
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Rehabil. Sci., 28 March 2022
Sec. Translational Research in Rehabilitation
Volume 3 - 2022 | https://doi.org/10.3389/fresc.2022.825147
This article is part of the Research TopicImplementation of Physical, Psychosocial, and Mind-body Approaches for the Management of OsteoarthritisView all 5 articles
 Yixue Mei1
Yixue Mei1 Jennifer S. Williams1
Jennifer S. Williams1 Erin K. Webb1
Erin K. Webb1 Alison K. Shea2
Alison K. Shea2 Maureen J. MacDonald1
Maureen J. MacDonald1 Baraa K. Al-Khazraji1*
Baraa K. Al-Khazraji1*Osteoarthritis (OA) is a highly prevalent condition characterized by degradation of the joints. OA and cardiovascular disease (CVD) are leading contributors to disease burden worldwide, with a high level of overlap between the risk factors and occurrence of both conditions. Chief among the risk factors that contribute to OA and CVD are sex and age, which are both independent and interacting traits. Specifically, the prevalence of both conditions is higher in older women, which may be mediated by the occurrence of menopause. Menopause represents a significant transition in a women's life, and the rapid decline in circulating sex hormones, estrogen and progesterone, leads to complex physiological changes. Declines in hormone levels may partially explain the increase in prevalence of OA and CVD in post-menopausal women. In theory, the use of hormone therapy (HT) may buffer adverse effects of menopause; however, it is unclear whether HT offers protective effects for the onset or progression of these diseases. Studies have shown mixed results when describing the influence of HT on disease risk among post-menopausal women, which warrants further exploration. The roles that increasing age, female sex, HT, and CVD play in OA risk demonstrate that OA is a multifaceted condition. This review provides a timely consolidation of current literature and suggests aims for future research directions to bridge gaps in the understanding of how OA, CVD, and HT interact in post-menopausal women.
Osteoarthritis (OA) is a progressive musculoskeletal disease involving the breakdown of cartilage and bone, and is one of the leading causes of pain and disability worldwide (1). An estimated 654 million individuals globally have OA, with higher prevalence in women (~22%) than in men (~12%), and sex-differences becoming apparent only after age 50 (2). Recent literature has found that up to 60% of individuals with OA had additional chronic conditions (3). Specifically, numerous studies have reported that individuals with OA have a higher risk for cardiovascular disease (CVD) (4–6), a disease which leads to over 17.3 million deaths per year worldwide (7). Collectively OA and CVD create a considerable healthcare burden and share complex risk factors that contribute to a large fraction of disability, and to a greater extent in the older population. Post-menopausal women are especially vulnerable to the onset of both OA and CVD, which may suggest that the occurrence of menopause and the associated physiological changes play a mediating role in the increased co-existence of OA and CVD.
Menopause represents a significant transition in a woman's life and is characterized by the cessation of the menstrual cycle and the subsequent dramatic decrease in levels of sex hormones estrogen and progesterone. Though testosterone also decreases in post-menopausal women, this decrease is more gradual, and the effects of androgens on women's health following the menopause transition is not fully understood (8, 9). The roles of testosterone and testosterone replacement therapy on OA and CVD risk in post-menopausal women fall outside the scope of this narrative review.
The relationship between OA and menopause was first described by Drs. Cecil and Harper in 1925 (10), and by 1952 was termed “menopausal arthritis” (11). Since then, several research studies have investigated the influence of the changing sex hormone profile during the menopausal transition on OA onset and progression. The higher prevalence of OA in older women is, in part, a result of estrogen deficiency (12), making hormone replacement therapy (HT) an important avenue to explore with consideration to preservation of joint health in OA (13, 14). Additionally, the apparent role of estrogen in maintaining cardiovascular health may influence CVD risk in older women with estrogen deficiency; however, prior research studies have presented mixed findings (15). The link between OA and CVD in the context of menopause is unclear, and the impact of HT for women with these conditions requires further consolidation of current literature. In addition, the relationships between OA and CVD and their associated risk factors may be facilitated by both biological and societal determinants of health. Specifically, factors such as gender identity (16, 17) and social disadvantage (18) are influencers of disease risk and health outcomes, but are often overlooked in biomedical research.
The purpose of this review is to synthesize existing literature examining the influence of menopause and HT on OA and CVD in women from a biomedical perspective, while applying an intersectional lens that considers socially-constructed gender identity and expression, age, ethnicity and socioeconomic factors. Specifically, biomedical literature pertaining to OA, CVD, menopause, and HT use within the recent decades were reviewed. Further searches were conducted to examine socioeconomic factors on OA and CVD, and how they interacted with menopause and HT primarily from a risk and prevalence perspective. It is important to note that this is a broad topic, thus the scope of the paper has been limited to certain factors of influence, though the authors acknowledge the vast spanning of this research field.
Given that women are disproportionately impacted by both OA and CVD and have been historically under-studied in biomedical and clinical research fields (19), this review provides a timely overview of current literature and highlights future research directions required to address knowledge gaps in the role of HT on OA, and subsequent CVD comorbidities, in women following menopause. Specifically, this review will cover literature on CVD and HT as important considerations when implementing interventions for OA management.
Osteoarthritis (OA) is one of the leading causes of chronic pain and disability, and is primarily characterized by excessive cartilage degradation, abnormal subchondral bone growth and sclerosis, and synovial inflammation (13). An abundance of evidence highlights the significant influence OA has on accelerating the onset of functional limitations and disability (20). The decline in physical activity in those living with OA subsequently contributes to both physical limitations (21) and diminished mental health (22, 23). The most prevalent sites of OA, namely with increasing age, are in the hand, knee, and hip (24, 25). It is important to acknowledge that these different locations of OA may potentially have different risk associations with menopause due to their differing etiology (24). Specifically, OA of the knee or hip, or weight-bearing OA, has greater incidence in post-menopausal women (24) and is more greatly associated with CVD risk than hand OA (26, 27). This review distinguishes between the subgroups of OA where possible.
Incidence and prevalence of OA are significantly greater in women than in men after the age of 50 (28–30), and there is a greater difference in the loss of cartilage volume between the sexes that becomes more prominent with age (31). Particularly in knee and hip OA, the prevalence and risk rapidly increase from menopausal age (around 50 years) to 75 years when compared to men of similar age (32). One large retrospective study conducted in Spain of over 5 million people reported that in adults who were 50 to 75 years of age, a diagnosis of hip and/or knee OA was 1.5–2 times greater in women than men (27). The increased OA prevalence and cartilage decline in post-menopausal women suggests that there could be a role of sex hormones on maintenance of joint health (33). Several earlier reviews investigating the effect(s) of endogenous sex hormones in post-menopausal women on OA concluded that there is an association between decreased endogenous estrogen levels and an increased risk and incidence of OA (12, 29, 30, 34). More recently, a study found that lower serum estradiol and progesterone were associated with increased knee effusion-synovitis in post-menopausal women, whereas higher levels of endogenous progesterone were associated with greater cartilage volume (35).
Animal studies provide a unique lens to study OA as these studies can isolate and manipulate individual variables (e.g., knock-out of one specific gene, influence of one specific hormone) and assess outcomes, which is challenging to accomplish even in controlled human trials. Ovaries are commonly removed in animal models to simulate post-menopausal conditions. A systematic review identified 16 high-quality animal studies (rodents, pigs, sheep, monkeys) that utilized the ovariectomy (OVX) technique to study the effects of reduced sex hormones on the development of OA (36). Of the 16 studies examined, 11 showed a detrimental effect of OVX on the outcome measures for OA (cartilage damage/degradation and mechanical defects), 4 showed no effect, and 1 showed a positive effect (36). Notably, several of these studies found that CTX-II (a biomarker of collagen type II breakdown) was elevated in mice that underwent OVX compared to the control animals, consistent with data previously reported in post-menopausal women (37, 38). Recent animal studies have provided mechanistic insight into how sex hormones may mediate the progression of OA in animal models. One study determined that OVX induced more severe OA in AMP-activated protein kinase (AMPK) knock-out mice, suggesting that estrogen may be acting through AMPK signaling cascades to protect articular cartilage (39).
Estrogen and progesterone receptors are present on many bone and cartilage tissues (40), where sex hormones bind and elicit downstream signaling effects. Several excellent reviews detail the role that estrogen and, to a lesser degree, progesterone, play in regulating joint and bone health (13, 40, 41). Briefly, estrogen increases proteoglycan production (42), decreases inflammation and reactive oxygen species (43, 44), and regulates calcium signaling in chondrocytes (13, 45). Estrogen also regulates subchondral bone turnover. It has been shown that OVX monkeys experience higher indices of bone turnover in subchondral bone than cancellous bone in comparison to the controls (46). In fact, OA is worsened in OVX-treated rabbits following methyl-prednisone-induced osteoporosis compared to controls, indicating that estrogen might have a dual effect on OA: a more direct effect on cartilage, and an indirect effect on subchondral bone (13). In comparison, the role of progesterone is not well understood; however, it may contribute to the maintenance of cartilage volume through suppressing production of matrix metalloproteinases (47). The role of sex hormones in moderating joint health in the context of OA risk requires future studies to isolate for the mechanistic effects and warrants further research in both animal and human populations.
Social disadvantage determinants, sometimes studied through racial differences, socioeconomic determinants, or perceived pain, have been proposed to influence OA outcomes (48, 49). Ethnicity has been suggested to further augment OA risk and disability (50, 51). Specifically in post-menopausal women, African-American, Hispanic, or Aboriginal ancestry are most susceptible to OA (50). Early research investigating factors such as education, income, and occupation have found that lower socioeconomic statuses and nonprofessional occupations were more strongly associated with arthritis outcomes, even after controlling for individual factors such as female sex and age (48). The severity of pain experienced in OA is associated with the degree of bone attrition and effusion-synovitis, both of which appear to be influenced by sex hormones (52). The role of estrogen in pain modulation is complex, as it may attenuate acute inflammatory pain but it has both anti- and pro-nociceptive effects (53). A foundational study reported that while women with OA experience significantly higher levels of pain and disability than men with OA, after adjusting for catastrophizing tendencies, or the tendency to exaggerate painful stimuli and take on a helpless role, the gender differences were eliminated (16). Recent work has shown that lower levels of emotional expression were associated with more pain catastrophizing in people with OA. However, when effective communication of pain is high, the act of pain catastrophizing becomes weaker, which may suggest that communication of pain is influenced by sociocultural factors, alongside the underlying biological sex factors (54). We recognize that social determinants of health are complex, multi-tiered with many aspects, and are not specifically or solely associated with OA. However, when considering post-menopausal women who may be at an increased risk of experiencing pain associated with OA due to attenuated sex hormones or general social disadvantage, better understanding of non-biomedical aspects of disease could have the potential to improve treatment and care strategies that women receive, and thereby further improve prognosis.
Men have a higher prevalence of CVD in comparison to women until the menopause transition (7). According to the INTERHEART global case-control study conducted across 52 countries, women experience their first acute myocardial infarction ~9 years later than men (55). The INTERHEART study identified that there are nine modifiable risk factors associated with CVD, some of which are differentially associated with risk between sexes (55). Further, this study found that sex differences in acute myocardial infarction were largely (80%) explained by these modifiable risk factors in men compared to women (55). The risk factors may result in increased risks for conditions such as atherosclerosis (56–59) or metabolic syndrome (55, 59–61), which directly contribute CVD onset. However, sex differences in CVD prevalence cannot be examined simply through a lens of biological sex as disease progression is influenced and elevated by intersections of (but not limited to) age, sex, gender, ethnic background, socioeconomic status, and access to care (17, 18).
Recent research by Pelletier and colleagues (17) done in a cohort of middle-aged adults (mean age of 48 years) reported that higher gender scores associated with feminine gender, as opposed to female sex, are associated with risk for CVD, indicating that socially constructed gender roles and their expression play a mediating role in CVD onset. Furthermore, Anand and colleagues (18) examined the role of social disadvantage (as an index of social and economic factors) and ethnicity (i.e., South Asian, Chinese, Aboriginal, and European ancestry) on CVD risk across middle-age (mean age of 50 years). The authors identified social disadvantage as an independent predictor of CVD, independent of age, sex, and ethnicity. Further, this study reported that women, in particular older women across all ethnic groups, had a higher proportion of social disadvantages than men, where ethnicity further contributed to the unequal risk of CVD. Specifically, compared to European participants, Aboriginal and South Asian participants were at a significantly higher risk (trend for South Asian) of CVD, while Chinese participants were at a lower risk of CVD, even after social disadvantage was accounted for in the analysis (18). Taken together, it is important to consider both biomedical and sociocultural risk factors when examining sex differences in CVD risk; however, minimal research has been conducted examining the role of non-biomedical factors like gender and ethnicity in CVD risk across the menopause transition, thus warranting further research.
Changes associated with the menopause transition drastically increase the risk of CVD among women as compared to men, such as the loss of estrogen receptors in post-menopausal women (62), years since menopause, and comorbid diseases (63). Furthermore, CVD risk is increased during peri- and post-menopause due to changes in lipids (i.e., cholesterol, triglycerides, lipoprotein A), body composition, and vascular function, among other factors underlying CVD progression (64). A recent publication from the American Heart Association identified early age of menopause (i.e., <45 years) as a risk factor for various CVDs (64), even when adjusting for age, ethnicity, and other traditional risk factors (57). Surgical menopause (i.e., the removal of both ovaries) may further increase the risk of CVD, particularly if the surgery occurred before the age of 40 years (e.g., <35–39 years) and follow-up care did not include HT to replace the loss of endogenous hormones (65).
Data from both longitudinal and cross-sectional studies support the theory that the menopause transition, and specifically the reduction in endogenous estradiol, underlies the age-related sex differences observed in the prevalence of CVD (66). Estradiol plays a cardioprotective role in improving vascular function as highlighted by studies using cell, animal and human models (15, 67). Estradiol may exert protective influence on the vasculature through the estrogen receptor – endothelial nitric oxide synthase pathway, increasing the production of the potent vasodilator nitric oxide (68). Further mechanisms of action include the ability for estradiol to reduce oxidative stress and increase antioxidant protection, increase vascular cell survival, reduce arterial fibrosis development, and protect from vascular injury, as detailed extensively in previous reviews (15, 67).
Recent research by Iwamoto and colleagues (69) examining internal carotid artery shear-mediated dilation as an early predictor of CVD found that dilation was impaired in peri- and post-menopausal women, as compared to pre-menopausal women. Importantly, women included in this trial were not using any type of medication, although there was no exclusion of prior use of HT. Furthermore, this study reported that the carotid artery dilation was improved with higher serum estradiol levels, even after controlling for age. These results point to an intrinsic role of estrogen in the regulation of CVD risk, independent of aging, through the menopause transition (69). Additionally, investigations into subclinical markers of CVD progression indicative of vascular disturbance also suggest dysfunction emerges across the menopause transition. Cross-sectionally, brachial artery flow mediated dilation was found to be progressively lower in late peri-menopause and post-menopause compared to early peri-menopause and pre-menopause (70). Similarly, in studies that have performed repeated testing, the menopause transition was associated with preclinical indices of vascular dysfunction including increased carotid intima-media thickness and increased aortic pulse wave velocity at follow-up (71, 72). Overall, despite some conflicting evidence, estradiol may be mechanistically linked to the risk of CVD, particularly across the menopause transition.
The interrelationship of OA and CVD can be partially explained through shared risk factors, overlapping etiology, and various indirect causes (Figure 1) (6). This section aims to highlight the possible mechanisms that link the OA and CVD pathologies and concludes by proposing several ways menopause plays a role in the comorbidities.
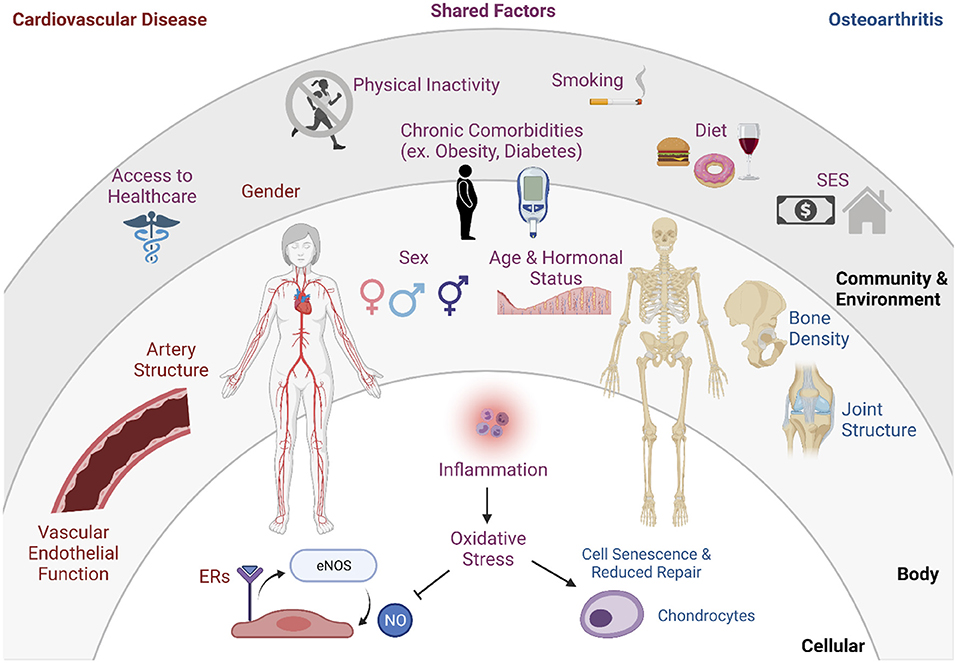
Figure 1. Interaction of risk factors of OA and CVD. Factors with research to support connection to OA alone: cell senescence & reduced repair, chondrocytes, bone density, joint structure. Factors with research to support connection to CVD alone: gender, artery structure, vascular endothelial function estrogen receptors (ERs), eNOS (endothelial nitric oxide synthase), nitric oxide (NO). Shared risk factors include access to healthcare, physical inactivity, chronic comorbidities, smoking, diet, socioeconomic status (SES), sex, age, hormonal status, inflammation, oxidative stress.
Several traditional CVD risk factors, including age, sex, obesity, cholesterol levels, and smoking, all elevate the risk of OA development and progression (5, 92). Aging affects the health of the heart and vasculature, and is associated with increased thickening and stiffening of vessels, which results in hypertension and heightens the risk of developing vascular-related conditions (92). As discussed previously, circulating sex hormone levels influence the levels of adiposity within the body, which in turn influences OA and CVD disease risk and etiology (92). For example, conditions such as diabetes and obesity are risk factors and precursors to the development of both OA and CVD, through inflammation and endothelial dysfunction mechanisms (92). Lifestyle habits, such as smoking and physical inactivity due to pain during movement, are also common risk factors for OA and CVD, and also act through altering endothelial health which contributes to atherosclerosis (93). Finally, another proposed underlying mechanism is the acute or chronic inflammation that is commonly associated with OA, which plays a role in the progressive degradation of joint health. This inflammation also affects the vasculature that supplies bone, and may play a role in increasing CVD-related incidents through the development of atherosclerosis (5, 92). Collectively, these shared risk factors and overlapping etiology point toward a potential disease phenotype that may result from these highly comorbid conditions, where various interactions and synergistic effects influence the severity of the conditions.
Disease management methods may, in part, contribute to the observed comorbidities. Pharmacological methods of managing OA may increase the risk of CVD. Namely, nonsteroidal anti-inflammatory drugs (NSAIDs) are frequently prescribed to relieve joint-related pain in OA patients and have been related to increased precursors of CVD (6, 92). NSAIDs act through inhibiting the production of prostaglandins; however, the interference of prostaglandin production results in adverse effects on vascular health, including altered kidney function resulting in increased blood pressure that subsequently increases the risk for heart failure (94, 95). Additionally, in the case of severe OA where surgical intervention is required, the event of surgery has been proposed to be a mediator between OA and CVD (96). One study found that hand, knee, and hip joint replacement increased the risk of venous thromboembolism, and subsequently the occurrence of cardiac events (96).
In post-menopausal women, the relationship between OA and CVD is further exacerbated through the decrease in circulating estrogen with menopause. A higher OA prevalence in women over 50 has been, in part, attributed to the absence of musculoskeletal-protective effects of estrogen (92). In addition to overlapping biomedical and physiological factors, it is important to consider the effects of environmental and societal contributors to disease. Very few studies have examined the intersection of OA and CVD through lenses of socioeconomic status, food accessibility, and access to care (Figure 1), but it is important to acknowledge these effects on disease onset, progression, and management (18).
Estrogen therapy is typically given either with progesterone, or alone (for women without a uterus), to mimic ovarian hormones and alleviate bothersome symptoms of menopause, particularly vasomotor symptoms. When prescribing HT, several factors are considered, including the patients' pre-existing medical conditions, contraindications (i.e., active thromboembolic disease). The risk for development and/or progression of certain diseases (i.e., CVD) may increase based on prior health factors, as well as with the type of HT used (i.e., oral versus transdermal estrogens) (79, 86). The following sections will detail the influence of HT independently on OA and CVD risk, and then discuss the interaction of the comorbidities with HT.
Since the 1980s, several studies have sought to determine the effects of HT on OA incidence, prevalence, and progression. As previous sections have highlighted, there is a strong rationale for utilizing exogenous sex hormones to mitigate the risk of disease: (1) greater OA prevalence in post-menopausal women, (2) the negative association between cartilage damage and circulating levels of sex hormones, and (3) the presence of estrogen and progesterone receptors on joint tissues.
Research on animals (rodents, sheep, miniature pigs) have highlighted that estradiol supplementation can inhibit changes in collagen turnover induced by OVX (36). Differences in the OA phenotype throughout the progression of the disease may influence the effect of ET on bone and cartilage function. Specifically, the early stages of OA are characterized by increased subchondral bone turnover and bone loss, whereas in the late stages of OA there is reduced subchondral bone turnover and sclerosis (97). It is possible that ET may have beneficial effects in early OA but may have limited or even harmful effects when treating late OA. The age when beginning HT (i.e., the timing) may be an important consideration in OA (98, 99), like the “timing hypothesis” prevalent in HT usage in CVD (64) (discussed in the following section).
The first longitudinal study to examine the effects of HT on OA diagnosis followed 551 older women, (aged 63–91) over a 10-year period and found that the current use of estrogen therapy (ET) had a moderate, but not statistically significant, protective effect against the worsening of radiographic knee OA (100). An additional and more recent study in 17,200 older women (3,440 women with OA, 13,760 women without OA, mean age ~51 years) found that menopause was a risk factor for OA, and women who initiated HT shortly after menopause were at reduced risk of OA, with the protective effects disappearing after HT cessation (98). In contrast, a case-control study comparing women with OA to age-matched controls found that current HT use was not associated with OA risk whereas past HT use modestly decreased the risk of developing OA (101). Both studies classified women as either never, current or past users of HT, but the type of HT or duration of treatment were not considered (100, 101). Therefore, based on these early incidence studies, it remains unclear if ET offers a protective effect on OA diagnosis and progression. The Women's Health Initiative conducted a blinded randomized control trial that included over 26, 000 women aged 50–79 assigned to receive either estrogen and progesterone, estrogen-only, or placebo in standardized dosages (conjugated equine estrogen 0.625 mg and medroxyprovera acetate (such as Depo-Provera) 2.5 mg) over an average duration of 7 years (102). The Women's Health Initiative found that the women receiving the estrogen-only treatment had significantly lower rates of overall arthroplasty (surgical management of severe OA), but when stratified by joint of interest, only hip arthroplasty remained slightly lower in the ET group; the occurrence of knee arthroplasty was not significantly different among the groups (103). In the estrogen and progesterone group, there was no association between total, knee, or hip arthroplasties with treatment (103). Similarly, in a separate randomized control trial, estrogen and progesterone HT over 4 years did not mitigate knee pain or degree of disability, measured by the WOMAC OA index, in 969 older women (mean age of 66 years) with CVD (compared to controls) (82). Together, these studies support a potential protective effect of ET on OA incidence which may be attenuated when progesterone is also administered; however, additional work should be completed to explore this relationship.
A systematic review conducted by Tanamas et al. in pre- and post-menopausal women suggested that ET may help with maintaining healthy cartilage and bone turnover, as measured by serum and urinary biochemical markers of bone and cartilage breakdown (CTX-I and CTX-II) (12). A different review conducted by Karsdal et al. summarized structural benefits of estrogens and protective effects for joint inflammation and degradation, suggesting associations between the roles of estrogen and the etiology of OA (104).
Several limitations are important to consider when interpreting the literature on HT and OA, such as duration of HT use, age of HT administration and cessation, type, and dosage of HT as well as general adherence to treatment. Aside from randomized control trials, many cross-sectional or longitudinal studies group HT usage into categories such as “current use,” “past use” or “never use” without taking into consideration additional information on HT. There appears to be differences in OA incidence in response to different types of HT (i.e., estrogen alone versus estrogen and progesterone) which is also not captured through this type of analysis. Not accounting for the details of HT usage introduces a considerable amount of variability. For example, women who utilize HT in their 70's are much more likely to present with significant cartilage damage than women in their 40's simply due to the “wear and tear” nature of the disease (101). Anecdotally, general musculoskeletal symptoms during menopause appear to improve with HT when used early in the menopausal transition (98, 99), but this has not been analyzed in randomized control trials (99).
Over the last two decades, the potential role of HT for the prevention of CVD has been thoroughly investigated. Several factors have been found as important determinants of the outcomes, such as age since menopause (i.e., “the timing hypothesis”), the composition of HT (e.g., estradiol alone, estradiol with progesterone/progestins), and the route of administration, which have been well detailed in a recent review (64). Longitudinal trials such as the Heart and Estrogen/Progestin Replacement Study (105) and the Women's Health Initiative (106) in postmenopausal women (mean age of ~67 and ~63 years, respectively) were conducted to examine the effects of HT on risk for disease and all-round mortality, but were not found to improve the CVD risk outcomes. However, from these trials, the age of the participants and time since menopause, along with the existing presence of CVD, were discovered to be important factors to consider when examining the potential benefits associated with HT.
Specifically, it appears that the beneficial impacts of HT on mitigating CVD risk are apparent within 10 years of menopause onset, and generally in those younger than 60 years old; this phenomenon has been termed the “timing effect” of HT (107). For example, recent meta-analyses conducted have reported a decrease in CVD risk and mortality with HT in early post-menopausal women (i.e., within 10 years of menopause onset), though there was an increased risk for venous thromboembolism compared to placebo or no treatment conditions (108, 109). In contrast, in late post-menopausal women (i.e., >10 years since menopause onset), there was no change in CVD risk and mortality with HT, though there was an increased risk of stroke and venous thromboembolism (108, 109). Further, the latter meta-analysis observed a ~25% reduction in mortality in younger post-menopausal women (i.e., <60 years old; average: 55 years) using HT compared to no treatment after an average of 5 years follow-up, providing further support to the notion that HT may benefit early post-menopausal women (109). However, this meta-analysis combined trials of women using oral and transdermal estradiol or conjugated equine estrogen, with or without the presence of progesterone or a progestin (e.g., medroxyprogesterone acetate), making it difficult to draw conclusions regarding the type and dose of HT and its impacts on mortality. More recent research such as the Early vs. Late Intervention Trial with Estradiol, reported reduced age-induced increases in carotid intima-media thickness, an early stage of atherosclerotic progression, with early post-menopausal women (i.e., <6 years after menopause) after ~5 years of oral 17β estradiol [(1 mg/day) with progesterone (45 mg vaginal gel) for 10 days in a 30-day cycle] in comparison to late post-menopausal women (i.e., more than 10 years after menopause) (110).
HT is a common treatment in helping manage bothersome vasomotor symptoms (e.g., night sweats, hot flashes) in post-menopausal women, with a large variation in the type, method of administration, duration of administration, pre-existing health risk factors, and time since menopause that all collectively affect the efficacy and disease risk (86, 87, 111) (Table 1). Current guidelines from the International Menopause Society, the North American Menopause Society, and the European Menopause and Andropause Society collectively state that HT remains the most effective treatment for menopausal vasomotor symptoms, with data to support greater benefits in earlier initiation of HT following menopause (~50–59 years of age) (112–115). This section serves to highlight key studies and reviews in this field examining OA and CVD health outcomes using different forms of HT. A newer option for HT is a selective estrogen receptor modulator (SERM), which has both estrogen receptor agonist and antagonist properties that are tissue dependent (85). SERMs have long been used for the treatment of osteoporosis but are infrequently considered for OA, with no trials published that have examined the effects (14, 104). Research investigating the effect of SERMs on CVD has been mixed (90, 91), and further research is needed. Finally, it is important to note that although this review focused on different types of hormonal treatments, there are also non-hormonal therapies used to improve menopausal symptoms which fall outside the scope of this review (87).
Future clinical directions in examining the role of HT on OA and CVD risk point to the benefits of an individualized approach to prescription, considering the age and time since menopause along with other risk factors (87). Additionally, examining differences across hormone sources, along with other hormone indicators, such as follicle-stimulating hormone and androgens, is warranted (63). Specifically in OA pathology, isolating the effects of sex hormones, such as through examining the effects of low estrogen in pre-menopausal women on OA risk, will clarify the role of sex hormones on maintaining joint health. Finally, given the renewed knowledge of the benefits associated with HT, especially when used early in the post-menopausal period (112), researchers have recommended the continued use of HT for joint and cardioprotective benefits; however, further research is needed on the duration of use and when to cease treatment in post-menopausal women using HT (116).
In addition to an incomplete understanding of the effects of HT with preclinical or existing disease conditions, mixed findings in the literature and inappropriate interpretations of these findings add confusion to the decisions made by women and clinicians (117). When considering the Women's Health Initiative results, initially published in 2002, there have been many misinterpretations of results that have led to significant declines in the prescription and use of HT to treat bothersome menopausal symptoms (117, 118). Future research should be done to further understand HT risks and benefits which would help clarify the information health care teams use to make recommendations and decisions regarding menopausal management.
OA and CVD collectively contribute to a considerable portion of morbidity and mortality worldwide. The risk for both conditions increase in women following menopause – an inevitable consequence of aging that affects nearly half the world's population for approximately one third of their lives. HT is a method by which women can manage menopausal symptoms during and after this transition; however, there is insufficient understanding of the precise effects that different forms of HT have on a woman's physiology and particularly in the context of OA and CVD risk. As discussed in this review, there exist mixed research findings surrounding effects of HT use on OA and CVD independently. The prevalent comorbidity of OA with CVD has been demonstrated through many studies linking OA and CVD risk factors and pathology, but there has been no research directly examining the intersection of these conditions with HT use. The research findings overlaps and gaps prompt future studies to investigate the relationships between HT with OA and CVD risk in the aging population of post-menopausal women.
Specifically, future research should examine dose, type, and duration of use of HT among women suffering from OA and CVD to help address differential findings in the varying methodologies of previous studies. This approach is of importance, as the earlier treatment and management of OA and CVD risk has the potential to offset morbidity in aging women. Longitudinal studies investigating these factors holistically will provide researchers and clinicians with a better understanding of the role and influence of HT on OA and CVD independently and together, as these different diseases may have different short- and long-term outcomes when considering HT administration. Studies should also focus on women who have undergone hysterectomy and/or ovariectomy procedures, as these are one of the most performed operations in women (119, 120), and the long-term interactions with HT in women who have undergone these procedures are still unclear. Finally, studies must address the influence of sociocultural factors on the pathophysiology and management of menopause and the influence of HT on OA and CVD. The limited research demonstrates significant influences of non-biological considerations that affect the health and quality of care that post-menopausal women receive. Through a comprehensive investigation of existing literature and development of novel research questions, healthcare can provide adequate individualized care. Specifically, the interaction of advanced age and female sex with OA and CVD in conjunction with the roles of HT and menopause in disease progressions prompts future questions to address these gaps in current understanding for health of older women at risk of OA and CVD.
YM, JW, and EW were responsible for drafting the article. All authors contributed to editing of the manuscript and contributed to the conception of the project, critical revision of the manuscript, and approving the final article for submission. All authors contributed to the article and approved the submitted version.
YM was supported by the McMaster Institute for Research on Aging (MIRA), JW was supported by NSERC CGS-D, EW was supported by MIRA, Canadian Frailty Network, and NSERC CGS-M. AS has received a Grant from Pfizer and has been on the advisory board for Pfizer and Bio-syent. BA-K has Natural Sciences and Engineering Research Council (NSERC) Discovery Grant Award funding (RGPIN-2020-07208). The funding sources were not involved in the study design, writing, reviewing, or decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kwoh CK. Epidemiology of osteoarthritis. In: The Epidemiology of Aging. Dordrecht: Springer (2012).
2. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. (2020) 29-30:100587. doi: 10.1016/j.eclinm.2020.100587
3. Swain S, Sarmanova A, Coupland C, Doherty M, Zhang W. Comorbidities in osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res. (2020) 72:991–1000. doi: 10.1002/acr.24008
4. Swain S, Coupland C, Mallen C, Kuo CF, Sarmanova A, Bierma-Zeinstra SMA, et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology. (2021) 60:4327–39. doi: 10.1093/rheumatology/keab067
5. Hall AJ, Stubbs B, Mamas MA, Myint PK, Smith TO. Association between osteoarthritis and cardiovascular disease: Systematic review and meta-analysis. Eur J Prev Cardiol. (2015) 23:938–46. doi: 10.1177/2047487315610663
6. Wang H, Bai J, He B, Hu X, Liu D. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep. (2016) 6:39672. doi: 10.1038/srep39672
7. Laslett Lawrence J, Alagona P, Clark Bernard A, Drozda Joseph P, Saldivar F, Wilson Sean R, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues. J Am Coll Cardiol. (2012) 60:S1–49. doi: 10.1016/j.jacc.2012.11.002
8. The role of testosterone therapy in postmenopausal women: position statement of The North American Menopause Society. Menopause. (2005) 12:497–511. doi: 10.1097/01.gme.0000177709.65944.b0
9. Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M. Androgen in postmenopausal women. J Med Investig JMI. (2012) 59:12–27. doi: 10.2152/jmi.59.12
10. Cecil RL, Archer BH. Arthritis of the menopause: a study of fifty cases. J Am Med Assoc. (1925) 84:75–9. doi: 10.1001/jama.1925.02660280001001
11. Kellgren JH, Moore R. Generalized osteoarthritis and heberden's nodes. Br Med J. (1952) 1:181–7. doi: 10.1136/bmj.1.4751.181
12. Tanamas SK, Wijethilake P, Wluka AE, Davies-Tuck ML, Urquhart DM, Wang Y, et al. Sex hormones and structural changes in osteoarthritis: a systematic review. Maturitas. (2011) 69:141–56. doi: 10.1016/j.maturitas.2011.03.019
13. Roman-Blas JA, Castañeda S, Largo R, Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. (2009) 11:241. doi: 10.1186/ar2791
14. Xiao YP, Tian FM, Dai MW, Wang WY, Shao LT, Zhang L. Are estrogen-related drugs new alternatives for the management of osteoarthritis? Arthritis Res Ther. (2016) 18:151. doi: 10.1186/s13075-016-1045-7
15. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. (2017) 8:33. doi: 10.1186/s13293-017-0152-8
16. Keefe FJ, Lefebvre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain. (2000) 87:325–34. doi: 10.1016/S0304-3959(00)00296-7
17. Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom Med. (2015) 77:517–26. doi: 10.1097/PSY.0000000000000186
18. Anand SS, Razak F, Davis AD, Jacobs R, Vuksan V, Teo K, et al. Social disadvantage and cardiovascular disease: development of an index and analysis of age, sex, and ethnicity effects. Int J Epidemiol. (2006) 35:1239–45. doi: 10.1093/ije/dyl163
19. Heart & Stroke 2018 Heart Report,. Ms Understood Women's Hearts are Victims of a System that is Ill-Equipped to Diagnose, Treat Support Them. (2018). p. 20. Available online at: https://www.heartandstroke.ca/-/media/pdf-files/canada/2018-heart-month/hs_2018-heart-report_en.ashx?la=en&hash=3BBC7F1DD1DA3EDC6B2B0E9BB31E855268C051EB
20. McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med. (2010) 26:387–9. doi: 10.1016/j.cger.2010.04.001
21. Veronese N, Stubbs B, Solmi M, Smith TO, Noale M, Cooper C, et al. Association between lower limb osteoarthritis and incidence of depressive symptoms: data from the osteoarthritis initiative. Age Ageing. (2017) 46:470–6. doi: 10.1093/ageing/afw216
22. Kye SY, Park K. Suicidal ideation and suicidal attempts among adults with chronic diseases: a cross-sectional study. Compr Psychiatry. (2017) 73:160–7. doi: 10.1016/j.comppsych.2016.12.001
23. Stubbs B, Hurley M, Smith T. What are the factors that influence physical activity participation in adults with knee and hip osteoarthritis? A systematic review of physical activity correlates. Clin Rehabil. (2015) 29:80–94. doi: 10.1177/0269215514538069
24. Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. (1995) 38:1134–41. doi: 10.1002/art.1780380817
25. Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. (2010) 26:355–69. doi: 10.1016/j.cger.2010.03.001
26. Hawker GA, Croxford R, Bierman AS, Harvey PJ, Ravi B, Stanaitis I, et al. All-Cause Mortality and Serious Cardiovascular Events in People with Hip and Knee Osteoarthritis: a Population Based Cohort Study. PLoS ONE. (2014) 9:e91286–e91286. doi: 10.1371/journal.pone.0091286
27. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. (2014) 73:1659–64. doi: 10.1136/annrheumdis-2013-203355
28. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. (2005) 13:769–81. doi: 10.1016/j.joca.2005.04.014
29. Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. (2000) 35:183–99. doi: 10.1016/S0378-5122(00)00118-3
30. Jung JH, Bang CH, Song GG, Kim C, Kim JH, Choi SJ. Knee osteoarthritis and menopausal hormone therapy in postmenopausal women: a nationwide cross-sectional study. Menopause. (2019) 26:598–602. doi: 10.1097/GME.0000000000001280
31. Ding C, Cicuttini F, Blizzard L, Scott F, Jones G. A longitudinal study of the effect of sex and age on rate of change in knee cartilage volume in adults. Rheumatology. (2007) 46:273–9. doi: 10.1093/rheumatology/kel243
32. Mahajan A, Patni R. Menopause and Osteoarthritis: any association ? J Midlife Health. (2018) 9:171–2. doi: 10.4103/jmh.JMH_157_18
33. Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. (2003) 70:257–62. doi: 10.1016/S1297-319X(03)00067-8
34. Sowers M, Hochberg M, Crabbe JP, Muhich A, Crutchfield M, Updike S. Association of bone mineral density and sex hormone levels with osteoarthritis of the hand and knee in premenopausal women. Am J Epidemiol. (1996) 143:38–47. doi: 10.1093/oxfordjournals.aje.a008655
35. Jin X, Wang BH, Wang X, Antony B, Zhu Z, Han W, et al. Associations between endogenous sex hormones and MRI structural changes in patients with symptomatic knee osteoarthritis. Osteoarthritis Cartilage. (2017) 25:1100–6. doi: 10.1016/j.joca.2017.01.015
36. Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JPTM, van Osch GJVM. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment - a systematic approach. Osteoarthritis and Cartilage. (2008) 16:533–41. doi: 10.1016/j.joca.2008.01.002
37. Oestergaard S, Sondergaard BC, Hoegh-Andersen P, Henriksen K, Qvist P, Christiansen C, et al. Effects of ovariectomy and estrogen therapy on type II collagen degradation and structural integrity of articular cartilage in rats: implications of the time of initiation. Arthritis Rheum. (2006) 54:2441–51. doi: 10.1002/art.22009
38. Ravn P, Warming L, Christgau S, Christiansen C. The effect on cartilage of different forms of application of postmenopausal estrogen therapy: comparison of oral and transdermal therapy. Bone. (2004) 35:1216–21. doi: 10.1016/j.bone.2004.07.017
39. Ge Y, Zhou S, Li Y, Wang Z, Chen S, Xia T, et al. Estrogen prevents articular cartilage destruction in a mouse model of AMPK deficiency via ERK-mTOR pathway. Ann Transl Med. (2019) 7:336. doi: 10.21037/atm.2019.06.77
40. Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. (2013) 70:4023–37. doi: 10.1007/s00018-013-1317-1
41. Martín-Millán M, Castañeda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine. (2013) 80:368–73. doi: 10.1016/j.jbspin.2012.11.008
42. Talwar RM, Wong BS, Svoboda K, Harper RP. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J Oral Maxillofac Surg. (2006) 64:600–9. doi: 10.1016/j.joms.2005.12.006
43. Morisset S, Patry C, Lora M, De Brum-Fernandes AJ. Regulation of cyclooxygenase-2 expression in bovine chondrocytes in culture by interleukin 1α, tumor necrosis factor-α, glucocorticoids, and 17β-estradiol. J Rheumatol. (1998) 25:1146–53.
44. Claassen H, Schünke M, Kurz B. Estradiol protects cultured articular chondrocytes from oxygen-radical-induced damage. Cell Tissue Res. (2005) 319:439–45. doi: 10.1007/s00441-004-1029-9
45. Ekstein J, Nasatzky E, Boyan BD, Ornoy A, Schwartz Z. Growth-plate chondrocytes respond to 17β-estradiol with sex-specific increases in IP3 and intracellular calcium ion signalling via a capacitative entry mechanism. Steroids. (2005) 70:775–86. doi: 10.1016/j.steroids.2005.04.007
46. Ham KD, Loeser RF, Lindgren BR, Carlson CS. Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum. (2002) 46:1956–64. doi: 10.1002/art.10406
47. Hashem G, Zhang Q, Hayami T, Chen J, Wang W, Kapila S. Relaxin and β-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: Progesterone prevents matrix loss. Arthritis Res Ther. (2006) 8:R98. doi: 10.1186/ar1978
48. Luong MLN, Cleveland RJ, Nyrop KA, Callahan LF. Social determinants and osteoarthritis outcomes. Aging health. (2012) 8:413–37. doi: 10.2217/ahe.12.43
49. McClendon J, Essien UR, Youk A, Ibrahim SA, Vina E, Kwoh CK, et al. Cumulative disadvantage and disparities in depression and pain among veterans with osteoarthritis: the role of perceived discrimination. Arthritis Care Res. (2021) 73:11–7. doi: 10.1002/acr.24481
50. Wluka AE. Ethnicity may be a risk modifier for knee osteoarthritis. Int J Clin Rheumatol. (2009) 4:29–32. doi: 10.2217/17584272.4.1.29
51. Shih VC, Song J, Chang RW, Dunlop DD. Racial differences in activities of daily living limitation onset in older adults with arthritis: a national cohort study. Arch Phys Med Rehabil. (2005) 86:1521–6. doi: 10.1016/j.apmr.2005.02.009
52. De Kruijf M, Stolk L, Zillikens MC, De Rijke YB, Bierma-Zeinstra SMA, Hofman A, et al. Lower sex hormone levels are associated with more chronic musculoskeletal pain in community-dwelling elderly women. Pain. (2016) 157:1425–31. doi: 10.1097/j.pain.0000000000000535
53. Craft RM. Modulation of pain by estrogens. Pain. (2007) 132 (Suppl 1):S3–12. doi: 10.1016/j.pain.2007.09.028
54. Van Denburg AN, Shelby RA, Caldwell DS, O'Sullivan ML, Keefe FJ. Self-Efficacy for Pain Communication Moderates the Relation Between Ambivalence Over Emotional Expression and Pain Catastrophizing Among Patients With Osteoarthritis. J Pain. (2018) 19:1006–14. doi: 10.1016/j.jpain.2018.04.001
55. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. (2008) 29:932–40. doi: 10.1093/eurheartj/ehn018
56. Koutroumpas A, Giannoukas A, Zintzaras E, Exarchou E, Baliakos A, Makaritsis K, et al. Erosive hand osteoarthritis is associated with subclinical atherosclerosis and endothelial dysfunction. Int J Biomed Sci IJBS. (2013) 9:217–23.
57. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause N Y N. (2012) 19:1081–7. doi: 10.1097/gme.0b013e3182517bd0
58. Fouda N, Abd-Elaziz H, Fouda EM. Assessment of subclinical carotid atherosclerosis in patients with primary osteoarthritis: correlation with disease severity and insulin resistance. Egypt Rheumatol. (2014) 36:85–91. doi: 10.1016/j.ejr.2013.12.001
59. Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. (2006) 444:875–80. doi: 10.1038/nature05487
60. Bennasar-Veny M, Lopez-Gonzalez AA, Tauler P, Cespedes ML, Vicente-Herrero T, Yañez A, et al. Body adiposity index and cardiovascular health risk factors in caucasians: a comparison with the body mass index and others. PLoS ONE. (2013) 8:e63999. doi: 10.1371/journal.pone.0063999
61. Al-Khazraji BK, Appleton CT, Beier F, Birmingham TB, Shoemaker JK. Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: a narrative review. Osteoarthritis Cartilage. (2018) 26:462–70. doi: 10.1016/j.joca.2018.01.011
62. Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. (1994) 89:1501–10. doi: 10.1161/01.CIR.89.4.1501
63. El Khoudary SR. Gaps, limitations and new insights on endogenous estrogen and follicle stimulating hormone as related to risk of cardiovascular disease in women traversing the menopause: a narrative review. Maturitas. (2017) 104:44–53. doi: 10.1016/j.maturitas.2017.08.003
64. Khoudary SR, El Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. (2020) 142:e506–32. doi: 10.1161/CIR.0000000000000912
65. Zhu D, Chung HF, Dobson AJ, Pandeya N, Brunner EJ, Kuh D, et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod. (2020) 35:1933–43. doi: 10.1093/humrep/deaa124
66. Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc Res. (2005) 66:295–306. doi: 10.1016/j.cardiores.2004.12.012
67. Murphy E, Kelly DP. Estrogen signaling and cardiovascular disease. Circ Res. (2011) 109:687–96. doi: 10.1161/CIRCRESAHA.110.236687
68. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. (2009) 94:3513–20. doi: 10.1210/jc.2009-0278
69. Iwamoto E, Sakamoto R, Tsuchida W, Yamazaki K, Kamoda T, Neki T, et al. Effects of menstrual cycle and menopause on internal carotid artery shear-mediated dilation in women. Am J Physiol Heart Circ Physiol. (2021) 320:H679–89. doi: 10.1152/ajpheart.00810.2020
70. Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. (2012) 97:4692–700. doi: 10.1210/jc.2012-2244
71. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. (2013) 20:8–14. doi: 10.1097/gme.0b013e3182611787
72. Khan ZA, Janssen I, Mazzarelli JK, Powell LH, Dumasius A, Everson-Rose SA, et al. Serial Studies in Subclinical Atherosclerosis During Menopausal Transition (from the Study of Women's Health Across the Nation). Am J Cardiol. (2018) 122:1161–8. doi: 10.1016/j.amjcard.2018.06.039
73. Stevens-Lapsley, JE, Kohrt, WM. Osteoarthritis in Women: Effects of Estrogen, Obesity and Physical Activity | EndNote Click. Available online at: https://click.endnote.com/viewer?doi=10.2217%2Fwhe.10.38&token=WzI3MjEzNjUsIjEwLjIyMTcvd2hlLjEwLjM4Il0.-6jvcZRGbdpZnZWwhfRkQxcqjAc (accessed June 25, 2021).
74. Keck C, Taylor M. Emerging Research on the Implications of Hormone Replacement Therapy on Coronary Heart Disease. Curr Atheroscler Rep. (2018) 20:57. doi: 10.1007/s11883-018-0758-2
75. Speth RC. D'ambra M, Ji H, Sandberg K. A heartfelt message, estrogen replacement therapy: use it or lose it. Am J Physiol Heart Circ Physiol. (2018) 315:H1765–78. doi: 10.1152/ajpheart.00041.2018
76. Conjugated estrogens. Available online at: https://go.drugbank.com/drugs/DB00286 (accessed June 25, 2021).
77. Chlebowski RT, Cirillo DJ, Eaton CB, Stefanick ML, Pettinger M, Carbone LD, et al. Estrogen Alone and Joint Symptoms in the Women's Health Initiative Randomized Trial. Menopause. (2013) 20: 600–8. doi: 10.1097/GME.0b013e31828392c4
78. Bhavnani BR, Stanczyk FZ. Pharmacology of conjugated equine estrogens: Efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol. (2014) 142:16–29. doi: 10.1016/j.jsbmb.2013.10.011
79. Harper-Harrison G, Shanahan MM. Hormone Replacement Therapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2021).
80. Seifert-Klauss V, Schmidmayr M, Hobmaier E, Wimmer T. Progesterone and bone: a closer link than previously realized. Climacteric. (2012) 15 Suppl 1:26–31. doi: 10.3109/13697137.2012.669530
81. Prior JC, Elliott TG, Norman E, Stajic V, Hitchcock CL. Progesterone Therapy, Endothelial Function and Cardiovascular Risk Factors: A 3-Month Randomized, Placebo-Controlled Trial in Healthy Early Postmenopausal Women. PLoS ONE. (2014) 9:e84698. doi: 10.1371/journal.pone.0084698
82. Nevitt MC, Felson DT, Williams EN, Grady D. The effect of estrogen plus progestin on knee symptoms and related disability in postmenopausal women: The Heart and Estrogen/Progestin Replacement Study, a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. (2001) 44:811–8. doi: 10.1002/1529-0131(200104)44:4<811::AID-ANR137>3.0.CO;2-F
83. Yang XP, Reckelhoff JF. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr Opin Nephrol Hypertens. (2011) 20:133–8. doi: 10.1097/MNH.0b013e3283431921
84. Barton M, Meyer MR. Postmenopausal hypertension. Hypertension. (2009) 54:11–8. doi: 10.1161/HYPERTENSIONAHA.108.120022
85. Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. (2014) 143:207–22. doi: 10.1016/j.jsbmb.2014.03.003
86. Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, et al. Prevention of diseases after menopause. Climacteric. (2014) 17:540–56. doi: 10.3109/13697137.2014.933411
87. Fait T. Menopause hormone therapy: latest developments and clinical practice. Drugs Context. (2019) 8:1–9. doi: 10.7573/dic.212551
88. Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, et al. The Effects of Tibolone in Older Postmenopausal Women. N Engl J Med. (2008) 359:697–708. doi: 10.1056/NEJMoa0800743
89. Lugo L, Villalvilla A, Largo R, Herrero-Beaumont G, Roman-Blas JA. Selective estrogen receptor modulators (SERMs): new alternatives for osteoarthritis? Maturitas. (2014) 77:380–4. doi: 10.1016/j.maturitas.2014.01.016
90. Fuggle NR, Cooper C, Harvey NC, Al-Daghri N, Brandi ML, Bruyere O, et al. Assessment of Cardiovascular Safety of Anti-Osteoporosis Drugs. Drugs. (2020) 80:1537–52. doi: 10.1007/s40265-020-01364-2
91. Cano A, Hermenegildo C, Oviedo P, Tarín JJ. Selective estrogen receptor modulators and risk for coronary heart disease. Climacteric J Int Menopause Soc. (2007) 10:97–111. doi: 10.1080/13697130701258804
92. Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest. (2015) 45:405–14. doi: 10.1111/eci.12413
93. Prevention (US) C for DC Promotion (US) NC for CDP H Health Health (US) O on S Cardiovascular Diseases. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Centers for Disease Control and Prevention (US) (2010). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK53012/ (accessed June 25, 2021).
94. Khan S, Andrews KL, Chin-Dusting JPF. Cyclo-Oxygenase (COX) Inhibitors and Cardiovascular Risk: Are Non-Steroidal Anti-Inflammatory Drugs Really Anti-Inflammatory? Int J Mol Sci. (2019) 20:4262. doi: 10.3390/ijms20174262
95. Varga Z, Sabzwari S rafay ali, Vargova V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus. (2017) 9: e1144. doi: 10.7759/cureus.1144
96. Zeng C, Bennell K, Yang Z, Nguyen USDT, Lu N, Wei J, et al. Risk of venous thromboembolism in knee, hip and hand osteoarthritis: a general population-based cohort study. Ann Rheum Dis. (2020) 79:1616–24. doi: 10.1136/annrheumdis-2020-217782
97. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. (2012) 8:665–73. doi: 10.1038/nrrheum.2012.130
98. Burkard T, Rauch M, Spoendlin J, Prieto-Alhambra D, Jick SS, Meier CR. Risk of hand osteoarthritis in new users of hormone replacement therapy: a nested case-control analysis. Maturitas. (2020) 132:17–23. doi: 10.1016/j.maturitas.2019.11.006
99. Watt FE. Hand osteoarthritis, menopause and menopausal hormone therapy. Maturitas. (2016) 83:13–8. doi: 10.1016/j.maturitas.2015.09.007
100. Zhang Y, McAlindon TE, Hannan MT, Chaisson CE, Klein R, Wilson PWF, et al. Estrogen replacement therapy and worsening of radiographic knee osteoarthritis: the Framingham study. Arthritis Rheum. (1998) 41:1867–73. doi: 10.1002/1529-0131(199810)41:10<1867::AID-ART20>3.0.CO;2-W
101. Oliveria SA, Felson DT, Klein RA, Reed JI, Walker AM. Estrogen replacement therapy and the development of osteoarthritis. Epidemiology. (1996) 7:415–9. doi: 10.1097/00001648-199607000-00013
102. Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, et al. Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. (1998) 19:61–109. doi: 10.1016/s0197-2456(97)00078-0
103. Cirillo DJ, Wallace RB, Wu L, Yood RA. Effect of hormone therapy on risk of hip and knee joint replacement in the Women's Health Initiative. Arthritis Rheum. (2006) 54:3194–204. doi: 10.1002/art.22138
104. Karsdal MA, Bay-Jensen AC, Henriksen K, Christiansen C. The Pathogenesis of Osteoarthritis Involves Bone, Cartilage and Synovial Inflammation: May Estrogen Be a Magic Bullet? Menopause Int. (2012) 18:139–46. doi: 10.1258/mi.2012.012025
105. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. (1998) 280:605–13. doi: 10.1001/jama.280.7.605
106. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. The Women's Health Initiative Hormone Therapy Trials: Update and Overview of Health Outcomes During the Intervention and Post-Stopping Phases. JAMA J Am Med Assoc. (2013) 310:1353–68. doi: 10.1001/jama.2013.278040
107. Manson JE, Bassuk SS. Invited commentary: hormone therapy and risk of coronary heart disease why renew the focus on the early years of menopause? Am J Epidemiol. 2007/07/25 ed. 2007;166:511–7. doi: 10.1093/aje/kwm213
108. Boardman HM, Hartley L, Eisinga A, Main C, Roqué i Figuls M, Bonfill Cosp X, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst Rev. (2015) Cd002229. doi: 10.1002/14651858.CD002229.pub4
109. Salpeter SR, Cheng J, Thabane L, Buckley NS, Salpeter EE. Bayesian Meta-analysis of Hormone Therapy and Mortality in Younger Postmenopausal Women. Am J Med. (2009) 122:1016–1022.e1. doi: 10.1016/j.amjmed.2009.05.021
110. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, et al. Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. N Engl J Med. (2016) 374:1221–31. doi: 10.1056/NEJMoa1505241
111. Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. (2017) 13:220–31. doi: 10.1038/nrendo.2016.164
112. Pines A. Guidelines and recommendations on hormone therapy in the menopause. J Midlife Health. (2010) 1:41–2. doi: 10.4103/0976-7800.66990
113. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause.(2017) 24:728–53. doi: 10.1097/GME.0000000000000921
114. Baber RJ, Panay N, Fenton A. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. (2016) 19:109–50. doi: 10.3109/13697137.2015.1129166
115. Gompel A, Rozenberg S, Barlow DH, EMAS board members. The EMAS 2008 update on clinical recommendations on postmenopausal hormone replacement therapy. Maturitas. (2008) 61:227–32. doi: 10.1016/j.maturitas.2008.10.009
116. Naftolin F, Friedenthal J, Nachtigall R, Nachtigall L. Cardiovascular health and the menopausal woman: the role of estrogen and when to begin and end hormone treatment. F1000Research. (2019) 8:1576. doi: 10.12688/f1000research.15548.1
117. Manson JE, Kaunitz AM. Menopause management — getting clinical care back on track. N Engl J Med. (2016) 374:803–6. doi: 10.1056/NEJMp1514242
118. Newson LR. Best practice for HRT: unpicking the evidence. Br J Gen Pract. (2016) 66:597–8. doi: 10.3399/bjgp16X687097
Keywords: menopause, osteoarthritis, cardiovascular disease, hormone therapy, aging/ageing, cardiovascular disease risk factors, woman/women
Citation: Mei Y, Williams JS, Webb EK, Shea AK, MacDonald MJ and Al-Khazraji BK (2022) Roles of Hormone Replacement Therapy and Menopause on Osteoarthritis and Cardiovascular Disease Outcomes: A Narrative Review. Front. Rehabilit. Sci. 3:825147. doi: 10.3389/fresc.2022.825147
Received: 30 November 2021; Accepted: 07 March 2022;
Published: 28 March 2022.
Edited by:
Jocelyn L. Bowden, The University of Sydney, AustraliaReviewed by:
Fiona Watt, University of Oxford, United KingdomCopyright © 2022 Mei, Williams, Webb, Shea, MacDonald and Al-Khazraji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baraa K. Al-KhazrajiS, YWxraGF6cmJAbWNtYXN0ZXIuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.