- Gene Regulation Section, Laboratory of Molecular Biology and Immunology, National Institute on Aging, Baltimore, MD, United States
Lymphocyte development culminates with generation of mature B and T cells that express unique antigen receptors on the cell surface. Genes that encode the two chains of B or T cell receptors are generated via DNA recombination and expressed sequentially during development, guided by locus activating enhancer sequences. In this review we summarize our understanding of molecular mechanisms that activate these enhancers in a lineage and developmental stage-specific manner. We draw attention to 1) the distinction between chromatin accessibility and transcriptional activation of these loci, 2) incomplete understanding of mechanisms that regulate B versus T cell-specific enhancer activity and 3) transcription factors that contribute to stage-specific enhancer activation within each lineage.
Introduction
B and T lymphocytes of the adaptive immune system protect against a variety of pathogens via unique receptors expressed on the cell surface. The wide diversity of such antigen receptor specificities ensures high likelihood of recognizing newly emerging, or previously encountered, pathogens. Antibodies, that constitute B cell antigen receptors (BCRs) are heterotetramers of two identical heavy chains (IgH) and two identical light chains (IgL) of either kappa (Igκ) or lambda (Igλ) type. T cell receptors (TCRs) confer antigen specificity to T lymphocytes via heterodimers consisting of alpha (TCRα) and beta (TCRβ) chains or gamma (TCRγ) and delta (TCRδ) chains. The unique recognition specificity of each lymphocyte is determined by variable N-terminal domains in BCRs and TCRs. These domains, and thereby receptor diversity, are generated during lymphocyte development.
Antigen receptor genes are assembled by V(D)J recombination
Unlike all other mammalian genes, loci that encode antigen receptors are composed of gene segments rather than fully functional genes. The variety, and thereby diversity, of antigen receptors is generated in part by randomly assorting hundreds of gene segments during lymphocyte development (Figure 1A). The mouse Igh locus, for example, contains several hundred variable (VH) gene segments (C57BL/6 mice have 110 functional VH genes and 85 VH pseudogenes), 8–12 diversity (DH) gene segments and 4 joining (JH) gene segments distributed over 3 Mb (Johnston et al., 2006) (Figure 1B). The N-terminal variable domain of antibody heavy chains is assembled by genomic juxtaposition of one VH, one DH and one JH gene segment by a process known as V(D)J recombination (Chowdhury and Sen, 2004; Jung and Alt, 2004; Jung et al., 2006; Kumari and Sen, 2015; Proudhon et al., 2015). Variable domains of TCRβ and TCRδ chains are also assembled by recombining three gene segments. By contrast, Ig light chain (Igκ or Igλ) and TCRα and TCRγ chain genes require only one recombination event between a variable and a joining gene segment to generate functional genes (Krangel, 2009; Collins and Watson, 2018). These gene rearrangements are mediated by the identical nuclear enzymatic machinery in both lineages. Key amongst these are the recombination-activating gene products, RAG1 and RAG2, that introduce double-strand DNA breaks to initiate the process and are expressed together only in developing lymphocytes (Schatz et al., 1989; Oettinger et al., 1990; Teng and Schatz, 2015; Lescale and Deriano, 2017; Lin et al., 2018; Christie et al., 2022). Thereafter, ubiquitously expressed proteins of the non-homologous end joining pathway are recruited to complete the process (Lieber, 2010; Wang et al., 2020; Zhao et al., 2020).
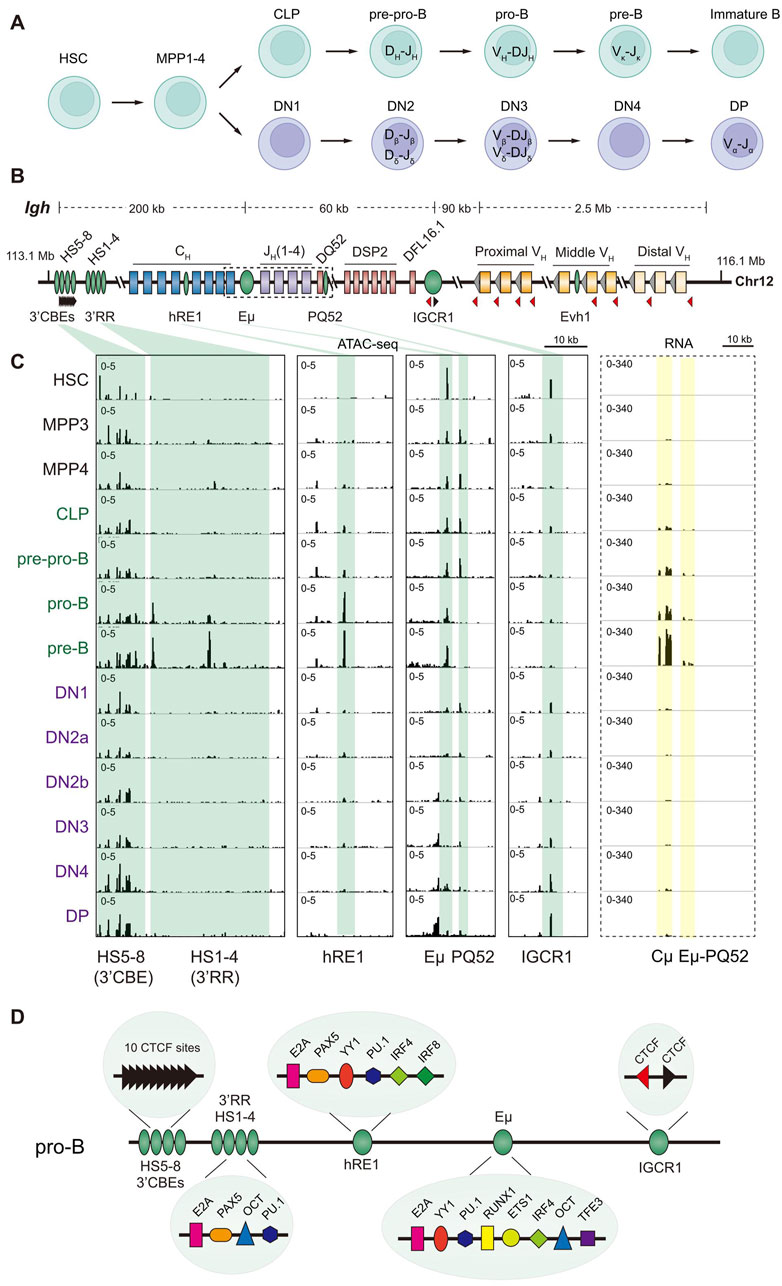
Figure 1. Igh locus regulatory sequences and transcription during B cell development. (A) Overview of adult hematopoiesis in mice. Long term reconstituting hematopoietic stem cells (HSC) differentiate via multipotential progenitors (MPP1-4) to B lymphocytes in the bone marrow (top row) and T lymphocytes (bottom row) in the thymus. Common lymphoid progenitors (CLP), though multipotent, are largely B cell precursors that differentiate via several intermediate stages to immunoglobulin (Ig) expressing mature B cells in the bone marrow. Multipotency is lost around the pre-pro-B cell stage where immunoglobulin heavy chain (Igh) gene rearrangements initiate. Ig light chain rearrangements occur in the pre-B cells. Multipotent cells that migrate to the thymus (DN1, a heterogenous cell subset) commit to T lineage differentiation in DN2 cells where T cell receptor β (Tcrb) gene rearrangements initiate. Tcra rearrangements occur in DP cells. (B) Schematic representation of the 3 Mb mouse Igh locus located on chromosome 12 (coordinates shown are mm10). Variable (VH), diversity (DQ52, DSP2 and DFL16.1), and joining (JH) gene segments are shown as yellow, pink, and purple boxes. Blue boxes represent constant region (CH) exons. Grey triangles adjacent to the VH gene segments indicate recombinase signal sequences (RSS) required for gene rearrangements. RSS adjacent to diversity and joining segments are not shown. Cis-regulatory sequences discussed in this review are shown as green ovals, including enhancers (Evh1, Eµ, hRE1, and 3′RR), intergenic CTCF site IGCR1 and 3′CBEs. CTCF binding sites and orientation are shown by black and red triangles. The region highlighted by a dashed box is expanded in the right panel of part C to display RNA-seq data. (C) ATAC-seq (left panel) and RNA-seq (right panel) profiles of the 3′ part of the Igh locus during hematopoiesis obtained from the Immunological Genome project (Yoshida et al., 2019). ATAC-seq patterns covering known cis regulatory sequences are shown as identified below the tracks. For RNA-seq the pattern of the locus in the dashed box in part B is shown. (D) Transcription factors that binds to previously identified regulatory sequences in the 3′ Igh domain in pro-B cells are shown. The summary combines in vitro protein binding studies and in vivo analysis by chromatin immunoprecipitation assays (ChIP) (Kumari et al., 2018; Medvedovic et al., 2013; Kleiman et al., 2016; Ernst and Smale, 1995; Henderson and Calame, 1998; Lin et al., 2010; Khamlichi et al., 2000; Loguercio et al., 2018), and does not represent the numbers of each protein binding site.
Despite a shared rearrangement mechanism, BCR genes recombine fully only in the B cells and TCR genes rearrange only in T cells. This lineage specificity has been understood in term of the accessibility hypothesis (Yancopoulos and Alt, 1985; Stanhope-Baker et al., 1996; Krangel, 2003). Namely, RAG proteins can access and act upon BCR loci, but not TCR loci, in B cell precursors and, conversely, only TCR loci (but not BCR loci) in precursor T cells. Additionally, antigen receptor gene recombination is developmentally segregated within each lineage. During B cell development, rearrangement and expression of Igh genes occurs first at the pro-B cell stage, followed by Igk and Igl genes in pre-B cells (Figure 1A) (Lescale, 2016; Borghesi et al., 2004; Hardy et al., 1991). In the T cell lineage, Tcrb rearrangements and expression in CD4−CD8− (double negative, DN) cells precede Tcra rearrangements which occur at the later CD4+CD8+ (double positive, DP) stage (Figure 1A) (Krangel, 2009; Christie et al., 2022; Rothenberg and Taghon, 2005). The lineage-specific accessibility hypothesis can be extended to account for stage-specificity of rearrangements within each lineage. For this, the idea is that Igh loci rearrange in pro-B cells when RAG proteins do not have access to Igk or Igl loci. The latter become accessible only at the pre-B cell stage once Igh rearrangements are completed. Similarly, only the Tcrb locus, but not Tcra locus, is accessible to RAG proteins in DN cells, the latter becoming accessible to RAGs at the subsequent DP stage. Igl, Tcrg and Tcrd loci are similarly regulated but will not be discussed in detail in this review due to space constraints.
Enhancers regulate antigen receptor gene assembly and expression
Enhancers were identified as regulatory sequences that activated transcription in a position (5′ or 3′ of a gene promoter) and orientation (relative to gene transcription) independent manner (Banerji et al., 1981). They function by recruiting DNA binding proteins to specific regions of the genome. These proteins (also referred to as transcription factors), in turn, recruit accessory proteins that result in the formation of multi-protein complexes on enhancers (Zabidi and Stark, 2016; Haberle and Stark, 2018; Jindal and Farley, 2021; Panigrahi and O'Malley, 2021). The numbers and layers of accessory proteins recruited likely varies between enhancers and has not been fully described for any enhancer. One of the best-known accessory proteins is the CREB binding protein (CBP) and its closely related family member, p300. CBP/p300 are histone acetyl transferases (HATs) that acetylate lysine 27 on histone H3 leading to the epigenetic modification H3K27ac (Heintzman et al., 2007; Creyghton et al., 2010; Rada-Iglesias et al., 2011; Calo and Wysocka, 2013). This mark is associated with gene transcription and active enhancers, and CBP/p300 are also referred to as co-activators (Weinert et al., 2018; Narita et al., 2021). Active enhancers are also associated with high chromatin accessibility as measured by sensitivity to endonucleases. The most recent iteration of this is the assay for transposase accessible chromatin followed by sequencing (ATAC-seq) that closely aligns with DNase I hypersensitivity assays (Buenrostro et al., 2015). Enhancer sequences that lack H3K27ac but are marked by H3K4me1 have been referred to as poised enhancers that are ready for activation (Creyghton et al., 2010; Lesch and Page, 2014; Crispatzu et al., 2021; Jenuwein and Allis, 2001; Klemm et al., 2019). DNA-bound transcription factors may also recruit co-repressor complexes, such as NcoR/SMRT and mSin3 that contain histone deacetylases, that are associated with gene repression (Adams et al., 2018; Watson et al., 2012; Wong et al., 2014). Because co-activators and co-repressors are expressed in most cell types, their tissue-specific utilization likely resides in the spectrum of transcription factors recruited to tissue-specific enhancers. Enhancer sequences have been identified in murine and human antigen receptors gene loci (Rodriguez-Caparros et al., 2020; Kasprzyk et al., 2021). Most of the analyses have been carried out with the murine enhancers, which bear all hallmarks of traditional transcriptional enhancers, that are the focus of this review.
Several observations substantiate the role of enhancers in determining lineage and developmental timing of antigen receptor gene activation. First, deletion of enhancers associated with these loci demonstrates that they are necessary for developmentally appropriate activation of each locus (further discussed below). Second, genetic substitution of enhancers partially recapitulates regulatory features of the locus from which it is derived. For example, replacement of an enhancer in the Tcrb locus (Eβ) by one from Igh (Eµ) induces Tcrb transcription in B lymphocytes (Bories et al., 1996). In another study, substitution of Eβ by an enhancer associated with the later activated Tcra locus reduced Tcrb transcription in early stage DN cells but activated transcription in DP cells where Tcra genes rearrange (Senoo et al., 2001). Similarly, substitution of the iEκ enhancer associated with the Igk locus that is activated later in B cell development with Eµ led to premature Igk transcription and rearrangements in pro-B cells (Inlay et al., 2006). Third, antigen receptor gene enhancers direct lineage and developmentally stage-specific activation of transgenes in mice. Eμ, for example, is necessary and sufficient to activate Ig or heterologous genes in B cells of transgenic mice (Adams et al., 1985). Similarly, Eβ, Eα and Eµ have been shown to activate transcription and V(D)J recombination in transgenic mini loci (Capone et al., 1993; Okada et al., 1994; Ferrier et al., 1990). Eβ has also been used to activate other transgenes in T cells. In a notable exception, Eµ activates transgenes in both B and T cell lineages (Ferrier et al., 1990). Eμ promiscuity is also reflected in DH to JH rearrangements and transcription of the Igh locus in a large proportion of thymocytes in wild type mice (Kurosawa et al., 1981; Born et al., 1988; Allman et al., 2003; Kumari et al., 2018). These transgenic experiments show that antigen receptor gene enhancers are sufficient to activate transgenes integrated at different genomic locations, reminiscent of the properties of locus control regions (Jenuwein et al., 1993). Thus, a few hundred nucleotides constituting these enhancers carry the information content that specifies tissue-specific gene activation. The sections below address mechanisms by which such specificity is achieved.
Enhancers that regulate immunoglobulin gene rearrangements
Enhancer control of Igh expression
B cells develop from hematopoietic stem cells (HSC) through intermediates that retain various levels of multipotentiality. Commitment to differentiation into B cells occurs close to the pre-pro-B cell differentiation stage (Figure 1A). Igh rearrangements initiate in these cells with DH rearrangements, followed by VH rearrangements at the pro-B cells stage (Alt et al., 1984). IgH expression is a checkpoint during B cell development. Only IgH-expressing pro-B cells differentiate to pre-B cells where Ig light chain genes (Igk and Igl) rearrange. Expression of light chain permits immature B cells to express membrane antibody molecules of the IgM isotype.
The 3′ Igh domain, extending from the intergenic control region 1 (IGCR1) to 3′ CTCF binding elements (3′CBE), within which the first rearrangements occur is marked by several regions of high chromatin accessibility in pro-B cells (Figure 1C, left panel). At the 5′ boundary two CTCF binding sites within IGCR1 regulate Igh rearrangements. Their mutation or deletion accentuates use of the closest VH gene segments thereby severely compromising Igh diversity, as well as permits VH rearrangements to unrearranged DH gene segments (Featherstone et al., 2010; Guo et al., 2011; Lin et al., 2015; Qiu et al., 2018; Giallourakis et al., 2010). The intronic enhancer, Eµ, and a promoter (PQ52) associated with the 3′-most DH gene segment, DQ52, are marked by two closely associated ATAC-sensitive regions. In pro-B cells Eµ regulates histone modifications in the 3′ Igh domain (Chakraborty et al., 2009), induces transcription of the unrearranged (germline) locus and activates rearrangements. Deletion of Eµ reduces DH recombination substantially (∼80%) and virtually abolishes VH recombination (Chakraborty et al., 2009; Perlot et al., 2005; Afshar et al., 2006; Bolland et al., 2007).
Several additional chromatin accessible sites are evident moving 3′ from Eµ (Figure 1C, left panel). hRE1 is an enhancer located between Cγ1 and Cγ2b IgH isotypes (Medvedovic et al., 2013; Predeus et al., 2014). hRE1 is not required for Igh rearrangements in pro-B cells but promotes class switch recombination (CSR) to IgG3, IgG2b and IgG2a isotypes during immune responses (Amoretti-Villa et al., 2019). The 3′ regulatory region (3′RR), located 3′ of the last Cα exons, consists of a cluster of four B cell-specific transcriptional enhancers that span 28 kb (Lieberson et al., 1991; Giannini et al., 1993; Matthias and Baltimore, 1993; Madisen and Groudine, 1994; Michaelson et al., 1995). Like hRE1, the 3′RR contributes primarily to CSR, but not to control of Igh rearrangements in pro-B cells (Vincent-Fabert et al., 2010; Rouaud et al., 2012; Saintamand et al., 2015; Bruzeau et al., 2021; Oudinet et al., 2020). Finally, 3′CBE is a cluster of CTCF binding chromatin accessible regions that mark the 3′ boundary of the Igh topologically associated domain (TAD) (Garrett et al., 2005; Vian et al., 2018). Accordingly, its deletion leads to transcriptional activation of genes located further 3′ that are not normally expressed in pro-B cells (Volpi et al., 2012; Zhang et al., 2021). Recently, an additional enhancer has been identified within the VH genes. Its deletion affects recombination of closely positioned VH gene segments (Bhat et al., 2023). Thus, Eµ constitutes the only validated regulatory sequence associated with both Igh locus activation and rearrangements in pre-pro- and pro-B cells.
Though originally identified as a transcriptional enhancer and proposed to be important for transcription of rearranged Igh alleles, several lines of evidence suggest that Eµ is not required for Igh expression in mature B cells or during immune responses. Eckhardt and colleagues first demonstrated that VDJ ‘knock in’ Igh alleles that lacked Eµ permitted normal B cell development and function (Li and Eckhardt, 2009; Li et al., 2010). Other studies in germline Eµ-deficient mice indicate that immune responses are not affected substantially despite smaller numbers of mature B cells in these strains (Perlot et al., 2005; Marquet et al., 2014). However, Eμ is essential for B cell-specific transcription of functionally rearranged Igh transgenes in mice. Eμ has also been shown to be a diversity activating sequence (DIVAC) that promotes activation-induced deaminase dependent somatic hypermutation of Ig sequences, a process that occurs only during peripheral immune responses (Buerstedde et al., 2014). We believe that additional studies of the role of Eμ in mature B cells are warranted.
Mechanisms of Eµ activation
Eµ binds many transcription factors (Figure 1D) (Kumari et al., 2018; Kleiman et al., 2016; Ernst and Smale, 1995; Henderson and Calame, 1998; Lin et al., 2010). However, none of these easily explain lineage- or developmental stage-specificity of Igh activation. Most of these proteins are widely expressed in hematopoietic cells, such YY1, RUNX family members, ETS proteins, OCT proteins and bHLH-zip proteins. Others, like PU.1 and E2A have more restricted tissue distribution. PU.1 is expressed at highest levels in myeloid cells where it has been proposed to act as a pioneer factor (Heinz et al., 2010). Lower levels of PU.1 present at early hematopoietic stages (such as HSC, CMP and CLP) are maintained throughout B cell development but extinguished during T cell differentiation (Heinz et al., 2010; Iwasaki et al., 2005; Dakic et al., 2007; Pang et al., 2018). E2A is also expressed in HSC through CLP stages but further up-regulated in pre-pro-B cells and thereafter (Semerad et al., 2009; Fischer et al., 2020; Aubrey et al., 2022). Based on early transfection experiments it was proposed that Eµ function is generated by combinatorial activity of different Eµ-binding proteins, especially combinations of ETS proteins and E2A (Ernst and Smale, 1995; Nelsen and Sen, 1992; Nelsen et al., 1993). Which combinations are most important in the endogenous context have not been identified.
Eμ function is also modulated by 5′ and 3′ flanking matrix attachment regions (MARs) (Scheuermann and Garrard, 1999). These are A/T-rich sequences that bind several transcription factors, including Cux/CDP, Satb1 and Bright (Romig et al., 1992; Weitzel et al., 1997; Dickinson et al., 1992; Herrscher et al., 1995; Alvarez et al., 2000; Dobreva et al., 2003). In transgenic studies MAR sequences are essential to fully reveal Eμ activity as reflected in transcription activation, especially at a distance, and extension of chromatin accessibility (Jenuwein et al., 1997; Forrester et al., 1999; Fernández et al., 2001; Forrester et al., 1994). These effects of MARS are independent of transcription factor binding to Eμ, suggesting that factor binding is not sufficient for Eμ function (Fernández et al., 2001). Unlike the effects of deleting Eμ, however, deletion of one or both Igh MARs from the endogenous locus does not affect V(D)J recombination regulated by Eμ or B cell development (Sakai et al., 1999). The role of MARs and their relationship to enhancer function awaits further studies.
While Eµ is both necessary and sufficient for gene activation, examination of chromatin accessibility throughout hematopoiesis reveals hitherto unstudied complexities. First, accessibility at closely positioned Eµ and DQ52 promoter is evident in HSC and maintained throughout developmental stages that precede B lineage commitment (Figure 1C, left panel). Absence of the DQ52 ATAC peak in pro-B cells likely reflects loss of that region by DH and VH recombination. The most parsimonious explanation is that Eµ is not B lineage specific though it drives transgenic expression largely in B and T lymphocytes. Alternatively, it is possible that though Eµ is accessible at earlier stages, it does not have enhancer activity until pre-pro- or pro-B cell stages. This is reminiscent of poised enhancers that are chromatin accessible but lack H3K27ac. Consistent with the latter hypothesis, transcription (Figure 1C, right panel) and H3K27ac modifications (not shown) are higher in pro-B cells compared to HSC (Choukrallah et al., 2015). We surmise that binding of hematopoietic transcription factors to Eµ marks this site for later activation in B lineage cells. Comprehensive analyses of enhancer function throughout hematopoiesis will be required to understand the underlying mechanisms.
Lack of simple concordance between ATAC sensitivity and enhancer function raises two questions. First, what transforms a chromatin accessible, but transcriptionally silent, enhancer into a functionally active enhancer in the B lineage? Our working hypothesis is that chromatin accessibility throughout hematopoiesis marks a poised enhancer that is transcriptionally activated in pre-pro- and pro-B cells. How many transcription factor binding sites are required to poise the enhancer for B lineage-specific activation and which factors convert a pre-marked but inactive enhancer to an active one remain to be discovered. Second, why does Eµ bind so many different transcription factors? One possibility is that some of these factors may suppress enhancer activity in other hematopoietic lineages. The most closely related one is developing T cells where Tcr genes recombine. Eµ chromatin accessibility and associated transcription is considerably lower in T lineage precursors (DN1-DP stages) compared to pro-B cells (Figure 1C, left panel), though they express many of the same transcription factors. This reduced accessibility drives low levels of Igh DH rearrangements in DP cells. We hypothesize that sub-optimal activation of Eµ in T cell precursors precludes compete V(D)J recombination and, thereby, the possibility of co-expressing functional IgH and TCRα/β chains in the same cell.
Enhancer control of Igk expression
Surface Ig heavy chain expression in pro-B cells triggers a proliferative burst that culminates with production of pre-B cells (Figure 1A). The bulk of Ig light chain gene rearrangements take place in these cells. In this review we focus on the Igk locus that encodes more than 90% of light chain protein in mice. The mouse Igk locus spans 3.2 Mb, most of which encodes 96 functional Vκ gene segments (Figure 2A). Clustered at the 3′ end are 4-5 Jκ gene segments, one exon encoding the constant region of Igκ (Cκ) and several regulatory sequences (Figure 2A). One recombination event creates VκJκ junctions that encode Igκ.
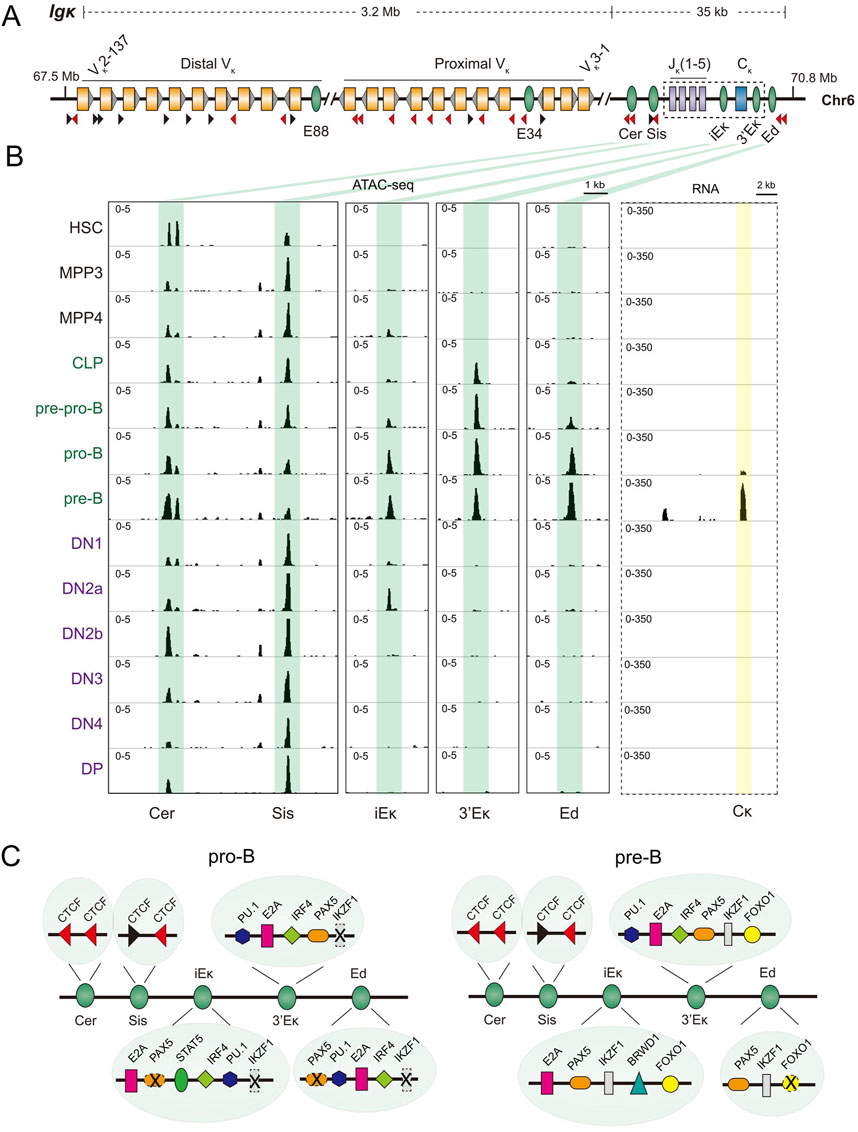
Figure 2. Igk locus regulatory sequences and transcription during B cell development. (A) Schematic representation of the mouse immunoglobulin kappa chain (Igk) locus, spanning approximately 3 Mb on chromosome 6 (coordinates are in mm10). Variable (Vκ) and joining (Jκ), segments, and the Cκ exon are depicted as yellow, purple, and blue boxes, respectively. Grey triangles adjacent to the Vκ gene segments indicate RSSs. Cis-regulatory elements, including enhancers (E88, E34, iEκ, 3′Eκ, Ed), contracting element for recombination (Cer), and silencer in the intervening sequence (Sis), are shown as green ovals. CTCF binding sites and orientations are shown by black and red triangles. The 3′ region of the locus, highlighted by a dashed box, is enlarged in part B to display RNA-seq data. (B) ATAC-seq (left panel) and RNA-seq (right panel) profiles of the 3′ region of the Igk locus during hematopoiesis obtained from the Immunological Genome project (Yoshida et al., 2019). ATAC-seq patterns covering known cis regulatory sequences are shown as identified below the tracks. For RNA-seq, the pattern of the locus in the dashed box in part (A) is shown. (C) Transcription factors that bind to previously identified regulatory sequences in the 3′ Igk domain in pro-B and pre-B cells are shown. The summary combines in vitro protein binding studies and in vivo analysis by chromatin immunoprecipitation (ChIP) (Lin et al., 2010; Mandal et al., 2011; Mandal et al., 2015; Shaffer et al., 1997; Lu et al., 2003; Ochiai et al., 2012; Revilla-I-Domingo et al., 2012; Schwickert et al., 2014; Stadhouders et al., 2014; Ferreiros-Vidal et al., 2013; Loguercio et al., 2018), and does not represent the numbers of each protein binding site. Sites depicted with dashed lines and a cross identify unoccupied sites at the specified developmental stage (Ribeiro de Almeida et al., 2015).
Multiple enhancers control Igk recombination. The intronic enhancer, iEκ, appears most important. iEk is marked by repressive H3K27me3 modification in pro-B cells that is replaced by acetylated histone 4 in pre-B cells where Igk genes recombine (Inlay et al., 2006). In its absence Vκ recombination is reduced about 10-fold (Xu et al., 1996). Residual recombination is lost upon additionally deleting 3′Eκ (Inlay et al., 2002), though deletion of 3′Eκ by itself has little/no effect (Inlay et al., 2002). A third enhancer, Ed, located further 3′ also has little effect when deleted alone, but the double deletion of Ed with 3′Eκ abolishes Igk rearrangements (Zhou et al., 2010). Thus, iEκ is insufficient for recombination, whereas 3′Eκ plus Ed together are relatively weak activators of Igk rearrangements. 3′Eκ and Ed may function as shadow enhancers alongside an active iEκ (Hobert, 2010). Loss of 3′Ek is accompanied by substantially reduced H3 acetylation at the Jκ gene segments in pre-B cells. Ed deletion has a smaller effect, but loss of both 3′Ek and Ed abolishes H3ac at Jκs (Zhou et al., 2010). Though histone acetylation status of iEκ deleted alleles has not been characterized, these results cumulatively suggest that enhancer activation reflected in active histone modifications correlates with induction of rearrangements. To what extent such modifications contribute to V(D)J recombination per se remains to be determined. Moreover, because different measures of locus activation have been used (many of which preceded the chromatin immunoprecipitation (ChIP) era), systematic studies of WT and mutated loci are needed to understand molecular connections between enhancers, chromatin states, transcriptional activation and recombination.
In addition to the classical enhancers, two elements, the contracting element for recombination (Cer) and silencer in the intervening sequence (Sis), regulate Igk rearrangements (Xiang et al., 2011; Xiang et al., 2013). Deletion of these elements skews the rearranged repertoire of Vκ genes to those located closer to the 3′ end of the locus (proximal Vκ genes). Specifically, Vκ genes lying within 100 kb of Jκs account for 25% of the repertoire in the absence of Sis and 62% of the repertoire in the absence of Cer, compared to 10% in the wild type context (Xiang et al., 2011; Xiang et al., 2013). The effects are even more pronounced when both elements are missing (Xiang et al., 2014). Their activity is likely conferred by oriented CTCF binding sites in each element, much like IGCR1 in Igh. However, the role of CTCF has not been directly confirmed by point mutations of these sites. Like Igh, additional enhancers within the Vκ region promote recombination of nearby gene segments (Barajas-Mora et al., 2019; Barajas-Mora et al., 2023). The greater effects of enhancers located near the joining gene segments (JH and Jκ) at both immunoglobulin loci for gene rearrangements may be via their role in establishing RAG1/2-rich recombination centers at which antigen receptor gene rearrangements are initiated.
Mechanisms of Igk activation
To what extent can developmental timing of Igk rearrangements be explained by enhancer activation by transcription factors? iEκ has been best studied in this regard. Early studies showed that mutating the NF-κB binding site in iEκ had little effect on rearrangements, whereas mutating two ‘E’ elements significantly reduced iEκ function (Inlay et al., 2004). These E elements bind E2A and bHLH-zip proteins such as TFE3 in vitro (Staudt and Lenardo, 1991). However, the importance of these motifs for iEκ function does not provide a ready explanation for developmental timing of Igk activation, in part because similar motifs are also found in Eμ that is activated at an earlier developmental stage. Additionally, genes encoding E2A and bHLH-zip proteins are not selectively expressed in pre-B cells. Timing of iEκ activation is now attributed to a combination of chemokine and cytokine activity that moves pre-B cells away from an IL-7-rich milieu essential for pro-B cell differentiation and proliferation. Clark and colleagues have proposed that STAT5 activated in response to IL-7 signals binds to iEκ and suppresses its activity in pro-B cells by competing with E2A binding and/or recruitment of EZH2, a writer of repressive H3K27me3 modification (Mandal et al., 2009; Malin et al., 2010; Mandal et al., 2011). In the IL-7-poor pre-B cells niche, phospho-STAT5 binding is reduced, permitting iEκ activation (Clark et al., 2014). STAT5 is also implicated in repressing BRWD1, a transcription factor that is up-regulated in the transition to pre-B cells, which was recently shown to bind iEκ and regulate Igk locus contraction (Mandal et al., 2015; Mandal et al., 2024). Loss of STAT5 from iEκ also coincides with recruitment of Ikaros to this enhancer which may also regulate its developmental stage-specific activation (see below). Finally, iEκ is also associated with a MAR on its 5′ flank (Cockerill and Garrard, 1986). Though systematic transgenic studies have not been done to investigate the contribution of this MAR to iEκ function, deletion of the MAR from the endogenous locus does not affect Igk rearrangements or B cell development (Yi et al., 1999).
Less is known about factors that activate 3′Eκ and Ed. ChIP studies show 3′Eκ binds to PU.1, E2A, IRF and PAX5 in pro- and pre-B cells (Figure 2C) (Lin et al., 2010; Shaffer et al., 1997; Lu et al., 2003; Ochiai et al., 2012; Revilla-I-Domingo et al., 2012; Schwickert et al., 2014; Stadhouders et al., 2014). Because 3′Eκ function has only been demonstrated in combination with either iEκ or Ed, it is not clear at which developmental stage 3′Eκ is activated. Published studies show that Ed binds PU.1, E2A, and IRF4 in pro-B cells (Lin et al., 2010; Shaffer et al., 1997; Lu et al., 2003; Schwickert et al., 2014; Stadhouders et al., 2014), though it is uncertain if this binding occurs in pre-B cells as well. In contrast, Ed binds selectively to Ikaros and PAX5 in pre-B cells (Ochiai et al., 2012; Revilla-I-Domingo et al., 2012; Schwickert et al., 2014; Ferreiros-Vidal et al., 2013). How these dynamically shifting interactions contribute to Ed function is not understood. An interesting unifying feature of all three Igk-associated enhancers is that they bind Ikaros selectively in pre-B cells (Ribeiro de Almeida et al., 2015). Ikaros has been shown to regulate pre-B cell differentiation and proposed to induce an enhancer hub in the 3′ Igk locus (Hu et al., 2023). This hub promotes interactions with Vκ gene segments leading to locus contraction required for distal Vκ rearrangements. Spatial proximity of 5′ Vκs and the 3′ Igk region is reduced in Ikaros-deficient pre-B cells with concomitant reduction in Vκ gene rearrangements. These observations are consistent with Ikaros playing a crucial role in timing Igk locus activation and recombination in pre-B cells (Hu et al., 2023). The possible interplay between BRWD1 and Ikaros, both of which induce locus contraction of Igk, remains to be discovered.
A global view of the chromatin structure of the Igk locus reveals similarities and dissimilarities with Igh. Like Igh, chromatin accessibility of all three recombination-related enhancers precedes developmental stage-specific functional activation. This is reflected in strong ATAC peaks at iEκ, 3′Eκ and Ed in pro- as well as pre-B cells (Figure 2B, left panel). However, activity as reflected by germline transcription is most prominent in pre-B cells (Figure 2B, right panel). Thus, developmental timing is strictly enforced at the level of function but not at the level of chromatin accessibility. Unlike Igh, however, Igk enhancers are not pre-marked in early hematopoiesis (Figure 2B, left panel). 3′Eκ gains accessibility first at the CLP stage whereas iEκ and Ed are most prominently accessible at pro- and pre-B cell stages (Figure 2B, left panel). The close coincidence of transcriptional activation with Ikaros binding strongly suggests that transformation of accessible but functionally inactive enhancers to a transcriptionally active state is mediated by recruitment of Ikaros to pre-marked chromatin regions.
It is interesting to note that mutation of E elements in iEκ or absence of Ikaros in pre-B cells attenuate iEκ function (Inlay et al., 2004; Hu et al., 2023). E2A binds to iEκ in both pro- and pre-B cells, suggesting that pre-marking of iEκ in pro-B cells may be mediated by this protein. Furthermore, a two-fold increase of E2A transcripts was observed in pre-B cells compared to pro-B cells (ImmGen) (Heng et al., 2008), indicating a greater abundance of E2A at iEκ. The mechanism of Ikaros recruitment to iEκ could be via direct interactions with E2A or to the altered chromatin state induced by E2A. Chromatin structure analyses of E-mutated iEκ in pro-B cells and point mutation of Ikaros binding sites will help to disentangle functions of these proteins at iEκ. It is also interesting that the spectrum of transcription factors that bind to 3′Eκ is very similar to that at iEκ. Yet, 3′Eκ does not effectively compensate for genetic deletion of iEκ. The basis for this distinction is not clear but may relate to its location beyond Cκ and associated inefficiency in inducing a recombination center near the Jκ gene segments.
Comparing stage-specific activity of Eμ and iEκ
In summary, multiple enhancers regulate developmental stage-specific transcription and rearrangements of Igh and Igk loci. Of these, Eμ and iEκ enhancers appear to be the most important because deletion of either element alone substantially impairs activation of the associated locus. A comparison of the two enhancers highlights several features:
1) Chromatin accessibility and transcriptional activation are temporally distinct for both enhancers. Accessibility at Eμ is evident throughout early hematopoiesis, whereas iEκ gains most accessibility in B lineage committed cells. Yet, transcriptional activation occurs precisely at pro- and pre-B stages, respectively (Figures 1C, 2B).
2) Both enhancers bind many of the same transcription factors, such as PU.1, IRF proteins, E2A and OCT proteins. Though these factors are important for B cell development they are unlikely to direct stage-specific enhancer activation. However, factors specific for each enhancer, such as YY1, RUNX, ETS for Eμ and Ikaros, BRWD1 and FOXO1 for iEκ are expressed in other tissues, suggesting more complex mechanisms at play than simple DNA binding. It also remains entirely possible that additional, currently unidentified, DNA binding proteins confer stage-specific enhancer activation.
3) Both enhancers are functionally inactive in the T lineage (there is residual activity of Eμ in DP cells as discussed above), despite binding transcription factors that are largely expressed in both B and T lineages. PU.1 is the exception to this rule. Its low-level expression is necessary for B cell differentiation and its extinction is essential for T cell differentiation. Further studies will be needed to understand mechanisms by which the same transcription factors activate enhancers selectively in one or the other lineage.
Enhancers that regulate T cell receptor gene rearrangements
Eβ control of Tcrb expression
Multipotential cells that migrate to the thymus undergo sequential rearrangements of Tcrb and Tcra genes. Developmental stages in the thymus are defined based on the expression of CD4 and CD8 cell surface proteins. The earliest stages lack both and are referred to as double negative (DN) cells (Figure 1A). DN cells are further divided into DN1, 2a/b, 3a/b and 4 (Krangel, 2009; Rodriguez-Caparros et al., 2020; Hosokawa and Rothenberg, 2021) subsets. DN1 cells are a mixed population that include multipotential cells. Commitment to T cell differentiation program is imposed in DN2 subsets by the combined action of transcription factors TCF1, BCl11b and GATA3 (Rothenberg and Taghon, 2005; Hosokawa and Rothenberg, 2021). Tcrb rearrangements initiate in DN2 cells and are completed by the DN3 stage. Only cells expressing TCRβ protein differentiate to CD4+CD8+ double positive (DP) stage via the intermediate DN4 stage. Tcra rearrangements occur in DP cells leading to generation of T cell receptor-expressing progenitor cells.
Organization of the germline Tcrb locus is shown in Figure 3A. The Tcrb locus spans approximately 0.65 Mb of mouse chromosome 6. The 5′ part contains 33 Vβ gene segments (Trbv) of which 21 are functional. Multiple trypsinogen genes are located between Vβ and Dβ genes. The 3′ end contains two Dβ-Jβ-Cβ clusters. Each cluster has one Dβ gene segment (Dβ1 or Dβ2), six Jβ gene segments and exons encoding the constant parts of TCRβ chains (Cβ1 or Cβ2). One Vβ gene segment, Vβ31, is located beyond the Dβ-Jβ-Cβ clusters. Despite being organized differently from Igh, V(D)J recombination proceeds similarly at both loci. Dβs rearranges first in DN2 cells followed by Vβ rearrangements to DJβ junctions in DN3 cells (Krangel, 2009). Three regulatory sequences control Tcrb rearrangements. Eβ, an enhancer located between Cβ2 and Vβ31, is essential for Tcrb recombination. Its deletion abrogates all Tcrb rearrangements (Bories et al., 1996; Bouvier et al., 1996). Absence of Eβ leads to loss of activating histone modifications (H3ac and H3K4me2) in a 25 kb region extending to a boundary element located 5′ of Dβ (Majumder et al., 2015; Mathieu et al., 2000; Carabana et al., 2011). Coordinately, this region gains repressive histone modifications, H3K9me2 and H3K27me3, on Eβ-deleted alleles (Majumder et al., 2015; Carabana et al., 2011). One side of Eβ contains a MAR, however, its deletion from the locus does not affect Tcrb transcription in thymocytes (Chattopadhyay et al., 1998).

Figure 3. Tcrb locus regulatory sequences and transcription during T cell development (A) Schematic representation of the 0.65 Mb mouse T cell receptor β (Tcrb) locus, located on chromosome 6 (coordinates shown are mm10). The variable (Vβ), diversity (Dβ1 and Dβ2), joining (Jβ1 and Jβ2) gene segments are depicted as yellow, pink and purple boxes. Blue boxes represent constant region (Cβ) exons. Trypsinogen genes located between Vβ and Dβ genes are indicated as grey rectangles. Grey triangles adjacent to the Vβ gene segments indicate recombinase signal sequences (RSS) required for gene rearrangements. RSS adjacent to diversity and joining segments are not shown. Cis-regulatory elements, including enhancers (Eβ, PDβ), 5′PC CTCF site are represented as green ovals. CTCF binding sites are indicated by black and red triangles, marking opposite orientations. The region highlighted by a dashed box is expanded in the right panel of part B to display RNA-seq data. (B) ATAC-seq data (left panel) and RNA-seq (right panel) profiles of the 3′ part of the Tcrb locus during hematopoiesis obtained from the Immunological Genome project (Yoshida et al., 2019). ATAC-seq patterns covering known cis regulatory sequences are shown as identified below the tracks. For RNA-seq the pattern of the locus in the dashed box in part (A) is shown. (C) Transcription factors that binds to previously identified regulatory sequences in the 3′ Tcrb domain in DN cells are shown. The summary combines in vitro protein binding studies and in vivo analysis by chromatin immunoprecipitation assays (ChIP) (Majumder et al., 2015; Spicuglia et al., 2002; Zhao et al., 2017; Loguercio et al., 2018), and does not represent the numbers of each protein binding site.
PDβ1 and PDβ2 are promoters located 5′ of the respective Dβ gene segments. Deletion of PDβ1 promoter attenuates recombination of the nearest Dβ1 gene segment (Whitehurst et al., 1999; Whitehurst et al., 2000). PDβ1 lies within the domain of influence of Eβ, and its histone modification state is altered on Eβ-deleted Tcrb alleles (Spicuglia et al., 2002). The effects of mutating or deleting PDβ2 alone have not been investigated. 5′PC, located within the trypsinogen cluster, is a CTCF binding site. Deletion of a large genomic region between Dβ2-Jβ2 and Trbv5 gene segments that includes 5′PC permits rearrangement of the remaining Trbv5 to Dβ2. The authors concluded that 5′PC plays a regulatory role in ordered assembly of Tcrb genes (Senoo et al., 2003).
ATAC profile of the 3′ end of Tcrb shows that Eβ is accessible in bone marrow progenitors as noted above for Eµ (Figure 3B, left panel). Accessibility of 5′PC in HSC and all developmental stages leading to B and T lymphocytes probably reflects lineage non-specific binding of CTCF. Accessibility at a region near the Vβ31 promoter parallels the pattern of Eβ (Figure 3B, left panel). This region also contains a CTCF binding site which, unlike 5′PC, may require Eβ accessibility for CTCF binding. Coincident with T cell precursors reaching the thymus (DN1 cells), PDβ1 and PDβ2 become accessible. We surmise this represents Eβ activation and Eβ-dependent activation of Dβ-associated promoters. Accordingly, non-coding transcription of Tcrb alleles is also first evident in DN1 cells (Figure 3B, right panel). This is reminiscent of the distinction between enhancer accessibility and enhancer activation observed with Eµ. Thereafter, PDβ1 and PDβ2 remain accessible in DN2 cells as Tcrb rearrangements proceed. Loss of ATAC accessibility of these regions in DN3 cells likely represents their loss from the genome by Vβ recombination. Eβ accessibility and activity is maintained in further differentiated T cells, but not in other mature hemopoietic lineages such as B cells and myeloid cells (Figure 3B, left panel). Taken together, these observations are consistent with developmental timing of Tcrb transcription and rearrangements being determined by Eβ activation.
Mechanisms of Eβ activation
The most prominent transcription factor motifs identified within Eβ are two composite ETS/RUNX binding sites (Figure 3C) (Hollenhorst et al., 2009). Targeted mutation of both RUNX sites abolishes enhancer activity and blocks T cell development as seen with Eβ deletion (Majumder et al., 2015). Oltz and colleagues dissected the requirements for ETS and RUNX proteins using mutated enhancers in which RUNX binding sites were replaced with GAL4 binding sites. Recruitment of RUNX1/GAL4 fusion proteins to the mutated enhancer activated Eβ-like function with regard to PDβ1 and PDβ2 transcription even in the absence of the adjacent ETS binding sites (Zhao et al., 2017). The ETS family member proposed to work at Eβ is ETS1, a gene whose expression is increased in T cell progenitors undergoing Tcrb rearrangements (Rodriguez-Caparros et al., 2020; Rothenberg et al., 2008; Cauchy et al., 2016). ETS1 and RUNX proteins bind cooperatively to ETS/RUNX composite motifs via neutralization of autoinhibitory domains in each factor leading to the following model for Eβ activation by these factors (Wotton et al., 1994; Kim et al., 1999; Goetz et al., 2000; Gu et al., 2000). RUNX1 is expressed throughout hematopoiesis, however it does not bind and activate Eβ until ETS1 levels rise in DN2 cells close to T lineage commitment (Rothenberg et al., 2008). Cooperative RUNX1-ETS1 binding to Eβ permits recruitment of co-activators such as CBP/p300 to establish transcriptional competence (Hollenhorst et al., 2009; Yang et al., 1998).
Several questions remain to be addressed. First, it has not been established whether the two ETS/RUNX motifs are sufficient for Eβ activity. Other transcription factors, such as E2A and the related bHLH protein HEB, have been shown to bind Eβ (Spicuglia et al., 2002) (Figure 3C), however their functional significance in the context of the Tcrb locus has not been explored. Second, it is not clear what makes Eβ T lineage specific because ETS and RUNX family proteins are widely expressed in hematopoietic cells. One possibility is that negative regulatory elements within Eβ suppress its activity in the wrong lineage. However, such elements have not been identified. Third, what factors make Eβ ATAC sensitive in bone marrow progenitors? Amongst key Eβ binding proteins identified to date the obvious candidate is RUNX1 (or a related RUNX family member). However, if RUNX1 proteins can bind and increase chromatin accessibility, then what prevents them from activating transcription as shown by the GAL4 fusion recruitment studies? Perhaps RUNX proteins bind with other (non-ETS) proteins in uncommitted progenitors in a configuration that precludes transcriptional coactivator recruitment. ETS1 (or other functionally similar ETS proteins) may replace these factors in DN1/2 cells to cooperatively activate Eβ with pre-bound RUNX proteins. Alternatively, progenitor accessibility may be mediated by currently unknown Eβ binding proteins. We expect that additional functional Eβ binding proteins will be identified.
Eα control of Tcra expression
Productive rearrangement of Tcrb alleles in DN cells leads to proliferation and differentiation of TCRβ chain-expressing T cell precursors to the CD4+CD8+ (double positive, DP) stage (Figure 1A). Tcra gene rearrangements take place in DP cells, poising these cells to express the complete αβ T cell receptor. Following additional selection events in the thymus, CD4+ and CD8+ single positive cells capable of mounting immune responses emerge. Tcra genes arise from a single recombination event between one of ∼80 Vα gene segments (Trav) and one of approximately 60 Jα gene segments (Traj) located close to exons that encode the constant part (Cα, Trac) of TCRα chains (Figure 4A). A 1.7 Mb region of mouse chromosome 14 houses the Tcra locus with interspersed gene segments that will recombine to generate TCRδ chains of the γδ T cell receptor (Figure 4A). Tcrd diversity (Dδ, Trdd), joining (Jδ, Trdj) and a few variable (Vδ, Trdv) gene segments, along with constant parts of the TCRδ chains (Cδ, Trdc), are embedded between Traj and most of the Tcra variable gene segments (Trav) (Figure 4A). Despite being located within the Tcra locus, Tcrd gene rearrangements occur in DN cells guided by the Eδ enhancer located near Trdc. Loss of Eδ selectively abolishes Tcrd rearrangements without affecting Tcra rearrangements (Monroe et al., 1999). Though Eδ will not be further discussed in this review it is interesting to note that its activity, as measured by Trdc transcription, is restricted precisely to DN cells where Tcrd rearrangements occur (Figure 4B).
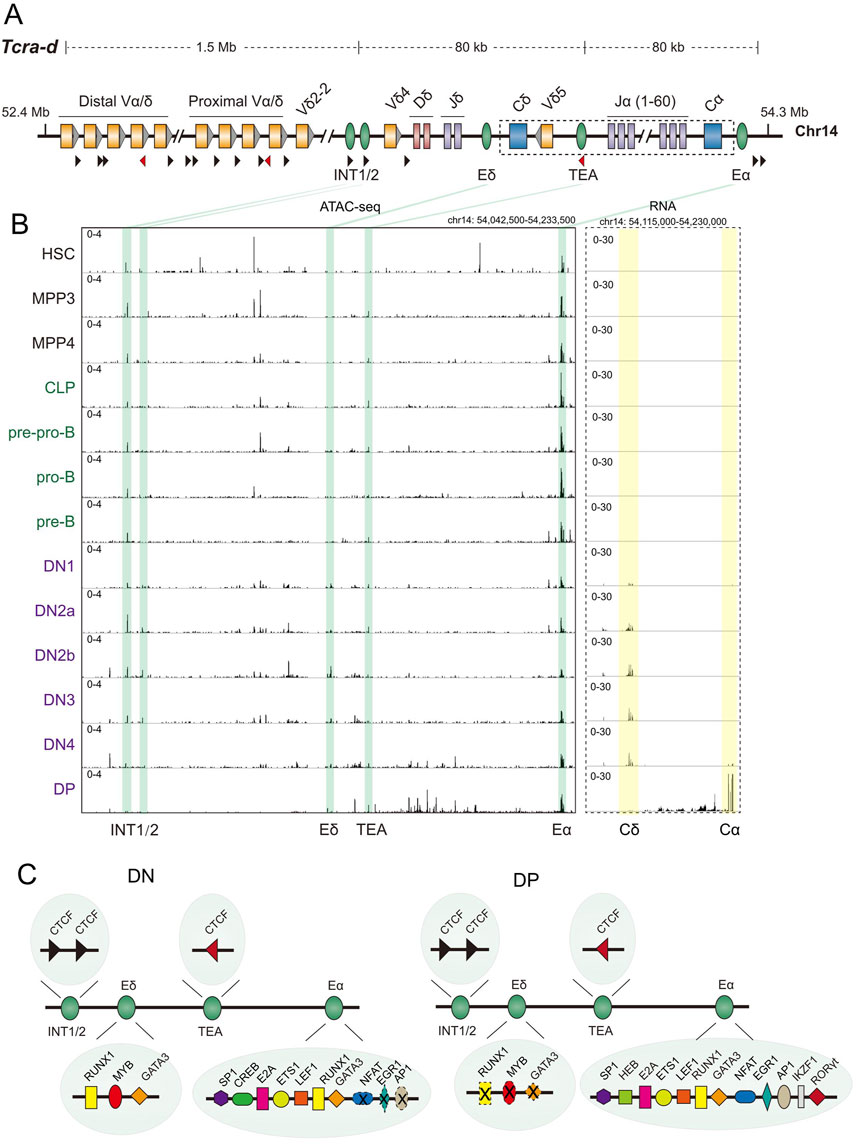
Figure 4. Tcra-d locus regulatory sequences and transcription during T cell development. (A) Schematic representation of the mouse T cell receptor α/δ (Tcra-d) locus, spanning approximately 1.6 Mb on chromosome 14 (coordinates are in mm10). Variable (Vα/δ (Trav and Trdv)), diversity (Dδ (Trdd)) and joining (Jα/δ (Traj and Trdj)) gene segments are shown as yellow, pink and purple boxes. Blue boxes represent constant region (Cα/δ (Trac and Trdc)) exons. Grey triangles adjacent to the Vα/δ gene segments indicate the RSSs. Cis-regulatory elements, including enhancers (Eδ and Eα), intergenic CBEs 1 and 2 (INT1 and INT2), and T early alpha (TEA) promoter, are represented as green ovals. CTCF binding sites and orientations are shown by black and red triangles. The region highlighted by the dashed box is expanded in the right panel of part B to display RNA-seq data. (B) ATAC-seq (left panel) and RNA-seq (right panel) profiles of the 3′ region of the Tcra-d locus during hematopoiesis obtained from the Immunological Genome project (Yoshida et al., 2019). ATAC-seq patterns covering known cis regulatory sequences are shown as identified below the tracks. For RNA-seq, the pattern of the locus in the dashed box in part (A) is shown. (C) Transcription factors that bind to previously identified in the 3′ Tcra-d domain in double negative (DN) and double positive (DP) cells are shown (Schwickert et al., 2014; Hernandez-Munain, 2015; del Blanco et al., 2012; Naik et al., 2024; Loguercio et al., 2018; Wei et al., 2011; Mihai et al., 2023). The summary combines in vitro protein binding studies and in vivo analysis by chromatin immunoprecipitation (ChIP) and does not represent the numbers of each protein binding site. Sites depicted with dashed lines and a cross identify unoccupied sites at the specified developmental stage.
The Eα enhancer located 3′ of Cα is essential for Tcra rearrangements. This enhancer is marked by an ATAC peak at all hematopoietic stages, including HSC (Figure 4B, left panel). Indeed, accessibility appears stronger in B cell precursors than in T cell precursors. Yet, its activity, as inferred by Cα RNA, is exquisitely specific to the developmental stage (DP cells) at which Tcra genes rearrange. Eα and approximately 80 kb of 5′ sequence that include Cα exons and all Jα gene segments gain histone H3 acetylation during transition to DP cells (McMurry and Krangel, 2000), closely correlating with transcriptional activation of Tcra. This domain of active histone modifications is lost in DP cells from mice that lack Eα, showing that the enhancer regulates long-range chromatin state that correlates with gene recombination (McMurry and Krangel, 2000). A MAR has not been identified near Eα (Scheuermann and Garrard, 1999). The ATAC pattern is reminiscent of those at Igh and Tcrb loci and distinct from that at Igk, insofar as enhancers in Igk are not accessible in early bone marrow precursors. Thus, locus activating enhancers at three out of four major antigen receptor loci are pre-marked early in hematopoiesis but activated in the appropriate lineage at the correct developmental stage.
In addition to Eα, several CTCF-binding regulatory elements modulate Tcra rearrangements. The T early alpha (TEA) promoter guides use of Jα gene segments. Its deletion results in reduced rearrangements of several 5′ Jα segments closest to TEA, but no effect on Jα segments located further 3’ (Villey et al., 1996). Intergenic CBEs 1 and 2 (INT1 and INT2) separate the large genomic region that contains Trav and Trdv gene segments from the rest of the Tcra-d locus. Orientation of CTCF binding sites in each element define an 80 kb domain that extends till the CTCF binding site in TEA (Chen et al., 2015). This domain, containing Dδ, Jδ and Cδ under control of Eδ, is thereby effectively segregated from the rest of the locus. Double deletion of INT1 and INT2 alters the Tcra repertoire and leads to defective γδ T cell development. The two elements are partially redundant as mutation of INT2 alone has minor effects (Chen et al., 2015).
Mechanisms of Eα activation
Many transcription factors bind Eα both in DN cells (where it is inactive) and in DP cells (where it is active, Figure 4C) (Hernandez-Munain, 2015). Amongst these are the first examples of T lineage-restricted factors such as LEF1/TCF1 and GATA3. These factors are expressed concomitant with T cell commitment in DN2 cells simultaneously with Tcrb activation by Eβ. However, their binding (along with other proteins) is apparently insufficient to activate Eα. By contrast, three factors that are widely associated with cell activation and pre-T cell receptor (pre-TCR) signaling, NFAT, EGR and AP1, bind selectively to Eα in late DN4 cells and DP cells (King et al., 1999; Aifantis et al., 2001; Carter et al., 2007; del Blanco et al., 2012). Hernández-Munain and colleagues proposed that constitutive but lymphoid-restricted transcription factors, such as E2A and ETS1, pre-mark Eα before its activation. The pre-marked enhancer recruits additional factors induced by pre-TCR signaling, as well as the CREB-binding protein/p300 coactivators, to fully activate Eα (del Blanco et al., 2012).
RORγt and Ikaros are two other proteins that were recently shown to bind to Eα in DP cells by ChIP experiments (Schwickert et al., 2014; Naik et al., 2024); whether they bind to Eα at earlier stages has not yet been explored. RORγt is important for lifetime of DP cells which, in turn, impacts the Vα repertoire (Sun et al., 2000; Guo et al., 2002). However, a direct role for RORγt in regulating recombination via Eα activity has not been demonstrated. It is noteworthy that Ikaros binds to both late-activated antigen receptor gene enhancers (iEκ and Eα) at the appropriate developmental stage. Because Ikaros deficiency perturbs T cell development at the earliest stages (Georgopoulos, 2002), it is not known to what extent Ikaros binding to Eα is required for enhancer function in DP cells. Point mutational analyses of Eα will be needed to sort through transcription factor dynamics and functions in developing T cells.
Comparing stage-specific activity of Eβ and Eα
Unlike Igh and Igk where multiple enhancers guide developmental stage-specific locus activation, Tcrb and Tcra loci each rely on only one (known) enhancer to initiate developmentally appropriate transcription and rearrangements. A comparison of Eβ and Eα is therefore pertinent for identifying mechanisms that guide T cell stage-specific gene expression. Additionally, a comparison of BCR- and TCR-associated enhancers provides a perspective into lineage-restricted gene expression. Several interesting features can be highlighted:
1) Both Eβ and Eα are ATAC sensitive from early hematopoietic stages. Thus, chromatin accessibility can be dissociated from transcriptional activation at all antigen receptor gene enhancers. This raises interesting questions regarding the role of pioneer factors in lineage- and stage-specific activation of antigen receptor gene enhancers. It is possible that these enhancers are atypical because of their role in recombination regulation beyond classical transcription activation. It is also interesting to note that both Eβ and Eα remain accessible in B lineage precursors, whereas both Eμ and iEκ are mostly inaccessible in T cell precursors.
2) Organization of Eβ, controlled by RUNX and ETS proteins, appears much simpler than that of Eα. However, both these transcription factors and (and many others) bind Eα in DN cells without apparently activating transcription. One possibility is that the numbers of RUNX/ETS motifs matter, to confer activity (of Eβ) or inactivity (of Eα) in DN cells. Additional factors, such as bHLH proteins, also bind to both Eβ and Eα in DN cells, leaving Eα inactivity in DN cells a mystery.
3) Both iEκ and Eα are transcriptionally activated as pre-BCR- or pre-TCR-selected progenitors complete a proliferative burst and regain quiescence as pre-B or DP cells, respectively. Their mechanisms of activation, however, appear to be quite different. iEκ activation has been attributed to pre-BCR-dependent reduced sensitivity to IL-7, whereas Eα activation coincides with recruitment of activation-induced transcription factors. Loss of IL-7 signaling is also associated with DN to DP transition of T cell progenitors and, conversely, activation-induced transcription factors are likely to be in play during pro- to pre-B transition of B cell progenitors. It is intriguing that apparently different strategies are adopted in the two lineages to accomplish the same end.
Organization and transcription factor utilization at Eμ and Eβ
It is interesting to examine the mechanisms by which Eμ and Eβ are transcriptionally activated coincident with B or T lineage commitment from multipotential progenitors. Both Eμ and Eβ contain multiple binding sites for bHLH, RUNX and ETS proteins, yet each largely activates transcription and recombination in distinct lineages. One possibility is that this is due to different organization of binding sites within each enhancer. For example, only one of the four RUNX binding sites in Eμ has the configuration of ETS/RUNX composite elements that dominate Eβ. ETS binding sites in Eμ are also distinct in both family member selectivity (Eμ has two PU.1 binding sites whose sequence specificities are different from that of ETS1 and related factors) and distribution across the enhancer. Both Eμ and Eβ are also pre-marked by chromatin accessibility much earlier in hematopoiesis than their functions are manifest (Figures 1C, 3B). We hypothesize that pre-marking identifies genomic sites at which functional factors will be recruited in the appropriate lineage and at the appropriate developmental stage. It will be interesting to identify other such regulatory sites that are pre-marked early in hematopoiesis for later functional activation to understand the underlying regulatory logic. It will also be interesting to compare such regulatory sequences to those at which accessibility and functional activation coincide. In the context of pioneer factors these observations suggest that factors that drive Eμ and Eβ activity may not be capable of pioneering correctly.
Concluding remarks
In this review we have summarized features of developmental regulation of antigen receptor loci from the perspective of enhancers associated with immunoglobulin (Ig) and T cell receptor genes. Though analyses of these enhancers led to identification of some of the key transcription factors required for B and T cell development, it is apparent that mechanisms by which they direct lineage- and developmental stage-specific activation remain poorly understood. It is also apparent that ‘simple’ identification of transcription factors binding sites within enhancers will not suffice to explain how overlapping sets of factors yield developmentally precise gene activation. The concept of combinatorial control was evoked to explain this but how it is imposed remains a challenge for the future. It is also intriguing that enhancer accessibility is distinct from enhancer activity, perhaps explaining in part their complex organization. To what extent this is true of other tissue-specific control elements remains unclear, as does the underlying reason. Finally, MARs are associated with three out of the four antigen receptor enhancers that control recombination. Functions of these enigmatic regulatory sequences remain to be elucidated.
Author contributions
FM: Writing–original draft, Writing–review and editing. FB: Writing–original draft, Writing–review and editing. RS: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Intramural Research Program of the National Institute on Aging.
Acknowledgments
We thank Noah Ollikainen for help with genomic analyses of the RNA-seq and ATAC-seq data from Immunological Genome project database and Drs. Rudolf Grosschedl (Max Planck Institute) and Ellen Rothenberg (Caltech) for valuable input during preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, G. E., Chandru, A., and Cowley, S. M. (2018). Co-repressor, co-activator and general transcription factor: the many faces of the Sin3 histone deacetylase (HDAC) complex. Biochem. J. 475, 3921–3932. doi:10.1042/bcj20170314
Adams, J. M., Harris, A. W., Pinkert, C. A., Corcoran, L. M., Alexander, W. S., Cory, S., et al. (1985). The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538. doi:10.1038/318533a0
Afshar, R., Pierce, S., Bolland, D. J., Corcoran, A., and Oltz, E. M. (2006). Regulation of IgH gene assembly: role of the intronic enhancer and 5'DQ52 region in targeting DHJH recombination. J. Immunol. 176, 2439–2447. doi:10.4049/jimmunol.176.4.2439
Aifantis, I., Gounari, F., Scorrano, L., Borowski, C., and von Boehmer, H. (2001). Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-κB and NFAT. Nat. Immunol. 2, 403–409. doi:10.1038/87704
Allman, D., Sambandam, A., Kim, S., Miller, J. P., Pagan, A., Well, D., et al. (2003). Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4, 168–174. doi:10.1038/ni878
Alt, F. W., Yancopoulos, G., Blackwell, T., Wood, C., Thomas, E., Boss, M., et al. (1984). Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 3, 1209–1219. doi:10.1002/j.1460-2075.1984.tb01955.x
Alvarez, J. D., Yasui, D. H., Niida, H., Joh, T., Loh, D. Y., and Kohwi-Shigematsu, T. (2000). The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes and Dev. 14, 521–535. doi:10.1101/gad.14.5.521
Amoretti-Villa, R., Rogier, M., Robert, I., Heyer, V., and Reina-San-Martin, B. (2019). A novel regulatory region controls IgH locus transcription and switch recombination to a subset of isotypes. Cell Mol. Immunol. 16, 887–889. doi:10.1038/s41423-019-0267-4
Aubrey, M., Warburg, Z. J., and Murre, C. (2022). Helix-Loop-helix proteins in adaptive immune development. Front. Immunol. 13, 881656. doi:10.3389/fimmu.2022.881656
Banerji, J., Rusconi, S., and Schaffner, W. (1981). Expression of a β-globin gene is enhanced by remote SV40 DNA sequences. Cell 27, 299–308. doi:10.1016/0092-8674(81)90413-x
Barajas-Mora, E. M., Kleiman, E., Xu, J., Carrico, N. C., Lu, H., Oltz, E. M., et al. (2019). A B-Cell-Specific enhancer orchestrates nuclear architecture to generate a diverse antigen receptor repertoire. Mol. Cell 73, 48–60.e5. doi:10.1016/j.molcel.2018.10.013
Barajas-Mora, E. M., Lee, L., Lu, H., Valderrama, J. A., Bjanes, E., Nizet, V., et al. (2023). Enhancer-instructed epigenetic landscape and chromatin compartmentalization dictate a primary antibody repertoire protective against specific bacterial pathogens. Nat. Immunol. 24, 320–336. doi:10.1038/s41590-022-01402-z
Bhat, K. H., Priyadarshi, S., Naiyer, S., Qu, X., Farooq, H., Kleiman, E., et al. (2023). An Igh distal enhancer modulates antigen receptor diversity by determining locus conformation. Nat. Commun. 14, 1225. doi:10.1038/s41467-023-36414-2
Bolland, D. J., Wood, A. L., Afshar, R., Featherstone, K., Oltz, E. M., and Corcoran, A. E. (2007). Antisense intergenic transcription precedes igh D-to-J recombination and is controlled by the intronic enhancer Eμ. Mol. Cell. Biol. 27, 5523–5533. doi:10.1128/mcb.02407-06
Borghesi, L., Hsu, L. Y., Miller, J. P., Anderson, M., Herzenberg, L., Herzenberg, L., et al. (2004). B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J. Exp. Med. 199, 491–502. doi:10.1084/jem.20031800
Bories, J. C., Demengeot, J., Davidson, L., and Alt, F. W. (1996). Gene-targeted deletion and replacement mutations of the T-cell receptor beta-chain enhancer: the role of enhancer elements in controlling V(D)J recombination accessibility. Proc. Natl. Acad. Sci. 93, 7871–7876. doi:10.1073/pnas.93.15.7871
Born, W., White, J., Kappler, J., and Marrack, P. (1988). Rearrangement of IgH genes in normal thymocyte development. J. Immunol. 140, 3228–3232. doi:10.4049/jimmunol.140.9.3228
Bouvier, G., Watrin, F., Naspetti, M., Verthuy, C., Naquet, P., and Ferrier, P. (1996). Deletion of the mouse T-cell receptor beta gene enhancer blocks alphabeta T-cell development. Proc. Natl. Acad. Sci. U S A. 93, 7877–7881. doi:10.1073/pnas.93.15.7877
Bruzeau, C., Moreau, J., Le Noir, S., and Pinaud, E. (2021). Panorama of stepwise involvement of the IgH 3' regulatory region in murine B cells. Adv. Immunol. 149, 95–114. doi:10.1016/bs.ai.2021.03.004
Buenrostro, J. D., Wu, B., Chang, H. Y., and Greenleaf, W. J. (2015). ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 109 (21), 29 21–21. doi:10.1002/0471142727.mb2129s109
Buerstedde, J. M., Alinikula, J., Arakawa, H., McDonald, J. J., and Schatz, D. G. (2014). Targeting of somatic hypermutation by immunoglobulin enhancer and enhancer-like sequences. PLoS Biol. 12, e1001831. doi:10.1371/journal.pbio.1001831
Calo, E., and Wysocka, J. (2013). Modification of enhancer chromatin: what, how, and why? Mol. Cell 49, 825–837. doi:10.1016/j.molcel.2013.01.038
Capone, M., Watrin, F., Fernex, C., Horvat, B., Krippl, B., Wu, L., et al. (1993). TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J. 12, 4335–4346. doi:10.1002/j.1460-2075.1993.tb06118.x
Carabana, J., Watanabe, A., Hao, B., and Krangel, M. S. (2011). A barrier-type insulator forms a boundary between active and inactive chromatin at the murine TCRβ locus. J. Immunol. 186, 3556–3562. doi:10.4049/jimmunol.1003164
Carter, J. H., Lefebvre, J. M., Wiest, D. L., and Tourtellotte, W. G. (2007). Redundant role for early growth response transcriptional regulators in thymocyte differentiation and survival. J. Immunol. 178, 6796–6805. doi:10.4049/jimmunol.178.11.6796
Cauchy, P., Maqbool, M. A., Zacarias-Cabeza, J., Vanhille, L., Koch, F., Fenouil, R., et al. (2016). Dynamic recruitment of Ets1 to both nucleosome-occupied and -depleted enhancer regions mediates a transcriptional program switch during early T-cell differentiation. Nucleic Acids Res. 44, 3567–3585. doi:10.1093/nar/gkv1475
Chakraborty, T., Perlot, T., Subrahmanyam, R., Jani, A., Goff, P. H., Zhang, Y., et al. (2009). A 220-nucleotide deletion of the intronic enhancer reveals an epigenetic hierarchy in immunoglobulin heavy chain locus activation. J. Exp. Med. 206, 1019–1027. doi:10.1084/jem.20081621
Chattopadhyay, S., Whitehurst, C. E., and Chen, J. (1998). A nuclear matrix attachment region upstream of the T cell receptor β gene enhancer binds Cux/CDP and SATB1 and modulates enhancer-dependent reporter gene expression but not endogenous gene expression. J. Biol. Chem. 273, 29838–29846. doi:10.1074/jbc.273.45.29838
Chen, L., Carico, Z., Shih, H. Y., and Krangel, M. S. (2015). A discrete chromatin loop in the mouse Tcra-Tcrd locus shapes the TCRδ and TCRα repertoires. Nat. Immunol. 16, 1085–1093. doi:10.1038/ni.3232
Choukrallah, M. A., Song, S., Rolink, A. G., Burger, L., and Matthias, P. (2015). Enhancer repertoires are reshaped independently of early priming and heterochromatin dynamics during B cell differentiation. Nat. Commun. 6, 8324. doi:10.1038/ncomms9324
Chowdhury, D., and Sen, R. (2004). Regulation of immunoglobulin heavy-chain gene rearrangements. Immunol. Rev. 200, 182–196. doi:10.1111/j.0105-2896.2004.00177.x
Christie, S. M., Fijen, C., and Rothenberg, E. V. (D. (2022). V(D)J recombination: recent insights in formation of the recombinase complex and recruitment of DNA repair machinery. Front. Cell Dev. Biol. 10, 886718. doi:10.3389/fcell.2022.886718
Clark, M. R., Mandal, M., Ochiai, K., and Singh, H. (2014). Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat. Rev. Immunol. 14, 69–80. doi:10.1038/nri3570
Cockerill, P. N., and Garrard, W. T. (1986). Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell 44, 273–282. doi:10.1016/0092-8674(86)90761-0
Collins, A. M., and Watson, C. T. (2018). Immunoglobulin light chain gene rearrangements, receptor editing and the development of a self-tolerant antibody repertoire. Front. Immunol. 9, 2249. doi:10.3389/fimmu.2018.02249
Creyghton, M. P., Cheng, A. W., Welstead, G. G., Kooistra, T., Carey, B. W., Steine, E. J., et al. (2010). Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U S A. 107, 21931–21936. doi:10.1073/pnas.1016071107
Crispatzu, G., Rehimi, R., Pachano, T., Bleckwehl, T., Cruz-Molina, S., Xiao, C., et al. (2021). The chromatin, topological and regulatory properties of pluripotency-associated poised enhancers are conserved in vivo. Nat. Commun. 12, 4344. doi:10.1038/s41467-021-24641-4
Dakic, A., Wu, L., and Nutt, S. L. (2007). Is PU.1 a dosage-sensitive regulator of haemopoietic lineage commitment and leukaemogenesis? Trends Immunol. 28, 108–114. doi:10.1016/j.it.2007.01.006
del Blanco, B., Garcia-Mariscal, A., Wiest, D. L., and Hernandez-Munain, C. (2012). Tcra enhancer activation by inducible transcription factors downstream of pre-TCR signaling. J. Immunol. 188, 3278–3293. doi:10.4049/jimmunol.1100271
Dickinson, L. A., Joh, T., Kohwi, Y., and Kohwishigematsu, T. (1992). A tissue-specific mar/sar DNA-binding protein with unusual binding-site recognition. Cell 70, 631–645. doi:10.1016/0092-8674(92)90432-c
Dobreva, G., Dambacher, J., and Grosschedl, R. (2003). SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin μ gene expression. Genes and Dev. 17, 3048–3061. doi:10.1101/gad.1153003
Ernst, P., and Smale, S. T. (1995). Combinatorial regulation of transcription II: the immunoglobulin mu heavy chain gene. Immunity 2, 427–438. doi:10.1016/1074-7613(95)90024-1
Featherstone, K., Wood, A. L., Bowen, A. J., and Corcoran, A. E. (2010). The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J. Biol. Chem. 285, 9327–9338. doi:10.1074/jbc.m109.098251
Fernández, L. A., Winkler, M., and Grosschedl, R. (2001). Matrix attachment region-dependent function of the immunoglobulin μ enhancer involves histone acetylation at a distance without changes in enhancer occupancy. Mol. Cell. Biol. 21, 196–208. doi:10.1128/mcb.21.1.196-208.2001
Ferreiros-Vidal, I., Carroll, T., Taylor, B., Terry, A., Liang, Z., Bruno, L., et al. (2013). Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood 121, 1769–1782. doi:10.1182/blood-2012-08-450114
Ferrier, P., Krippl, B., Blackwell, T., Furley, A., Suh, H., Winoto, A., et al. (1990). Separate elements control DJ and VDJ rearrangement in a transgenic recombination substrate. EMBO J. 9, 117–125. doi:10.1002/j.1460-2075.1990.tb08087.x
Fischer, U., Yang, J. J., Ikawa, T., Hein, D., Vicente-Dueñas, C., Borkhardt, A., et al. (2020). Cell fate decisions: the role of transcription factors in early B-cell development and leukemia. Blood Cancer Discov. 1, 224–233. doi:10.1158/2643-3230.bcd-20-0011
Forrester, W. C., Fernández, L. A., and Grosschedl, R. (1999). Nuclear matrix attachment regions antagonize methylation-dependent repression of long-range enhancer-promoter interactions. Genes and Dev. 13, 3003–3014. doi:10.1101/gad.13.22.3003
Forrester, W. C., Vangenderen, C., Jenuwein, T., and Grosschedl, R. (1994). Dependence of enhancer-mediated transcription of the immunoglobulin-mu gene on nuclear matrix attachment regions. Science 265, 1221–1225. doi:10.1126/science.8066460
Garrett, F. E., Emelyanov, A. V., Sepulveda, M. A., Flanagan, P., Volpi, S., Li, F., et al. (2005). Chromatin architecture near a potential 3' end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol. Cell. Biol. 25, 1511–1525. doi:10.1128/mcb.25.4.1511-1525.2005
Georgopoulos, K. (2002). Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat. Rev. Immunol. 2, 162–174. doi:10.1038/nri747
Giallourakis, C. C., Franklin, A., Guo, C., Cheng, H. L., Yoon, H. S., Gallagher, M., et al. (2010). Elements between the IgH variable (V) and diversity (D) clusters influence antisense transcription and lineage-specific V(D)J recombination. Proc. Natl. Acad. Sci. U S A. 107, 22207–22212. doi:10.1073/pnas.1015954107
Giannini, S. L., Singh, M., Calvo, C. F., Ding, G., and Birshtein, B. K. (1993). DNA regions flanking the mouse Ig 3' alpha enhancer are differentially methylated and DNAase I hypersensitive during B cell differentiation. J. Immunol. 150, 1772–1780. doi:10.4049/jimmunol.150.5.1772
Goetz, T. L., Gu, T. L., Speck, N. A., and Graves, B. J. (2000). Auto-inhibition of ets-1 is counteracted by DNA binding cooperativity with core-binding factor α2. Mol. Cell. Biol. 20, 81–90. doi:10.1128/mcb.20.1.81-90.2000
Gu, T. L., Goetz, T. L., Graves, B. J., and Speck, N. A. (2000). Auto-inhibition and partner proteins, core-binding factor β (CBFβ) and ets-1, modulate DNA binding by CBFα2 (AML1). Mol. Cell. Biol. 20, 91–103. doi:10.1128/mcb.20.1.91-103.2000
Guo, C., Yoon, H. S., Franklin, A., Jain, S., Ebert, A., Cheng, H. L., et al. (2011). CTCF-binding elements mediate control of V(D)J recombination. Nature 477, 424–430. doi:10.1038/nature10495
Guo, J., Hawwari, A., Li, H., Sun, Z., Mahanta, S. K., Littman, D. R., et al. (2002). Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat. Immunol. 3, 469–476. doi:10.1038/ni791
Haberle, V., and Stark, A. (2018). Eukaryotic core promoters and the functional basis of transcription initiation. Nat. Rev. Mol. Cell Biol. 19, 621–637. doi:10.1038/s41580-018-0028-8
Hardy, R. R., Carmack, C. E., Shinton, S. A., Kemp, J. D., and Hayakawa, K. (1991). Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225. doi:10.1084/jem.173.5.1213
Heintzman, N. D., Stuart, R. K., Hon, G., Fu, Y., Ching, C. W., Hawkins, R. D., et al. (2007). Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318. doi:10.1038/ng1966
Heinz, S., Benner, C., Spann, N., Bertolino, E., Lin, Y. C., Laslo, P., et al. (2010). Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589. doi:10.1016/j.molcel.2010.05.004
Henderson, A., and Calame, K. (1998). Transcriptional regulation during B cell development. Annu. Rev. Immunol. 16, 163–200. doi:10.1146/annurev.immunol.16.1.163
Heng, T. S., Painter, M. W., Elpek, K., Lukacs-Kornek, V., Mauermann, N., Turley, S. J., et al. (2008). The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 9, 1091–1094. doi:10.1038/ni1008-1091
Hernandez-Munain, C. (2015). Recent insights into the transcriptional control of the Tcra/Tcrd locus by distant enhancers during the development of T-lymphocytes. Transcription 6, 65–73. doi:10.1080/21541264.2015.1078429
Herrscher, R. F., Kaplan, M. H., Lelsz, D. L., Das, C., Scheuermann, R., and Tucker, P. W. (1995). The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes and Dev. 9, 3067–3082. doi:10.1101/gad.9.24.3067
Hobert, O. (2010). Gene regulation: enhancers stepping out of the shadow. Curr. Biol. 20, R697–R699. doi:10.1016/j.cub.2010.07.035
Hollenhorst, P. C., Chandler, K. J., Poulsen, R. L., Johnson, W. E., Speck, N. A., and Graves, B. J. (2009). DNA specificity determinants associate with distinct transcription factor functions. PLoS Genet. 5, e1000778. doi:10.1371/journal.pgen.1000778
Hosokawa, H., and Rothenberg, E. V. (2021). How transcription factors drive choice of the T cell fate. Nat. Rev. Immunol. 21, 162–176. doi:10.1038/s41577-020-00426-6
Hu, Y., Salgado Figueroa, D., Zhang, Z., Veselits, M., Bhattacharyya, S., Kashiwagi, M., et al. (2023). Lineage-specific 3D genome organization is assembled at multiple scales by IKAROS. Cell 186, 5269–5289.e22. doi:10.1016/j.cell.2023.10.023
Inlay, M., Alt, F. W., Baltimore, D., and Xu, Y. (2002). Essential roles of the kappa light chain intronic enhancer and 3' enhancer in kappa rearrangement and demethylation. Nat. Immunol. 3, 463–468. doi:10.1038/ni790
Inlay, M. A., Lin, T., Gao, H. H., and Xu, Y. (2006). Critical roles of the immunoglobulin intronic enhancers in maintaining the sequential rearrangement of IgH and Igk loci. J. Exp. Med. 203, 1721–1732. doi:10.1084/jem.20052310
Inlay, M. A., Tian, H., Lin, T., and Xu, Y. (2004). Important roles for E protein binding sites within the immunoglobulin κ chain intronic enhancer in activating κ rearrangement. J. Exp. Med. 200, 1205–1211. doi:10.1084/jem.20041135
Iwasaki, H., Somoza, C., Shigematsu, H., Duprez, E. A., Iwasaki-Arai, J., Mizuno, S., et al. (2005). Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 106, 1590–1600. doi:10.1182/blood-2005-03-0860
Jenuwein, T., and Allis, C. D. (2001). Translating the histone code. Science 293, 1074–1080. doi:10.1126/science.1063127
Jenuwein, T., Forrester, W. C., Fernández-Herrero, L. A., Laible, G., Dull, M., and Grosschedl, R. (1997). Extension of chromatin accessibility by nuclear matrix attachment regions. Nature 385, 269–272. doi:10.1038/385269a0
Jenuwein, T., Forrester, W. C., Qiu, R. G., and Grosschedl, R. (1993). The immunoglobulin-mu enhancer core establishes local factor access in nuclear chromatin independent of transcriptional stimulation. Genes and Dev. 7, 2016–2032. doi:10.1101/gad.7.10.2016
Jindal, G. A., and Farley, E. K. (2021). Enhancer grammar in development, evolution, and disease: dependencies and interplay. Dev. Cell 56, 575–587. doi:10.1016/j.devcel.2021.02.016
Johnston, C. M., Wood, A. L., Bolland, D. J., and Corcoran, A. E. (2006). Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J. Immunol. 176, 4221–4234. doi:10.4049/jimmunol.176.7.4221
Jung, D., and Alt, F. W. (2004). Unraveling V(D)J recombination. Cell 116, 299–311. doi:10.1016/s0092-8674(04)00039-x
Jung, D., Giallourakis, C., Mostoslavsky, R., and Alt, F. W. (2006). Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu. Rev. Immunol. 24, 541–570. doi:10.1146/annurev.immunol.23.021704.115830
Kasprzyk, M. E., Sura, W., and Dzikiewicz-Krawczyk, A. (2021). Enhancing B-cell malignancies-on repurposing enhancer activity towards cancer. Cancers (Basel) 13, 3270. doi:10.3390/cancers13133270
Khamlichi, A. A., Pinaud, E., Decourt, C., Chauveau, C., and Cogné, M. (2000). The 3′ IgH regulatory region:: a complex structure in a search for a function. Adv. Immunol. 75 (75), 317–345. doi:10.1016/s0065-2776(00)75008-5
Kim, W. Y., Sieweke, M., Ogawa, E., Wee, H. J., Englmeier, U., Graf, T., et al. (1999). Mutual activation of Ets-1 and AML1 DNA binding by direct interaction of their autoinhibitory domains. EMBO J. 18, 1609–1620. doi:10.1093/emboj/18.6.1609
King, L. B., Tolosa, E., Lenczowski, J. M., Lu, F., Lind, E. F., Hunziker, R., et al. (1999). A dominant-negative mutant of c-Jun inhibits cell cycle progression during the transition of CD4(-)CD8(-) to CD4(+)CD8(+) thymocytes. Int. Immunol. 11, 1203–1216. doi:10.1093/intimm/11.8.1203
Kleiman, E., Jia, H. Q., Loguercio, S., Su, A. I., and Feeney, A. J. (2016). YY1 plays an essential role at all stages of B-cell differentiation. Proc. Natl. Acad. Sci. U. S. A. 113, E3911–E3920. doi:10.1073/pnas.1606297113
Klemm, S. L., Shipony, Z., and Greenleaf, W. J. (2019). Chromatin accessibility and the regulatory epigenome. Nat. Rev. Genet. 20, 207–220. doi:10.1038/s41576-018-0089-8
Krangel, M. S. (2003). Gene segment selection in V(D)J recombination: accessibility and beyond. Nat. Immunol. 4, 624–630. doi:10.1038/ni0703-624
Krangel, M. S. (2009). Mechanics of T cell receptor gene rearrangement. Curr. Opin. Immunol. 21, 133–139. doi:10.1016/j.coi.2009.03.009
Kumari, G., Gerasimova, T., Du, H., De, S., Wood, W. H., Becker, K. G., et al. (2018). Misregulation of the IgH locus in thymocytes. Front. Immunol. 9, 2426. doi:10.3389/fimmu.2018.02426
Kumari, G., and Sen, R. (2015). Chromatin interactions in the control of immunoglobulin heavy chain gene assembly. Adv. Immunol. 128, 41–92. doi:10.1016/bs.ai.2015.08.001
Kurosawa, Y., von Boehmer, H., Haas, W., Sakano, H., Trauneker, A., and Tonegawa, S. (1981). Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature 290, 565–570. doi:10.1038/290565a0
Lescale, C., and Deriano, L. (2017). The RAG recombinase: beyond breaking. Mech. Ageing Dev. 165, 3–9. doi:10.1016/j.mad.2016.11.003
Lescale, C., and Deriano, L. (2016). L V(D)J recombination: orchestrating diversity without damage. Encycl. Cell Biol. 3, 550–566. doi:10.1016/B978-0-12-821618-7.00013-4
Lesch, B. J., and Page, D. C. (2014). Poised chromatin in the mammalian germ line. Development 141, 3619–3626. doi:10.1242/dev.113027
Li, F., Yan, Y., Pieretti, J., Feldman, D. A., and Eckhardt, L. A. (2010). Comparison of identical and functional igh alleles reveals a nonessential role for Eμ in somatic hypermutation and class-switch recombination. J. Immunol. 185, 6049–6057. doi:10.4049/jimmunol.0902992
Li, F. B., and Eckhardt, L. A. (2009). A role for the IgH intronic enhancer Eμ in enforcing allelic exclusion. J. Exp. Med. 206, 153–167. doi:10.1084/jem.20081202
Lieber, M. R. (2010). The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 79, 181–211. doi:10.1146/annurev.biochem.052308.093131
Lieberson, R., Giannini, S. L., Birshtein, B. K., and Eckhardt, L. A. (1991). An enhancer at the 3' end of the mouse immunoglobulin heavy chain locus. Nucleic Acids Res. 19, 933–937. doi:10.1093/nar/19.4.933
Lin, S. G., Ba, Z., Alt, F. W., and Zhang, Y. (2018). RAG chromatin scanning during V(D)J recombination and chromatin loop extrusion are related processes. Adv. Immunol. 139, 93–135. doi:10.1016/bs.ai.2018.07.001
Lin, S. G., Guo, C., Su, A., Zhang, Y., and Alt, F. W. (2015). CTCF-binding elements 1 and 2 in the Igh intergenic control region cooperatively regulate V(D)J recombination. Proc. Natl. Acad. Sci. U S A. 112, 1815–1820. doi:10.1073/pnas.1424936112
Lin, Y. C., Jhunjhunwala, S., Benner, C., Heinz, S., Welinder, E., Mansson, R., et al. (2010). A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat. Immunol. 11, 635–643. doi:10.1038/ni.1891
Loguercio, S., Barajas-Mora, E. M., Shih, H. Y., Krangel, M. S., and Feeney, A. J. (2018). Variable extent of lineage-specificity and developmental stage-specificity of cohesin and CCCTC-binding factor binding within the immunoglobulin and T cell receptor loci. Front. Immunol. 9, 425. doi:10.3389/fimmu.2018.00425
Lu, R., Medina, K. L., Lancki, D. W., and Singh, H. (2003). IRF-4,8 orchestrate the pre-B-to-B transition in lymphocyte development. Genes Dev. 17, 1703–1708. doi:10.1101/gad.1104803
Madisen, L., and Groudine, M. (1994). Identification of a locus control region in the immunoglobulin heavy-chain locus that deregulates c-myc expression in plasmacytoma and Burkitt's lymphoma cells. Genes Dev. 8, 2212–2226. doi:10.1101/gad.8.18.2212
Majumder, K., Koues, O. I., Chan, E. A., Kyle, K. E., Horowitz, J. E., Yang-Iott, K., et al. (2015). Lineage-specific compaction of Tcrb requires a chromatin barrier to protect the function of a long-range tethering element. J. Exp. Med. 212, 107–120. doi:10.1084/jem.20141479
Malin, S., McManus, S., Cobaleda, C., Novatchkova, M., Delogu, A., Bouillet, P., et al. (2010). Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol. 11, 171–179. doi:10.1038/ni.1827
Mandal, M., Hamel, K. M., Maienschein-Cline, M., Tanaka, A., Teng, G., Tuteja, J. H., et al. (2015). Histone reader BRWD1 targets and restricts recombination to the Igk locus. Nat. Immunol. 16, 1094–1103. doi:10.1038/ni.3249
Mandal, M., Maienschein-Cline, M., Hu, Y., Mohsin, A., Veselits, M. L., Wright, N. E., et al. (2024). BRWD1 orchestrates small pre-B cell chromatin topology by converting static to dynamic cohesin. Nat. Immunol. 25, 129–141. doi:10.1038/s41590-023-01666-z
Mandal, M., Powers, S. E., Maienschein-Cline, M., Bartom, E. T., Hamel, K. M., Kee, B. L., et al. (2011). Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nat. Immunol. 12, 1212–1220. doi:10.1038/ni.2136
Mandal, M., Powers, S. E., Ochiai, K., Georgopoulos, K., Kee, B. L., Singh, H., et al. (2009). Ras orchestrates exit from the cell cycle and light-chain recombination during early B cell development. Nat. Immunol. 10, 1110–1117. doi:10.1038/ni.1785
Marquet, M., Garot, A., Bender, S., Carrion, C., Rouaud, P., Lecardeur, S., et al. (2014). The Eμ enhancer region influences H chain expression and B cell fate without impacting IgVH repertoire and immune response in vivo. J. Immunol. 193, 1171–1183. doi:10.4049/jimmunol.1302868
Mathieu, N., Hempel, W. M., Spicuglia, S., Verthuy, C., and Ferrier, P. (2000). Chromatin remodeling by the T cell receptor (TCR)-beta gene enhancer during early T cell development: implications for the control of TCR-beta locus recombination. J. Exp. Med. 192, 625–636. doi:10.1084/jem.192.5.625
Matthias, P., and Baltimore, D. (1993). The immunoglobulin heavy chain locus contains another B-cell-specific 3' enhancer close to the alpha constant region. Mol. Cell Biol. 13, 1547–1553. doi:10.1128/mcb.13.3.1547
McMurry, M. T., and Krangel, M. S. (2000). A role for histone acetylation in the developmental regulation of VDJ recombination. Science 287, 495–498. doi:10.1126/science.287.5452.495
Medvedovic, J., Ebert, A., Tagoh, H., Tamir, I., Schwickert, T. A., Novatchkova, M., et al. (2013). Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity 39, 229–244. doi:10.1016/j.immuni.2013.08.011
Michaelson, J. S., Giannini, S. L., and Birshtein, B. K. (1995). Identification of 3α-hs4, a novel Ig heavy chain enhancer element regulated at multiple stages of B cell differentiation. Nucleic Acids Res. 23, 975–981. doi:10.1093/nar/23.6.975
Mihai, A., Roy, S., Krangel, M. S., and Zhuang, Y. (2023). E protein binding at the Tcra enhancer promotes Tcra repertoire diversity. Front. Immunol. 14, 1188738. doi:10.3389/fimmu.2023.1188738
Monroe, R. J., Sleckman, B. P., Monroe, B. C., Khor, B., Claypool, S., Ferrini, R., et al. (1999). Developmental regulation of TCR delta locus accessibility and expression by the TCR delta enhancer. Immunity 10, 503–513. doi:10.1016/s1074-7613(00)80050-3
Naik, A. K., Dauphars, D. J., Corbett, E., Simpson, L., Schatz, D. G., and Krangel, M. S. (2024). RORγt up-regulates RAG gene expression in DP thymocytes to expand the Tcra repertoire. Sci. Immunol. 9, eadh5318. doi:10.1126/sciimmunol.adh5318
Narita, T., Ito, S., Higashijima, Y., Chu, W. K., Neumann, K., Walter, J., et al. (2021). Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol. Cell 81, 2166–2182.e6. doi:10.1016/j.molcel.2021.03.008
Nelsen, B., and Sen, R. (1992). Regulation of immunoglobulin gene transcription. Int. Rev. Cytol. 133, 121–149. doi:10.1016/s0074-7696(08)61859-8
Nelsen, B., Tian, G., Erman, B., Gregoire, J., Maki, R., Graves, B., et al. (1993). Regulation of lymphoid-specific immunoglobulin mu heavy chain gene enhancer by ETS-domain proteins. Science 261, 82–86. doi:10.1126/science.8316859
Ochiai, K., Maienschein-Cline, M., Mandal, M., Triggs, J. R., Bertolino, E., Sciammas, R., et al. (2012). A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nat. Immunol. 13, 300–307. doi:10.1038/ni.2210
Oettinger, M. A., Schatz, D. G., Gorka, C., and Baltimore, D. (1990). RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science 248, 1517–1523. doi:10.1126/science.2360047
Okada, A., Mendelsohn, M., and Alt, F. (1994). Differential activation of transcription versus recombination of transgenic T cell receptor beta variable region gene segments in B and T lineage cells. J. Exp. Med. 180, 261–272. doi:10.1084/jem.180.1.261
Oudinet, C., Braikia, F. Z., Dauba, A., and Khamlichi, A. A. (2020). Mechanism and regulation of class switch recombination by IgH transcriptional control elements. Adv. Immunol. 147, 89–137. doi:10.1016/bs.ai.2020.06.003
Pang, S. H. M., de Graaf, C. A., Hilton, D. J., Huntington, N. D., Carotta, S., Wu, L., et al. (2018). PU.1 is required for the developmental progression of multipotent progenitors to common lymphoid progenitors. Front. Immunol. 9, 1264. doi:10.3389/fimmu.2018.01264
Panigrahi, A., and O'Malley, B. W. (2021). Mechanisms of enhancer action: the known and the unknown. Genome Biol. 22, 108. doi:10.1186/s13059-021-02322-1
Perlot, T., Alt, F. W., Bassing, C. H., Suh, H., and Pinaud, E. (2005). Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc. Natl. Acad. Sci. U S A. 102, 14362–14367. doi:10.1073/pnas.0507090102
Predeus, A. V., Gopalakrishnan, S., Huang, Y., Tang, J., Feeney, A. J., Oltz, E. M., et al. (2014). Targeted chromatin profiling reveals novel enhancers in Ig H and Ig L chain Loci. J. Immunol. 192, 1064–1070. doi:10.4049/jimmunol.1302800
Proudhon, C., Hao, B., Raviram, R., Chaumeil, J., and Skok, J. A. (2015). Long-range regulation of V(D)J recombination. Adv. Immunol. 128, 123–182. doi:10.1016/bs.ai.2015.07.003
Qiu, X., Kumari, G., Gerasimova, T., Du, H., Labaran, L., Singh, A., et al. (2018). Sequential enhancer sequestration dysregulates recombination center formation at the IgH locus. Mol. Cell 70, 21–33.e6. doi:10.1016/j.molcel.2018.02.020
Rada-Iglesias, A., Bajpai, R., Swigut, T., Brugmann, S. A., Flynn, R. A., and Wysocka, J. (2011). A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283. doi:10.1038/nature09692
Revilla-I-Domingo, R., Bilic, I., Vilagos, B., Tagoh, H., Ebert, A., Tamir, I. M., et al. (2012). The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 31, 3130–3146. doi:10.1038/emboj.2012.155
Ribeiro de Almeida, C., Hendriks, R. W., and Stadhouders, R. (2015). Dynamic control of long-range genomic interactions at the immunoglobulin kappa light-chain locus. Adv. Immunol. 128, 183–271. doi:10.1016/bs.ai.2015.07.004
Rodriguez-Caparros, A., Álvarez-Santiago, J., del Valle-Pastor, M. J., Suñé, C., López-Ros, J., and Hernández-Munain, C. (2020). Regulation of T-cell receptor gene expression by three-dimensional locus conformation and enhancer function. Int. J. Mol. Sci. 21, 8478. doi:10.3390/ijms21228478
Romig, H., Fackelmayer, F. O., Renz, A., Ramsperger, U., and Richter, A. (1992). Characterization of saf-a, a novel nuclear-DNA binding-protein from hela-cells with high-affinity for nuclear matrix scaffold attachment DNA elements. EMBO J. 11, 3431–3440. doi:10.1002/j.1460-2075.1992.tb05422.x
Rothenberg, E. V., Moore, J. E., and Yui, M. A. (2008). Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21. doi:10.1038/nri2232
Rothenberg, E. V., and Taghon, T. (2005). Molecular genetics of T cell development. Annu. Rev. Immunol. 23, 601–649. doi:10.1146/annurev.immunol.23.021704.115737
Rouaud, P., Vincent-Fabert, C., Fiancette, R., Cogné, M., Pinaud, E., and Denizot, Y. (2012). Enhancers located in heavy chain regulatory region (hs3a, hs1,2, hs3b, and hs4) are dispensable for diversity of VDJ recombination. J. Biol. Chem. 287, 8356–8360. doi:10.1074/jbc.m112.341024
Saintamand, A., Rouaud, P., Garot, A., Saad, F., Carrion, C., Oblet, C., et al. (2015). The IgH 3' regulatory region governs mu chain transcription in mature B lymphocytes and the B cell fate. Oncotarget 6, 4845–4852. doi:10.18632/oncotarget.3010
Sakai, E., Bottaro, A., Davidson, L., Sleckman, B. P., and Alt, F. W. (1999). Recombination and transcription of the endogenous Ig heavy chain locus is effected by the Ig heavy chain intronic enhancer core region in the absence of the matrix attachment regions. Proc. Natl. Acad. Sci. U S A. 96, 1526–1531. doi:10.1073/pnas.96.4.1526
Schatz, D. G., Oettinger, M. A., and Baltimore, D. (1989). The V(D)J recombination activating gene, RAG-1. Cell 59, 1035–1048. doi:10.1016/0092-8674(89)90760-5
Scheuermann, R. H., and Garrard, W. T. (1999). MARs of antigen receptor and co-receptor genes. Crit. Reviews™ Eukaryot. Gene Expr. 9, 295–310. doi:10.1615/critreveukargeneexpr.v9.i3-4.140
Schwickert, T. A., Tagoh, H., Gültekin, S., Dakic, A., Axelsson, E., Minnich, M., et al. (2014). Stage-specific control of early B cell development by the transcription factor Ikaros. Nat. Immunol. 15, 283–293. doi:10.1038/ni.2828
Semerad, C. L., Mercer, E. M., Inlay, M. A., Weissman, I. L., and Murre, C. (2009). E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc. Natl. Acad. Sci. U S A. 106, 1930–1935. doi:10.1073/pnas.0808866106
Senoo, M., Mochida, N., Wang, L., Matsumura, Y., Suzuki, D., Takeda, N., et al. (2001). Limited effect of chromatin remodeling on Dβ-to-Jβ recombination in CD4+CD8+ thymocyte: implications for a new aspect in the regulation of TCR β gene recombination. Int. Immunol. 13, 1405–1414. doi:10.1093/intimm/13.11.1405
Senoo, M., Wang, L., Suzuki, D., Takeda, N., Shinkai, Y., and Habu, S. (2003). Increase of TCR Vβ accessibility within Eβ regulatory region influences its recombination frequency but not allelic exclusion. J. Immunol. 171, 829–835. doi:10.4049/jimmunol.171.2.829
Shaffer, A. L., Peng, A., and Schlissel, M. S. (1997). In vivo occupancy of the kappa light chain enhancers in primary pro- and pre-B cells: a model for kappa locus activation. Immunity 6, 131–143. doi:10.1016/s1074-7613(00)80420-3
Spicuglia, S., Kumar, S., Yeh, J. H., Vachez, E., Chasson, L., Gorbatch, S., et al. (2002). Promoter activation by enhancer-dependent and -independent loading of activator and coactivator complexes. Mol. Cell 10, 1479–1487. doi:10.1016/s1097-2765(02)00791-8
Stadhouders, R., de Bruijn, M. J. W., Rother, M. B., Yuvaraj, S., de Almeida, C. R., Kolovos, P., et al. (2014). Pre-B cell receptor signaling induces immunoglobulin kappa locus accessibility by functional redistribution of enhancer-mediated chromatin interactions. PLoS Biol. 12, e1001791. doi:10.1371/journal.pbio.1001791
Stanhope-Baker, P., Hudson, K. M., Shaffer, A. L., Constantinescu, A., and Schlissel, M. S. (1996). Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell 85, 887–897. doi:10.1016/s0092-8674(00)81272-6
Staudt, L. M., and Lenardo, M. J. (1991). Immunoglobulin gene transcription. Annu. Rev. Immunol. 9, 373–398. doi:10.1146/annurev.iy.09.040191.002105
Sun, Z., Unutmaz, D., Zou, Y. R., Sunshine, M. J., Pierani, A., Brenner-Morton, S., et al. (2000). Requirement for RORγ in thymocyte survival and lymphoid organ development. Science 288, 2369–2373. doi:10.1126/science.288.5475.2369
Teng, G., and Schatz, D. G. (2015). Regulation and evolution of the RAG recombinase. Adv. Immunol. 128, 1–39. doi:10.1016/bs.ai.2015.07.002
Vian, L., Pękowska, A., Rao, S. S., Kieffer-Kwon, K. R., Jung, S., Baranello, L., et al. (2018). The energetics and physiological impact of cohesin extrusion. Cell 175, 292–294. doi:10.1016/j.cell.2018.09.002
Villey, I., Caillol, D., Selz, F., Ferrier, P., and de Villartay, J. P. (1996). Defect in rearrangement of the most 5′ TCR–jα following targeted deletion of T early α (TEA): implications for TCR α locus accessibility. Immunity 5, 331–342. doi:10.1016/s1074-7613(00)80259-9
Vincent-Fabert, C., Fiancette, R., Pinaud, E., Truffinet, V., Cogné, N., Cogné, M., et al. (2010). Genomic deletion of the whole IgH 3' regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood 116, 1895–1898. doi:10.1182/blood-2010-01-264689
Volpi, S. A., Verma-Gaur, J., Hassan, R., Ju, Z., Roa, S., Chatterjee, S., et al. (2012). Germline deletion of Igh 3' regulatory region elements hs 5, 6, 7 (hs5-7) affects B cell-specific regulation, rearrangement, and insulation of the Igh locus. J. Immunol. 188, 2556–2566. doi:10.4049/jimmunol.1102763
Wang, X. S., Lee, B. J., and Zha, S. (2020). The recent advances in non-homologous end-joining through the lens of lymphocyte development. DNA Repair (Amst) 94, 102874. doi:10.1016/j.dnarep.2020.102874
Watson, P. J., Fairall, L., and Schwabe, J. W. R. (2012). Nuclear hormone receptor co-repressors: structure and function. Mol. Cell. Endocrinol. 348, 440–449. doi:10.1016/j.mce.2011.08.033
Wei, G., Abraham, B., Yagi, R., Jothi, R., Cui, K., Sharma, S., et al. (2011). Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311. doi:10.1016/j.immuni.2011.08.007
Weinert, B. T., Narita, T., Satpathy, S., Srinivasan, B., Hansen, B. K., Schölz, C., et al. (2018). Time-resolved analysis reveals rapid dynamics and broad scope of the CBP/p300 acetylome. Cell 174, 231–244.e12. doi:10.1016/j.cell.2018.04.033
Weitzel, J. M., Buhrmester, H., and Stratling, W. H. (1997). Chicken MAR-binding protein ARBP is homologous to rat methyl-CpG-binding protein MeCP2. Mol. Cell. Biol. 17, 5656–5666. doi:10.1128/mcb.17.9.5656
Whitehurst, C. E., Chattopadhyay, S., and Chen, J. (1999). Control of V(D)J recombinational accessibility of the Dβ1 gene segment at the TCRβ locus by a germline promoter. Immunity 10, 313–322. doi:10.1016/s1074-7613(00)80031-x
Whitehurst, C. E., Schlissel, M. S., and Chen, J. (2000). Deletion of germline promoter PDβ1 from the TCRβ locus causes hypermethylation that impairs Dβ1 recombination by multiple mechanisms. Immunity 13, 703–714. doi:10.1016/s1074-7613(00)00069-8
Wong, M. M., Guo, C., and Zhang, J. (2014). Nuclear receptor corepressor complexes in cancer: mechanism, function and regulation. Am. J. Clin. Exp. Urol. 2, 169–187.
Wotton, D., Ghysdael, J., Wang, S., Speck, N. A., and Owen, M. J. (1994). Cooperative binding of Ets-1 and core binding factor to DNA. Mol. Cell. Biol. 14, 840–850. doi:10.1128/mcb.14.1.840-850.1994
Xiang, Y., Park, S. K., and Garrard, W. T. (2013). Vκ gene repertoire and locus contraction are specified by critical DNase I hypersensitive sites within the vκ-jκ intervening region. J. Immunol. 190, 1819–1826. doi:10.4049/jimmunol.1203127
Xiang, Y., Park, S. K., and Garrard, W. T. (2014). A major deletion in the vκ–jκ intervening region results in hyperelevated transcription of proximal Vκ genes and a severely restricted repertoire. J. Immunol. 193, 3746–3754. doi:10.4049/jimmunol.1401574
Xiang, Y., Zhou, X., Hewitt, S. L., Skok, J. A., and Garrard, W. T. (2011). A multifunctional element in the mouse Igκ locus that specifies repertoire and Ig loci subnuclear location. J. Immunol. 186, 5356–5366. doi:10.4049/jimmunol.1003794
Xu, Y., Davidson, L., Alt, F. W., and Baltimore, D. (1996). Deletion of the Ig kappa light chain intronic enhancer/matrix attachment region impairs but does not abolish V kappa J kappa rearrangement. Immunity 4, 377–385. doi:10.1016/s1074-7613(00)80251-4
Yancopoulos, G. D., and Alt, F. W. (1985). Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell 40, 271–281. doi:10.1016/0092-8674(85)90141-2
Yang, C., Shapiro, L. H., Rivera, M., Kumar, A., and Brindle, P. K. (1998). A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol. Cell. Biol. 18, 2218–2229. doi:10.1128/mcb.18.4.2218
Yi, M., Wu, P., Trevorrow, K. W., Claflin, L., and Garrard, W. T. (1999). Evidence that the Igκ gene MAR regulates the probability of premature V-J joining and somatic hypermutation. J. Immunol. 162, 6029–6039. doi:10.4049/jimmunol.162.10.6029
Yoshida, H., Lareau, C. A., Ramirez, R. N., Rose, S. A., Maier, B., Wroblewska, A., et al. (2019). The cis-regulatory atlas of the mouse immune system. Cell 176, 897–912.e20. doi:10.1016/j.cell.2018.12.036
Zabidi, M. A., and Stark, A. (2016). Regulatory enhancer-core-promoter communication via transcription factors and cofactors. Trends Genet. 32, 801–814. doi:10.1016/j.tig.2016.10.003
Zhang, X., Yoon, H. S., Chapdelaine-Williams, A. M., Kyritsis, N., and Alt, F. W. (2021). Physiological role of the 3'IgH CBEs super-anchor in antibody class switching. Proc. Natl. Acad. Sci. U S A. 118, e2024392118. doi:10.1073/pnas.2024392118
Zhao, B., Rothenberg, E., Ramsden, D. A., and Lieber, M. R. (2020). The molecular basis and disease relevance of non-homologous DNA end joining. Nat. Rev. Mol. Cell Biol. 21, 765–781. doi:10.1038/s41580-020-00297-8
Zhao, J. Y., Osipovich, O., Koues, O. I., Majumder, K., and Oltz, E. M. (2017). Activation of mouse Tcrb: uncoupling RUNX1 function from its cooperative binding with ETS1. J. Immunol. 199, 1131–1141. doi:10.4049/jimmunol.1700146
Keywords: enhancers, antigen receptor gene, lymphocyte development, lineage specific, stage specific, transcription factors (TF)
Citation: Ma F, Braikia FZ and Sen R (2024) Lineage- and stage-specific activity of antigen receptor gene enhancers during lymphocyte development. Front. Epigenet. Epigenom. 2:1489362. doi: 10.3389/freae.2024.1489362
Received: 01 September 2024; Accepted: 02 December 2024;
Published: 16 December 2024.
Edited by:
Ramon Y. Birnbaum, Ben-Gurion University of the Negev, IsraelReviewed by:
Vasco Barreto, New University of Lisbon, PortugalBoxun Li, Duke University, United States
Copyright © 2024 Ma, Braikia and Sen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjan Sen, cmFuamFuLnNlbkBuaWguZ292
†These authors have contributed equally to this work
 Fei Ma
Fei Ma Fatima Zohra Braikia
Fatima Zohra Braikia Ranjan Sen
Ranjan Sen