
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Arachn. Sci., 15 March 2024
Sec. Arachnid Ecology and Behavior
Volume 3 - 2024 | https://doi.org/10.3389/frchs.2024.1383339
 Alexander Wirth*
Alexander Wirth* Gaby Schulemann-Maier
Gaby Schulemann-MaierIn Germany, Zoropsis spinimana (Dufour, 1820) is an introduced, likely synanthropic spider species. Here, we report the results of a nationwide mapping appeal conducted by the citizen science platform NABU-naturgucker.de, used to assemble live distributional data for the species in Germany. With the help of media interest in this species, we gathered a valuable dataset and a large image gallery of the species. In just five weeks, we received more than 15,000 records, representing a 2.3-fold increase in occupied territory compared to previous knowledge. By analyzing the data in detail, we obtained novel insights into the ecology and eco-geography of Z. spinimana in Germany, including information on prey, coloration, potential predators, altitudinal distribution and temporal appearance, along with two cases of accidental human translocation.
The use of datasets generated by citizen scientists has the potential to be a powerful tool for studying changes in the geographic distribution of species, as well as various ecological phenomena, and this utility has already been demonstrated in the literature (e.g., Pocock et al., 2015; Pocock et al., 2017b; Sumner et al., 2019). Citizen science has also proven to be a useful tool for studying invasive species (Pocock et al., 2017a; Phillips et al., 2021), which are among the causes of threats to biodiversity (Doherty et al., 2016). However, the reason for the redistribution of species isn’t necessarily because they are invasive. In general, anthropogenic activities such as land-use change, pollution, anthropogenic climate change and overexploitation are the key drivers of biodiversity loss (Brook et al., 2008). All these parameters can affect species in different ways, inter alia leading to species redistribution or even extinction (Chowdhury et al., 2021; Stevens et al., 2023). Citizen science can contribute to the investigation of changes in species behavior in the context of anthropogenic pressure (Arroyo-Solís et al., 2013; Bermúdez-Cuamatzin et al., 2020). Properly curated image galleries from citizen scientists can be especially useful in mapping species distribution and studying biological traits (Suzuki-Ohno et al., 2017). Nowadays, social media networks such as Instagram or Facebook can be a valuable source of images that could be of interest for conservation or the enhancement of knowledge about biology of species (Edwards et al., 2021; Chowdhury et al., 2023, 2024), but are not further considered here. Citizen science data has also already been used in arachnology to better understand species distributions and patterns of spatiotemporal occurrence (Campbell and Engelbrecht, 2018; Hart et al., 2018; Wang et al., 2018). However, gathering observational data on less charismatic species, such as spiders, can be challenging. Attracting participants to report observations is mainly based on the observers’ motivation, e.g. sharing their sightings with other people (Munzinger, 2015) as well as a ‘fear factor’ regarding taxa such as spiders (Campbell and Engelbrecht, 2018).
The spiders of the genus Zoropsis Simon, 1878 (family Zoropsidae) generally show a circum-Mediterranean distribution, although a number of species can also be found in the Canary Islands, China, the Middle East and Eurasia (Natural History Museum Bern, 2023). Zoropsis spinimana – which is now called ‘Nosferatu-Spinne’ in Germany due to its prominent carapace markings that resemble the face of the main character of the German movie Nosferatu (Breitling et al., 2020) – is known from Italy (Pantini and Isaia, 2019), Croatia (Thaler and Knoflach, 1998), France (Simon, 1914), and the Iberian Peninsula (Branco et al., 2019). The first record of Z. spinimana north of the Alps dates back to a sighting in Basel in 1994 (Hänggi, 2003). Today, the species is well-established there and in other urban places in Switzerland (Hänggi and Zuercher, 2013). In October 1997, Thaler and Knoflach added an observation from Innsbruck, Austria, on the wall of a house (Thaler and Knoflach, 1998). Finally, in 2005 the species was documented for the first time in Germany, in Freiburg im Breisgau (Hänggi and Bolzern, 2006). Today, Z. spinimana is introduced in many countries including the United Kingdom (Harvey, 2012)), Netherlands (van Helsdingen, 2015) and Luxemburg (Massard and Greimer, 2018) as well as Georgia (Marusik and Kovblyuk, 2004), Crimea (Nadolny, 2016), and even the U.S.A (Griswold, 2009). (for further details see Nentwig et al., 2022).
Naturally, Z. spinimana is found in open pine forests beneath stones and bark, but in urban habitats, it has been reported from both the inside and outside of buildings (Thaler and Knoflach, 1998). Because of its relatively large size, Z. spinimana is easily identified in buildings and there appears to be an increase in sightings indoors in spring as well as late summer/early autumn (Hänggi and Zuercher, 2013). The species has been previously reported as a crepuscular and nocturnal spider, and even though it is cribellate (with a fully developed cribellum and calamistrum) it is a cursorial hunter and does not appear to build a capture web (Foelix et al., 2015). Nevertheless, little is known about its prey, although various insects have been reported (Eggs et al., 2015; Foelix et al., 2015).
Here we used a combined strategy based on citizen science data including images, which were 82% correct, as well as observations without images to study the geographical range of Z. spinimana in Germany. We used the data to elaborate details of the biology in non-native habitats, such as temporal occurrence including reproductive period, prey and predators. In addition, the huge amount of images can reflect the color diversity of this species which has never been shown before. Furthermore, we demonstrate that images can also be used to investigate the body size, a parameter typically not addressed by such data. Our approach answers different questions and highlights both the value of using citizen science data and its suitability for addressing such topics.
The Zoropsis survey started on 30 August 2022 when the Naturschutzbund Deutschland (NABU) Baden-Württemberg issued a press release asking for sightings of Z. spinimana, following a significant amount of German media attention paid to the species at the time. On the same day, this request was advertised through national mass media and the gathering of the observations on NABU-naturgucker.de started immediately, with up to 1,020 observations received per day. Launched in 2008, NABU-naturgucker.de is the leading German citizen science platform for nature observations and contributes data to the GBIF database. The Z. spinimana observations were assembled either via the web application, with GPS coordinates and local time directly tagged (https://nabu-naturgucker.de/app/Nosferatu), or via the main observation website (https://NABU-naturgucker.de), in which details such as observation time or GPS coordinates needed to be added manually.
For the analysis of spatial and temporal occurrence, we used the observation data collected from 30 August until 3 October 2022 (in total five weeks). Observations were verified based on images, and geographically analyzed and compared based on TK25 grid cells (geographical unit: ~10 km x 10 km area) – a standard topographic unit in Germany, rendering the data comparable to the Atlas of European Arachnids henceforth referred to as the reference dataset (Arachnologische Gesellschaft, 2022). Observations handed in without images but with additional descriptions were checked for terms like ‘in web’ and phrases expressing uncertainty, for example ‘could you please confirm ID’, words like ‘maybe’, or question marks. These observations were in turn omitted from the analyzed dataset. In addition, any observation without an image was only taken into account if it was made in a TK25 grid cell in which at least one observation evidenced by a plausibilized image already existed, submitted by any observer.
Since data from NABU-naturgucker.de are fluid and due to ongoing user activities changing over time, we downloaded the used dataset (Supplementary Table 1) on 5 October 2022. To increase the value and to check the validity of our dataset, we compared it to the expert-curated dataset hosted by the Arachnologische Gesellschaft (AraGes) – a standard reference in Germany. We received the dataset of the reference database ‘the Atlas of European Arachnids’ including cumulative Z. spinimana observations until 3 October 2022 on 28 October 2022.
A large number of observations tagged with at least one image (during the five weeks: in total n = 8,905 observations with at least one image, 14,581 images in total) were checked manually and carefully assigned and quantified according to the following criteria (based on 4,298 annotated, representative images): sex (male, female, uncertain/not determinable); location (inside or associated with a building, outside, transiently caught by the observer [not reported if indoors or outside], not determinable); prey (with prey); reproduction (reproducing); size (a scale within the image). The quantification of this set of representative images was intended to cover a large spatial distribution and was carried out by six people on a daily basis spending in total 486 working hours. The visual evaluation, looking for human translocation, prey and predators as well as different developmental stages of the spider, was later extended to all images showing Z. spinimana being uploaded to the citizen science platform NABU-naturgucker.de until the end of the year 2022 (n = 20,177). All images were checked for the correct identification based on the identification criteria outlined by Thaler and Knoflach (Thaler and Knoflach, 1998) and, if they did not meet these criteria, were omitted from the analyses.
Images including scales were further analyzed with Fiji 12 (Schindelin et al., 2012) by first calibrating the scale according to the image and subsequently measuring the carapace and body as well as the length of femur I (see Figure 1A).

Figure 1 Quantification of body size measurements: quantification of measured parameters separately for all (n = 50), male (n = 32), and female (n = 11) individuals. p < 0.001; 2-way ANOVA with Tukey’s multiple comparisons test.
Statistical analyses of the size of individual spiders (variables: sex, body part), as well as the temporal occurrence of individuals (variables: sex, calendar week), were analyzed using a two-way ANOVA with Tukey’s multiple comparisons test using GraphPad Prism 8. All analyzed data can be found in the Supplementary Material. Data were analyzed using MatLab, GraphPad Prism 8, Fiji 12, Microsoft Excel 2016, and Google Earth.
In total, we analyzed 15,997 observations reported between 30 August and 3 October 2022, of which 8,905 included at least one image. Within these five weeks, we gathered 14,581 images of Z. spinimana from Germany, which increased to 20,177 images by the end of the year 2022. In comparison, other publicly accessible database entries addressing the species were low in numbers at that time (Table 1). Hänggi and Zuercher (2013) based their findings of Z. spinimana in Switzerland on 100 observations of spiders (mostly images) gathered over five years (2008–2012), of which 68 were identified as Z. spinimana (68% correct) (Hänggi and Zuercher, 2013). Our approach reached 82% image accuracy during the five-week period, a result which will be the topic of a future publication and not further discussed here.
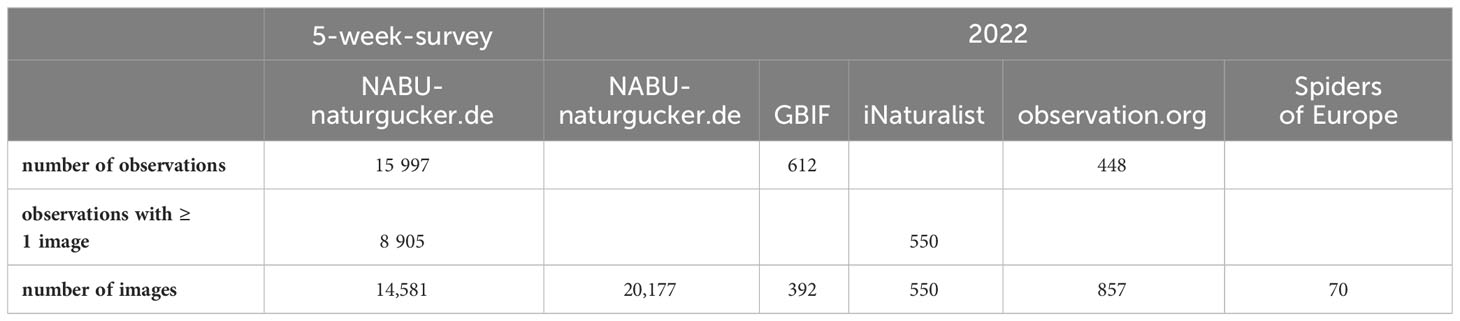
Table 1 Number of observations and images of Z. spinimana in online repositories and citizen science databases (GBIF, 2022; iNaturalist, 2022; Nentwig et al., 2022; observation.org, 2022).
By analyzing the images, we noticed a broad color spectrum and variation in Z. spinimana. The individuals varied from very light brown, nearly yellowish individuals with light markings on the carapace and more or less prominent alternating-colored leg bands (Figure 2A), through to another color type with a reddish opisthosoma and light brown to gray carapace, with very light markings and light banding on the legs (Figure 2B). Many individuals showed a medium color spectrum, ranging from darker brown to lighter brown, with contrasting visible markings on the carapace and distinct alternating-colored leg bands (Figure 2C). There were also some individuals which combined several color features such as a dark reddish opisthosoma and dark carapace with yellowish/whitish markings, and light brown/yellowish legs interrupted by black bands (Figure 2D).

Figure 2 Color variation in Z. spinimana individuals: (A), representative images of individuals with a yellowish basis from lighter colors (left) to darker shades (right); (B), representative images of individuals with a reddish basis in a similar way as in (A); (C), representative images of a darker brown individuals; (D), individuals rich in contrast and displaying several colors.
Several high-quality images were taken with a visible scale, for example, a ruler or a coin. We used these items to calibrate the images as described above and measured in total 50 adult individuals (♂ = 32, ♀ = 11, sex unknown = 7). Zoropsis spinimana individuals in Germany ranged between 10.3 mm to 19.6 mm, with females being significantly larger than males (mean ♂ = 13.8 mm ± 1.8 mm, ♀ = 16.0 mm ± 2.4 mm, p < 0.001; 2-way ANOVA with Tukey’s multiple comparisons test). The carapace size varied between 4.5 mm and 9.2 mm in length and 4.1 mm to 7.1 mm in width. The length of the femur I varied between 4.5 mm and 8.1 mm, resulting in a femur I to carapace ratio of 0.7 to 1.5 (Figure 1; Table 2).
At first glance, the two datasets have 191 TK25 grid cells in common. The Atlas of European Arachnids shows 32 unique TK25 grid cells, which are not covered by our data, whereas our dataset adds 326 unique TK25 grid cells to the occurrence of Z. spinimana in Germany (Figure 3A). Our data equate to a 10-fold increase in unique TK25 grid cells and a 2.3-fold increase in TK25 grid cells in total compared to the reference dataset. These differences become evident by focusing on the spatial distribution of TK25 grid cells with observations of Z. spinimana (Figures 3C, D). The maps show a high abundance of Z. spinimana in the Upper Rhine Plain, the Lower Rhine region, and the Cologne Lowland, but also in cities like Stuttgart and to a lesser extent Munich, Berlin, Hamburg, and Bremen. This is also reflected by the TK25 grid cells with the highest abundance of observations which are mainly cities. Looking at different federal states in Germany, most observations were reported from Baden-Württemberg (9,599 observations), followed by North Rhine-Westphalia (2,402 observations) and Hesse (1,916 observations). Of note, compared to the reference database which shows no data for Hamburg, Schleswig-Holstein, and Thuringia, we here report the appearance of Z. spinimana in these German federal states for the first time. In addition, our data show that Z. spinimana populations in all federal states except Mecklenburg-Vorpommern are larger than the reference data suggest (Table 3). There is only one photo-documented observation reported from Mecklenburg-Vorpommern in 2022, which to our knowledge is the first case of a documented accidental human translocation to this state and is described in more detail below. Even observations made in 2023 reveal only a single photo-documented observation of Z. spinimana in Mecklenburg-Vorpommern, reported from a holiday cottage on the island of Rügen (https://NABU-naturgucker.de/?bild=-1641469348). A number of observations also spread east from Freiburg im Breisgau into the Black Forest, or south of Stuttgart towards the Swabian Jura. Since both, the Black Forest and Swabian Jura, are mountainous areas, Z. spinimana can be found in Germany at altitudes above 1,000 m, which extends its range in altitude, too. The distribution of Z. spinimana along main roads towards more remote villages around Freiburg im Breisgau is also striking (Figure 3B).

Figure 3 Spatial distribution of Z. spinimana in Germany: (A) Venn diagram of TK25 grid cells, occupied by Z. spinimana reported to ‘the Atlas of European Arachnids’ (red) and to NABU-naturgucker.de (blue); (B) GPS-tagged observations around Freiburg im Breisgau; scale bar: 5 km; (C) accumulated observations of Zoropsis spinimana in the reference database ‘the Atlas of European Arachnids’; (D) distribution of observations reported to NABU-naturgucker.de within five weeks; scale bar (C, D): dark blue one observation, dark magenta > 100 observations in a TK25 grid cell;.
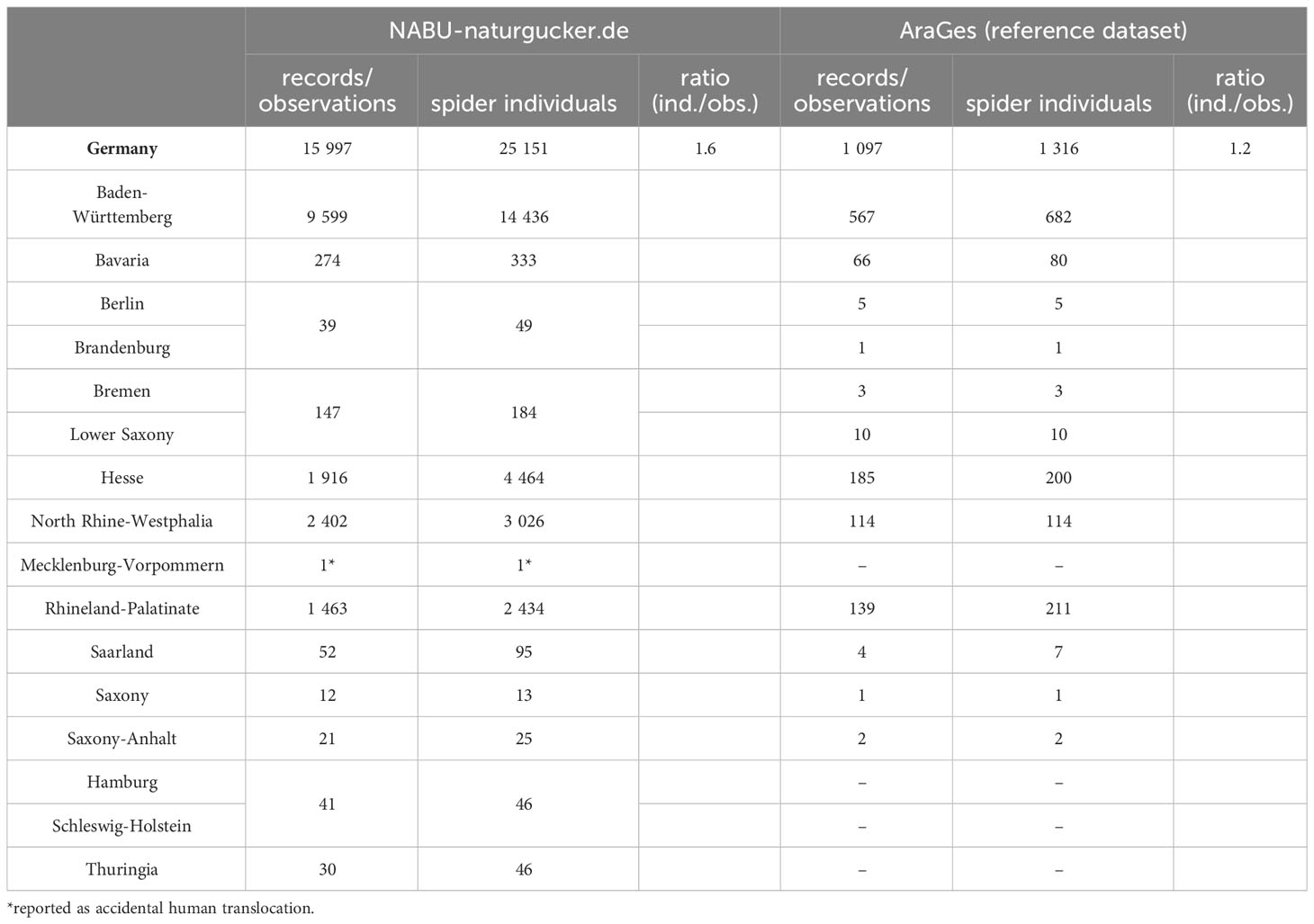
Table 3 Comparison between observations and individuals of Z. spinimana during the 5-week survey (NABU-naturgucker.de) and the cumulative expert-curated data (AraGes) separated by federal states.
Focusing on single TK25 grid cells, our dataset shows 12.5 × (median) more observations in the same grid cells than the reference. The maximum difference for a single TK25 grid cell is 116 × more observations than the reference. In Freiburg in the Breisgau, from where Z. spinimana was first described in Germany, our dataset shows 37.7 × more observations (adding up data from all four TK25 grid cells: 7912, 7913, 8012, 8013) gathered in only five weeks compared to the cumulative data from the Atlas of European Arachnids (Supplementary Table 3).
Most of the reported observations including images were made in or associated with buildings or other human-made structures (49%, n = 2,089), whereas only 8% (n = 340) of the images were taken outside, even though this might still mean in a garden or urban landscape. In total, 33% (n = 1,401) of the images show captured spiders (i.e., in a live insect catcher or glass), and 10% of the analyzed images could not be assigned to any category (n = 438).
To study temporal details of the occurrence of Z. spinimana, we first evaluated the time of day tagged to the observations (which might not necessarily correspond with the observation time). The data showed that the majority of the observations tagged with times were reported between 8 pm and 11 pm (Figure 4A). Next, we analyzed the total number of observations in each calendar week of the five weeks and added the temporal appearance of male and female spiders (Figure 4B), which appeared in total to a similar extent in the analyzed data (♂ = 51% (n = 1,349), ♀ = 49% (n = 1,296)). Only in calendar weeks 36 (CW 36) and 39 (CW 39), there were slightly more reports of females. During the other weeks, more male individuals were reported, which did not reach statistical significance (Figure 4B, p > 0.05; 2-way ANOVA with Tukey’s multiple comparisons test).
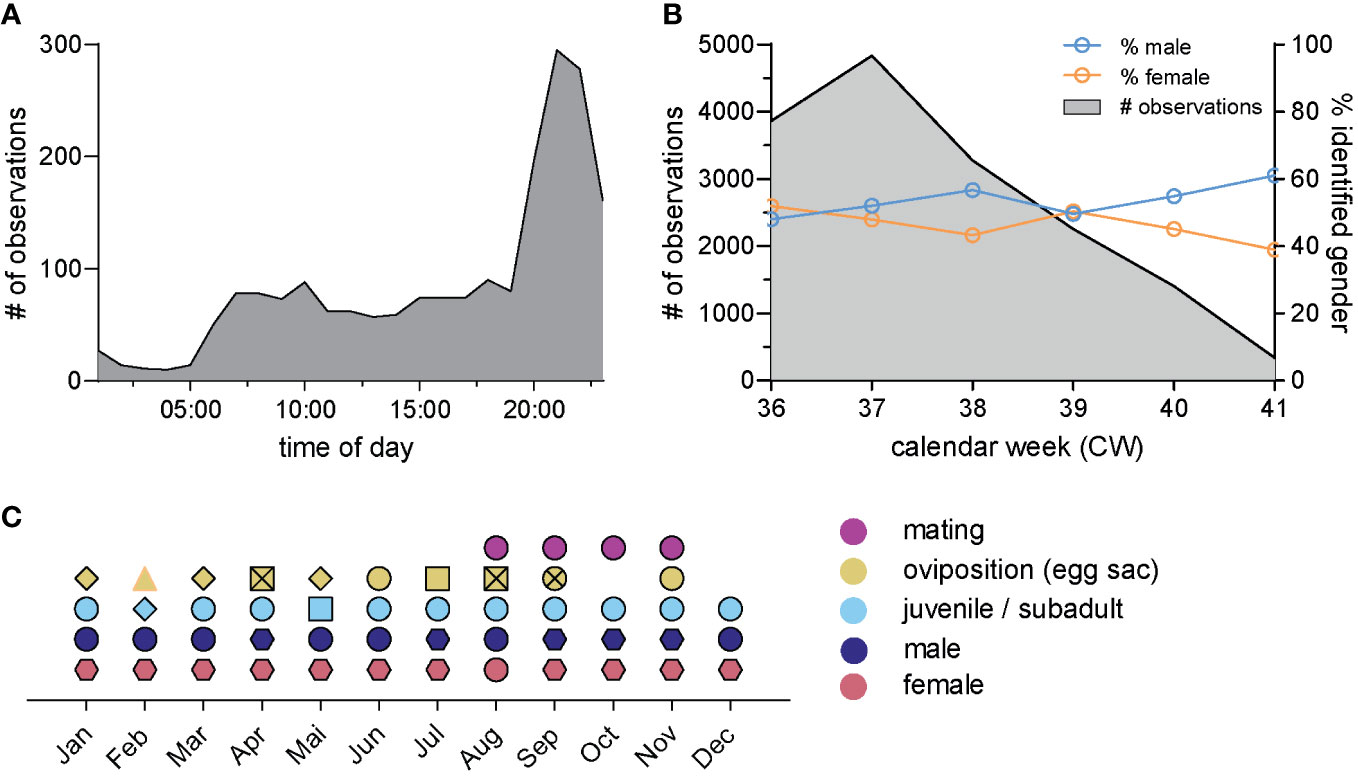
Figure 4 Temporal distribution of Z. spinimana in Germany: (A) histogram of the number of observations with respect to the clock time, the observation was reported to NABU-naturgucker.de (total n = 2,004 observations); (B) quantification of the observations of Z. spinimana per week (total n = 15,997) including a separate quantification of male (blue) and female (orange) individuals (ngender-ID = 2,908), p > 0.05; 2-way ANOVA with Tukey’s multiple comparisons test; (C) phenology of Z. spinimana. black framed circles (○) originate from this study; hexagons (⬡) data from Spiders of Europe (Nentwig et al., 2022), diamond-shaped icons (◇) data by captured individuals by Hänggi and Zuercher (Hänggi and Zuercher, 2013); square-shaped icons (□) data by captured individuals by Berland (Berland, 1927) supported by this study (black framed squares); black crossings (X) data from iNaturalist (iNaturalist, 2022); triangle (△) data from observation.org (observation.org, 2022).
Finally, we screened images from the years 2022 and 2023 of Z. spinimana (https://naturwerke.net/?beitrag=2134) and checked whether they showed egg sacs, mating spiders, or reproduction success by juveniles/subadult spider individuals. In total, we found 19 images with potential mating Z. spinimana individuals as well as more than 50 images with egg sacs. In addition, the database holds more than 70 images of juvenile/subadult spiders (Supplementary Table 2). The temporal occurrence of those events is depicted in Figure 4C. All data points depicted as circles are obtained in this study, whereas any icon framed in black highlights data of this study corroborating existing records. Icons other than the circles summarize data published elsewhere (see figure legend). The data show that both adult male and female, and even subadult spiders can be found throughout the year. Correlating with year-round observations of subadult spiders, egg sacs can nearly be found throughout the year, too. Of note, most data on egg sacs obtained in this study represent the first documented cases of natural, non-captive oviposition, except data in November. In contrast, natural mating events were reported from August to November. We further report here the first details of Z. spinimana development in natural habitats. In total, we found three observations with consecutive images of the same egg sac, enabling us to estimate the developing time, which depends on the environmental temperature. Z. spinimana is still able to develop into spiderlings at a temperature of 10.5°C, leading to a developmental time of 66 days. When the temperature rises above 20°C, the developmental time shortens to 24 days (Table 4). If not in captivity, observers report that the egg sac is left alone in the last days of the development. In addition, nesting sites are reused for several times and even years.
We report here two potential cases of human-mediated dispersal of Z. spinimana individuals. To gather these data, we used the whole image repository. The first case shows an adult individual which traveled within a parcel from Basel, Switzerland, to Schöttlingen/Lindhorst, Lower Saxony (GPS: 52.34768° N, 9.276838° E), 6 March 2020; image by N. Hundertmarck: https://NABU-naturgucker.de/?bild=-976232786.
The second case traces an immature Z. spinimana from Stuttgart, Baden-Württemberg to Kühlungsborn, Mecklenburg-Vorpommern (GPS: 54.15331° N, 11.76363° E). This individual traveled as a stowaway in the luggage (personal communication with the observer), 10 September 2022; image by K. Watter: https://NABU-naturgucker.de/?bild=-1888531485.
Together with other images uploaded to NABU-naturgucker.de showing Z. spinimana individuals standing on cars, bicycles as well as post-drop stations, this is clear evidence for human-mediated long- and short-range dispersal of Z. spinimana.
While analyzing the images we came across some interesting behaviors of Z. spinimana, which we think are worth reporting. One image shows a female individual on a mirror, secured with a silk filament (Figure 5A, image by R. Mettlach: https://NABU-naturgucker.de/?bild=-887474885). Our collection of images also shows some Z. spinimana individuals that were photographed while they were standing on water (Figure 5B; image by E. Junginger: https://NABU-naturgucker.de/?bild=-543277651, image by W. Gehrig: https://NABU-naturgucker.de/?bild=329749451). The third image shows a Z. spinimana individual close to the water line which was reported to have walked on the water (observer comment; image by V. Rosenbaum https://NABU-naturgucker.de/?bild=-1845641811), a potential behavior which – to our knowledge – has not been described before and could be of interest.

Figure 5 Pictures of particular behaviors: (A) images of Zoropsis spinimana secured with a silk line; (B) Zoropsis spinimana standing on water or close to a water line.
Zoropsis spinimana is described as polyphagous in literature, but little information on its prey is available. In particular, there is hardly any information on the prey hunted in the spiders’ habitat, since the data on diet usually derived from experiments in captivity.
Based on 30 images (Supplementary Table 4), in which prey of Z. spinimana were identifiable down to taxonomic order, we found 37% belonging to Arachnida, comprising 82% Araneae and 18% Opiliones. Insects accounted for 63% of prey, including Diptera (42%), Heteroptera (11%), Dermaptera (5%), and Lepidoptera (32%). Orthoptera could only be found on images showing captured Z. spinimana (Figure 6A).
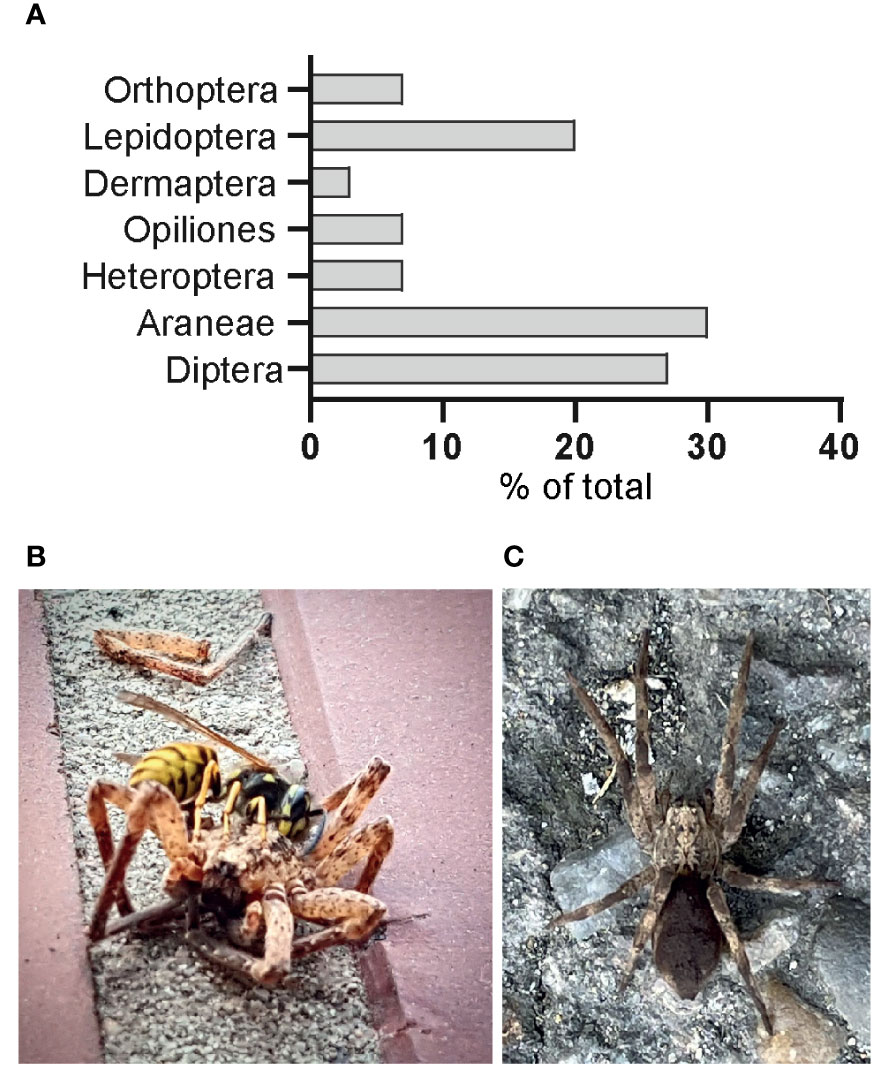
Figure 6 Prey and predation: (A) quantification of an image-based prey analysis. The amount of class-specified prey is related to the total prey; (B, C) potential predators in Germany.
There is not only poor knowledge about the prey of Z. spinimana in natural habitats but also a lack of information on predation pressure on Z. spinimana itself. Especially since this species is not native to Germany, it is of particular interest if Z. spinimana faces natural enemies. Noteworthy are two images that show a) a common wasp (Vespula vulgaris (Linnaeus, 1758)) dissecting a Z. spinimana individual (image by D. Heines: https://NABU-naturgucker.de/?bild=-771345568) (Figure 6B) and b) a Z. spinimana individual showing a down-like covering on its opisthosoma, structures that resembles fungal infections in other invertebrates (Figure 6C; image by NABU Sammelmelder: https://NABU-naturgucker.de/?bild=520356467.
Since members of the public became interested in spiders following the NABU call for reports, some spiders originally intended as Z. spinimana turned out to be rare observations. The following identifications are just based on the presented images.
Eusparassus walckenaeri (Audouin, 1826):
GERMANY, Bavaria, Schwebheim, TK25 grid cell 6027/1, GPS: 49.98072° N, 10.23840° E, single ♀ adult, 7 September 2022, logistics and freight transport area, arrived with herbs from the Mediterranean area, image by R. Parzinger: https://NABU-naturgucker.de/?bild=967083820 (personal communication with the observer) (Figure 7A).
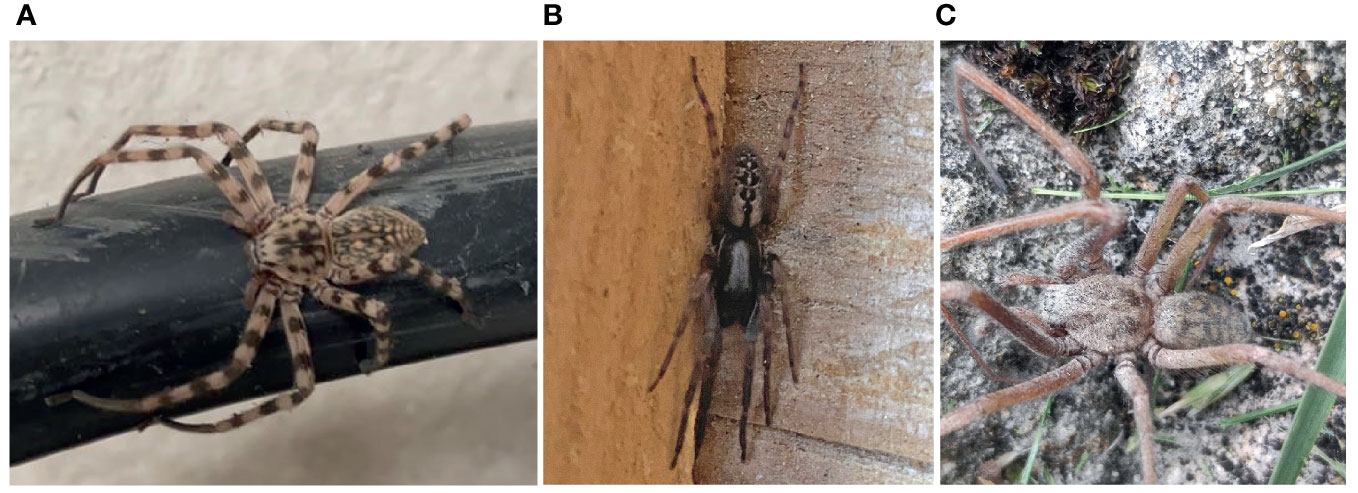
Figure 7 Spider findings of interest: (A) image of Eusparassus walckenaeri; (B) image of Segestria bavarica (finding 2); (C) image of Tegenaria parietina; all reported to NABU-naturgucker.de.
Segestria bavarica C.L. Koch, 1843:
GERMANY, Nothrine-Westfalia, Berrendorf, TK25 grid cell 5005/4, GPS: 50.923° N, 6.59° E, single ♂ adult, 13 April 2022, images by R. Hirsch: https://NABU-naturgucker.de/?bild=101678866.
GERMANY, Baden-Württemberg, Freiburg im Breisgau, TK25 grid cell 8013/1, GPS: 47.998° N, 7.859° E, 5 September 2022, image by M. Tschaffon: https://NABU-naturgucker.de/?bild=1811157861.
GERMANY, Baden-Württemberg, Ingelheim am Rhein, TK25 grid cell 6014/1, 7 September 2022, image by B. Schwenk: https://NABU-naturgucker.de/?bild=-2134103148 (Figure 7B).
Tegenaria parietina (Fourcroy, 1785)
GERMANY, Baden-Württemberg, Bad Überkingen, TK25 grid cell 7424/2, GPS: 48.598° N, 9.795° E, 2 September 2022, image by J. Scholz: https://NABU-naturgucker.de/?bild=711865965 (Figure 7C).
Zoropsis spinimana is one of the largest spiders in Germany and is thus easy to observe. From time to time it is the subject of extensive media attention, especially following first records in newly colonized locations. Media attention is also based on the fact that Z. spinimana is one of the few spiders in Germany with a bite that is able to penetrate the skin of humans; local symptoms are usually described as comparable to the stings of wasps (Nentwig et al., 2013), even though extremely rare cases can be of greater medical significance (Bertlich et al., 2019).
Here we report on a large collection of Z. spinimana observations, images and videos from Germany. Our citizen science survey gathered more than 15,000 observations throughout Germany in just five weeks, again highlighting the power of citizen science in species distribution mapping (Sumner et al., 2019). By chance and by being motivated to support the survey, the citizen scientists also contributed to other interesting spider records from Germany, such as the second observation of Eusparassus walckenaeri in Germany, and new localities for Segestria bavarica and Tegenaria parietina. The evaluation of all of these data demonstrates certain deficiencies in the distributional data currently in the reference database ‘the Atlas of European Arachnids’ (Arachnologische Gesellschaft, 2022).
The image library examined for this study demonstrates the different color variations of Z. spinimana, from light brown to dark individuals (Hänggi et al., 2020). This variability in coloration could potentially mask the presence of another, not yet detected Zoropsis species in Germany. There are several different reasons why spider individuals can differ in color (Oxford and Gillespie, 1998), but these causes were not studied for Z. spinimana. In addition, we could show that Z. spinimana males with a mean size of 13.8 mm in Germany are significantly smaller than females with a mean size of 16 mm, but can grow larger than previously reported (Thaler and Knoflach, 1998). The size of a spider likely impacts the range of available prey since Z. spinimana is reported to be polyphagous and can handle prey up to its own size (Eggs et al., 2015; Hänggi et al., 2020). Until now, reports about the prey taken by Z. spinimana are limited to experiments in captivity in which the spiders were fed with house crickets (Acheta domesticus (Linnaeus, 1758) or house flies (Musca domestica Linnaeus, 1758) (Eggs et al., 2015). Our analysis is the first quantification of natural prey and highlights the range of prey items taken by Z. spinimana, ranging from small flies to rather big butterflies. It also indicates that, in rare cases, prey larger than the spider itself can be hunted (Supplementary Table 4).
The most important finding of our analyses is the broad distribution of Z. spinimana throughout Germany. We evaluated the TK25 grid cell occupancy for both our dataset and the reference dataset and found a 2.3-fold increase in occupied TK25 grid cells. The comparison of both maps (Figures 3C, D) shows a range extension mainly towards the east and north, which is further corroborated by an observation of Z. spinimana in Denmark back in 2020 (Kjær, 2020). Our data reveal a nearly nationwide appearance of Z. spinimana except in the remote northeastern part of Germany (Mecklenburg-Vorpommern). Here, we report a case of accidental human translocation of a spider via luggage to Mecklenburg-Vorpommern, and even a single accidental transportation event like this might establish local populations of Z. spinimana if females have previously mated. For example, once fertilized, females can produce fertile eggs in several egg sacs over the course of at least six months (Klesser and Ruge, 2021). It is believed that Z. spinimana is mainly accidentally translocated by humans and transported along main roads (Hänggi and Zuercher, 2013). This is supported by images in the gallery which show Z. spinimana individuals standing on cars and bikes. Our data also show that Z. spinimana has large populations in many TK25 grid cells with up to 531 observations in a single grid cell within five weeks pointing to well-established populations. These populations might well be able to drive species range extension naturally without the help of humans. This important question should be addressed in further studies to better judge the situation of Z. spinimana in Germany. There are already some hints to the use of bird nest boxes (image by A. Scheuermann: https://NABU-naturgucker.de/?bild=332906038) (Hänggi et al., 2020). Indeed, observations in the Black Forest and also from the Swabian Jura demonstrate that Z. spinimana is able to occupy habitats in Germany up to 1,000 m above sea level, raising questions about its ecological potential in newly occupied territories.
Why Z. spinimana is now well-established in most parts of Germany and why it might be actively extending its range is still not clear. In the literature, climate change is often debated as a possible reason, but there is no direct evidence that climate change is driving range expansion in Z. spinimana. However, our data indicate that reproduction is occurring nearly all year around in some areas, which could indicate a less strict annual reproduction cycle in anthropogenic habitats leading to a successful population manifestation or expansion. This idea is supported by the successful development of Z. spinimana at a temperature of 10.5°C. The temperature dependency of Z. spinimana points to a warm-adapted species (Li and Jackson, 1996). Future work is needed to find the temperature limits for proper development - a criterion important in the context of climate change and range expansion.
A big advantage of data derived by citizen science projects like ours is that people from all over the country contributed, so the project resulted in an enormous quantity of data that would be impossible to obtain through targeted scientific mapping of a species’ distribution by only a handful of researchers. However, the data obtained this way are not ‘ready to use’ (Gardiner et al., 2012) and its usefulness and quality are often disputed. As is usual for nature observation data, a certain proportion of the reported observations may be based on misidentifications (here only 18%). Therefore, it is necessary to check the dataset before processing it. If there are no attached images, observations cannot be verified. For this reason we have mainly focused on observations with at least one image attached. We carefully checked these images for identification errors and found them to be 82% correct. Also, we will continue to monitor the distribution of Z. spinimana and kindly request individuals to further submit observations to NABU-naturgucker.de. There will be regular updates posted to all volunteers which can be accessed at https://naturwerke.net/?beitrag=2134 and https://www.naturwerke.net/?beitrag=2131. At present it is still unclear what factors are driving range extension and what role climate change might be playing. We need to better understand the biology of this species to characterize its biological niche in a non-natural habitat.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AW: Conceptualization, Data curation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GS: Data curation, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
First of all, we thank all volunteers and contributors, who submitted their observations and images of Z. spinimana and other spider species to NABU-naturgucker.de. Furthermore, we thank the NABU-naturgucker.de community for the engagement to comment and report misidentified Z. spinimana observations. We thank Peter Mielke, Stephan Pflume and Andreas Hille for reporting details of oviposition and development. We thank Dorina Neumann and Martina Limprecht for their help in assigning spider images, Dieter Schneider for the help in prey identification and Reinhard Naumann, Michael Hohner and Christoph Muster for proofreading the manuscript. The authors are further greatly thankful to the Arachnologische Gesellschaft e. V. (AraGes) for access to their data. We express our special thanks to the Naturschutzbund Deutschland (NABU) Landesverband Baden-Württemberg e. V., who chaired the Z. spinimana survey.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frchs.2024.1383339/full#supplementary-material
Arachnologische Gesellschaft (2022). Atlas der spinnentiere Europas. Atlas Spinn. Eur. Available at: https://atlas.arages.de/
Arroyo-Solís A., Castillo J. M., Figueroa E., López-Sánchez J. L., Slabbekoorn H. (2013). Experimental evidence for an impact of anthropogenic noise on dawn chorus timing in urban birds. J. Avian Biol. 44, 288–296. doi: 10.1111/j.1600-048X.2012.05796.x
Berland L. (1927). Contributions à l’étude de la biologie des arachnides (2e Mémoire). Arch. Zool. Expérimentale Générale 66, 7–31.
Bermúdez-Cuamatzin E., Delamore Z., Verbeek L., Kremer C., Slabbekoorn H. (2020). Variation in diurnal patterns of singing activity between urban and rural great tits. Front. Ecol. Evol. 8. doi: 10.3389/fevo.2020.00246
Bertlich I., Enk A., Haenssle H. A., Höfer H., Haus G. (2019). Extensive local reaction after bite of the Mediterranean spider Zoropsis spinimana. JDDG J. Dtsch. Dermatol. Ges. 17, 76–78. doi: 10.1111/ddg.13717
Branco V. V., Morano E., Cardoso P. (2019). An update to the Iberian spider checklist (Araneae). Zootaxa 4614, 201–254. doi: 10.11646/zootaxa.4614.2.1
Breitling R., Merches E., Muster C., Duske K., Grabolle A., Hohner M., et al. (2020). List of German names for the spiders of Germany (Araneae). Arachnol. Mitt. Arachnol. Lett. 59, 38–62. doi: 10.30963/aramit5907
Brook B. W., Sodhi N. S., Bradshaw C. J. A. (2008). Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. doi: 10.1016/j.tree.2008.03.011
Campbell H., Engelbrecht I. (2018). The Baboon Spider Atlas – using citizen science and the ‘fear factor’ to map baboon spider (Araneae: Theraphosidae) diversity and distributions in Southern Africa. Insect Conserv. Divers. 11, 143–151. doi: 10.1111/icad.12278
Chowdhury S., Aich U., Rokonuzzaman M., Alam S., Das P., Siddika A., et al. (2023). Increasing biodiversity knowledge through social media: A case study from tropical Bangladesh. BioScience 73, 453–459. doi: 10.1093/biosci/biad042
Chowdhury S., Braby M. F., Fuller R. A., Zalucki M. P. (2021). Coasting along to a wider range: niche conservatism in the recent range expansion of the Tawny Coster, Acraea terpsicore (Lepidoptera: Nymphalidae). Divers. Distrib. 27, 402–415. doi: 10.1111/ddi.13200
Chowdhury S., Fuller R. A., Ahmed S., Alam S., Callaghan C. T., Das P., et al. (2024). Using social media records to inform conservation planning. Conserv. Biol. 38, e14161. doi: 10.1111/cobi.14161
Doherty T. S., Glen A. S., Nimmo D. G., Ritchie E. G., Dickman C. R. (2016). Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. U. S. A. 113, 11261. doi: 10.1073/pnas.1602480113
Edwards T., Jones C. B., Perkins S. E., Corcoran P. (2021). Passive citizen science: The role of social media in wildlife observations. PloS One 16, e0255416. doi: 10.1371/journal.pone.0255416
Eggs B., Wolff J. O., Kuhn-Nentwig L., Gorb S. N., Nentwig W. (2015). Hunting without a web: how lycosoid spiders subdue their prey. Ethology 121, 1166–1177. doi: 10.1111/eth.12432
Foelix R., Erb B., Eggs B. (2015). Morphologische Besonderheiten der Kräuseljagdspinne Zoropsis spinimana. ARACHNE 20, 4–14.
Gardiner M. M., Allee L. L., Brown P. M., Losey J. E., Roy H. E., Smyth R. R. (2012). Lessons from lady beetles: accuracy of monitoring data from US and UK citizen-science programs. Front. Ecol. Environ. 10, 471–476. doi: 10.1890/110185
GBIF (2022). Zoropsis spinimana (Dufour, 1820) in GBIF Secretariat (2022) Checklist dataset. doi: 10.15468/39ome
Griswold C. (2009). Zoropsidae: a spider family newly introduced to the USA (Araneae, Entelegynae, Lycosoidea). J. Arachnol. 29, 111–113. doi: 10.1636/0161-8202(2001)029[0111:ZASFNI]2.0.CO;2
Hänggi A. (2003). Nachträge zum “Katalog der schweizerischen Spinnen”. 3. Neunachweise von 1999 bis 2002 und Nachweise synanthroper Spinnen. Arachnol. Mitt. 26, 36–54. doi: 10.5431/aramit2604
Hänggi A., Bolzern A. (2006). Zoropsis spinimana (Araneae: Zoropsidae) neu für Deutschland. Arachnol. Mitt. 32, 8–10. doi: 10.5431/aramit3202
Hänggi A., Inches S., Brunner S. (2020). Zoropsis spinimana - eine gebietsfremde Spinnenart aus dem Mittelmeerraum besiedelt auch Vogelnistkästen 117, 262–266.
Hänggi A., Zuercher I. (2013). Zoropsis spinimana - eine mediterrane Spinne ist in Basel (NW-Schweiz) heimisch geworden. Mitt. Naturforschenden Gesellschaften Beider Basel 14, 125–134.
Hart A. G., Nesbit R., Goodenough A. E. (2018). Spatiotemporal variation in house spider phenology at a national scale using citizen science. Arachnology 17, 331–334. doi: 10.13156/arac.2017.17.7.331
Harvey P. (2012). Zoropsis spinimana (Dufour 1820) established indoors in Britain. Newsl. Br. Arachnol. Soc 125, 20–21.
iNaturalist (2022). inaturlist.org - zoropsis spinimana. iNaturalist. Available at: https://www.inaturalist.org/observations?nelat=55.08149999602162&nelng=15.04189619759281,15.04189619759281&place_id=any&swlat=47.27011146235569,47.27011146235569&swlng=5.86634248758437,5.86634248758437&taxon_id=127112
Kjær H. W. (2020). Nosferatu-spinne (Zoropsis spinimana). iNaturalist. Available at: https://www.inaturalist.org/observations/41186655
Klesser R., Ruge E. (2021). Erstnachweis von Zoropsis spinimana (DUFOUR 1820) (Araneae: Zoropsidae) in Sachsen und Beobachtungen zur Reproduktionsstrategie 40, 141–145. ResearchGate. Available at: https://www.researchgate.net/publication/355874036_Erstnachweis_von_Zoropsis_spinimana_DUFOUR_1820_Araneae_Zoropsidae_in_Sachsen_und_Beobachtungen_zur_Reproduktionsstrategie
Li D., Jackson R. R. (1996). How temperature affects development and reproduction in spiders: A review. J. Therm. Biol. 21, 245–274. doi: 10.1016/0306-4565(96)00009-5
Marusik Y. M., Kovblyuk M. M. (2004). New and interesting cribellate spiders from Abkhazia (Aranei: Amaurobiidae, Zoropsidae). Arthropoda Sel. 13, 55–61.
Massard J. A., Greimer G. (2018). Neu für Luxemburg - Kräuseljagdspinne in Echternach entdeckt - keine Gefahr für Menschen. Letzebuerger J. 268:18 Available at: journal.lu
Munzinger S. (2015). Citizen science: qualitätssicherung durch motivation. Entomolgy Heute 27, 171–176. doi: 10.1007/s00484-012-0598-7
NABU-naturgucker.de (2022) Zoropsis spinimana - NABU-naturgucker.de. Available online at: https://www.NABU-naturgucker.de/?art=-1361070917 (Accessed December 31, 2022).
Nadolny A. A. (2016). The first record of Zoropsis spinimana (Aranei, Zoropsidae) in the Crimea. Zool. Ecol. 26, 127–128. doi: 10.1080/21658005.2016.1150623
Nentwig W., Blick T., Bosmans R., Gloor D., Hänggi A., Kropf C. (2022) Spiders of Europe. Available online at: https://araneae.nmbe.ch/ (Accessed October 4, 2022).
Nentwig W., Gnädinger M., Fuchs J., Ceschi A. (2013). A two year study of verified spider bites in Switzerland and a review of the European spider bite literature. Toxicon 73, 104–110. doi: 10.1016/j.toxicon.2013.07.010
observation.org (2022) observation.org - Zoropsis spinimana (Observation.org). Available online at: https://observation.org/species/210093/ (Accessed December 6, 2022).
Oxford G. S., Gillespie R. G. (1998). EVOLUTION AND ECOLOGY OF SPIDER COLORATION. Annu. Rev. Entomol. 43, 619–643. doi: 10.1146/annurev.ento.43.1.619
Pantini P., Isaia M. (2019). Araneae.it: the online Catalog of Italian spiders, with addenda on other Arachnid Orders occurring in Italy (Arachnida: Araneae, Opiliones, Palpigradi, Pseudoscorpionida, Scorpiones, Solifugae). Fragmenta Entomologica, 51:127–152. doi: 10.4081/fe.2019.374
Phillips T. B., Bailey R. L., Martin V., Faulkner-Grant H., Bonter D. N. (2021). The role of citizen science in management of invasive avian species: What people think, know, and do. J. Environ. Manage. 280, 111709. doi: 10.1016/j.jenvman.2020.111709
Pocock M. J. O., Roy H. E., Fox R., Ellis W. N., Botham M. (2017a). Citizen science and invasive alien species: Predicting the detection of the oak processionary moth Thaumetopoea processionea by moth recorders. Biol. Conserv. 208, 146–154. doi: 10.1016/j.biocon.2016.04.010
Pocock M. J. O., Roy H. E., Preston C. D., Roy D. B. (2015). The Biological Records Centre: a pioneer of citizen science. Biol. J. Linn. Soc 115, 475–493. doi: 10.1111/bij.12548
Pocock M. J. O., Tweddle J. C., Savage J., Robinson L. D., Roy H. E. (2017b). The diversity and evolution of ecological and environmental citizen science. PloS One 12, e0172579. doi: 10.1371/journal.pone.0172579
Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019
Simon E. (1914). Les arachnides de France. Synopsis générale et catalogue des espèces françaises de l’ordre des Araneae (Paris, France: Roret). doi: 10.5281/zenodo.1067644
Stevens T. K., Hale A. M., Williams D. A. (2023). Environmental and anthropogenic variables influence the distribution of a habitat specialist (Sylvilagus aquaticus) in a large urban forest. Conserv. Sci. Pract. 5, e12882. doi: 10.1111/csp2.12882
Sumner S., Bevan P., Hart A. G., Isaac N. J. B. (2019). Mapping species distributions in 2 weeks using citizen science. Insect Conserv. Divers. 12, 382–388. doi: 10.1111/icad.12345
Suzuki-Ohno Y., Yokoyama J., Nakashizuka T., Kawata M. (2017). Utilization of photographs taken by citizens for estimating bumblebee distributions. Sci. Rep. 7, 11215. doi: 10.1038/s41598-017-10581-x
Thaler K., Knoflach B. (1998). Zoropsis spinimana (Dufour), eine für Österreich neue Adventivart (Araneae, Zoropsidae). Berichte Naturwissenschaftlich - Med. Ver. Innsbr. 85, 173–185.
Wang Y., Casajus N., Buddle C., Berteaux D., Larrivée M. (2018). Predicting the distribution of poorly-documented species, Northern black widow (Latrodectus variolus) and Black purse-web spider (Sphodros Niger), using museum specimens and citizen science data. PloS One 13, e0201094. doi: 10.1371/journal.pone.0201094
Keywords: spatio-temporal species distribution, biogeography, alien species, neozoon, human dispersal
Citation: Wirth A and Schulemann-Maier G (2024) Updated distribution of Zoropsis spinimana (Dufour, 1820; Araneae: Zoropsidae) in Germany and novel insights into its ecology based on a citizen science survey. Front. Arachn. Sci. 3:1383339. doi: 10.3389/frchs.2024.1383339
Received: 07 February 2024; Accepted: 05 March 2024;
Published: 15 March 2024.
Edited by:
Thomas Hesselberg, University of Oxford, United KingdomReviewed by:
Shawan Chowdhury, German Centre for Integrative Biodiversity Research (iDiv), GermanyCopyright © 2024 Wirth and Schulemann-Maier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Wirth, YS53aXJ0aEBOQUJVLW5hdHVyZ3Vja2VyLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.