
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Dement. , 15 January 2024
Sec. Genetics and Biomarkers of Dementia
Volume 2 - 2023 | https://doi.org/10.3389/frdem.2023.1320329
APOE-ε4 allele[s] is a risk factor for Alzheimer's disease (AD) and Amyloid-Related Imaging Abnormalities (ARIA) in anti-amyloid beta therapy, and is also associated with cerebrovascular risk factors such as hyperlipidemia or atherosclerosis. During AD clinical trials, APOE-ε4 carriers may experience neuropsychiatric adverse events (AEs) related to these risks, complicating the differentiation of ARIA from cerebrovascular events based on symptoms. This study aimed to examine the hypothetical impact of considering the APOE-ε4 allele's risk for non-ARIA AEs during AD clinical trials. We used data from the Critical Path for Alzheimer's Disease (CPAD) from the placebo arm of randomized controlled trials (RCT) for AD treatment. We determined whether AEs were reported more frequently in APOE-ε4 carriers, quantifying with reporting odds ratio (ROR) using a mixed effect model. We also evaluated the association between ROR levels and the prior probability that an AE is symptomatic ARIA. We analyzed 6,313 patients with AD or mild cognitive impairment in 28 trials. Of the prespecified 35 neuropsychiatric or related AEs, several had a significantly high ROR: “delusion” (ROR = 4.133), “confusional state” (ROR = 1.419), “muscle spasms” (ROR = 9.849), “irritability” (ROR = 12.62), “sleep disorder” (ROR = 2.944), or “convulsion” (ROR = 13.00). However, none remained significant after adjusting for Mini-Mental State Examination scores. There is no strong evidence to suggest that specific neuropsychiatric AEs occur more frequently without drug treatment association among APOE-ε4 carriers. The influence of APOE-ε4 allele[s] on the clinicians' assessment of the likelihood of ARIA during safety monitoring in anti-amyloid beta monoclonal antibody treatment might be unchanged, thus maintaining the current level of awareness of clinicians of AEs.
APOE-ε4 allele[s] is associated with a higher risk of developing Alzheimer's disease (AD) (Lumsden et al., 2020) and its earlier onset, making it a critical focus in observational studies on AD or dementia. It is also associated with an increased risk of developing Amyloid-Related Imaging Abnormalities (ARIA) in clinical trials involving anti-amyloid beta therapy, which is a disease-modifying therapy (DMT) aimed at treating or preventing AD (Sperling et al., 2011; Cummings et al., 2021; Barakos et al., 2022). Notable examples of these therapies include aducanumab (Cummings et al., 2021) and lecanemab (Cummings et al., 2023), which have recently been approved. Consequently, the APOE genotype is of considerable importance to clinicians and investigators during safety monitoring in clinical trials or clinical practice.
ARIA is typically classified into two main types based on imaging findings: ARIA-H (hemorrhage) and ARIA-E (edema/effusion), and they can coexist with each other. ARIA-H includes microhemorrhages and superficial siderosis, manifested as hypointense foci on T2*-weighted gradient-recalled echo and susceptibility-weighted (SWI) magnetic resonance imaging (MRI) scans (Roytman et al., 2023). ARIA-E represents vasogenic edema or sulcal effusion, identified by hyperintensities on fluid-attenuated inversion recovery (FLAIR) MRI scans (Roytman et al., 2023). While many ARIA findings are asymptomatic, some present with specific or non-specific symptoms such as headahe, confusion, nausea, visual disturbanes, or seizures (Cummings et al., 2023). Routine MRI scans need to be scheduled for patients who are to receive aducanumab or lecanemab treatment to detect even asymptomatic ARIA at the appropriate time.
Furthermore, the presence of the APOE-ε4 allele[s] has been reported to increase the risk of various disease statuses, such as hyperlipidemia (HL) (e.g., weighted mean difference +0.1~0.3 mmol/L compared to those with ε3/ε3) (Bennet et al., 2007; Khan et al., 2013), increased carotid artery intima-media thickness (e.g., by +0.3 mm compared to those with ε3) (Elosua et al., 2004), carotid and coronary atherosclerosis (Granér et al., 2008), increased white matter lesions (de Leeuw et al., 2004), or increased risk of coronary diseases (e.g., odds ratio 1.06 compared to those with ε3/ε3) (Bennet et al., 2007). These conditions are also risk factors for cerebrovascular diseases. Despite inconsistent findings in earlier studies, ischemic stroke has also been associated with the APOE-ε4 allele[s] (e.g., odds ratio of approximately 2 compared to those with ε3) (Kokubo et al., 2000; MacLeod et al., 2001; Abboud et al., 2008; Chen and Hu, 2016). In light of this, clinical trial participants with APOE-ε4 allele[s] may experience additional adverse events (AEs) related to cerebrovascular diseases (Tai et al., 2016), a risk that remains regardless of their group allocation in the trials.
This risk of cerebrovascular or other AEs due to APOE-ε4, which is referred to here as the “inherent risk of APOE-ε4,” has not been seriously recognized. However, it may pose challenges to clinical inference during safety monitoring in trials or clinical practice of DMT drugs. This is due to the wide variety of ARIA symptoms that can mimic cerebrovascular events or other neuropsychiatric symptoms and vice versa. For example, suppose that there is a participant with APOE-ε4 allele[s] who has recently developed symptomatic AE that is typically observable as one of the ARIA symptoms (e.g., seizure or confusion) during a randomized controlled trial (RCT); elective MRI scans may be required even if it is out-of-schedule. Meanwhile, when there is a participant with APOE-ε4 allele[s] who has recently developed a symptomatic AE that is not typical as one of the ARIA symptoms (e.g., fatigue or tremor), it is up to the clinicians to decide whether to perform MRI scans. Furthermore, suppoe there is a patient with APOE-ε4 allele[s] who has recently developed symptomatic AE that may be an ARIA symptom but is highly suggestive of acute ischemic stroke (e.g., hemiplegia and dysarthria); in this case, urgent brain imaging may be required immediately.
If such AE terms actually turned out to be highly reported in clinical trials in association with having APOE-ε4 allele[s] itself, regardless of its actual causality, the AE observed in participants with APOE-ε4 allele[s] shall be less attributed to ARIA compared to the conventional assumption in which the “inherent AE risk of APOE-ε4 allele[s]” is not considered or ignored. This means that clinicians' assessment of the need to perform brain imaging for ARIA detection can be influenced in the case of such AEs.
Therefore, understanding the degree of contribution of having APOE-ε4 allele[s] to the observed reporting of AEs, including cerebrovascular symptoms during clinical trials, might be informative for clinicians and investigators of clinical trials for AD, in a viewpoint to help distinguish some potentially confusing AEs from the symptoms due to ARIA. This could be especially true in some trials designed to concentrate participants with APOE-ε4 allele[s] (Vandenberghe et al., 2016). In this study, we evaluated these points using a large database of data collected from a placebo arm of RCT for the treatment of AD.
This retrospective study analyzed publicly available databases and was approved by our institutional review board. Informed consent was not obtained from participants in this study. The purpose of this study is to measure the degree of contribution of having APOE-ε4 allele[s] to the reporting of AEs, including cerebrovascular symptoms during clinical trials, discussing the actual necessity of considering such APOE-ε4-related risk of AEs in safety monitoring. For this purpose, we will utilize data from the placebo arms of earlier RCT for AD. By using data from placebo groups, we can avoid the need to consider the potential confounding effects of the adiministered active agents on the observed AEs, which could otherwise complicate the discussion.
First, we will present rationales for our purpose in the following formulations, in terms of the above consideration of the inherent APOE-ε4-related risk of AE involved in the safety monitoring—symptom-based vigilance of ARIA. The data used will also be described in this section.
First, consider a participant with APOE-ε4 allele[s] who recently developed a particular AE (referred to here as “AEX”), which may or may not be attributed to ARIA in the brain. Examples of AEx include headaches, nausea, and dizziness (Sperling et al., 2011; Barakos et al., 2022). These events occurred during an RCT in which participants with mild cognitive impairment (MCI) or AD, with or without APOE-ε4 allele[s], are randomly assigned to an active arm (e.g., receiving anti-amyloid monoclonal antibody) or a placebo arm in an arbitrary proportion. At the end of the trial, all participants were classified into two AE types (Table 1): (a) cases who had ever reported an AEX during the trial and (b) cases who had never reported an AEX.
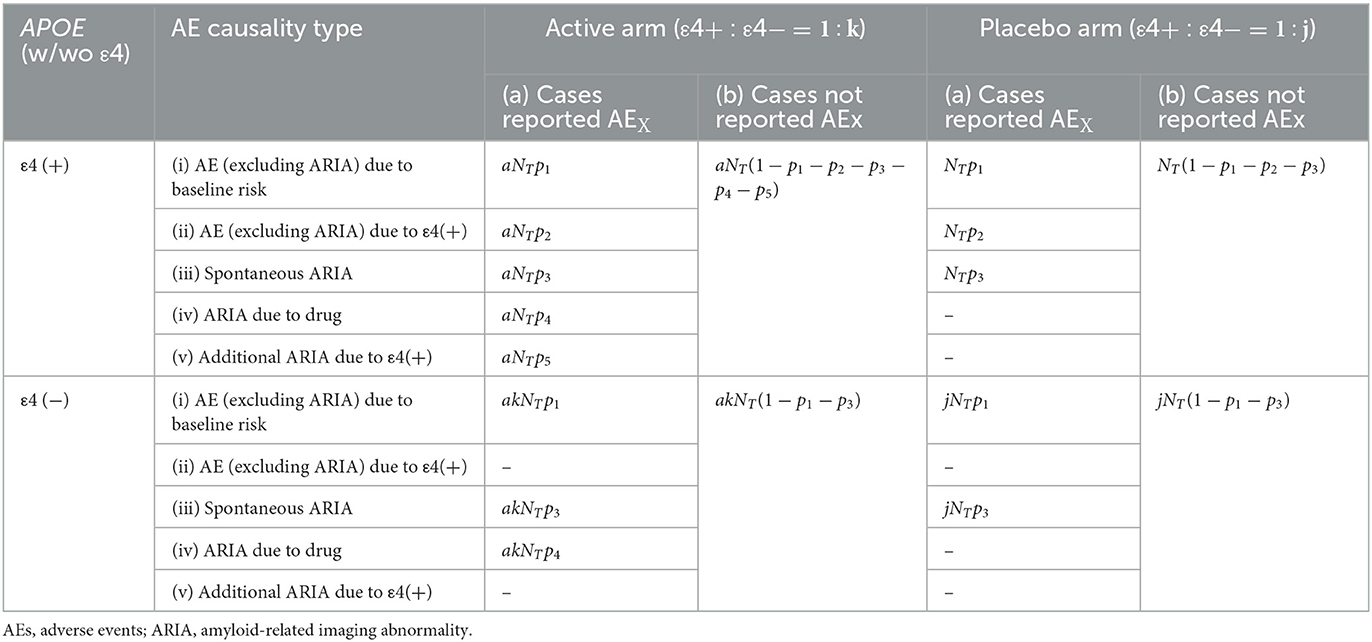
Table 1. Classification of participants and their frequency by trial arm, APOE status, and AE reporting.
Participants in the active arm and fall under the AE-type (a) are further subdivided based on a the hypothetical assumption that the causes of the AEX can be categorized into one of five causality types: (i) AEs attributed to baseline risk due to age or comorbidities; (ii) additional AEs (other than ARIA) attributed to having APOE-ε4 allele[s]; (iii) spontaneous ARIA observed regardless of the use of DMT drugs (Antolini et al., 2021); (iv) symptomatic ARIA induced by the use of DMT; and (v) additional symptomatic ARIA as a result of interaction between the anti-amyloid monoclonal antibody and having APOE-ε4 allele[s] (Sperling et al., 2011; Barakos et al., 2022). Please note that participants who did not report AEX during the clinical trial must be categorized as AE-type (b), even if they had unrecognized “asymptomatic” ARIA in their brains.
We suppose that the total number of trial participants in ε4 (+)-placebo arm is NT, the total number of trial participants in ε4 (+)-active arm is aNT, and the proportion of participants who are classified as causality-type (i)–(v) in AE-type (a) is p1 ~ p5 (0 ≤ pi ≤ 1 for i = 1 to 5, 0 ≤ p1 + p2 + p3 + p4 + p5 ≤ 1). The number of remaining participants with AE-type (b) in the active arm is aNT(1 − p1 − p2 − p3 − p4 − p5).
The total number of trial participants in ε4(–) arms is determined to akNT (k ≥ 0) in the active arm and jNT (j ≥ 0) in the placebo arm. AEs observed in the placebo arms are limited to AE-types (i), (ii), or (iii) because no active drugs are administered. In contrast, the AEs observed in ε4(–) arms should be AE-types (i), (iii), or (iv), thus defining the numbers in the left cells accordingly. Under the traditional assumption, which does not account for or neglect the inherent risk of AE due to APOE-ε4, AE-type (ii) becomes negligible, implying p2 = 0 in Table 1. It is important to emphasize that the causality-type categorizations (i)–(v) are theoretical and are intended to explain the rationale of our study. This classification is practically impossible; thus, it is not applied to the actual data analysis.
Based on the contingency table (Table 1), our goal is to estimate how many of the presented AEX are attributable to ARIA in the context of safety monitoring. Specifically, we calculate the “positive predictive value (PPV)” of AE-based vigilance for ARIA. PPV is obtained using the following formula, which varies depending on whether the APOE-ε4 status of each participant is known to clinicians:
• PPV in a group of which participants are known to have APOE-ε4 allele[s]:
• PPV in a group of which participants are known not to have APOE-ε4 allele[s]:
• PPV in a group in which the participants' APOE status is unknown to investigators or clinicians:
We define p2 = r(p1+p3) (r ≥ 0) for ease of subsequent calculations, in which r = 0 corresponds to the conventional assumption where inherent AE risk due to APOE-ε4 is not considered or is ignored. By incorporating the variable “r,” the degree of inherent risk of AE due to APOE-ε4 can be captured as a ratio to the baseline risk of AE not related to APOE-ε4. In the above equations, a, k, j, and p1-p5 depend on the background population of the study participants, as well as the characteristics of the administered drugs; therefore, we regard them other than r (and p2) as constant. The ratio of the PPVs with respect to those at r = 0 (= PPVr = 0) are as follows:
•
•
•
All PPV ratios are equal to 1 when r = 0. Because of the underlined terms, both PPV Ratio1 and Ratio3 monotonically decrease as r increases (r ≥ 0). This means that, in a trial group consisting of participants with APOE-ε4(+) or in a group where the APOE-ε4 status of participants is unknown, the PPV for predicting the likelihood of ARIA in participants reporting the AEX will invariably decrease to an uncertain extent when r > 0, compared to r = 0.
We turn our attention to Table 2, which is derived from the placebo arms presented in Table 1. The items in Table 2 are condensed into a 2-by-2 contingency table. This is done by grouping all participants in the placebo arm based on whether they reported AEX (either with or without) and on their status of APOE (with or without ε4 allele[s]). Subsequently, the association of AE development with the APOE-ε4 status can be quantified as the reporting odds ratio (ROR). This metric is commonly used in pharmacovigilance studies (Sato et al., 2020) and is calculated using the following formula with the values of NA-ND in Table 2:
•
A significantly high ROR means that AE is reported more frequently among individuals with APOE-ε4 allele[s] compared to those without it, regardless of a direct causal relationship. Because the ROR reflects only reported AEs, it is susceptible to reporting bias. Consequently, the ROR differs from the OR typically measured in observational studies. The ROR was calculated using the values in Table 2 as follows:
•
Because p2 = r(p1+p3) and therefore p1+p2+p3 = (1+r)(p1+p3 ),
•
We can ascertain that ROR = 1 when r = 0.
Because (r > 0), the ROR exhibits a monotonic increase along with the increasing r (r > 0). If we assume that (p1+p3) is sufficiently small that it can be approximated as 1−(p1+p3) ≅ 1, then above ROR can be further simplified as follows:
• ROR ≅ 1+r.
Because the PPV ratio and r mentioned above cannot be directly obtained from actual data, we relied on another reference point: ROR = 1. When analyzing a specific AE with ROR > 1, it is inferred that r > 0 for that AE. Concurrently, the PPV ratio of the corresponding AE should be lower than that at r = 0. Specifically, discovering an AE with an ROR significantly >1 through statistical analysis of the actual data indicates a non-negligible r value (i.e., greater than zero). Consequently, the AE-based PPV should be lower than it would be under the conventional assumption where the inherent risk of APOE-ε4 is not considered or is ignored, regardless of the p1 or p3 values. It should be noted that, in this approach, determining the actual degree of PPV decline remains elusive as it depends on the values of p2, p4, and p5. Despite this limitation, this methodology can offer valuable insights for clinicians and help to identify AEs that require less vigilance when monitoring ARIA.
We used data from the Critical Path for Alzheimer's Disease (CPAD) (Ito et al., 2013; Neville et al., 2015; Arnerić et al., 2018). This dataset comprises thousands of participants with AD or MCI from the placebo arms of numerous RCTs aimed at AD treatment. The names of the administered drugs or trials have not been disclosed. Most participants were diagnosed with AD at baseline, while a small proportion had MCI (c.f., PRIMARY DIAGNOSIS in the data file named “MH”). The diagnostic criteria for AD or MCI are uncertain and may vary across studies, and we assumed that many of the enrolled participants were clinically diagnosed with AD as defined by the NINCDS-ADRDA criteria (McKhann et al., 1984). We also retrieved baseline data on the use of symptomatic anti-dementia drugs prior to the beginning of trials, including donepezil, galantamine, rivastigmine, and memantine. The severity of AD or cognitive scores, such as Mini-Mental State Examination (MMSE) at baseline, was provided only in a subset of the included studies (c.f., the variable QSSTRESC in the data file named “QS”).
AEs that appeared in the early phase would hardly be ARIA, as most of the development of ARIA was observed in aducanumab or lecanemab trials 8–9 weeks after the beginning of drug administration (Barakos et al., 2022; Honig et al., 2023). Consequently, we excluded AEs that emerged 4 weeks after the start of the study from the analysis. We included AEs of any severity, although ARIA symptoms observed in the aducanumab or lecanemab trials were reportedly mostly mild (Barakos et al., 2022; Honig et al., 2023).
We want to obtain the ROR of having APOE-ε4 to the reporting/development of AE (in binary: with/without) while adjusting for age at baseline, sex, and other variables. Among the reported AEs, we arbitrarily selected 35 that may be associated with neuropsychiatric or cerebrovascular symptoms. The 35 AEs are listed in Supplementary Table 1. Among them, we analyzed AEs, the frequency of which was ≥10 in the examined data.
We used a mixed effects model (Bates et al., 2015) by appointing the individual study ID as a random intercept because the CPAD database used is the aggregated data of different RCTs with which the inclusion criteria, background population, length of period, or administered drugs differ one by one, and there should be some heterogeneity in the AE reports of each RCT study. The equations for the generalized linear mixed model are as follows:
• Model (1): log(Odds) = β0 + Age·β1 + Sex·β2 + APOE(e4)·β3 + Drug·β4 + Diagnosis·β5 + Interaction(age × APOE)·β6 + Study·γ0
• Model (2): log(Odds) = β0 + Age·β1 + Sex·β2 + APOE(e4)·β3 + Drug·β4 + Diagnosis·β5 + Interaction(age × APOE)·β6 + MMSE·β7 + Study·γ0
where β0 is the fixed intercept, Age is the age of participants at index starting of the trial, Sex is a binary variable (male or female), APOE(ε4) is a binary variable whether each participant has APOE-ε4 allele[s], Drug refers to a binary variable showing a medical history of taking any drugs related to dementia treatment (i.e., donepezil, galantamine, rivastigmine, or memantine), Diagnosis refers to binary variable showing diagnosis of dementia of each participant, MMSE refers to baseline MMSE total score of each participant, and γ0 denotes random intercept by each study (Sato et al., 2021). We included the interaction term between age and APOE genotype to separately analyze APOE-related amyloid burden, which should be exacerbated with age. In Model (2), we included MMSE scores, as different cognitive statuses may lead to different symptoms, represented as AEs. As underlined in the formulas, β3 is the coefficient we want to obtain. When the lower 95% confidence interval (CI) of the exp(β3) is higher than 1, the ORAEX is considered significantly high.
A total of 28 clinical trial data were included in the eligible CPAD data, with 6,313 participants (Supplementary Table 2). Approximately 85% of the participants had AD at baseline and the remaining had MCI. Their median age was 75.0 years old (95% CI, 69.0–80.0), and 56.2% of them were female. Among all participants, 24.4% had one or two APOE-ε4 allele[s], and those with APOE-ε4 allele[s] were slightly older than those without them (median 75.0 years old vs. 74.0 years old, p = 0.001, Wilcoxon rank sum test), while no association was observed between the frequency of sex and APOE-ε4 allele[s] (p = 0.097, chi-square test).
In addition, we summarized the basic characteristics of the subgroups in models (1) and (2) (Supplementary Table 3). In summary, the cases included in model (2) were more prevalent in having APOE-e4 carriers (24.4% vs. 47.7%) and had a primary diagnosis of AD (86.1% vs. 100%) compared to those in model (1). The MMSE scores for cases in model (2) had a median of 20 (IQR: 19 ~ 22).
Among the 35 prespecified AEs examined (Supplementary Table 1), 29 AEs with a frequency of ≥10 within the examined data were examined with Model (1), and only 15 AEs with a frequency of ≥10 within the examined data were examined with Model (2). Please note that Model (2) requires the MMSE score so that participants whose MMSE was not recorded were excluded from the analysis. Some AEs such as “delusion” (ROR = 4.133), “confusional state” (ROR = 1.419), “muscle spasms” (ROR = 9.849), “irritability” (ROR = 12.62), “sleep disorder” (ROR = 2.944), and “convulsion” (ROR = 13.00) were identified as those with significantly high ROR (lower 95% >1) by Model (1). However, none of them were confirmed to be significant when examined using Model (2) (Table 3).
In this study, we presented a hypothetical formulation to examine how the “inherent APOE-ε4 related risk of AE” may potentially influence the symptom-based vigilance of ARIA during safety monitoring. We then assessed the degree of impact using actual data analysis. As a result, regardless of their causal relationship, we found that certain neuropsychiatric AEs, namely “delusion,” “confusional state,” “muscle spasms,” “irritability,” “sleep disorder,” or “convulsion,” may be reported more frequently by individuals with the APOE-ε4 allele[s] among placebo arm during RCTs [Table 3, Model (1)]. However, these findings were not consistent when adjusted for baseline MMSE scores [Table 3, Model (2)].
Although the cases included in model (2) were more prevalent in having APOE-e4 carriers and had a primary diagnosis of AD compared to those in model (1), the differences in the characteristics of the participants included in the models (1) and (2) do not undermine our conclusion; instead, they contribute to enhancing its robustness. Furthermore, although the cases included in model (2) (i.e., MMSE median 20) may be slightly more severe than those for whom currently available DMT drug such as lecanemab is typically indicated (e.g., MMSE 22–30) (Cummings et al., 2023), we consider this justifiable since such a level of change in MMSE might be observed over the course of the disease, even in those who began treatment with lecanemab.
Collectively, these results suggest that there is currently no solid statistical evidence indicating that some neuropsychiatric AEs are more likely to be reported by individuals with the APOE-ε4 allele[s] during RCTs solely in association with the APOE-ε4 allele[s] but not with the development of ARIA. According to our formulation, this means that there is no reliable AE whose r > 0, then it is implied that AEs due to APOE-ε4 by itself might not influence investigators to consider the probability of being ARIA in safety monitoring during clinical trials with anti-amyloid beta monoclonal antibodies (i.e., PPVratio = 1). The level of alertness required for clinicians to these AEs might be unchanged even when considering the inherent APOE-ε4-related risk of AE after all.
The unique contribution of our study lies in its focus on the AE risk associated solely with APOE-ε4, a factor that has often been overlooked. In clinical trials involving anti-amyloid monoclonal antibody medications, the predominant concern has been the interactive risk of ARIA with APOE-ε4: the development of ARIA is a significant safety concern when monitoring anti-amyloid monoclonal antibodies (Sperling et al., 2011; Cummings et al., 2021, 2023; Barakos et al., 2022). Although asymptomatic ARIA occurs more frequently than symptomatic ARIA, many cases of symptomatic ARIA have been reported to be mild (Barakos et al., 2022). Regular MRI evaluations are typically set on predefined schedules (Cummings et al., 2021, 2023), but additional MRI scans may be warranted, especially when the observed AEs resemble ARIA symptoms during trials. PPV evaluated in this study served as a metric to measure the likelihood of ARIA in these clinical scenarios. In this study, we confirmed that clinicians' judgment on the need for brain imaging to detect ARIA remains unchanged.
The sensitivity and specificity to identify ARIA among individuals with certain AEs should be considered instead of PPV, as in this study. However, because not all ARIAs are detected/reported in clinical trials due to the varying frequency of MRI assessments, it is challenging to compare the development of AEs between participants with and without ARIA. Furthermore, asymptomatic ARIA cannot be captured using our current AE-based approach. Therefore, we chose not to use sensitivity or specificity as metrics to measure the utility of our approach. In future research, it will be essential to obtain sensitivity, specificity, or other classification metrics for individual AEs when predicting symptomatic ARIA, especially if evidence is available on the timing of ARIA development for each anti-amyloid agent or consistent MRI scheduling protocols.
There are some limitations to this study. In particular, we at first wanted to incorporate variables associated with the severity of AD pathology because such variables might also be associated with the development of ARIA or related AEs. However, they were not available in the used data. Instead, we referred to MMSE, a cognitive score, as a surrogate variable which has correspondence with the clinical severity of AD. Incorporating MMSE scores as a result greatly reduced the number of cases available for analysis [n = 6,313 in Model (1) and n = 1,303 in Model (2)]. Participants were clinically diagnosed with AD or MCI; however, they were not always diagnosed based on specific AD biomarkers. The prevalence or degree of AD pathology shall be higher in a subgroup with APOE-ε4 allele[s] than in those without, which means that the ROR of AEs measured in this study may partially reflect the effect of developing AE by mixed-in non-AD pathology or by the degree of amyloid or tau burden (Sato et al., 2019). We attempted to ameliorate this potential confounding by including an interaction term between age and APOE genotype, although we could not consider other factors, such as hypertension or diabetes mellitus, that can exacerbate cerebrovascular damage due to APOE-ε4 (Tai et al., 2016) in the models because of the lack of these variables.
In future studies, replicating the current results could be beneficial using datasets from the placebo arms of RCTs for various anti-amyloid drugs similar to those of the CPAD, such as the YODA Project (https://yoda.yale.edu), which includes the placebo arms from two RCTs of bapineuzumab, one of the anti-amyloid drugs for AD. In future, we would like to conduct a comprehensive validation study, including the placebo arms of RCTs for several kinds of anti-amyloid drugs, as soon as more RCT data becomes publicly available.
Furthermore, although we examined the association between having one or more APOE-ε4 alleles and the development of AEs, the association between possessing two APOE-ε4 alleles and the subsequent development of AEs also requires evaluation. This is because the frequency of ARIA increases significantly in individuals homozygous for the APOE-ε4 allele when administered anti-amyloid drugs, which results in a matter of critical decision-making regarding the initiation of treatment with DMTs for those carrying the APOE-ε4 homozygous genotype. Due to the limitations of the formulations we used, we could not assess this; therefore, we may need to develop new formulations that can take into account the number of APOE-ε4 alleles and the development of AEs in future studies.
In conclusion, we presented a formulation to determine how the inherent APOE-ε4 related risk of AE might potentially influence the symptom-based vigilance of ARIA during safety monitoring. As a result, there is no strong evidence suggesting that specific neuropsychiatric AEs are more common in APOE-ε4 carriers of the placebo arm during RCTs. The APOE-ε4 allele's influence on the likelihood of ARIA during safety monitoring in anti-amyloid beta monoclonal antibody trials could be negligible, after all, maintaining the current level of clinician alertness.
Publicly available datasets were analyzed in this study. This data can be found at: https://c-path.org/programs/cpad/.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent was not required to participate in this study in accordance with the local legislation and institutional requirements.
KSa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft. YN: Writing – review & editing. RI: Writing – review & editing. KSu: Writing – review & editing. AI: Writing – review & editing. TI: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by AMED Grant Numbers JP21dk0207048 and JP21dk0207054 and JSPS KAKENHI Grant Number 21K20891.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frdem.2023.1320329/full#supplementary-material
Abboud, S., Viiri, L. E., Lütjohann, D., Goebeler, S., Luoto, T., Friedrichs, S., et al. (2008). Associations of apolipoprotein E gene with ischemic stroke and intracranial atherosclerosis. Eur. J. Hum. Genet. 16, 955–960. doi: 10.1038/ejhg.2008.27
Antolini, L., DiFrancesco, J. C., Zedde, M., Basso, G., Arighi, A., Shima, A., et al. (2021). Spontaneous ARIA-like events in cerebral amyloid angiopathy-related inflammation: a multicenter prospective longitudinal cohort study. Neurology 97, e1809–e1822. doi: 10.1212/WNL.0000000000012778
Arnerić, S. P., Kern, V. D., and Stephenson, D. T. (2018). Regulatory-accepted drug development tools are needed to accelerate innovative CNS disease treatments. Biochem. Pharmacol. 151, 291–306. doi: 10.1016/j.bcp.2018.01.043
Barakos, J., Purcell, D., Suhy, J., Chalkias, S., Burkett, P., Marsica Grassi, C., et al. (2022). Detection and management of amyloid-related imaging abnormalities in patients with alzheimer's disease treated with anti-amyloid beta therapy. J. Prev. Alzheimers Dis. 9, 211–220. doi: 10.14283/jpad.2022.21
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bennet, A. M., Di Angelantonio, E., Ye, Z., Wensley, F., Dahlin, A., Ahlbom, A., et al. (2007). Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298, 1300–1311. doi: 10.1001/jama.298.11.1300
Chen, C., and Hu, Z. (2016). ApoE polymorphisms and the risk of different subtypes of stroke in the chinese population: a comprehensive meta-analysis. Cerebrovasc. Dis. 41, 119–138. doi: 10.1159/000442678
Cummings, J., Aisen, P., Apostolova, L. G., Atri, A., Salloway, S., Weiner, M., et al. (2021). Aducanumab: appropriate use recommendations. J. Prev. Alzheimers Dis. 8, 398–410. doi: 10.14283/jpad.2021.41
Cummings, J., Apostolova, L., Rabinovici, G. D., Atri, A., Aisen, P., Greenberg, S., et al. (2023). Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10, 362–377. doi: 10.14283/jpad.2023.30
de Leeuw, F. E., Richard, F., de Groot, J. C., van Duijn, C. M., Hofman, A., Van Gijn, J., et al. (2004). Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke 35, 1057–1060. doi: 10.1161/01.STR.0000125859.71051.83
Elosua, R., Ordovas, J. M., Cupples, L. A., Fox, C. S., Polak, J. F., Wolf, P. A., et al. (2004). Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J. Lipid Res. 45, 1868–1875. doi: 10.1194/jlr.M400114-JLR200
Granér, M., Kahri, J., Varpula, M., Salonen, R. M., Nyyssönen, K., Jauhiainen, M., et al. (2008). Apolipoprotein E polymorphism is associated with both carotid and coronary atherosclerosis in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 18, 271–277. doi: 10.1016/j.numecd.2007.01.003
Honig, L. S., Barakos, J., Dhadda, S., Kanekiyo, M., Reyderman, L., Irizarry, M., et al. (2023). ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer's disease. Alzheimers Dement. 9, e12377. doi: 10.1002/trc2.12377
Ito, K., Corrigan, B., Romero, K., Anziano, R., Neville, J., Stephenson, D., et al. (2013). Understanding placebo responses in Alzheimer's disease clinical trials from the literature meta-data and CAMD database. J. Alzheimers. Dis. 37, 173–183. doi: 10.3233/JAD-130575
Khan, T. A., Shah, T., Prieto, D., Zhang, W., Price, J., Fowkes, G. R., et al. (2013). Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int. J. Epidemiol. 42, 475–492. doi: 10.1093/ije/dyt034
Kokubo, Y., Chowdhury, A. H., Date, C., Yokoyama, T., Sobue, H., Tanaka, H., et al. (2000). Age-dependent association of apolipoprotein E genotypes with stroke subtypes in a Japanese rural population. Stroke 31, 1299–1306. doi: 10.1161/01.STR.31.6.1299
Lumsden, A. L., Mulugeta, A., Zhou, A., and Hyppönen, E. (2020). Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 59, 102954. doi: 10.1016/j.ebiom.2020.102954
MacLeod, M. J., De Lange, R. P., Breen, G., Meiklejohn, D., Lemmon, H., Clair, D. S., et al. (2001). Lack of association between apolipoprotein E genoype and ischaemic stroke in a Scottish population. Eur. J. Clin. Invest. 31, 570–573. doi: 10.1046/j.1365-2362.2001.00851.x
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, E. M., et al. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944. doi: 10.1212/WNL.34.7.939
Neville, J., Kopko, S., Broadbent, S., Avilés, E., Stafford, R., Solinsky, C. M., et al. (2015). Coalition against major diseases. development of a unified clinical trial database for Alzheimer's disease. Alzheimers. Dement. 11, 1212–1221. doi: 10.1016/j.jalz.2014.11.005
Roytman, M., Mashriqi, F., Al-Tawil, K., Schulz, P. E., Zaharchuk, G., Benzinger, T. L. S., et al. (2023). Amyloid-related imaging abnormalities: an update. Am. J. Roentgenol. 220, 562–574. doi: 10.2214/AJR.22.28461
Sato, K., Mano, T., Iwata, A., and Toda, T. (2020). Subtype-dependent reporting of stroke with SGLT2 inhibitors: implications from a Japanese Pharmacovigilance Study. J. Clin. Pharmacol. 60, 629–635. doi: 10.1002/jcph.1561
Sato, K., Mano, T., Matsuda, H., Senda, M., Ihara, R., Suzuki, K., et al. (2019). Visualizing modules of coordinated structural brain atrophy during the course of conversion to Alzheimer's disease by applying methodology from gene co-expression analysis. Neuroimage Clin. 24, 101957. doi: 10.1016/j.nicl.2019.101957
Sato, K., Mano, T., Niimi, Y., Iwata, A., Toda, T., Iwatsubo, T., et al. (2021). The impact of COVID-19 pandemic on the utilization of ambulatory care for patients with chronic neurological diseases in Japan: evaluation of an administrative claims database. Biosci. Trends. 15, 219–230. doi: 10.5582/bst.2021.01194
Sperling, R. A., Jack, C. R. Jr., Black, S. E., Frosch, M. P., Greenberg, S. M., Hyman, B. T., et al. (2011). Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers. Dement. 7, 367–385. doi: 10.1016/j.jalz.2011.05.2351
Tai, L. M., Thomas, R., Marottoli, F. M., Koster, K. P., Kanekiyo, T., Morris, A. W., et al. (2016). The role of APOE in cerebrovascular dysfunction. Acta Neuropathol. 131, 709–723. doi: 10.1007/s00401-016-1547-z
Keywords: adverse event, APOE, Amyloid-Related Imaging Abnormalities, ARIA, Alzheimer's disease, disease-modifying therapy
Citation: Sato K, Niimi Y, Ihara R, Suzuki K, Iwata A and Iwatsubo T (2024) APOE-ε4 allele[s]-associated adverse events reported from placebo arm in clinical trials for Alzheimer's disease: implications for anti-amyloid beta therapy. Front. Dement. 2:1320329. doi: 10.3389/frdem.2023.1320329
Received: 12 October 2023; Accepted: 20 December 2023;
Published: 15 January 2024.
Edited by:
Shunsuke Koga, Hospital of the University of Pennsylvania, United StatesReviewed by:
Luke William Bonham, University of California, San Francisco, United StatesCopyright © 2024 Sato, Niimi, Ihara, Suzuki, Iwata and Iwatsubo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenichiro Sato, a2VuaXNhdG91QG0udS10b2t5by5hYy5qcA==; Takeshi Iwatsubo, aXdhdHN1Ym9AbS51LXRva3lvLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.