
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Rehabil. Sci., 25 October 2021
Sec. Pulmonary Rehabilitation
Volume 2 - 2021 | https://doi.org/10.3389/fresc.2021.729190
This article is part of the Research TopicEmerging Technologies and Add-on Interventions in Pulmonary RehabilitationView all 5 articles
Background: The COVID-19 pandemic brought numerous challenges, namely in routine assessment of people with chronic obstructive pulmonary disease (COPD). The COPD Assessment Test (CAT), the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS) and the St. George's respiratory questionnaire (SGRQ) are important patient-reported outcome measures used to assess people with COPD, but its face-to-face application has been compromised. The telephone interview offers a simple and effective alternative, yet uncertainty regarding its equivalence remains. This study aimed to establish the reliability and validity of the CAT, the FACIT-FS and the SGRQ administered by telephone interview in people with COPD.
Methods: Data from an observational prospective study including people with COPD were analyzed. Participants answered to the CAT, FACIT-FS and SGRQ questionnaires in person and by telephone, with a maximum interval of 48-h. Participants were randomly selected to answer first to the in-person questionnaire followed by telephone or vice versa. Reliability measures included internal consistency with Cronbach's alpha, test-retest reliability with the intraclass correlation coefficient (ICC2,1), test-retest measurement error with the standard error of measurement (SEM) and agreement with the Bland and Altman 95% limits of agreement. Validity was assessed with the Spearman correlation (rho).
Results: Fifty-five people with COPD (44 men; 68.1 ± 7.9 years; FEV1: 59.1 ± 20.3% predicted) were included. Similar internal consistency was observed between in person vs. telephone interview for the CAT (0.82 vs. 0.84), the FACIT-FS (0.83 vs. 0.84) and the SGRQ (0.92 vs. 0.93). Test-retest reliability was excellent, with an ICC2,1 of 0.77 (95% CI: 0.65; 0.86), 0.86 (95% CI: 0.77; 0.92) and 0.94 (95% CI: 0.90; 0.96) for the CAT, FACIT-FS and SGRQ total scores, respectively. The SEM showed a low level of associated measurement error and the Bland and Altman plots illustrated a good level of agreement between both modes of administration, with no evidence of systematic bias. Robust positive correlations (rho 0.87–0.94, p < 0.001) were found for the CAT, FACIT-FS and SGRQ total scores applied by both methods.
Conclusion: The telephonic administration of the CAT, the FACIT-FS and the SGRQ are a valid and reliable alternative approach to in person interviews for monitoring symptoms and health-related quality of life in people with COPD. The telephone might be an important add-on for personalized assessment and management of COPD thru remote monitoring.
Chronic obstructive pulmonary disease (COPD) is a major worldwide health problem due to its high prevalence (about 10% of the adult population), rising mortality and huge burden (1–3). Most of the burden is driven by the impact of COPD symptoms on individuals' daily life (4–6). A symptom-centered treatment approach might be key to improve quality of life, health status and prognosis of people with COPD (4–6) by optimizing symptom control and reducing future risks, such as acute exacerbations, mortality, comorbidities and the long-term consequences of disease (7).
The COVID-19 pandemic impacted patient care significantly (2, 8). The main reason was the reduction in face-to-face appointments which severely limited routine assessments to monitor patients' health status and the delivery of personalized interventions, such as pulmonary rehabilitation (2, 8). Patients' health status is commonly assessed using clinical examination, physical measures and self-reported questionnaires on symptoms and health-related quality of life (HRQoL). While it is understandable that clinical examination and assessment using physical measures can be difficult outside scheduled face-to-face appointments, the same may not be true for self-reported questionnaires. In fact, the routine application of valid and reliable patient-reported outcome measures (PROMS) assessing COPD symptoms and daily functioning is important to prevent and identify complications early (1, 9–11). It also allows optimisation of outcomes for each person (10, 11) thus, should be maintained even in challenging times, with innovative approaches (12, 13).
Evidence suggests that some healthcare can be provided remotely, for instance, by gathering information on symptoms and health status providing education for disease self-management and counseling, e.g., on smoking cessation. Specifically, in the context of the pandemic, multiple actions were driven to implement remote services, including teleconsultation of people with COPD (14–16) and telerehabilitation (8, 17, 18). Therefore, alternative methods to maintain routine assessment of this population and simultaneously reduce social interactions as a way to prevent SARS-CoV-2 propagation are important and deserve to be further explored (8, 17).
Technologies of information and communication, namely the telephone, offer a simple and effective alternative to paper-forms to keep monitoring these individuals (17, 19). Evidence has shown that telephone administration of some health instruments for symptoms control (20) and measurement of HRQoL (21, 22) is comparable to the in-person application. However, the psychometric properties of specific questionnaires to people with COPD still need to be explored; specifically, the reliability and validity of the telephone administration of the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS) and the St. George's Respiratory Questionnaire (SGRQ) have never been tested and only one study assessed these properties in the COPD Assessment Test (CAT) (23). Therefore, the equivalence of assessing PROMs, namely the CAT, the FACIT-FS and the SGRQ, by telephone in comparison to paper-forms in people with COPD, remains uncertain.
This information will be fundamental for rethinking alternative models of remote assessment and/or follow-up of people with COPD, both in clinical and research settings, in a context where social interactions should be (e.g., due to health reasons) or are (e.g., due to geographical constraints) reduced, but individuals' continuity of care remains essential (8, 24). Therefore, this study aimed to establish the reliability and validity of the CAT, FACIT-FS and SGRQ administered by telephone interview in people with COPD.
This study was conducted between July 2020 and April 2021, as part of an observational prospective study (PRIME - PTDC/SAU-SER/28806/2017) aiming to conduct pulmonary rehabilitation and follow-up participants with COPD over a 6-months period. The following Ethics Committees (Centro Hospitalar do Médio Ave reference 09/2016 and 10/2018; Unidade Local de Saúde de Matosinhos reference 10/CES/JAS 17/02/2017 and 73/CE/JAS 12/10/2018; Centro Hospitalar Baixo Vouga reference 777638 and 086892; Hospital Distrital da Figueira da Foz reference 1807/2017 and 27/05/2019; Administração Regional de Saúde do Centro reference 64/2016 and 85/2018) approved the study.
This study followed the guidelines on measurement properties for patient-reported outcomes of the COnsensus-based Standards for selection of health status Measurement Instruments (COSMIN) initiative (25) and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (26).
People with COPD living in the community were recruited by clinicians/pulmonologists at two hospitals (Centro Hospitalar Baixo Vouga and Hospital Distrital da Figueira da Foz) during their routine appointments. Individuals were eligible if diagnosed with COPD (1) and clinically stable for 1 month prior to the study [i.e., no hospital admissions or exacerbations, nor changes in medication, according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) (1)]. Exclusion criteria included the presence of other respiratory diseases or significant cardiovascular, neurologic, or musculoskeletal disease that precluded their participation in the study. Eligible participants were invited to participate in a 12-weeks pulmonary rehabilitation programme which includes supervised exercise training twice per week, education and psychosocial support every 2 weeks (27) and/or to have their health status monitored monthly over a 6-months period. More detailed information on the structure of the pulmonary rehabilitation programme is available elsewhere (28). Before enrolment and data collection, written and verbal description of the study was provided to all participants and the written informed consent was obtained.
Sociodemographic (age, sex and educational level), anthropometric (height and weight to compute body mass index-BMI) and clinical data (smoking habits, baseline dyspnoea, comorbidities and number of acute exacerbations and hospitalisations in the preceding year) were first collected using a structured questionnaire administered during a baseline face-to-face visit. Lung function values were obtained from participants' medical records and used to establish the severity of airway obstruction according to the GOLD report (1).
Participants' educational level was measured as completed years of schooling and classified into three categories according to the International Standard Classification of Education (ISCED) (29): ≤ 4 years (ISCED 0–1); 5–9 years (ISCED 2); ≥10 years (ISCED 3–8), following current Portuguese study cycles. The smoking status were classified as never, former and current smokers and the pack-years were also computed by multiplying each pack of 20 cigarettes smoked per day by the number of years the person has smoked.
The modified British medical research council (mMRC) questionnaire was used to assess activity-related dyspnoea and scored from 0 (no trouble with breathlessness) to 4 (too breathless to leave the house) (30). The severity of comorbid diseases was scored according to Charlson Comorbidity Index (CCI) (i.e., mild: 1–2; moderate: 3–4; severe: ≥5 scores) (31).
Participants were then asked to answer the CAT, the FACIT-FS and the SGRQ. All questionnaires were administered twice, in-person and by telephone by an interviewer, with a maximum interval of 48-h between the two modes of administration. In person administration occurred during evaluations at the Respiratory Research and Rehabilitation Laboratory (Lab3R) or during a home visit for follow-up assessments. Participants were randomly selected to answer first to the in-person interview followed by telephone interview or vice versa, to ensure that participants had the same opportunity of answering either formats in the first assessment. Some participants had never answered these instruments previously, and others might have answered them in previous assessments occurring at least 3-months ago, which reduced the possibility of a learning effect. A well-trained physiotherapist on COPD assessment and management conducted all the telephone and the in-person interviews. Participants and the physiotherapist were blinded to the answers/scores of the instruments in their first application.
The CAT is a brief and simple questionnaire for assessing and monitoring COPD (32, 33). It consists of eight items covering the most burdensome symptoms and limitations of COPD, for instance cough, phlegm, chest tightness, breathlessness, activity limitations, confidence, sleep, and energy (32, 33). The score for each question ranges from zero to five, and the total score ranges from zero to 40, with higher scores indicating worse health status. A cut-off ≥10 points is associated with a considerable impact of the disease in individuals' daily life (34). The CAT is also recommended to classify participants according to the ABCD assessment tool (1).
The FACIT-FS evaluates tiredness, weakness and difficulty in handling daily activities due to fatigue over the prior 7 days (35, 36). This scale is a multidimensional 13-item questionnaire in which items are classified according to a five-point Likert scale from “not at all” to “very much” (36). The scores range from zero to 52, with higher scores indicating less fatigue (36). Individuals scoring below the cut-off of 43 points were considered to have clinically relevant fatigue (37).
The SGRQ is a disease-specific instrument measuring HRQoL in people with chronic lung disease (38). The questionnaire has three domains: symptoms, activities, and impact (38). Each domain and the total questionnaire is scored from zero (no impairment) to 100 (maximum impairment), with lower scores representing a better HRQoL (39). A cut-off ≥ 25 points is indicative of the disease impact on HRQoL.
The psychometric properties assessed for the CAT, the FACIT-FS and the SGRQ were reliability and validity.
Reliability is the ability to reproduce a consistent result in time and space, or from different observers, presenting aspects of coherence, stability, equivalence and homogeneity (25, 40). Reliability estimates may be used to evaluate the equivalence of a set of items from the same test (internal consistency) and the stability of measures administered at different times to the same individuals or using the same standard (test–retest reliability) (40). Relative and absolute reliability are complementary methods and thus, should both be tested (25). Reliability coefficients range from zero to one, with higher coefficients indicating higher levels of reliability (40).
Validity is defined as the extent to which an instrument measures what it intends to measure (25, 40). Specifically, for establishing concurrent validity, scores of an instrument are correlated with scores of other measure which assesses the same construct and in the same subjects (40). Validity requires that an instrument is reliable, but an instrument can be reliable without being valid (40).
Descriptive statistics were used to characterize the sample. Normal distribution of the CAT, FACIT-FS and SGRQ scores was tested using the Kolmogorov–Smirnov test. Differences between the questionnaires scores applied in person vs. by telephone were tested using the Wilcoxon signed rank test.
The internal consistency was assessed using the Cronbach's alpha and a value higher than 0.70 was considered an acceptable level of consistency (41). Relative and absolute reliability were established. Relative reliability in test-retest was explored with the Intraclass Correlation Coefficient (ICC) equation (1, 2) and the respective 95% confidence intervals (95%CI) (42). The ICC was interpreted as excellent (>0.75), moderate to good (0.4–0.75) or poor (<0.4) (43). Absolute reliability, i.e., the agreement between the scores obtained in person vs. by telephone interview, was tested with the Bland and Altman method and the standard error of measurement (SEM). The first was used for visual judgement of how well the scores obtained by the two modes of administration agreed (44) and the second indicated the extent to which the scores varied on repeated measurements, providing a value for measurement error in the same units as the measurement itself (42). The SEM formula is: SEM = SD√ (1-ICC), where SD is the standard deviation of the scores obtained from the total sample of each scale.
The concurrent validity of the questionnaires applied in person and by telephone was assessed with the Spearman correlation coefficient (rho). This coefficient was interpreted as strong (≥0.70), moderate (0.30–0.70) or weak (≤0.30) (45).
A sample size of at least 50 participants are needed to test the psychometric properties of PROMs, although a sample size of 100 participants is desirable according to the COSMIN initiative (25).
All statistical analyses were conducted using IBM SPSS Statistics, version 24 (IBM Corporation, Armonk, NY, USA). The level of significance was set at p-value (p) ≤ 0.05.
Fifty-nine people with COPD were invited to participate in the study. Four individuals were excluded due to the presence of significant cognitive (n = 1) and musculoskeletal (n = 1) impairment, and absence of a confirmed diagnosis of COPD (n = 2). Table 1 shows the baseline characteristics of the 55 participants included in the analysis. Participants' mean age was 68.1 (± 7.9) years, 80% were male and presented a mean BMI of 27.3 (± 4.6) kg/m2. About 38.2% of participants had less or equal to four years of education (ISCED 0–1), were mostly former smokers (80%), presented a median mMRC grade of 2 [1; 3] and mild to moderate airflow limitation (65.4%). No significant differences were observed in the average scores of the CAT [11.3 (± 4.6) vs. 10.6 (± 7.4), p = 0.191], the FACIT-FS [41.4 (± 8.1) vs. 42.6 (± 7.0), p = 0.058] and the SGRQ [31.7 (± 20.1) vs. 32.1 (± 20.9), p = 0.459] assessed by in person vs. telephone interview (Table 2).
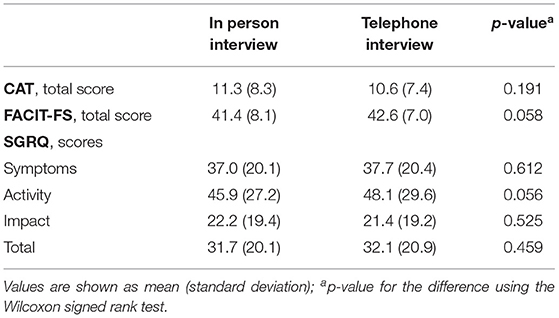
Table 2. The COPD Assessment Test (CAT), the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS) and the St. George's Respiratory Questionnaire (SGRQ) scores applied in person and by telephone interview to people with chronic obstructive pulmonary disease (n = 55).
A similar and strong internal consistency was observed between the telephone vs. in person interview for the CAT (0.82 vs. 0.84), the FACIT-FS (0.83 vs. 0.84) and the SGRQ (0.92 vs. 0.93) (Table 3).
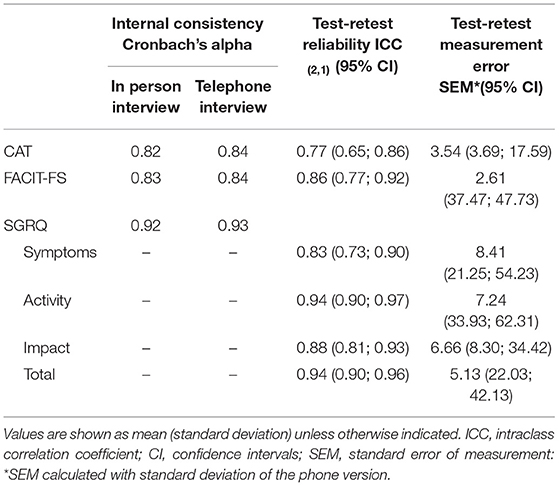
Table 3. Reliability of the COPD Assessment Test (CAT), the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS), St. George's Respiratory Questionnaire (SGRQ) applied in person and by telephone interview to people with chronic obstructive pulmonary disease (n = 55).
Excellent test-retest reliability was found for total scores of the CAT [ICC: 0.77 (95% CI: 0.65; 0.86)], the FACIT-FS [ICC: 0.86 (95% CI: 0.77; 0.92)] and the SGRQ [ICC: 0.94 (95% CI: 0.90; 0.96)], as well as for the scores of each SGRQ domain (Table 3). A reduced test-retest measurement error was found among the three questionnaires, ranging between 2.61 in FACIT-FS to 8.41 in SGRQ symptoms subscale (Table 3).
The Bland and Altman plots illustrated a good level of agreement between the two modes of administration of the questionnaires, with no evidence of systematic bias (Figure 1). The CAT plot showed a mean difference of 0.67 and upper and lower limits of 10.99 and −9.66, respectively (Figure 1A). The FACIT-FS plot illustrated a mean difference of −1.26 and upper and lower limits of 3.98 and −9.10, respectively (Figure 1B). The SGRQ plot showed a mean difference of −0.40 and upper and lower limits of 13.73 and −14.53, respectively (Figure 1C).
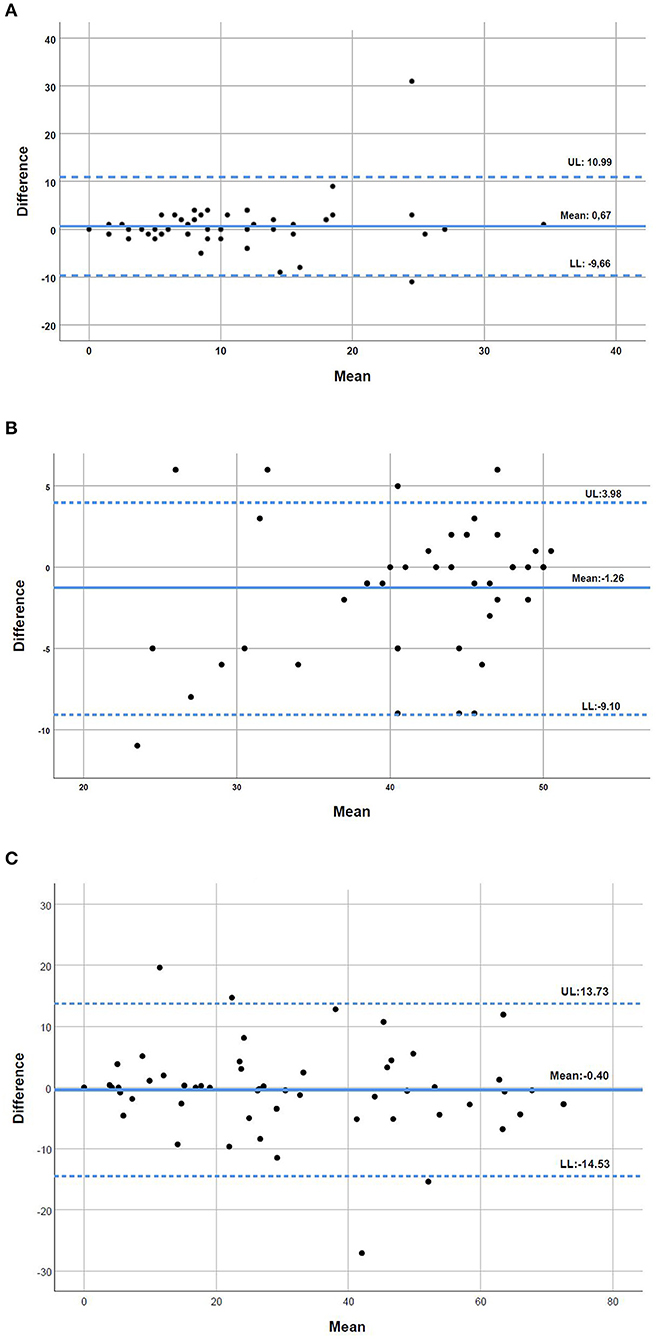
Figure 1. (A–C) Bland and Altman plots for the level of agreement between the COPD Assessment Test (CAT), the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS) and the St. George's Respiratory Questionnaire (SGRQ) applied in person and by telephone interview to people with chronic obstructive pulmonary disease (n = 55). The bold line represents the mean difference in the two types of interview and the dotted lines the 95% upper and lower limits of agreement (UL and LL, respectively).
Robust positive correlations were found between the total scores of the CAT (rho = 0.88, p < 0.001), the FACIT-FS (rho = 0.87, p < 0.001) and the SGRQ (rho = 0.94, p < 0.001) collected in person vs. by telephone interview (Figure 2). A slightly higher dispersion toward higher values of fatigue was observed in the FACIT-FS plot (Figure 2B).
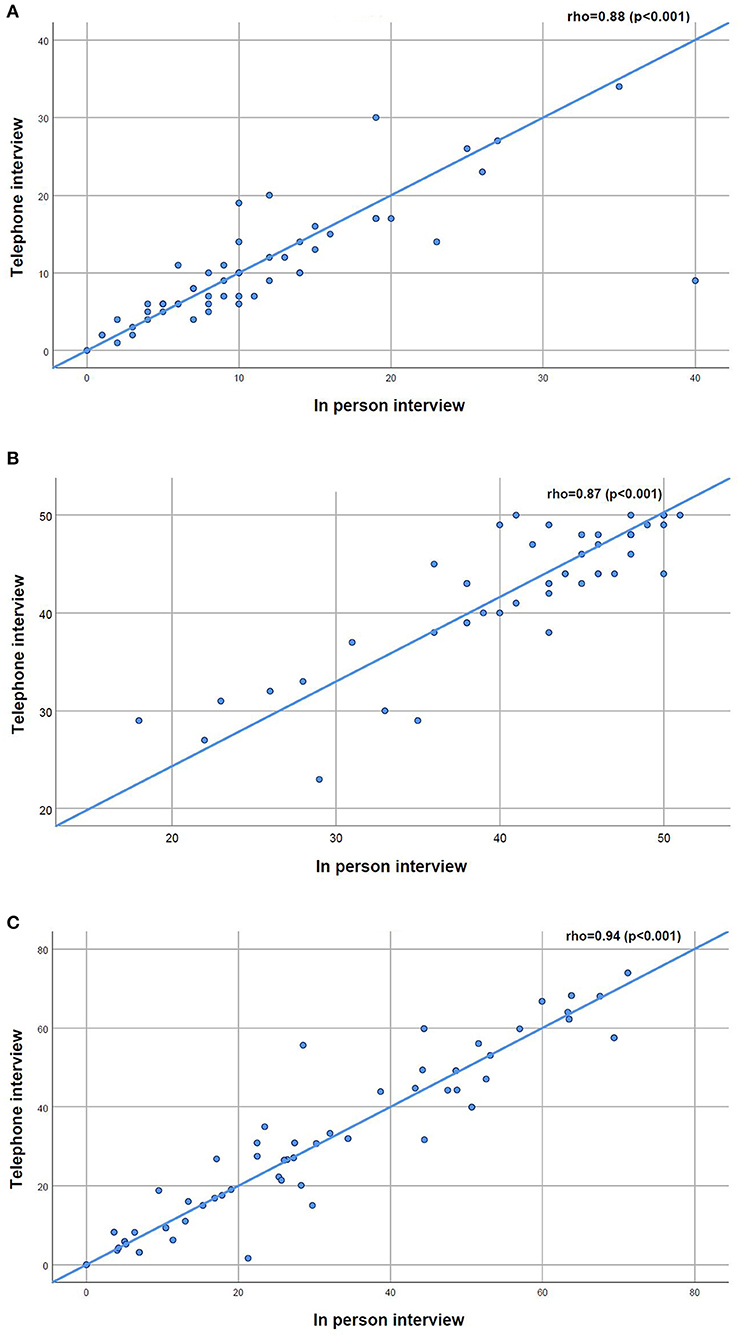
Figure 2. (A–C) Scatterplots for the relationship between the COPD Assessment Test (CAT), the Functional Assessment of Chronic Illness-Fatigue-Subscale (FACIT-FS) and the St. George's Respiratory Questionnaire (SGRQ) applied in person and by telephone interview to people with chronic obstructive pulmonary disease (n = 55).
Our study shows that the telephonic administration of the CAT, the FACIT-FS and the SGRQ is a reliable and valid alternative approach to in person interviews for monitoring symptoms and HRQoL in people with COPD.
The similar values of the Cronbach's alpha found between the two modes of administration showed their equivalent application. The excellent test–retest reliability, the high agreement and the lack of systematic bias further reinforced the use of telephonic interviews to assess these important PROMs. A strong correlation (>0.70) between the two modes of administration has also been found in all three questionnaires, confirming the validity of telephone interview to assess symptoms and HRQoL in this population (25, 46, 47). These results are in line with prior studies (21, 22) showing that the interview mode (telephone vs. in person) did not influence the health surveys' outcomes. Furthermore, previous evidence (36, 48, 49) demonstrated that the CAT, the FACIT and the SGRQ scores and its psychometric properties do not seem to be influenced by the method of administration. Thus, researchers and clinicians may be confident in applying these questionnaires by telephone, and its use in remote monitoring is encouraged.
The SGRQ showed the greatest reliability and validity when compared to the other two questionnaires, but the subscale of symptoms presented the highest values of associated measurement error. This finding might be explained by the questionnaires' different structure and recall period (33, 36, 38, 50). Specifically in the SGRQ (38) symptoms subscale, questions rely on the presence of cough, sputum, dyspnoea, etc. in the last 3 months and answers are “most days a week,” “several days a week,” “some days a month,” “only with respiratory infections,” or “not at all.” These questions require each participant to report symptoms occurring over a long period of time in contrast to the CAT (33) and the FACIT-FS (36) which refer to the presence and/or frequency of symptoms in the present moment or in the last 7 days, respectively. Evidence suggests that patients tend to value more and consequently overreport more severe symptoms that occurred in the last few days (51, 52). Additionally, it is consensual that symptoms in COPD vary significantly throughout the day and over the weeks and/or months (52, 53). It is, therefore, likely that individuals find difficult to provide a precise report over a 3-months recall period, leading to a higher measurement error.
We also observed that the FACIT-FS showed the higher difference (−1.26) between the in person and telephone interviews compared to the CAT (0.67) and the SGRQ (0.40), as well as a slightly dispersion of values toward higher values of fatigue. A possible explanation might be that the difference between the two modes of administration tended to increase as the scores of the scale increase, suggesting that participants tend to report with more consistency fatigue levels when they were high and caused a perceived higher impact on their daily life. The relatively small sample size and the high symptom variability, confirmed by the results of the SGRQ symptom domain, might also explain our findings. Thus, more research is needed to enhance our knowledge on this and other PROMs, namely exploring the agreement applied by telephone and in person.
The telephone interview might have additional advantages in comparison to in person questionnaires, since it may save costs associated with the participants' transportation, enhance adherence due to the flexibility for scheduling the interview, or improve confidence to speak without having the physical presence of the interviewer (8, 54). These advantages are important in the context of current and future pandemics, but also for researcher planning of observational studies and clinical trials, which may consider to collect some of the information using telephone interview. Nevertheless, in some cases people with COPD might prefer face-to-face contacts which allow a more thorough assessment. Thus, whenever possible the preferences of people with COPD on the mode of assessment should be considered. Additionally, further research exploring these preferences would be important for supporting the use of technologies of information and communication in people with COPD.
Strengths of our study include addressing a highly needed topic in the current pandemic, with implications for research and clinical practice, and the random order of the administration modes. Some limitations should also be acknowledged. Our sample size might have influenced our results, since according to the guidelines of the COSMIN initiative (25), a sample size over 100 individuals is desirable for testing reliability and validity of PROMs. Nevertheless, we were able to show the reliability and validity properties of the CAT, the FACIT-FS and the SGRQ including the minimal sample size required (more than 50 individuals), and we could hypothesize that a larger sample would strengthen our results. The interviewer presence applying the CAT and the SGRQ questionnaires, which were originally developed to be self-administered, may be perceived as a possible limitation, since it might bias individuals toward more favorable responses (55). This is however unlikely to have had a significant impact on our results, since the interviewer was present in both modes of administration, thus his/her possible influence was similar in both modes of administration. Moreover, both face-to-face and telephone questionnaires were applied by the same well-trained interviewer to improve consistency. The lack of standardization of the day period in which questionnaires were applied might have influenced our results, since people with COPD tend to report worse symptoms in the morning (56). Further studies should consider to collect this information and standardize the timing of application. Finally, our sample was composed by older adults (from 60 to 76 years old) mainly male and with a low level of education (i.e., ≤ 4 years of education). Thus, participants' characteristics limited external validity to all people with COPD.
The findings of our study have important implications for research and clinical practice highlighting the added-value of technologies of information and communication to ensure a safe follow-up of meaningful outcomes such as symptoms and health-related quality of life in people with COPD, when face-to-face contact needs to be reduced or is not possible. Moreover, our results also suggest that researchers might consider to collect some baseline and follow-up information using the telephone interview, without compromising the validity and reliability of the results. This is a valuable information in the context of the COVID-19 pandemic, but also in the case of future health outbreaks.
The telephone interview is a reliable and valid method to assess and monitor symptoms and HRQoL in people with COPD using recommended PROMs, such as the CAT, the FACIT-FS and the SGRQ. The telephone might be an important add-on for personalized assessment and management of chronic respiratory disease in the context of remote monitoring. Nevertheless, the level of adherence and satisfaction of people with COPD with the replacement/addition of these technologies to the standard care should be explored.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Centro Hospitalar do Médio Ave reference 09/2016 and 10/2018; Unidade Local de Saúde de Matosinhos reference 10/CES/JAS 17/02/2017 and 73/CE/JAS 12/10/2018; Centro Hospitalar Baixo Vouga reference 777638 and 086892; Hospital Distrital da Figueira da Foz reference 1807/2017 and 27/05/2019; Administração Regional de Saúde do Centro reference 64/2016 and 85/2018. The patients/participants provided their written informed consent to participate in this study.
VR, CJ, and AM conceived the study. VR performed data collection, data analysis and interpretation, and drafted the manuscript. AM was responsible for obtaining the funding and ensured the project administration and resources. All authors critically revised the manuscript, ensured accuracy and integrity of the work, approved the final version to be published, and agreed to be accountable for all aspects of the work.
This work was supported by Programa Operacional de Competitividade e Internacionalização – POCI, through Fundo Europeu de Desenvolvimento Regional - FEDER (POCI-01-0145-FEDER-028806), by Fundação para a Ciência e Tecnologia (PTDC/SAU-SER/28806/2017), and under the project UIDB/04501/2020.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Global Initiative for Chronic Obstructive Lung Disease G. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2021 Report). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) (2021).
2. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Res Crit Care Med. (2021) 203:24–36. doi: 10.1164/rccm.202009-3533SO
3. Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Res Med. (2020) 8:585–96. doi: 10.1016/S2213-2600(20)30105-3
4. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Res Res. (2017) 18:67. doi: 10.1186/s12931-017-0548-3
5. van der Molen T, Miravitlles M, Kocks JWH. COPD management: role of symptom assessment in routine clinical practice. Int J Chron Obstruct Pulmon Dis. (2013) 8:461–71. doi: 10.2147/COPD.S49392
6. Machado A, Marques A, Burtin C. Extra-pulmonary manifestations of COPD and the role of pulmonary rehabilitation: a symptom-centered approach. Expert Rev Respir Med. (2021) 15:131–42. doi: 10.1080/17476348.2021.1854737
7. Postma D, Anzueto A, Calverley P, Jenkins C, Make BJ, Sciurba FC, et al. A new perspective on optimal care for patients with COPD. Prim Care Respir J. (2011) 20:205–9. doi: 10.4104/pcrj.2011.00041
8. Wu F, Burt J, Chowdhury T, Fitzpatrick R, Martin G, van der Scheer JW, et al. Specialty COPD care during COVID-19: patient and clinician perspectives on remote delivery. BMJ Open Res Res. (2021) 8:e000817. doi: 10.1136/bmjresp-2020-000817
9. Bellamy D, Bouchard J, Henrichsen S, Johansson G, Langhammer A, Reid J, et al. International primary care respiratory group (IPCRG) Guidelines: management of chronic obstructive pulmonary disease (COPD). Prim Care Respir J. (2006) 15:48–57. doi: 10.1016/j.pcrj.2005.11.003
10. Franssen FM, Alter P, Bar N, Benedikter BJ, Iurato S, Maier D, et al. Personalized medicine for patients with COPD: where are we? Int J Chron Obstruct Pulmon Dis. (2019) 14:1465–84. doi: 10.2147/COPD.S175706
11. Agusti A. The path to personalised medicine in COPD. Thorax. (2014) 69:857. doi: 10.1136/thoraxjnl-2014-205507
12. Dawson J, Doll H, Fitzpatrick R, Jenkinson C, Carr AJ. The routine use of patient reported outcome measures in healthcare settings. BMJ. (2010) 340:c186. doi: 10.1136/bmj.c186
13. Ekström M, Sundh J, Larsson K. Patient reported outcome measures in chronic obstructive pulmonary disease: which to use? Exp Rev Res Med. (2016) 10:351–62. doi: 10.1586/17476348.2016.1146595
14. Dechman G, Aceron R, Beauchamp M, Bhutani M, Bourbeau J, Brooks D, et al. Delivering pulmonary rehabilitation during the COVID-19 pandemic: a canadian thoracic society position statement. Can J Res Crit Care Sleep Med. (2020) 4:232–5. doi: 10.1080/24745332.2020.1828683
15. Alqahtani JS, Alghamdi SM, Aldhahir AM, Althobiani M, Oyelade T. Key toolkits of non-pharmacological management in COPD: during and beyond COVID-19. Front Biosci Landmark. (2021) 26:246–52. doi: 10.52586/4938
16. Tsutsui M, Gerayeli F, Sin DD. Pulmonary rehabilitation in a Post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2021) 16:379–91. doi: 10.2147/COPD.S263031
17. National Institute for Health Care Excellence N. COVID-19 Rapid Guideline: Community-Based Care of Patients With Chronic Obstructive Pulmonary Disease (COPD). London: National Institute for Health and Care Excellence (2020).
18. Gardiner L, Alderslade A, Butler F, Graham L, Harvey-Dunstan T, Ingram K, et al. Statement and Considerations for the Remote Delivery of Pulmonary Rehabilitation Services During the COVID-19 Pandemic. London: The Association of Chartered Physiotherapists in Respiratory Care (2020).
19. Pleguezuelos E, Del Carmen A, Moreno E, Ortega P, Vila X, Ovejero L, et al. The experience of COPD patients in lockdown due to the COVID-19 pandemic. Int J Chron Obstruct Pulmon Dis. (2020) 15:2621–7. doi: 10.2147/COPD.S268421
20. Jácome C, Pereira R, Almeida R, Amaral R, Correia MA, Mendes S, et al. Validation of app and phone versions of the control of allergic rhinitis and asthma test (CARAT). J Investig Allergol Clin Immunol. (2021) 31:270–3. doi: 10.18176/jiaci.0640
21. Chatterji R, Naylor JM, Harris IA, Armstrong E, Davidson E, Ekmejian R, et al. An equivalence study: ARE patient-completed and telephone interview equivalent modes of administration for the EuroQol survey? Health Quality Life Outcomes. (2017) 15:18. doi: 10.1186/s12955-017-0596-x
22. McPhail S, Lane P, Russell T, Brauer SG, Urry S, Jasiewicz J, et al. Telephone reliability of the frenchay activity index and EQ-5D amongst older adults. Health Quality Life Outcomes. (2009) 7:48. doi: 10.1186/1477-7525-7-48
23. da Silva GF, Morano MTA, Sales MPU, Olegário NB, Cavalcante AGM, Pereira EDB. Comparison of face-to-face interview and telephone interview administration of COPD assessment test: a randomized study. Quality Life Res. (2014) 23:1193–7. doi: 10.1007/s11136-013-0563-x
24. Rutkowski S. Management challenges in chronic obstructive pulmonary disease in the COVID-19 pandemic: telehealth and virtual reality. J Clin Med. (2021) 10:1261. doi: 10.3390/jcm10061261
25. Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Quality Res. (2010) 19:539–49. doi: 10.1007/s11136-010-9606-8
26. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Ann Int Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
27. World Health O. WHO Strategy for Prevention and Control of Chronic Respiratory Diseases. Geneva: World Health Organization (2002).
28. Marques A, Jácome C, Rebelo P, Paixão C, Oliveira A, Cruz J, et al. Improving access to community-based pulmonary rehabilitation: 3R protocol for real-world settings with cost-benefit analysis. BMC Public Health. (2019) 19:676. doi: 10.1186/s12889-019-7045-1
29. OECD/Eurostat/UNESCO Institute for Statistics. ISCED 2011 Operational Manual: Guidelines for Classifying National Education Programmes and Related Qualifications. Paris: OECD Publishing (2015). doi: 10.1787/9789264228368-en
30. Doherty DE, Belfer MH, Brunton SA, Fromer L, Morris CM, Snader TC. Chronic obstructive pulmonary disease: consensus recommendations for early diagnosis and treatment. J Family Prac. (2006) 55:S1+. Available online at: https://link.gale.com/apps/doc/A154691962/AONE?u=anon~48c0054d&sid=googleScholar&xid=e578703b
31. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. (1994) 47:1245–51. doi: 10.1016/0895-4356(94)90129-5
32. Jones PW, De Amaral-Marques RJC, Bone MF, Boulet LP, Brusasco V, Burke CM, et al. Quality of life, symptoms and pulmonary function in asthma: long-term treatment with nedocromil sodium examined in a controlled multicentre trial. Eur Res J. (1994) 7:55–62. doi: 10.1183/09031936.94.07010055
33. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Res J. (2009) 34:648. doi: 10.1183/09031936.00102509
34. Kon SSC, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Res Med. (2014) 2:195–203. doi: 10.1016/S2213-2600(14)70001-3
35. Antoniu SA, Ungureanu D. Measuring fatigue as a symptom in COPD: from descriptors and questionnaires to the importance of the problem. Chron Respir Dis. (2015) 12:179–88. doi: 10.1177/1479972315575716
36. Webster K, Cella D, Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Quality Life Outcomes. (2003) 1:79. doi: 10.1186/1477-7525-1-79
37. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. (2002) 94:528–38. doi: 10.1002/cncr.10245
38. Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. J Chronic Obst Pulmo Dis. (2005) 2:75–9. doi: 10.1081/COPD-200050513
39. Ferrer M, Villasante C, Alonso J, Sobradillo V, Gabriel R, Vilagut G, et al. Interpretation of quality of life scores from the St George's respiratory questionnaire. Eur Res J. (2002) 19:405. doi: 10.1183/09031936.02.00213202
40. Kimberlin CL, Winterstein AG. Validity and reliability of measurement instruments used in research. Am J Health Sys Pharmacy. (2008) 65:2276–84. doi: 10.2146/ajhp070364
41. Taber KS. The use of cronbach's alpha when developing and reporting research instruments in science education. Res Sci Educ. (2018) 48:1273–96. doi: 10.1007/s11165-016-9602-2
42. Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Stren Cond Res. (2005) 19:231–40. doi: 10.1519/15184.1
43. J. F. Reliability of measurements. In: Fleiss JE, editor. The Design and Analysis of Clinical Experiments. 1st. ed. New York, NY: John Wiley & Sons (1986).
44. Rankin G, Stokes M. Reliability of assessment tools in rehabilitation: an illustration of appropriate statistical analyses. Clin Rehabil. (1998) 12:187–99. doi: 10.1191/026921598672178340
45. Akoglu H. User's guide to correlation coefficients. Turkish J Emerg Med. (2018) 18:91–3. doi: 10.1016/j.tjem.2018.08.001
46. Terwee CB, Bot SDM, de Boer MR, van der Windt DAWM, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. (2007) 60:34–42. doi: 10.1016/j.jclinepi.2006.03.012
47. Weldam SWM, Schuurmans MJ, Liu R, Lammers J-WJ. Evaluation of quality of life instruments for use in COPD care and research: a systematic review. Int J Nurs Studies. (2013) 50:688–707. doi: 10.1016/j.ijnurstu.2012.07.017
48. Agusti A, Soler-Cataluña JJ, Molina J, Morejon E, Garcia-Losa M, Roset M, et al. Does the COPD assessment test (CATTM) questionnaire produce similar results when self- or interviewer administered? Quality Life Res. (2015) 24:2345–54. doi: 10.1007/s11136-015-0983-x
49. Hood K, Robling M, Ingledew D, Gillespie D, Greene G, Ivins R, et al. Mode of data elicitation, acquisition and response to surveys: a systematic review. Health Technol Assess. (2012) 16:27. doi: 10.3310/hta16270
50. Morishita-Katsu M, Nishimura K, Taniguchi H, Kimura T, Kondoh Y, Kataoka K, et al. The COPD assessment test and St George's respiratory questionnaire: are they equivalent in subjects with COPD? Int J Chron Obstruct Pulmon Dis. (2016) 11:1543–51. doi: 10.2147/COPD.S104947
51. Meek PM, Lareau SC, Anderson D. Memory for symptoms in COPD patients: how accurate are their reports? Eur Res J. (2001) 18:474. doi: 10.1183/09031936.01.00083501
52. Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. (2011) 37:264–72. doi: 10.1183/09031936.00051110
53. Miravitlles M, Worth H, Soler Cataluña JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respirat Res. (2014) 15:122. doi: 10.1186/s12931-014-0122-1
54. Newsom C, Schut H, Stroebe M, Birrell J, Wilson S. Telephone versus in-person intake assessment for bereavement intervention: does efficiency come at a cost? Death Studies. (2016) 40:71–9. doi: 10.1080/07481187.2015.1068244
55. Wu AW, Jacobson DL, Berzon RA, Revicki DA, van der Horst C, Fichtenbaum CJ, et al. The effect of mode of administration on medical outcomes study health ratings and EuroQol scores in AIDS. Qual Life Res. (1997) 6:3–10.
Keywords: chronic obstructive pulmonary disease, assessment and monitoring, COPD assessment test, functional assessment of chronic illness-fatigue subscale, St. George's respiratory questionnaire, telephone interview, patient-reported outcome measures
Citation: Rocha V, Jácome C, Martins V and Marques A (2021) Are in Person and Telephone Interviews Equivalent Modes of Administrating the CAT, the FACIT-FS and the SGRQ in People With COPD? Front. Rehabilit. Sci. 2:729190. doi: 10.3389/fresc.2021.729190
Received: 22 June 2021; Accepted: 24 September 2021;
Published: 25 October 2021.
Edited by:
Simone Dal Corso, Universidade Nove de Julho, BrazilReviewed by:
Linzy Houchen-Wolloff, University Hospitals of Leicester NHS Trust, United KingdomCopyright © 2021 Rocha, Jácome, Martins and Marques. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alda Marques, YW1hcnF1ZXNAdWEucHQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.