
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Dement. , 17 January 2025
Sec. Aging and Risk Factors for Dementia
Volume 3 - 2024 | https://doi.org/10.3389/frdem.2024.1496051
This article is part of the Research Topic Lifestyle and Healthy Aging to Prevent Cognitive Decline and Dementia View all 28 articles
 Tamlyn J. Watermeyer1,2*
Tamlyn J. Watermeyer1,2* Sarah Gregory1,3
Sarah Gregory1,3 Emmi Leetham2
Emmi Leetham2 Chinedu T. Udeh-Momoh4,5,6,7,8
Chinedu T. Udeh-Momoh4,5,6,7,8 Graciela Muniz-Terrera1,9 on behalf of the Female Brain & Endocrine Research (FemBER) Consortium
Graciela Muniz-Terrera1,9 on behalf of the Female Brain & Endocrine Research (FemBER) ConsortiumIntroduction: The impact of Hormone Replacement Therapy (HRT) on cognitive function in postmenopausal women remains a topic of considerable debate. Although estrogen's neuroprotective effects suggest potential cognitive benefits, empirical findings are mixed.
Methods: This study uses data from the Cognitive Function and Ageing Study Wales (CFAS Wales) cohort to explore the relationships between HRT use, age at menopause, APOE4 carrier status, lifestyle factors, comorbidities, and cognitive outcomes in older adult women. Two regression models were employed: one analyzing cognitive performance at follow-up and another examining changes in cognitive scores over time.
Results: Results indicate that while age, education, HRT use, age at menopause, alcohol consumption, and diet were associated with cognitive function at a single later time point, only age remained a significant predictor when modeling cognition over time.
Discussion: These findings suggest that while HRT, menopausal age and lifestyle factors may support cognitive stability, they do not necessarily predict cognitive decline in post-menopausal older women. A major limitation of the current work is the lack of detail regarding HRT use, such as formulation, timing and duration; caveats that future studies should address. The study underscores the need for longer follow-up periods, consideration of other female-specific risk factors, and more comprehensive lifestyle and health assessments to clarify the complex interplay between HRT use, reproductive history, lifestyle, comorbidities and cognitive aging in women.
The relationship between Hormone Replacement Therapy (HRT) and cognitive function in postmenopausal women is subject to ongoing investigation and debate (see Mills et al., 2023 for review). The rationale for HRT's potential cognitive benefits lies in the marked decline in estrogen levels during menopause, which is associated with age-related cognitive decline and an increased risk of neurodegenerative diseases, including Alzheimer's disease (AD) (Nerattini et al., 2023). Ovarian hormones are believed to play a crucial role in neurocognitive processes by supporting synaptic plasticity, neuroprotection, and cerebral blood flow regulation (Jett et al., 2022). Consequently, the potential for HRT to mitigate cognitive decline has generated significant interest, particularly in light of the higher prevalence of AD in women compared to men (Buckley et al., 2019; Mielke, 2018; Riedel et al., 2016), understanding the role of HRT in cognitive aging is of significant clinical importance (Ferretti et al., 2018) and relevant for secondary prevention strategies that target sex-specific risk factors (Udeh-Momoh and Watermeyer, 2021).
Despite these theoretical benefits, empirical findings on HRT's impact on cognition have been inconsistent (see Nerattini et al., 2023 for systematic review). Observational studies have frequently reported a positive association between HRT use and cognitive performance on global as well as domain scores, such as memory, processing speed and executive functions (Coughlan et al., 2022; Saleh et al., 2023; Yaffe et al., 1998). Similarly, HRT use has been associated with greater brain volumes in key AD-relevant regions, such as the entorhinal cortex, the hippocampus and prefrontal cortex (Saleh et al., 2023; Coughlan et al., 2023) and lower levels of AD-relevant biomarkers, such as phosphorylated tau (p-tau) and total tau (t-tau) (Lee et al., 2024). The influence of APOE4 carriership is controversial, with evidence suggesting that these effects are relevant only or more for women carrying the APOE4 allele (Saleh et al., 2023; Depypere et al., 2023), while other evidence suggests that only non-carriers derive cognitive benefits from HRT (Burkhardt et al., 2004). Still other evidence indicates no relevance of APOE4 status to female-specific factors and cognition (Lindseth et al., 2023). The influence of menopausal age alongside HRT use may also be relevant, with some studies noting that later menopausal age for HRT users is associated with better cognitive performance and reduced dementia risk in later life (Park et al., 2024), suggesting that longer exposure to endogenous and exogenous estrogen may be neuroprotective (Lee et al., 2024).
Conversely, randomized controlled trials (RCTs) have generally produced less favorable results in terms of cognitive benefits from HRT use. The Women's Health Initiative Memory Study (WHIMS, Shumaker et al., 1998), found that HRT was associated with an increased risk of dementia and cognitive decline when initiated in women aged 65 and older (Coker et al., 2010). This study significantly influenced clinical guidelines, leading to a more cautious approach to HRT use in older women. Additionally, the Kronos Early Estrogen Prevention Study (KEEPS) failed to demonstrate significant cognitive benefits of HRT in newly menopausal women (Gleason et al., 2015). More recently, a prospective trial of 6-months estrogen therapy in HRT-naïve cognitively healthy and younger menopausal women showed longitudinal changes in AB/p-tau ratio scores for women within the HRT arm; these effects strongest for APOE4 carriers (Depypere et al., 2023), indicating, for the first time, potential positive effects of HRT on biomarkers relating to AD pathophysiology. However, cognition was not assessed in this study due to the relatively short follow-up period.
The discordance between observational studies and RCTs could stem from differences in study populations, HRT formulations (i.e., estrogen-only, progesterone-only or combined preparations), and, crucially, the timing of HRT initiation. The “critical window hypothesis” posits that HRT is most effective when initiated near the onset of menopause, during a period when the brain may be more responsive to estrogen (Maki and Sundermann, 2009; Udeh-Momoh and Watermeyer, 2021). Similarly, the importance of considering cardiovascular health, metabolic status, and lifestyle factors when evaluating the cognitive effects of HRT has also been highlighted (Lee et al., 2024; Espeland et al., 2015; Liao et al., 2023; Rocca et al., 2024), despite several comorbidities representing exclusion criteria for HRT RCTs. RCT studies of the Mediterranean diet suggest significant improvements in cognitive domain composites and evidence for modulation of AD-relevant genotypes in dementia risk (Radd-Vagenas et al., 2018). Separately, adherence to this diet, which prescribes a high-intake of vegetables, nuts and their oils, has shown benefits to menopausal women's health, through weight and cardiovascular management (Gonçalves et al., 2024; Gregory et al., 2023b). Such benefits might occur through the estrogenic properties of recommended foods associated with the diet (Rispo et al., 2024).
While HRT remains a commonly prescribed treatment for menopausal symptoms, its impact on cognitive health remains controversial. Based on the existing body of literature, this study seeks to clarify the complex relationship between HRT and cognitive function in postmenopausal women, considering the potential influence of key factors such as age at menopause, APOE4 carrier status, lifestyle factors, and comorbidities. By leveraging data from the Cognitive Function and Ageing Study Wales (CFAS Wales) cohort of older-adults to explore whether HRT use is associated with better cognitive outcomes in older women, and how these outcomes are potentially moderated or mediated by such variables.
This study uses data from the Cognitive Function and Ageing Study Wales (CFAS Wales), a population-based longitudinal cohort study focused on individuals aged 65 and older residing in Wales. The study investigates various risk factors and health outcomes among this population, and previously has explored the influence of socio-economic factors, bilingualism, cognitive reserve and lifestyle on cognitive status in later life across the entire sample (men and women) (Gamble et al., 2022; The Medical Research Council Cognitive Function and Ageing Study, 1998; Clare et al., 2017; Jia et al., 2021). It was conducted in two distinct geographical areas: a rural region (Gwynedd and Ynys Môn) and an urban area (Neath Port Talbot). All participants were consented to the study using informed consent procedures. The study included two waves of data collection: baseline data were gathered between 2011 and 2014, with a follow-up conducted 2 years later, from 2013 to 2016. The study received ethical approval from the NHS North Wales—West Research Ethics Committee (REC Ref No: 10/Wno01/37; IRAS Project No: 40092). The original study inclusion criteria comprised being born before 1946 and English or Welsh proficiency. For the purposes of our study, we included only female participants without evidence of cognitive impairment at the baseline assessment, as indicated by a Mini-Mental State Examination (MMSE, Folstein et al., 1975) score of 25 or higher. The study sample included 629 individuals who met the inclusion criteria.
Age was recorded at baseline; education was quantified as number of years of formal education. At the baseline visit, participants were asked the year of their last period. From this year, 1 year was added to represent their menopausal age, in keeping with the STRAW criteria (Harlow et al., 2012). Whether menopause was natural or due to surgical intervention was not recorded. Also at the baseline visit, participants were asked if they had ever been prescribed HRT. Twenty-three participants responded that they were currently taking HRT. Due to the small sample size of the latter group, this was combined with the participants who had been prescribed HRT in the past, generating 264 participants in the group with HRT history and 365 participants with no HRT history. APOE4 status was obtained through blood donation, available for all 629 eligible participants.
Participants were asked if they had ever been diagnosed with cancer, hypertension or diabetes by a general or specialist practitioner. Self-reported endorsements of diagnosis of co-morbidities were chosen for this study based on data availability, power and previous HRT and cognition literature where such comorbidities were indicated as confounders.
Participants were assessed on self-report measures relating to lifestyle. A history of smoking was ascertained through the questions: “Do you smoke?” or “Have you ever smoked?”. For alcohol or drinking behavior, participants were categorized into four groups based on their self-reported frequency of alcohol consumption over the past 12 months: nearly abstinent (no alcohol consumption or drinking once or twice a year); infrequent drinkers (consuming alcohol once or twice a month or once every few months); frequent light-to-moderate drinkers (drinking once or twice a week or three to four times a week); and regular drinkers (drinking five to six times a week or almost daily).
Participants' level of physical activity was assessed based on how frequently participants engaged in 18 different activities classified by intensity: mild (such as light gardening, bowls, light housework, and home repairs), moderate (such as gardening, using an electric lawn mower, car cleaning, moderate-paced walking, dancing, stretching exercises, and heavy housework), and vigorous (such as jogging, swimming, cycling, aerobics or gym activities, tennis, heavy gardening, and manual lawn mowing). A continuous scale was created by multiplying the reported frequency of activity (scored as 0 = once a year or less, 1 = several times a year, 2 = several times a month, 3 = several times a week, and 4 = every day or nearly every day) by the intensity ratio (mild: moderate: vigorous = 1:2:3). This ratio was established according to the metabolic equivalent of task (MET) values recommended in the literature (Aaron et al., 1995).
Self-reported information on the frequency of alcohol consumption over the last 12 months was used to classify participants into four groups: nearly abstinent (not at all in the last 12 months or once or twice a year); infrequent drinkers (once or twice a month or once every couple of months); frequent light-to-moderate drinkers (once or twice a week or three or four times a week); and regular drinkers (five or six times a week or almost every day).
To capture the overall dietary pattern, a total healthy diet score was created. CFAS-Wales assessed the frequency (never, seldom, once a week, 2–4 times a week, 5–6 times a week, or daily) and the daily servings of various foods, including fresh fruit, green leafy vegetables, other vegetables, fatty fish, other fish, wholemeal/brown bread, starch foods, dairy foods, and sugary foods. This analysis specifically focused on the intake frequency of “Mediterranean style” foods, such as fresh fruit, green leafy vegetables, other vegetables, fatty fish, other fish, and wholemeal/brown bread. The frequency was categorized into six levels: never, seldom, once a week, 2–4 times a week, 5–6 times a week, or daily. As per Clare et al. (2017), the total healthy diet score was generated based on these six frequency levels, with scores ranging from 2 (least frequent intake) to 30 (most frequent intake).
Baseline cognitive function was assessed using the MMSE (Folstein et al., 1975) for screening (only participants with scores ≥25 were included in the analysis) and The Cambridge Cognitive Examination (CAMCOG, Roth et al., 1986), a standardized assessment tool comprising 67 items designed to evaluate cognitive function across eight domains. These domains include orientation, comprehension, expression, various aspects of memory (such as remote, recent, and learning), attention and calculation, praxis, abstract thinking, and perception. The total possible score ranges from 0 to 107, with lower scores indicating poorer cognitive performance. In this study, both the baseline and follow-up CAMCOG scores were used in these analyses. The change in CAMCOG scores was calculated as the difference between baseline and follow-up CAMCOG scores and were also included in the analyses.
Multiple linear regression models were employed to investigate the relationship between the independent variables and cognitive outcomes. Initially, a series of linear univariate regression models were fitted to study the association of the CAMCOG follow-up score, with menopausal age, education, history of HRT use, and APOE4 carrier status as predictors. Next, a multivariable linear regression model was fitted including all predictors. Finally, a fully adjusted model that also including an interaction term between HRT use and APOE carriership was also fitted.
Finally, we repeated the same steps considering change in CAMCOG scores between baseline and follow-up as the dependent variable, that is, we fitted a series of univariate models with menopausal age, education, history of HRT use, and APOE4 carrier status as predictors and then fitted a fully adjusted model. Significance of each predictor was assessed using p-values associated with the regression coefficients (p < 0.05). Results are reported with 95% confidence intervals, and assumptions of normality, linearity, and homoscedasticity were checked for each model via inspection of diagnostic plots. The model fit was evaluated using R-squared and adjusted R-squared values, and the overall model significance was tested using the F-statistic. Finally, Variation inflation factor (VIF) values were examined for issues with multicollinearity. All statistical analyses were conducted using Stata version 17, with a significance level set at p < 0.05.
Sample characteristics and averages for variables of interest are shown in Table 1 for all participants included in this study. Briefly, participants had a mean age of 72.95 years and were ~20 years post menopause (mean age of menopause 49.28 years). Nearly 42% of participants had a history of HRT use and about a quarter (25.25%) were APOE4 carriers.
Results of the first regression analysis modeling cognitive performance at follow-up showed that the overall model was statistically significant, F(12;615) = 13.68, p < 0.001, with an R2 = 0.21, indicating that ~21% of the variance in CAMCOG follow-up scores was explained by the predictors. Results are shown in Table 2. Menopausal age, history of HRT use, alcohol consumption, healthy diet, education in years and age at baseline were significant predictors (p < 0.05). A diagnosis of diabetes showed an association that approached significance (p = 0.08).
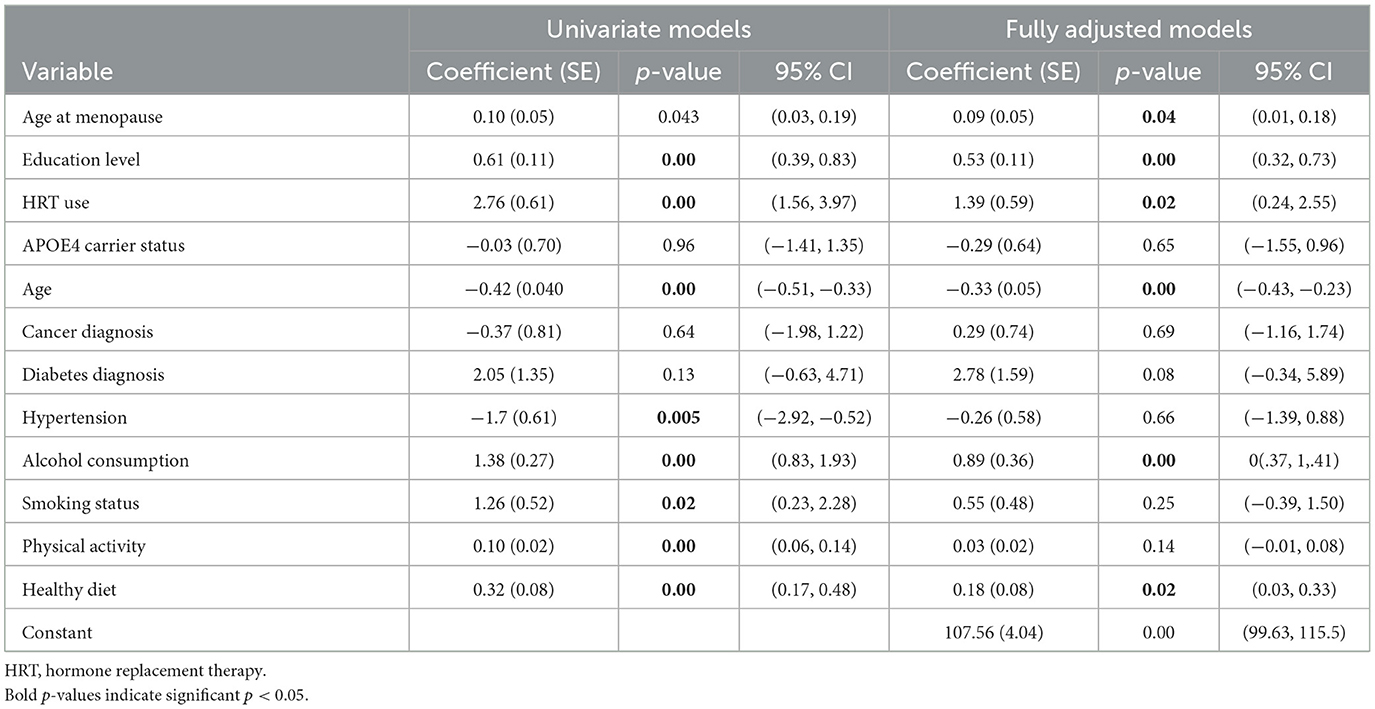
Table 2. Results from univariate and fully adjusted regression models fitted to cognitive function at follow-up.
Results of the second analysis modeling change overtime, operationalized as the difference between baseline and follow-up CAMCOG scores are shown in Table 3. The overall model was statistically significant, F(12, 615) = 2.58, p = 0.002, with an R2 = 0.048, indicating that ~4.8% of the variance in the change in cognitive scores was explained by the predictors. Age at baseline was the only significant predictor in this subsequent model (p < 0.05).
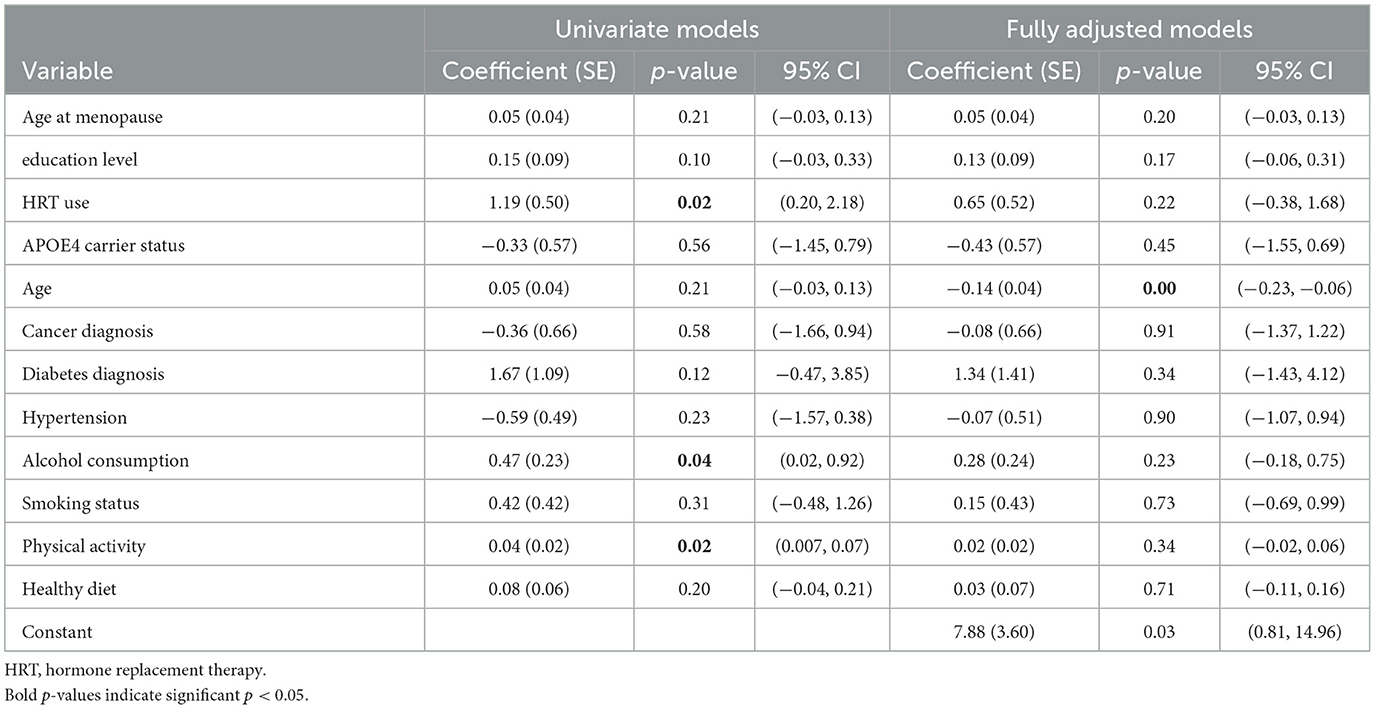
Table 3. Results from univariate and fully adjusted regression models fitted to cognitive change over time.
The interaction term of HRT history and APOE carriership did not emerge as statistically significant in either of the two fully adjusted models.
Due to criticisms regarding the sensitivity of the MMSE cut-off threshold of 25 as indicative of cognitive decline, we conducted sensitivity analyses with a revised cut-off threshold of 27 in keeping with previous work (Kukull et al., 1994). The results of this sensitivity analysis did not change the interpretation of our findings for the change in CAMCOG scores over time, but did affect interpretation of the CAMCOG at follow-up model (see Supplementary Table S1), with HRT use and age at menopause no longer contributing to the model with the revised MMSE cutoff score. We further assessed whether any participants transitioned from “normal cognition” to “cognitive impairment” based on MMSE scores at baseline and follow-up. Fifty-five women scoring above 25 on the MMSE at baseline, scored below 25 on the MMSE at the follow-up timepoint. A logistic regression was carried out to assess the relationship between our variables of interest and cognitive impairment. The final log-likelihood of the model was −165.73, and the model yielded a likelihood ratio chi-square (LR χ2) statistic of 31.98, p = 0.0014, indicating that the overall model was statistically significant. The Pseudo R2 value was 0.088, suggesting that ~8.8% of the variance in cognitive impairment was explained by the included predictors. Only education level and adherence to a healthy diet were significant predictors (see Table 4).
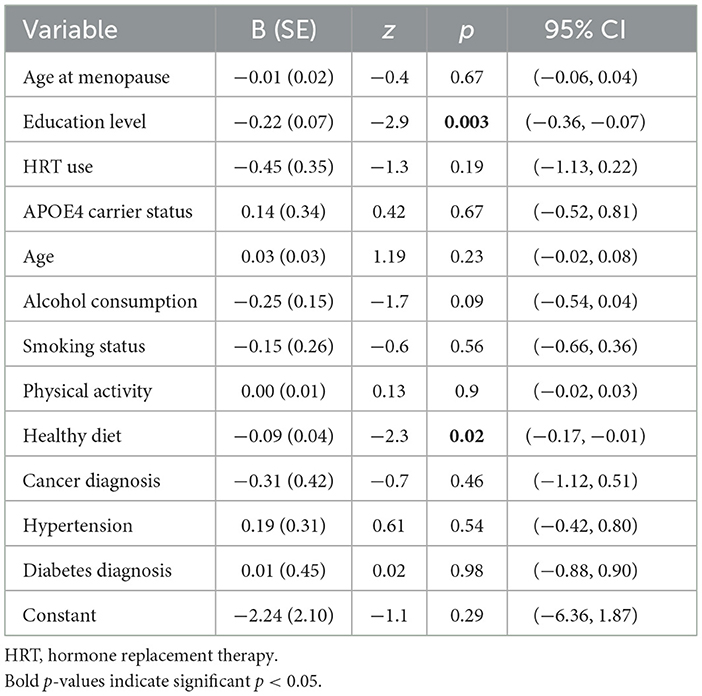
Table 4. Results of fully-adjusted logistic regression model to assess risk for cognitive impairment at follow-up.
This study explored the complex relationships between HRT use, age at menopause, APOE4 carrier status, lifestyle variables, presence of comorbidities and cognitive outcomes in postmenopausal older-adult women, contributing to the ongoing investigation regarding the efficacy and risks associated with HRT in the context of aging and cognitive decline. In the first analysis which modeled absolute cognitive performance at a specific timepoint, age at menopause, history of HRT, alcohol consumption and healthy diet, along with age and education levels, were significantly associated with cognitive performance. However, the second analysis modeling change in cognitive performance over time revealed that only age remained a significant predictor. The discrepancies in findings between these model approaches may differentiate between predictors that support stable cognitive performance and those that actively contribute to cognitive decline or improvement in older-adult women; these findings will be considered in turn in relation to previous research.
A significant positive association between HRT use and cognitive function, as measured by the CAMCOG scores at follow-up, is consistent with previous observational studies reporting better cognitive performance associated with HRT use (Saleh et al., 2023; Yaffe et al., 1998; Hogervorst et al., 2000); however, the finding that this association did not persist with change over time is in keeping with several RCT studies finding no evidence for HRT to delay cognitive decline in older-adult women (Espeland et al., 2010; Shumaker et al., 2003) as well as an observational study of post-menopausal older-adult women in which HRT history showed no effects on cognitive change over a 3-year follow-up (Wood Alexander et al., 2024). Instead, this shift in significance when change in cognitive scores over time is applied, indicates that while HRT and the other variables of interest, may be associated with cross-sectional cognitive function at a specific time point, they do not necessarily predict the cognitive change on trajectory toward dementia. Nonetheless, the follow-up period of 2 years in this study is relatively restricted and a longer follow-up period with more interim assessments might reveal long-term benefits in rate of cognitive change or decline.
Similarly, age at menopause showed an association with cognitive outcome at follow-up, with earlier menopause associated with poorer cognitive performance at this timepoint. This finding aligns with studies reporting that early menopause increases the risk of Alzheimer's disease (Liao et al., 2023), and suggests that early intervention might mitigate this risk (Coughlan et al., 2023). Again, as the association with menopausal age association did not persist in our model of change in cognitive performance this suggests that either the benefit of later menopausal age does not translate to reduced cognitive decline long-term or the underlying pathophysiological benefits of a longer fertile window could not be indicated by our global cognitive measure. Greater menopausal age may confer a longer fertile window—the period from menarche to menopause—and therefore might represent prolonged exposure to endogenous estrogens providing neuroprotection. Women with longer fertile windows show lower levels of p-tau and t-tau biomarkers relative to those with shorter windows (Lee et al., 2024), substantiating the role of reproductive or sex hormones in mediating AD pathology. Unfortunately, the age of first menses was not available in the current dataset, and thus we could not estimate participant's fertile window in this study. Lee et al. (2024) also considered the duration of HRT use, reporting that longer durations of HRT, particularly when initiated within the critical window of 5 years after menopause, were associated with better cognitive outcomes. Conversely, prolonged use of HRT when started later after menopause did not show the same cognitive benefits and, in some cases, could be associated with increased risks, as found in the (Shumaker et al., 1998) RCT study.
Given proposed caveats of the sensitivity of the MMSE to detect cognitive impairment, we conducted sensitivity analyses including participants with a cut-off score > 27 (Kukull et al., 1994). Under these conditions, HRT use and age at menopause no longer contributed to the model for cognitive performance at follow-up, indicating that the effects of HRT use and age at menopause might be more apparent for maintaining cognitive health in participants with significant impairment (< MMSE 25). The new model might also include participants across a broader range of cognitive abilities, potentially introducing more variability and obscuring the effects of HRT use and age at menopause. Alternatively, these discrepancies might reflect an artifact of the MMSE itself, with it not being sensitive enough to capture subtle cognitive changes in higher-functioning individuals (those scoring closer to 30). The ceiling effect could artificially dampen or obscure true associations in the model, making it appear as if certain factors are less relevant.
In our first model two lifestyle factors, current alcohol consumption and greater adherence to a Mediterranean-like healthy diet, were found to be associated with better cognitive performance. Interestingly, a traditional Mediterranean diet typically includes the moderate consumption of red wine and there may be cumulative benefits of the two components measured in our study. The relationship between alcohol consumption behaviors and later-life cognitive health is controversial, but similar evidence in older-adult women suggests that moderate drinkers (consuming < 15 g or approximately one drink per day) show better cognitive scores than non-drinkers (Stampfer et al., 2005). This finding has been further substantiated in moderate drinkers through systematic review (Ran et al., 2021) and through dose-response analysis for female drinkers, specifically (Brennan et al., 2020). Nonetheless, as the Lancet Commission report (Livingston et al., 2024) suggests there is a lack of definitive evidence that non-drinkers are at increased risk of dementia and that excess risk for non-drinkers may in fact be an artifact of current non-drinkers' abstinence at the time of study collection being a response to previously excessive consumption that was not captured. Alternatively, perhaps these relationships are influenced by sex and the heterogeneity in the literature reflects the lack of sex-specific reporting. The finding that more frequent intake of foods associated with the Mediterranean diet shows a positive association with cognitive performance resonates with several previous works (see Siervo et al., 2021 for review). Indeed, higher adherence to this dietary pattern has been associated with a significantly lower risk of mild cognitive impairment (RR = 0.91, 95% CI = 0.85–0.97) and lower risk of AD (RR = 0.89, 95% CI = 0.84–0.93) through dose-response meta-analysis (García-Casares et al., 2021). Specific to women, the diet's focus on plant-based foods with high degrees of polyphenolic and phytoestrogenic compounds and purported estrogenic properties (Barrea et al., 2021) may support the menopausal transition and possibly offset the influence of cumulating age-related and post-menopausal health conditions, such as cardiovascular and metabolic conditions (Szmidt et al., 2023). Our study did not find any influence of comorbidities, such as cancer, hypertension and diabetes, although the latter did show a trend toward significance (p = 0.08) in our first model.
Interestingly, no significant association with APOE4 status and cognitive outcome or change was demonstrated, and there was no evidence of an interaction of HRT and APOE4 upon cognition in this study, in contrast to previous studies of diverse older-adults (Saleh et al., 2023; Gharbi-Meliani et al., 2021). Although, the UK Biobank cohort study (N = 111,739) found significant interactions between APOE4 status with the presence of cardiometabolic diseases and older age on cognitive abilities, but these effects did not survive correction for confounders, including diabetes, cardiovascular disease and hypertension (Lyall et al., 2016); variables incorporated into our analyses. Longitudinal evidence from a 20 year follow-up study suggests that APOE4 heterozygote carriers showed poorer cognition only upon age 75 years and older (Gharbi-Meliani et al., 2021). Thus, it is possible that our sample may be, on average, too young to be demonstrating group level effects on cognition. Moreover, our study's sample size limitations could contribute to these non-significant findings. Although APOE4 status was recorded for all participants, the relatively small proportion of APOE4 carriers (~25%) may not have provided sufficient power to detect smaller effect sizes in interaction with other factors, such as HRT use and lifestyle behaviors. Nonetheless, the debate surrounding the influence of APOE4 genotype independently or in interaction with other reproductive (Saleh et al., 2023; Lindseth et al., 2023) or lifestyle and health factors (Dhana et al., 2021; Lyall et al., 2019) is deserving of further investigation to resolve these inconsistencies.
While this study contributes valuable insights to predictors of cognitive performance in older-adult post-menopausal women, there are notable limitations. Its cross-sectional design with a limited follow-up period of 2-years restricts its ability to draw causal conclusions. Further, it relied on self-report of health and lifestyle variables, in some instances reducing these factors to presence or history of comorbidities and health behaviors. More detailed and validated medical and lifestyle information (e.g., duration of illness, duration of smoking, history of alcohol consumption behaviors) might have rendered more nuanced or a different set of results. Similarly, the lack of detailed information on HRT formulations, dosages, as well as proxies for fertility, such as parity and length of fertile window may have compromised interpretations of the results obtained in relation to reproductive and fertility proxies. For example, our study could also not address whether the timing of HRT initiation following the menopause was a critical factor, as the HRT initiation date was not recorded as part of the interview. The timing of HRT initiation relative to menopause onset may indeed be crucial in determining its effects on cognitive outcomes and brain structure. In a recent study, women who initiated HRT closer to the onset of menopause, that is, within 5 years, tended to have better cognitive performance and more favorable brain volume outcomes compared to those who started HRT after a longer delay following menopause (Coughlan et al., 2023). Nonetheless, previous work failed to find evidence that HRT initiation close to menopause shows beneficial effects on cognitive function in later life (Ryan et al., 2009). Surgical and natural menopause could also not be differentiated in the dataset, which might have provided a more refined interpretation of these results and in relation to other findings. Women who have had an oophorectomy before their natural age of menopause have a faster rate of cognitive decline and a significantly higher risk of cognitive impairment and dementia later in life (Bove et al., 2014; Phung et al., 2010; Rocca et al., 2007), risks which are particularly pronounced in women who do not initiate HRT at all or soon after surgery (Bove et al., 2014; Rocca et al., 2014).
Importantly, while our sample comprises participants living in urban and rural Wales, offering some socio-economic and socio-linguistic diversity, it is greatly restricted by its lack of ethno-racial diversity. Ethno-racial variations in the prevalence, risk, presentation, and progression of AD have been documented (Demirovic et al., 2003; Guland et al., 1997; Howell et al., 2017; Kulminski et al., 2020). These disparities are likely the result of intricate interactions between genetic susceptibility and risk factors that are influenced by biological, lifestyle, and socio-cultural differences, which vary across ethno-racial groups (Brothers et al., 2019; Xiong et al., 2020). In relation to female-specific risk factors, both the timing of menopause and the associated symptoms, including psychological and vasomotor changes, have been found to vary among different ethnicities (Avis et al., 2001; Thurston and Joffe, 2011). Similarly, this is an older-adult sample (all were aged 65 years and older at baseline), and diversity in ages and thus stages of the fertile window, as well as variations in parity and experiences with HRT and other hormones, including exogenous estrogens, such as hormonal contraception (see Gregory et al., 2023a for review), may have further delineated the complex interplay of factors that influence cognitive outcomes in later life or potentially explain the heterogeneity observed in cognitive aging and Alzheimer's disease risk within the female population. Like many established cohorts, the selected dataset was not designed with sex differences or female-specific risks for dementia in mind, and thus the ability to comprehensively assess all purported sex-specific risks was limited. Nonetheless, there have been calls and initiatives to improve upon current and future data collection methods in cognitive aging cohorts with an embedded focus on female brain health (Udeh-Momoh and Watermeyer, 2021; Watermeyer et al., 2024; Udeh-Momoh et al., 2024).
Future research will benefit from this concerted effort to highlight female-specific dementia risks. These studies should focus on longitudinal follow-up with higher throughout to better understand the long-term effects of HRT on cognitive outcomes, particularly in relation to APOE4 genotype or other genetic factors or biomarkers. Studies exploring broader physiological responses of HRT and its interaction with female-specific risks and factors as well as lifestyle and health factors will also be crucial in providing a clearer picture of how these factors influence dementia risk.
In conclusion, the study provides some support for the influence of HRT, menopausal age and lifestyle factors on later life cognitive function or performance of older-adult women, but no evidence that these factors influence cognitive function over time. The discrepancy between the two analytic models highlights the importance of considering the choice of outcome when interpreting the effects of various predictors on cognitive health, a possible oversight in previous work leading to mixed findings surrounding the influences of HRT history and menopausal age in female cognitive health, in particular. Overall, the pattern of our findings emphasizes the complexity in delineating the roles of HRT and reproductive histories alongside lifestyle and health variables in cognitive aging in women. Future longitudinal work is needed to further illuminate these complex relationships to optimize tailored strategies that support cognitive health in women across the menopause spectrum.
Publicly available datasets were analyzed in this study. This data can be found at: https://portal.dementiasplatform.uk/CohortDirectory/Item?fingerPrintID=CFAS%20Wales.
The studies involving humans were approved by NHS North Wales – West Research Ethics Committee (REC Ref No: 10/Wno01/37; IRAS Project No: 40092). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
TW: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SG: Methodology, Writing – review & editing. EL: Writing – review & editing. CU-M: Conceptualization, Methodology, Supervision, Writing – review & editing. GM-T: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. TW was funded by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration (ARC) North-East and North Cumbria (NENC) (NIHR200173).
SG is an employee of Scottish Brain Sciences, an independent research organization, and the University of Edinburgh. Scottish Brain Sciences was not involved in the design, analysis, drafting or review of this work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frdem.2024.1496051/full#supplementary-material
Aaron, D. J., Dearwater, S. R., Anderson, R., Olsen, T., Kriska, A. M., Laporte, R. E., et al. (1995). Physical activity and the initiation of high-risk health behaviors in adolescents. Med. Sci. Sports Exerc. 27, 1639–1645. doi: 10.1249/00005768-199512000-00010
Avis, N. E., Stellato, R., Crawford, S., Bromberger, J., Ganz, P., Cain, V., et al. (2001). Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Soc. Sci. Med. 52, 345–356. doi: 10.1016/S0277-9536(00)00147-7
Barrea, L., Pugliese, G., Laudisio, D., Savastano, S., Colao, A., Muscogiuri, G., et al. (2021). Does Mediterranean diet could have a role on age at menopause and in the management of vasomotor menopausal symptoms? The viewpoint of the endocrinological nutritionist. Curr. Opin. Food Sci. 39, 171–181. doi: 10.1016/j.cofs.2021.02.018
Bove, R., Secor, E., Chibnik, L. B., Barnes, L. L., Schneider, J. A., Bennett, D. A., et al. (2014). Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 82, 222–229. doi: 10.1212/WNL.0000000000000033
Brennan, S. E., McDonald, S., Page, M. J., Reid, J., Ward, S., Forbes, A. B., et al. (2020). Long-term effects of alcohol consumption on cognitive function: a systematic review and dose-response analysis of evidence published between 2007 and 2018. Syst. Rev. 9:33. doi: 10.1186/s13643-019-1220-4
Brothers, R. M., Fadel, P. J., and Keller, D. M. (2019). Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am. J. Physiol. 317, H777–H789. doi: 10.1152/ajpheart.00126.2019
Buckley, R. F., Mormino, E. C., Rabin, J. S., Hohman, T. J., Landau, S., Hanseeuw, B. J., et al. (2019). Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 76, 542–551. doi: 10.1001/jamaneurol.2018.4693
Burkhardt, M. S., Foster, J. K., Laws, S. M., Baker, L. D., Craft, S., Gandy, S. E., et al. (2004). Oestrogen replacement therapy may improve memory functioning in the absence of APOE ε4. J. Alzheimers Dis. 6, 221–228. doi: 10.3233/JAD-2004-6302
Clare, L., Wu, Y. T., Teale, J. C., MacLeod, C., Matthews, F., Brayne, C., et al. (2017). Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: a cross-sectional study. PLoS Med. 14:e1002259. doi: 10.1371/journal.pmed.1002259
Coker, L. H., Espeland, M. A., Rapp, S. R., Legault, C., Resnick, S. M., Hogan, P., et al. (2010). Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS). J. Steroid Biochem. Mol. Biol. 118, 304–310. doi: 10.1016/j.jsbmb.2009.11.007
Coughlan, G. T., Betthauser, T. J., Boyle, R., Koscik, R. L., Klinger, H. M., Chibnik, L. B., et al. (2023). Association of age at menopause and hormone therapy use with tau and β-amyloid positron emission tomography. JAMA Neurol. 80, 462–473. doi: 10.1001/jamaneurol.2023.0455
Coughlan, G. T., Koscik, R. L., Betthauser, T. J., Boyle, R. T., Jonaitis, E. M., Wenzel, A., et al. (2022). Menopause age and hormone therapy use moderate PET tau and amyloid association: findings from the Wisconsin Registry for Alzheimer Prevention. Alzheimers Dement. 18:e062007. doi: 10.1002/alz.062007
Demirovic, J., Prineas, R., Loewenstein, D., Bean, J., Duara, R., Sevush, S., et al. (2003). Prevalence of dementia in three ethnic groups: the South Florida program on aging and health. Ann. Epidemiol. 13, 472–478. doi: 10.1016/S1047-2797(02)00437-4
Depypere, H., Vergallo, A., Lemercier, P., Lista, S., Benedet, A., Ashton, N., et al. (2023). Menopause hormone therapy significantly alters pathophysiological biomarkers of Alzheimer's disease. Alzheimers Dement. J. Alzheimers Assoc. 19, 1320–1330. doi: 10.1002/alz.12759
Dhana, K., Aggarwal, N. T., Rajan, K. B., Barnes, L. L., Evans, D. A., Morris, M. C., et al. (2021). Impact of the apolipoprotein E ε4 allele on the relationship between healthy lifestyle and cognitive decline: a population-based study. Am. J. Epidemiol. 190, 1225–1233. doi: 10.1093/aje/kwab033
Espeland, M. A., Brinton, R. D., Hugenschmidt, C., Manson, J. E., Craft, S., Yaffe, K., et al. (2015). Impact of type 2 diabetes and postmenopausal hormone therapy on incidence of cognitive impairment in older women. Diabetes Care 38, 2316–2324. doi: 10.2337/dc15-1385
Espeland, M. A., Brunner, R. L., Hogan, P. A., Rapp, S. R., Coker, L. H., Legault, C., et al. (2010). Long term effects of conjugated equine estrogens therapies on domain-specific cognitive function: results from the Women's Health Initiative Study of Cognitive Aging (WHISCA) Extension. J. Am. Geriatr. Soc. 58, 1263–1271. doi: 10.1111/j.1532-5415.2010.02953.x
Ferretti, M. T., Iulita, M. F., Cavedo, E., Chiesa, P. A., Schumacher Dimech, A., Santuccione Chadha, A., et al. (2018). Sex differences in Alzheimer disease - the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469. doi: 10.1038/s41582-018-0032-9
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Gamble, L. D., Matthews, F. E., Jones, I. R., Hillman, A. E., Woods, B., Macleod, C. A., et al. (2022). Characteristics of people living with undiagnosed dementia: findings from the CFAS Wales study. BMC Geriatr. 22:409. doi: 10.1186/s12877-022-03086-4
García-Casares, N., Gallego Fuentes, P., Barbancho, M. Á., López-Gigosos, R., García-Rodríguez, A., Gutiérrez-Bedmar, M., et al. (2021). Alzheimer's disease, mild cognitive impairment and mediterranean diet. A systematic review and dose-response meta-analysis. J. Clin. Med. 10:4642. doi: 10.3390/jcm10204642
Gharbi-Meliani, A., Dugravot, A., Sabia, S., Regy, M., Fayosse, A., Schnitzler, A., et al. (2021). The association of APOE ε4 with cognitive function over the adult life course and incidence of dementia: 20 years follow-up of the Whitehall II study. Alzheimers Res. Ther. 13:5. doi: 10.1186/s13195-020-00740-0
Gleason, C. E., Dowling, N. M., Wharton, W., Manson, J. E., Miller, V. M., Atwood, C. S., et al. (2015). Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. 12:e1001833. discussion e1001833. doi: 10.1371/journal.pmed.1001833
Gonçalves, C., Moreira, H., and Santos, R. (2024). Systematic review of mediterranean diet interventions in menopausal women. AIMS Public Health 11, 110–129. doi: 10.3934/publichealth.2024005
Gregory, S., Booi, L., Jenkins, N., Bridgeman, K., Muniz-Terrera, G., Farina, F. R., et al. (2023a). Hormonal contraception and risk for cognitive impairment or Alzheimer's disease and related dementias in young women: a scoping review of the evidence. Front. Glob. Womens Health 4:1289096. doi: 10.3389/fgwh.2023.1289096
Gregory, S., Ntailianis, G., Shannon, O., Stevenson, E., Ritchie, C., Wells, K., et al. (2023b). The Mediterranean diet is associated with better cardiometabolic health for women in mid-life but not men: A PREVENT dementia cohort cross-sectional analysis. Nutr. Metab. Cardiovasc. Dis. 33, 2251–2260. doi: 10.1016/j.numecd.2023.07.020
Guland, B., Wilder, D., Lantigua, R., Mayeux, R., Stern, Y., Chen, J., et al. (1997). “Differences in rates of dementia between ethno-racial groups,” in Racial and Ethnic Differences in the Health of Older Americans. National Academies Press (US). Available at: https://www.ncbi.nlm.nih.gov/books/NBK109847/ (accessed September 11, 2024).
Harlow, S. D., Gass, M., Hall, J. E., Lobo, R., Maki, P., Rebar, R. W., et al. (2012). Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19, 387–395. doi: 10.1097/gme.0b013e31824d8f40
Hogervorst, E., Williams, J., Budge, M., Riedel, W., and Jolles, J. (2000). The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience 101, 485–512. doi: 10.1016/S0306-4522(00)00410-3
Howell, J. C., Watts, K. D., Parker, M. W., Wu, J., Kollhoff, A., Wingo, T. S., et al. (2017). Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res. Ther. 9:88. doi: 10.1186/s13195-017-0315-1
Jett, S., Schelbaum, E., Jang, G., Boneu Yepez, C., Dyke, J. P., Pahlajani, S., et al. (2022). Ovarian steroid hormones: a long overlooked but critical contributor to brain aging and Alzheimer's disease. Front. Aging Neurosci. 14:948219. doi: 10.3389/fnagi.2022.948219
Jia, F., Liu, F., Li, X., Shi, X., Liu, Y., Cao, F., et al. (2021). Cognitive reserve, modifiable-risk-factor profile and incidence of dementia: results from a longitudinal study of CFAS Wales. Aging Ment. Health 25, 2286–2292. doi: 10.1080/13607863.2020.1828270
Kukull, W. A., Larson, E. B., Teri, L., Bowen, J., McCormick, W., Pfanschmidt, M. L., et al. (1994). The Mini-Mental State Examination score and the clinical diagnosis of dementia. J. Clin. Epidemiol. 47, 1061–1067. doi: 10.1016/0895-4356(94)90122-8
Kulminski, A. M., Shu, L., Loika, Y., Nazarian, A., Arbeev, K., Ukraintseva, S., et al. (2020). APOE region molecular signatures of Alzheimer's disease across races/ethnicities. Neurobiol. Aging 87, 141.e1–141.e8. doi: 10.1016/j.neurobiolaging.2019.11.007
Lee, J. K., Frank, R. D., Christenson, L. R., Fields, J. A., Rocca, W. A., Mielke, M. M., et al. (2024). Associations of reproductive factors and exogenous estrogens with global and domain-specific cognition in later life. Alzheimers Dement. J. Alzheimers Assoc. 20, 63–73. doi: 10.1002/alz.13394
Liao, H., Cheng, J., Pan, D., Deng, Z., Liu, Y., Jiang, J., et al. (2023). Association of earlier age at menopause with risk of incident dementia, brain structural indices and the potential mediators: a prospective community-based cohort study. EClinicalMedicine 60:102033. doi: 10.1016/j.eclinm.2023.102033
Lindseth, L. R. S., de Lange, A. M. G., van der Meer, D., Agartz, I., Westlye, L. T., Tamnes, C. K., et al. (2023). Associations between reproductive history, hormone use, APOE ε4 genotype and cognition in middle- to older-aged women from the UK Biobank. Front. Aging Neurosci. 14:1014605. doi: 10.3389/fnagi.2022.1014605
Livingston, G., Huntley, J., Liu, K. Y., Costafreda, S. G., Selbæk, G., Alladi, S., et al. (2024). Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 404, 572–628. doi: 10.1016/S0140-6736(24)01296-0
Lyall, D. M., Celis-Morales, C., Lyall, L. M., Graham, C., Graham, N., Mackay, D. F., et al. (2019). Assessing for interaction between APOE ε4, sex, and lifestyle on cognitive abilities. Neurology 92, e2691–e2698. doi: 10.1212/WNL.0000000000007551
Lyall, D. M., Ward, J., Ritchie, S. J., Davies, G., Cullen, B., Celis, C., et al. (2016). Alzheimer disease genetic risk factor APOE e4 and cognitive abilities in 111,739 UK Biobank participants. Age Ageing 45, 511–517. doi: 10.1093/ageing/afw068
Maki, P. M., and Sundermann, E. (2009). Hormone therapy and cognitive function. Hum. Reprod. Update 15, 667–681. doi: 10.1093/humupd/dmp022
Mielke, M. M. (2018). Sex and gender differences in Alzheimer's disease dementia. Psychiatr. Times 35, 14–17.
Mills, Z. B., Faull, R. L. M., and Kwakowsky, A. (2023). Is hormone replacement therapy a risk factor or a therapeutic option for Alzheimer's disease? Int. J. Mol. Sci. 24:3205. doi: 10.3390/ijms24043205
Nerattini, M., Jett, S., Andy, C., Carlton, C., Zarate, C., Boneu, C., et al. (2023). Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer's disease and dementia. Front. Aging Neurosci. 15:1260427. doi: 10.3389/fnagi.2023.1260427
Park, H. K., Marston, L., and Mukadam, N. (2024). The effects of estrogen on the risk of developing dementia: a cohort study using the UK biobank data. Am. J. Geriatr. Psychiatry 32, 792–805. doi: 10.1016/j.jagp.2024.01.025
Phung, T. K. T., Waltoft, B. L., Laursen, T. M., Settnes, A., Kessing, L. V., Mortensen, P. B., et al. (2010). Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement. Geriatr. Cogn. Disord. 30, 43–50. doi: 10.1159/000314681
Radd-Vagenas, S., Duffy, S. L., Naismith, S. L., Brew, B. J., Flood, V. M., Fiatarone Singh, M. A., et al. (2018). Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am. J. Clin. Nutr. 107, 389–404. doi: 10.1093/ajcn/nqx070
Ran, L. S., Liu, W. H., Fang, Y. Y., Xu, S. B., Li, J., Luo, X., et al. (2021). Alcohol, coffee and tea intake and the risk of cognitive deficits: a dose-response meta-analysis. Epidemiol. Psychiatr. Sci. 30:e13. doi: 10.1017/S2045796020001183
Riedel, B. C., Thompson, P. M., and Brinton, R. D. (2016). Age, APOE and sex: triad of risk of Alzheimer's disease. J. Steroid Biochem. Mol. Biol. 160, 134–147. doi: 10.1016/j.jsbmb.2016.03.012
Rispo, F., De Negri Atanasio, G., Demori, I., Costa, G., Marchese, E., Perera-del-Rosario, S., et al. (2024). An extensive review on phenolic compounds and their potential estrogenic properties on skin physiology. Front. Cell Dev. Biol. 11:1305835. doi: 10.3389/fcell.2023.1305835
Rocca, W. A., Bower, J. H., Maraganore, D. M., Ahlskog, J. E., Grossardt, B. R., de Andrade, M., et al. (2007). Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69, 1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6
Rocca, W. A., Grossardt, B. R., and Shuster, L. T. (2014). Oophorectomy, estrogen, and dementia: A 2014 update. Mol. Cell. Endocrinol. 389, 7–12. doi: 10.1016/j.mce.2014.01.020
Rocca, W. A., Kantarci, K., and Faubion, S. S. (2024). Risks and benefits of hormone therapy after menopause for cognitive decline and dementia: a conceptual review. Maturitas 184:108003. doi: 10.1016/j.maturitas.2024.108003
Roth, M., Tym, E., Mountjoy, C. Q., Huppert, F. A., Hendrie, H., Verma, S., et al. (1986). CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br. J. Psychiatry J. Ment. Sci. 149, 698–709. doi: 10.1192/bjp.149.6.698
Ryan, J., Carrière, I., Scali, J., Dartigues, J. F., Tzourio, C., Poncet, M., et al. (2009). Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology 73, 1729–1737. doi: 10.1212/WNL.0b013e3181c34b0c
Saleh, R. N. M., Hornberger, M., Ritchie, C. W., and Minihane, A. M. (2023). Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer's Disease (EPAD) cohort. Alzheimers Res. Ther. 15:10. doi: 10.1186/s13195-022-01121-5
Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., et al. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289, 2651–2662. doi: 10.1001/jama.289.20.2651
Shumaker, S. A., Reboussin, B. A., Espeland, M. A., Rapp, S. R., McBee, W. L., Dailey, M., et al. (1998). The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control. Clin. Trials 19, 604–621. doi: 10.1016/S0197-2456(98)00038-5
Siervo, M., Shannon, O. M., Llewellyn, D. J., Stephan, B. C., and Fontana, L. (2021). Mediterranean diet and cognitive function: From methodology to mechanisms of action. Free Radic. Biol. Med. 176, 105–117. doi: 10.1016/j.freeradbiomed.2021.09.018
Stampfer, M. J., Kang, J. H., Chen, J., Cherry, R., and Grodstein, F. (2005). Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 352, 245–253. doi: 10.1056/NEJMoa041152
Szmidt, M. K., Granda, D., Madej, D., Sicinska, E., and Kaluza, J. (2023). Adherence to the mediterranean diet in women and reproductive health across the lifespan: a narrative review. Nutrients 15:2131. doi: 10.3390/nu15092131
The Medical Research Council Cognitive Function and Ageing Study (1998). Cognitive function and dementia in six areas of England and Wales: the distribution of MMSE and prevalence of GMS organicity level in the MRC CFA Study. Psychol. Med. 28, 319–335. doi: 10.1017/S0033291797006272
Thurston, R. C., and Joffe, H. (2011). Vasomotor symptoms and menopause: findings from the study of women's health across the nation. Obstet. Gynecol. Clin. N Am. 38, 489–501. doi: 10.1016/j.ogc.2011.05.006
Udeh-Momoh, C., Maina, R. W., Bosire, E. N., Khakali, L. N., Gregory, S., Blackmon, K., et al. (2024). “The Female Brain Health and Endocrine Research in Africa Study (FemBER-AFRICA): identifying endocrinological, lifestyle, psychosocial and socio-cultural targets for Alzheimer's disease prevention in women of African ancestry,” in Alzheimer's Association International Conference.
Udeh-Momoh, C., and Watermeyer, T. (2021). Female specific risk factors for the development of Alzheimer's disease neuropathology and cognitive impairment: call for a precision medicine approach. Ageing Res. Rev. 71:101459. doi: 10.1016/j.arr.2021.101459
Watermeyer, T., Atkinson, E., Howatson, G., McGill, G., Dodds, C., Ansdell, P., et al. (2024). Female Brain and Endocrinological Research – Veteran (FemBER-Vet) Study: A Study Protocol for Identifying Endocrinological, Lifestyle and Psychosocial Determinants of Brain Health Outcomes in Female Veterans for Future Intervention Success. Available at: https://europepmc.org/article/PPR/PPR867558 (accessed September 12, 2024).
Wood Alexander, M., Wu, C. Y., Coughlan, G. T., Puri, T., Buckley, R. F., Palta, P., et al. (2024). Associations between age at menopause, vascular risk, and 3-year cognitive change in the Canadian longitudinal study on aging. Neurology 102:e209298. doi: 10.1212/WNL.0000000000209298
Xiong, C., Luo, J., Coble, D., Agboola, F., Kukull, W., Morris, J. C., et al. (2020). Complex interactions underlie racial disparity in the risk of developing Alzheimer disease dementia. Alzheimers Dement. J. Alzheimers Assoc. 16, 589–597. doi: 10.1002/alz.12060
Keywords: hormone replacement therapy (HRT), menopausal age, cognition, APOE4, cognitive aging, lifestyle factors, postmenopausal women, comorbidities
Citation: Watermeyer TJ, Gregory S, Leetham E, Udeh-Momoh CT and Muniz-Terrera G (2025) Hormone replacement therapy, menopausal age and lifestyle variables are associated with better cognitive performance at follow-up but not cognition over time in older-adult women irrespective of APOE4 carrier status and co-morbidities. Front. Dement. 3:1496051. doi: 10.3389/frdem.2024.1496051
Received: 13 September 2024; Accepted: 19 December 2024;
Published: 17 January 2025.
Edited by:
Ayse Kuspinar, McMaster University, CanadaReviewed by:
Claudia Duran-Aniotz, Adolfo Ibáñez University, ChileCopyright © 2025 Watermeyer, Gregory, Leetham, Udeh-Momoh and Muniz-Terrera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamlyn J. Watermeyer, dGFtbHluLndhdGVybWV5ZXJAbm9ydGh1bWJyaWEuYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.