the Short-LIMOS within 72 h from admission to the acute stroke unit by trained occupational and physical therapists. These therapists were familiar with the use of the LIMOS, because they have been working at the neurorehabilitation center as well as at the acute stroke unit of the same institution. After a presentation and workshop about the development of the Short-LIMOS with the occupational and physical therapy teams, the use of the Short-LIMOS was introduced at the acute stroke unit. The assessment of the Short-LIMOS is provided in the Appendix (see Supplementary Material 2).
The BI measures ten items of basic everyday functions in self-care and mobility (8). The total score ranges from 0 to 20, with a score of 20 points indicating full independency in ADLs (17). The BI is recorded as part of the clinical routine within the first 24 h after admission by the nurses of the acute stroke unit.
For all analyses, a two-tailed p value with a critical threshold of <0.05 was used for accepting statistical significance. Descriptive statistics for nominal data were expressed as number of patients and percentages. Descriptive statistics for non-normally distributed quantitative data included median as well as first and third quartiles (Q1–Q3). Descriptive statistics for normally distributed data included mean and standard deviation.
Cronbach's alpha was calculated for testing the degree of interrelatedness among items [i.e., internal consistency (11)] of the Short-LIMOS. In addition, Cronbach's alpha was calculated for each common dimension that resulted from the principal component analysis (PCA). A value above 0.70 was considered to reflect an acceptable homogeneity among items within the total scale (11, 18).
Test-retest reliability is “the extent to which scores for patients who have not changed are the same for repeated measurement under several conditions: for example, using different sets of items over time (test-retest)” (11). To determine this measurement property, data from patients transferred from the acute stroke unit to the neurorehabilitation center were used (Figure 2). For this purpose, the ten LIMOS items that were included in the Short-LIMOS at admission to the neurorehabilitation center were compared with the ones of the Short-LIMOS performed at the acute stroke unit. For this comparison, the Intraclass Correlation Coefficient (ICC: two-way random model with effect of absolute agreement) (19) was calculated in a subgroup of 85 patients. An ICC value above 0.70 was considered as acceptable (20).
Measurement error is defined as “the systematic and random error of a patient's score that is not attributed to true changes in the construct to be measured” (11). To assess the measurement error, data from patients transferred from the acute stroke unit to the neurorehabilitation center were analyzed (Figure 2). The measurement error was determined by calculating the standard error of measurement (SEM), which is a measure of the error in the scores that is not due to true changes. The SEM was calculated by dividing the standard deviation of the difference between test and retest scores by square root of two (21, 22).
Structural validity refers to “the degree to which the scores of a health-related patient reported instrument [or: outcome measure] are an adequate reflection of the dimensionality of the construct to be measured” (11). A PCA was performed to explore the underlying dimensions of the Short-LIMOS. With the PCA, the degree to which the ten items of the Short-LIMOS were most strongly correlated with each other was defined, which allowed to build common dimensions within the total observation scale. A minimum eigenvalue of 1 was specified as extraction criterion. The criterion for factor loading was set at 0.40 (11, 23).
Hypotheses testing refers to construct validity and is defined as “the degree to which the scores of a health-related patient reported instrument [or: outcome measure] are consistent with hypotheses (for instance with regard to internal relationships, relationships to scores of other instruments, or differences between relevant groups) based on the assumption that the health-related patient reported instrument [or: outcome measure] validly measures the construct to be measured” (11). To strengthen construct validity, the Short-LIMOS scores were compared with the ones of the BI. We hypothesized that the sum score of the Short-LIMOS would positively and strongly correlate (i.e., rs ≥ 0.60) with the total score of the BI. Due to the implementation of a new electronic patient record system in September 2019, the BI from this time point onwards could be extracted. Therefore, we calculated the Pearson's correlation in a subgroup of 278 patients. Correlation coefficients of < 0.25 indicate little or no relationship; 0.25–0.50 suggest a fair degree; values of 0.50–0.75 are moderate to good; and values above 0.75 are considered good to excellent (24).
To assess “the degree to which the scores of a health-related patient-reported outcome [or: outcome measure] are an adequate reflection of a ‘gold standard”’ (11), the Spearman Rank correlation coefficient between the LIMOS and Short-LIMOS was calculated.
Based on the SEM, the distribution-based responsiveness was determined by calculating the Minimum Detectable Change , which describes with 90% certainty the amount of true change in subject status beyond measurement error (25). A Bland-Altman plot analysis was used to display the within-subject variability as well as the systematic difference between the Short-LIMOS items at acute stroke unit and the same ten items at admission to the neurorehabilitation center. The bias (mean difference; MD), its standard deviation (SD), as well as the upper and lower limits of agreement (defined as MD ± 1.96 * SD) were calculated (26).
The floor and ceiling effects reflect the extent to which scores cluster at the bottom or at the top of the scale range, respectively. Floor and ceiling effects were considered as present if 15% of the respondents scored the lowest or highest score on a scale, respectively (27).
The patient flow for the first phase is displayed in Figure 1. Baseline characteristics of the patients included are summarized in Table 1.
The first linear regression analysis ANOVA showed that 12 LIMOS items were influencing variables: dressing (d540), maintaining a body position (d415), communicating with—receiving—written messages (reading) (d325), applying knowledge, remembering facts (d179), solving complex problems (d1751), basic interpersonal interactions (d710), changing basic body position (d410), calculating (d172), climbing stairs (d4551), eating (d550), undertaking a simple task (d2100), making simple decisions (d177). A significant regression equation was found [F(12,823) = 199.514, p < 0.000] with an R2 of 0.744. Two items were removed, as they were classified as misfits by the LIMOS scale in an earlier performed Rasch analysis of the LIMOS (5). These items were basic interpersonal interactions (d710) and calculating (d172). The subsequent second linear regression analysis with the inclusion of the remaining ten LIMOS items also resulted in a significant equation [F(10,825) = 232.083, p < 0.000] with an R2 of 0.738.
A total of 480 patients were included in the second phase (Figure 1). During the pilot study, all Short-LIMOS files were complete. In the second phase, 64 (13.3%) Short-LIMOS files were not or partially filled out (Figure 1). Comparing patients with complete and incomplete files, we noted that there was no significant difference in terms of age (p > 0.5) and sex (p > 0.5). However, the 64 patients with missing data showed a significantly lower NIHSS score at hospital admission (p < 0.001) and a shorter length of stay at the acute stroke unit (p = 0.004) compared to those whose Short-LIMOS files were completed. For further statistical analyses, data of 416 patients with completed Short-LIMOS files were used.
A total of 416 patients were included in the validation study. The demographics and the baseline characteristics of the patients are presented in Table 1.
An excellent internal consistency was found for the whole Short-LIMOS (Cronbach's alpha = 0.959) and its motor (Cronbach's alpha 0.948) and cognition/communication (Cronbach's alpha 0.952) subscales.
Eighty-three patients were transferred from the acute stroke unit to inpatient neurorehabilitation within 1–4 days after being assessed with the Short-LIMOS [median 3 (2–3) days] (Figure 2). The test-retest reliability of the Short-LIMOS within 4 days was excellent (ICC = 0.922; 95% confidence interval 0.856–0.955; p < 0.001).
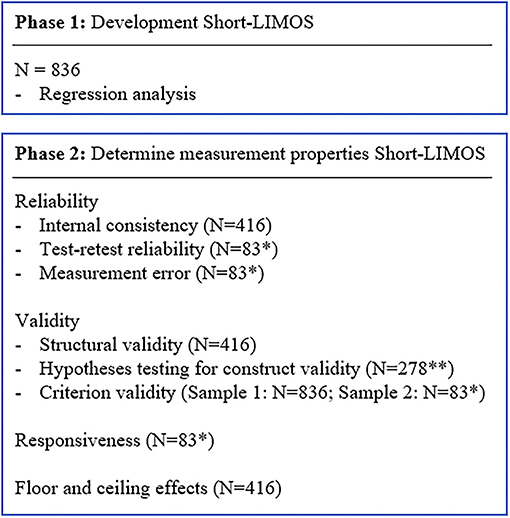
Figure 2. Flow chart of the statistical analyses for the development and validation phase. *Patients transferred from acute stroke unit to neurorehabilitation within 1–4 days; **Short-LIMOS total score and Barthel Index total score at admission acute stroke unit.
The SEM was 3.09 points. The Bland–Altman plot showed the comparison between the two measurement time points (Figure 3).
The PCA resulted in two components with an eigenvalue of above 1.0. A first, strong component had an eigenvalue of 7.41 and explained 74.12% of the variance and the second component had an eigenvalue of 1.09, which explained 10.91% of the variance. Both components together explained 85.03% of the variance.
The PCA revealed that all the ten items of the Short-LIMOS correlated at least with rs = 0.3 with at least one other item, suggesting reasonable factorability. Secondly, the Kaiser-Meyer-Olkin measure of sampling adequacy was of 0.922, which is above the recommended value of 0.6, and Barlett's test of sphericity was significant (χ2 (5,190.25), df 45, p < 0.001). The diagonals of the anti-image correlation matrix were also all over 0.5. The rotated factor loadings of the two components are presented in Table 2.
The hypothesis concerning construct validity was supported by a moderate correlation above 0.60 (rs = 0.67, p < 0.001) between Short-LIMOS total and BI total at admission to the acute stroke unit.
The comparison of the Short-LIMOS with the original LIMOS showed a high correlation of rs = 0.99 in Sample 1 and rs = 0.98 in Sample 2.
The MDC90 was 7.22 points, which indicates a reliable change of 18.05% on the total range of 40 points (range from 10 to 50 points).
With 3.4% of patients achieving the lowest score, and 1.7% achieving the highest score, no floor and ceiling effects were found for the total Short-LIMOS score.
The shortened version of the Lucerne ICF-Based Multidisciplinary Observation Scale (so called Short-LIMOS) developed in this study, for use at an acute stroke unit consists of 10 items that capture stroke patients' performance in daily activities from a motor, cognitive, and communication perspective. The Short-LIMOS was found to be reliable, valid, and responsive in the (hyper)acute phase (0 h to 7 days) (12) post-stroke.
The Short-LIMOS consists of two constructs, namely motor performance, and cognitive and communication performance, and structural validity reflects an adequate dimensionality of these two constructs. The test-retest reliability of the Short-LIMOS was excellent, meaning that the scale is stable by repeated measurement of patients who did not clinically change in their performance. This makes the test utmost suitable for repeated measures to track the patient's progress (i.e., follow-up). Based on the responsiveness, a change of 7.22 points can be interpreted with 90% confidence that the patient has indeed changed. The absence of floor and ceiling effects indicates that within the Short-LIMOS, there is room to detect improvement or deterioration at both ends of the scale.
The reduction in the number of the scale items from 45 to 10 resulted in an outcome measure that is feasible to perform in the highly dynamic acute stroke unit, at which the available time for assessments is limited. The items can be rated within one therapy session, making the administration time comparable to the one of the direct observations in the 10-item BI (8). However, in contrast to the BI, within this time window the short-LIMOS allows not only to assess the patients' motor performance in daily activities, but also their cognitive performance, including communication. Critically, this allows a more comprehensive assessment, which provides important information to determine the patients' treatment needs based on the identified impairments, but also for the planning of a suitable discharge destination. The correlation coefficient (rs) of 0.67 between the Short-LIMOS and the BI shows indeed that the Short-LIMOS is not merely a copy of this latter established test but provides additional information to the patient's motor function, namely the cognitive and communication abilities. Furthermore, the Short-LIMOS is a multidisciplinary observational scale, which has the advantage that none of the single disciplines is solely responsible for all observations, i.e., these can be divided across neurologists, nurses, occupational therapists, physical therapists, and speech therapists. This results in an administration burden of <5 mins per discipline.
The criterion validity showed a high correlation between the original LIMOS and its shortened version. This is not surprising, as the ten Short-LIMOS items were derived from the original LIMOS by means of a regression analysis and the corresponding R2 was 0.738. With the high correlation between the original and shortened LIMOS, one could raise the question whether both scales are needed? Our answer to this question is yes, as the target setting for each of the scales is different. The original LIMOS was developed for an inpatient neurorehabilitation setting (4) in which are more time and disciplines available to observe the patient's performance limitations during daily activities. In addition, the patients' situation allows a more extended examination when compared to the acute stroke unit setting. A more profound observation results in a more detailed profile of the patient's limitations, based on which focused rehabilitation goals can be defined and interventions be selected. Contrary to this, the newly developed Short-LIMOS's field of application is the stroke unit, where there is little time and space available to acquire a first objective assessment regarding the patient's performance with accompanying deficits.
Although we developed the Short-LIMOS for clinical purposes, this assessment could also be applied in acute stroke research projects to capture the patient's motor, cognitive and communication performance. The lack of floor and ceiling effects of the Short-LIMOS makes is utmost suitable for investigating changes over time, one point of criticism to which the BI is often subject to (28), in both clinical settings and research projects. Indeed, a sequential re-assessment of the Short-LIMOS would allow to profile the patients' progress over time, also beyond the acute phase. If a more detailed assessment is needed at later time points, then the full LIMOS (4) can be administered. A comparison with the Short-LIMOS scores would still be possible, as the present work shows that the two scales are highly correlated (rs = 0.99).
For determining the test-retest reliability and measurement error of the Short-LIMOS, the sample needs to be clinically stable in the interim period (11). Therefore, only data from patients who were transferred from the acute stroke unit to the internal neurorehabilitation center within 1–4 days after being assessed with the Short-LIMOS on the acute stroke unit were analyzed. As neurological improvement is particularly sustained early after stroke (29), this may also result in a change in daily activity performance. Nevertheless, we found an excellent ICC in the test-retest reliability analysis. Two other measurement properties for reliability, namely the intrarater as well as the interrater reliability, were not assessed in the present work and should be further investigated. However, for the original LIMOS, the interrater reliability on item-level ranged from moderate to almost perfect agreement for most items and therefore, it can be assumed that this is also the case for its shortened version (4).
With a value of 1.7%, the ceiling effect of the Short-LIMOS was very low. This value might be slightly underestimated, as 13.33% of the patients were not considered in the analysis due to no or incomplete data on the Short-LIMOS. This subset of patients did not differ significantly from those with complete Short-LIMOS data in terms of age and sex. They were, however, less severely neurologically impaired (NIHSS) and had a shorter length of hospital stay.
The Short-LIMOS allows a reliable, valid, responsive, and rapid multidisciplinary observation of stroke patients' motor, cognitive and communication performance in daily activities in the context of an acute stroke unit. With that, the scale could provide useful information for discharge planning after acute stroke unit stay and designing rehabilitation. Future work should study the predictive ability of the Short-LIMOS for, for example, discharge destination, to further increase the evidence-based application of this scale. Furthermore, capturing the recovery profile of the patient's actual performance in daily activities from a physical, cognitive and speech perspective in parallel—using the Short-LIMOS—would shed new light on stroke recovery.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Cantonal Ethics Committee Northwest and Central Switzerland (BASEC-ID 2017-00998). The patients/participants provided their written informed consent to participate in this study.
BO, TV, TN, and JV contributed to conception and design of the study. BO organized the database. BO and TV performed the statistical analysis. BO and JV drafted the manuscript. TN, TV, and DC critically revised the manuscript for important intellectual content. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the SNF Grant No. 32003B_196915.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the therapists working at the neurorehabilitation and acute stroke unit for having so easily integrated the standard assessments into the clinical routine.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2022.857955/full#supplementary-material
ADL, activities of daily living; ANOVA, analysis of variance; BI, Barthel Index; CI, confidence interval; COSMIN, Consensus-based Standards for the Selection of Health Measurement Instruments; ICC, Intra-class Correlation Coefficient; ICF, International Classification of Functioning, Disability and Health; LIMOS, Lucerne ICF-based Multidisciplinary Observation Scale; LUKS, Luzerner Kantonsspital; MD, mean difference; MDC, minimum detectable change; NIHSS, National Institutes of Health Stroke Scale; PCA, principal component analysis; SD, standard deviation; SDdiff, standard deviation of the difference; SEM, standard error of measurement; Short-LIMOS, Shortened Version of the Lucerne ICF-based Multidisciplinary Observation Scale; SRM, standardized response means; Q1, first quartile; Q3, third quartile.
1. Gialanella B, Santoro R, Ferlucci C. Predicting outcome after stroke: the role of basic activities of daily living predicting outcome after stroke. Eur J Phys Rehabil Med. (2013) 49:629–37. Available online at: https://www.minervamedica.it/
2. Saab A, Glass-Kaastra S, Gordon B, Young. Discharge destination from a rehabilitation unit after acute ischemic stroke. Can J Neurol Sci. 46, 209–215. doi: 10.1017/cjn.2018.386
3. Ottiger B, Lehnick D, Pflugshaupt T, Vanbellingen T, Nyffeler T. Can I discharge my stroke patient home after inpatient neurorehabilitation? LIMOS cut-off scores for stroke patients ‘living alone’ and ‘living with family.’ Front Neurol. 11:601725. doi: 10.3389/fneur.2020.601725
4. Ottiger B, Vanbellingen T, Gabriel C, Huberle E, Koenig-Bruhin M, Plugshaupt T, et al. Validation of the new lucerne ICF Based Multidisciplinary Observation Scale (LIMOS) for stroke patients. PLoS ONE. (2015) 10:1–8. doi: 10.1371/journal.pone.0130925
5. Van de Winckel A, Ottiger B, Bohlhalter S, Nyffeler T, Vanbellingen T. Comprehensive ADL outcome measurement after stroke: rasch validation of the Lucerne ICF-Based Multidisciplinary Observation Scale (LIMOS). Arch Phys Med Rehabil. (2019) 100:2314–23. doi: 10.1016/j.apmr.2019.02.012
6. Vanbellingen T, Ottiger B, Pflugshaupt T, Mehrholz J, Bohlhalter S, Nef T, et al. The responsiveness of the lucerne ICF-based multidisciplinary observation scale: a comparison with the functional independence measure and the Barthel Index. Front Neurol. (2016) 7:1–6. doi: 10.3389/fneur.2016.00152
7. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. (1987) 1:6–18.
8. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. (1965) 14:61–5. doi: 10.1037/t02366-000
9. Kwon S, Hartzema AG, Duncan PW, Lai SM. Disability measures in stroke: relationship among the Barthel Index. The Functional Independence Measure, and the Modified Rankin Scale. Stroke. (2004) 35:918–23. doi: 10.1161/01.STR.0000119385.56094.32
10. Desrosiers J, Rochette A, Noreau L, Bravo G, Hébert R, Boutin C. Comparison of two functional independence measure in post-stroke rehabilitation. Arch Gerontol Geriatr. (2003) 37:157–72. doi: 10.1016/S0167-4943(03)00044-X
11. Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy. Terminology, and defintions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. (2010) 63:737–45. doi: 10.1016/j.jclinepi.2010.02.006
12. Bernhardt J, Zorowitz RD, Becker KJ, Keller E, Saposnik G, Strbian D, et al. Advances in Stroke 2017. Stroke. (2018) 49:E174–99. doi: 10.1161/STROKEAHA.118.021380
13. Arbeitsgruppe Stroke Unit der Schweizerischen Hirnschlaggesellschaft. Stroke Units Und Stroke Centers in Der Schweiz: Richtlinien Und Anforderungsprofil—Schweizerische Hirngesellschaft. Schweiz Med Forum. (2012) 12:918–22. Available online at: https://www.neurovasc.ch/
14. WHO. World Health Organization. International Classficiation of Functioning, Disability and Health: ICF. Geneva: WHO (2001).
15. Lyden P, Brott T, Tilley B, Welch KMA, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke scale using video training. Stroke. (1994) 25:2220–26. doi: 10.1161/01.STR.25.11.2220
16. Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. (1989) 46:660–662. doi: 10.1001/archneur.1989.00520420080026
17. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. (1988) 10:61–3. doi: 10.3109/09638288809164103
19. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. (1979) 86:420–28.
20. Lance CE, Butts MM, Michels LC. The sources of four commonly reported cutoff criteria: what did they really say? Organ Res Methods. (2006) 9:202–20. doi: 10.1177/1094428105284919
21. de Vet. 6, 5.3 Cross-Cultural Validity. In Measurement in Medicine: A Practical Guide. (2011). Cambridge: Cambridge University Press, p. 181–85.
22. Harvill LM. Standard error of measurement: an NCME instructional module on. Educ Meas Issues Pract. (1991) 10:33–41. doi: 10.1111/j.1745-3992.1991.tb00195.x
23. Fleiss JL. Reliability of measurement. In: Fleiss JL, editors. The Design and Analysis of Clinical Experiments. New York: John Wiley and Sons p. 1–31.
24. Gross Portney L, Walkins MP. Criteria for evaluating correlation coefficients. In: Foundations of Clinical Research, 2nd ed. New Jersey: Prentice-Hall, Ltd (2000).
25. Haley SM, Fragala-Pinkham MA. Interpreting change scores of tests and measures used in physical therapy. Phys Ther. (2006) 86:735–43. doi: 10.1093/ptj/86.5.735
26. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. (1986) 8:307–10. doi: 10.1016/S0140-6736(86)90837-8
27. McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. (1995) 4:293–307. doi: 10.1007/BF01593882
28. Quinn TJ, Langhorne P, Stott DJ. Barthel Index for stroke trials: development, properties, and application. Stroke. (2011) 42:1146–51. doi: 10.1161/STROKEAHA.110.598540
Keywords: stroke, acute, ADL, assessment, short-LIMOS, reliability, validation, ICF
Citation: Ottiger B, Vanbellingen T, Cazzoli D, Nyffeler T and Veerbeek JM (2022) Development and Validation of the Short-LIMOS for the Acute Stroke Unit—A Short Version of the Lucerne ICF-Based Multidisciplinary Observation Scale. Front. Rehabilit. Sci. 3:857955. doi: 10.3389/fresc.2022.857955
Received: 19 January 2022; Accepted: 16 February 2022;
Published: 05 April 2022.
Edited by:
Jannis Papathanasiou, Medical University—Sofia, BulgariaReviewed by:
Alessandro Giustini, Istituto di Riabilitazione Santo Stefano, ItalyCopyright © 2022 Ottiger, Vanbellingen, Cazzoli, Nyffeler and Veerbeek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janne M. Veerbeek, amFubmUudmVlcmJlZWtAbHVrcy5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.