
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Arachn. Sci., 28 November 2023
Sec. Arachnid Morphology, Systematics and Evolution
Volume 2 - 2023 | https://doi.org/10.3389/frchs.2023.1211418
Introduction: It is generally thought that mating plugs, where present, impede or reduce the possibilities of female subsequent mating. Behavioral studies on numerous spiders, where mating plugs are common, have generally supported this function. However, mating plugs in spiders could plausibly serve other functions as well. Namely, the structure of entelegyne spermathecae—the morphology of most spiders—could require a mechanism that would prevent sperm from leakage, desiccation, and backflow. Although the form and function of mating plugs in several spider species imply their potential adaptation for sperm protection, this function has never been empirically tested.
Methods: Here, we test whether mating plugs in the sheet-web spider Neriene emphana serve as a sperm protective device by investigating its genital morphology, its copulation process, and the precise formation of its amorphous mating plugs.
Results: This species constructs secretion plugs through male-female cooperation. Additionally, we found sperm plugs to be formed as a side product of sperm transfer, as well as an intermediate type of secretion plugs. These plug materials are transferred in different mating stages as documented by variations in the rhythm of male palpal application during copulation. We showed that complete copulations always resulted in formation of secretion plugs at spermathecal entrances via laborious deposition of male materials.
Discussion: While our findings do not reject that secretion plugs in N. emphana prevent females from subsequent mating, we suggest that they must have evolved to provide sperm protection.
Mating plugs refer to solid materials (‘any mass or structure’ in Huber, 2005) deposited in female copulatory openings or on the way to the spermatheca after copulation (Huber, 2005; Eberhard and Huber, 2010). It is generally believed that mating plugs in spiders function as a male mating strategy to impede female remating and thus to protect paternity (Uhl et al., 2010). This ‘mating strategy hypothesis' has been tested in many spiders (Fromhage and Shneider, 2006; Eberhard and Huber, 2010; Uhl et al., 2010). Its variant, the ‘female mating strategy hypothesis' explains that through plug formation, females may reject unwanted, excessive copulations (Kuntner et al., 2012). Alternatively, the structural characteristics of the spermathecae of entelegyne spiders do not in itself present a sperm protection mechanism that would prevent sperm leakage, desiccation, and backflow. This ‘sperm protection hypothesis’ (Huber, 1995) that assumes that mating plugs might serve as a sperm protection mechanism has remained untested. It could well be that these hypotheses in most cases are not mutually exclusive.
Literature makes a distinction between two types of mating plugs in spiders. While a genital plug (GP) consists of severed male genital pieces, the more common type is the amorphous plug (AP) formed by hardened secretions (Uhl et al., 2010; Kuntner et al., 2012). The latter are further categorized as sperm plugs (SP) formed largely by sperm liquid (Huber, 1995; Eberhard, 2004; Eberhard and Huber, 2010; Uhl et al., 2010), or secretion plugs (SCP) formed by viscous secretions other than sperm. Of the mating plugs recorded in over 200 spider species, most are amorphous (Eberhard and Huber, 2010; Uhl et al., 2010), and even more amorphous plugs can be detected when perusing published SEM images (e.g., Hormiga, 2000; Miller, 2007; Álvarez-Padilla and Hormiga, 2011; Ramírez, 2014). However, the mating plug biology for the majority of taxa with amorphous plugs remains unknown (Kuntner et al., 2012).
The most widely accepted hypothesis regarding mating plugs is that they function as a male mating strategy to impede female remating and thus to protect male’s paternity (Snow and Andrade, 2005; Snow et al., 2006; Nessler et al., 2007; also see review by Uhl et al., 2010). This mating strategy hypothesis has been corroborated by behavioral tests in numerous spider species, however, conflicting evidence also exists. Oftentimes, females possess several male sexual organic parts inside one copulatory opening, and these cases strongly indicate that the females have mated with multiple males (e.g., Berendonck and Greven, 2002: Figure 16; Kuntner et al., 2009a: Figure 4E; Fromhage and Shneider, 2006). In some cases, mating plugs can be removed or overcome by a subsequent male (Jackson, 1980; Masumoto, 1993; Knoflach, 2004; Uhl and Busch, 2009; Sentenská et al., 2018). Increasing amounts of evidence show that amorphous plugs might consist of secretions coming from the female (Knoflach, 1998; Aisenberg and Barrantes, 2011), and if so, their formation should require female cooperation or control (Eberhard and Huber, 1998; Aisenberg and Eberhard, 2009; Sentenská et al., 2015; Sentenská et al., 2018; Kuntner et al., 2012). Moreover, females are able to alter the efficiency and the fate of mating plugs in many ways (Eberhard and Huber, 2010; Uhl et al., 2010; Sentenská et al., 2018). Kuntner et al. (2012) found that in Nephila pilipes females add amorphous plugs to the existing male genital plugs, likely to avoid unwanted, excessive copulations. Mating plugs would serve as a mating strategy, be it male’s or female’s, only in polyandrous mating systems. However, while polyandry may be common in spiders, it is not universal (van Helsdingen, 1965; Pollard and Jackson, 1982; Huber, 1993; Foelix, 2011; Wu et al., 2018).
Generally, the structural characteristics of entelegyne spermathecae (the anatomy of the vast majority of spider species) may dictate the need for a sperm protection mechanism. Namely, entelegyne spermathecae have dual openings, one to the copulatory tract for sperm to enter, and the other to the fertilization tract for sperm to exit (Uhl et al., 2010; Foelix, 2011; Zhan et al., 2019). The strongly sclerotized walls of spermathecae and copulatory tracts render the spermathecal entrances and copulatory openings perpetually open. Moreover, sperm in spiders are stored in spermathecae for extended time periods only to be released for egg laying and fertilization (van Helsdingen, 1965; Uhl et al., 2010; Sentenská et al., 2015). These features bring potential risks to the sperm in spermathecae, and these risks relate to leakage, desiccation, and backflow to copulatory tracts. Sperm protection mechanisms are therefore expected in entelegyne spiders, yet any such mechanisms remain unknown.
Mating plugs might solve the sperm protection problem (Huber, 1995). To serve that function, mating plugs should effectively block spermathecal entrances. While their presence is externally widespread in spiders, very few studies have shown that plugs block spermathecal entrances (e.g., Berendonck and Greven, 2002; Kuntner et al., 2009a). Since copulatory tracts in many spiders are short, amorphous plugs deposited in copulatory openings could easily also block spermathecal entrances (e.g., plugs in Diphya spinifera and Metleucauge eldorado in Álvarez-Padilla and Hormiga, 2011, Oedothorax retusus in Uhl et al., 2014). This is probably also the case in those species with long copulatory ducts (Berendonck and Greven, 2002; Kuntner et al., 2009a) and in the case of secretion materials in Micaria sociabilis (Sentenská et al., 2015). Although these mating plugs do decrease female copulation rates, one cannot rule out the possibility that they also serve as a sperm protection mechanism.
The aim of our study is to test the sperm protection function of mating plugs in our model species, Neriene emphana. By investigating the genital morphology and plug formation process, observing interactions between male and female genitalia during copulation, and analyzing variations in the rhythm of male palpal application, we depict the features of plug materials transferred in different mating stages. To this we add behavioral copulatory tests in the field and in the laboratory. Our results demonstrate that secretion plugs do impede female’s subsequent mating, but the elaborate formation process undertaken by spiders to form the secretion plugs suggests their function beyond being a mere mating obstacle.
Neriene emphana is a linyphiid spider that presents a suitable model system to test the predictions derived from the two hypotheses, the mating strategy and/or the sperm protection mechanism. The epigynum of Neriene has a pair of spiral, groove-like copulatory tracts (referred as copulatory groove hereafter) with a slit opening on the inner surface of epigynal atrium (Figures 1C, 2A), and the male palpal embolus has a pointed proper (Figure 3D) that inserts into female’s copulatory openings for insemination (Helsdingen, 1969). During copulation the male palp pumps sperm from the copulatory opening to the spermatheca through a long copulatory groove rather than directly through the embolus of equivalent length as in most other spiders (Foelix, 2011; Ramírez, 2014). Furthermore, the morphology of male palpal terminal apophysis fits with the cone-shaped epigynal atrium (Helsdingen, 1969). If the plug material is also pumped into copulatory tract, one may expect to directly observe from the groove slit whether the spermathecal entrance is blocked or not, with different biological implications. From the functional morphological perspective, we assume that hardening plug material lodged at copulatory opening or the copulatory groove would form an obstacle for the female subsequent mating. On the other hand, only if plug materials were to block the spermathecal entrance would they prevent sperm backflow, leakage and desiccation.
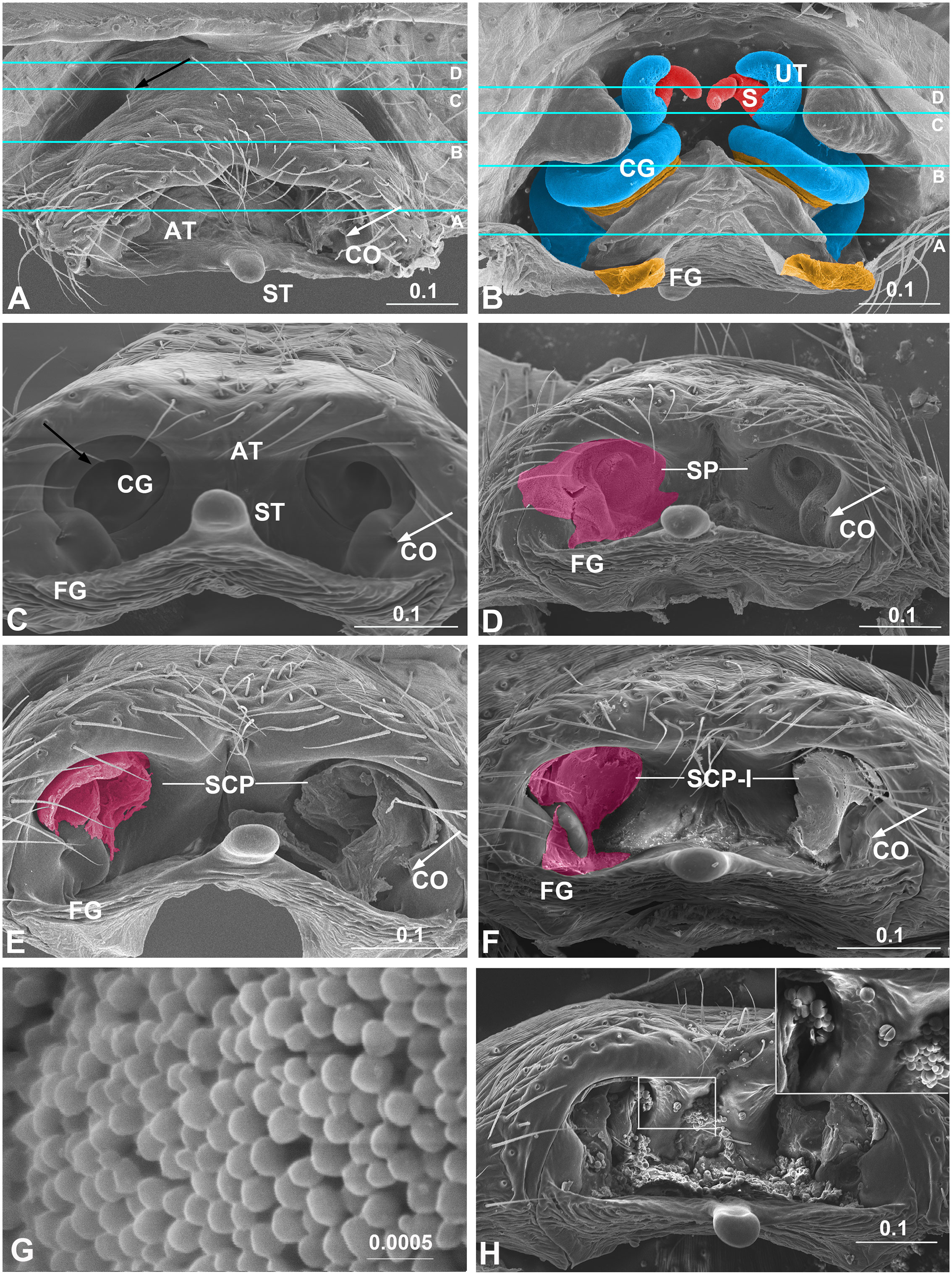
Figure 1 Epigynum and mating plugs of Neriene emphana. (A), ventral view, white arrow points to plugged CO, black arrow to the pit on epigynal surface; (B), inner view, shows tract cones with CG and FG spiral together; (C), caudal view, shows cone-shaped epigynal atrium, white arrow points to CO, black arrow to spiral CG slit; (D), same, shows SP on the atrial inner surface, arrow indicates SP plugged CO; (E), same, shows plug material of SCP spiraling along CG slits, arrow points to exposed CO; (F), same, shows plug material of SCP-I filling epigynal atrium with flat surface, arrow points to SCP-I in CO; (G), spilled spermatozoa, transfer form; (H), epigynum ventral view, shows spermatozoa, released form. Colors: light blue represents CG; magenta, amorphous plugs; red, spermatheca; yellow, FG. Lines in A and B indicate positions of sections in Figure 2 AT, atrium; CG, copulatory groove; CO, copulatory opening; FG, fertilization groove; S, spermatheca; SCP, secretion plug; SCP-I, secretion plug of intermediate type; SP, sperm plug; ST, stretcher; UT, U-tract. Scale bar: mm.
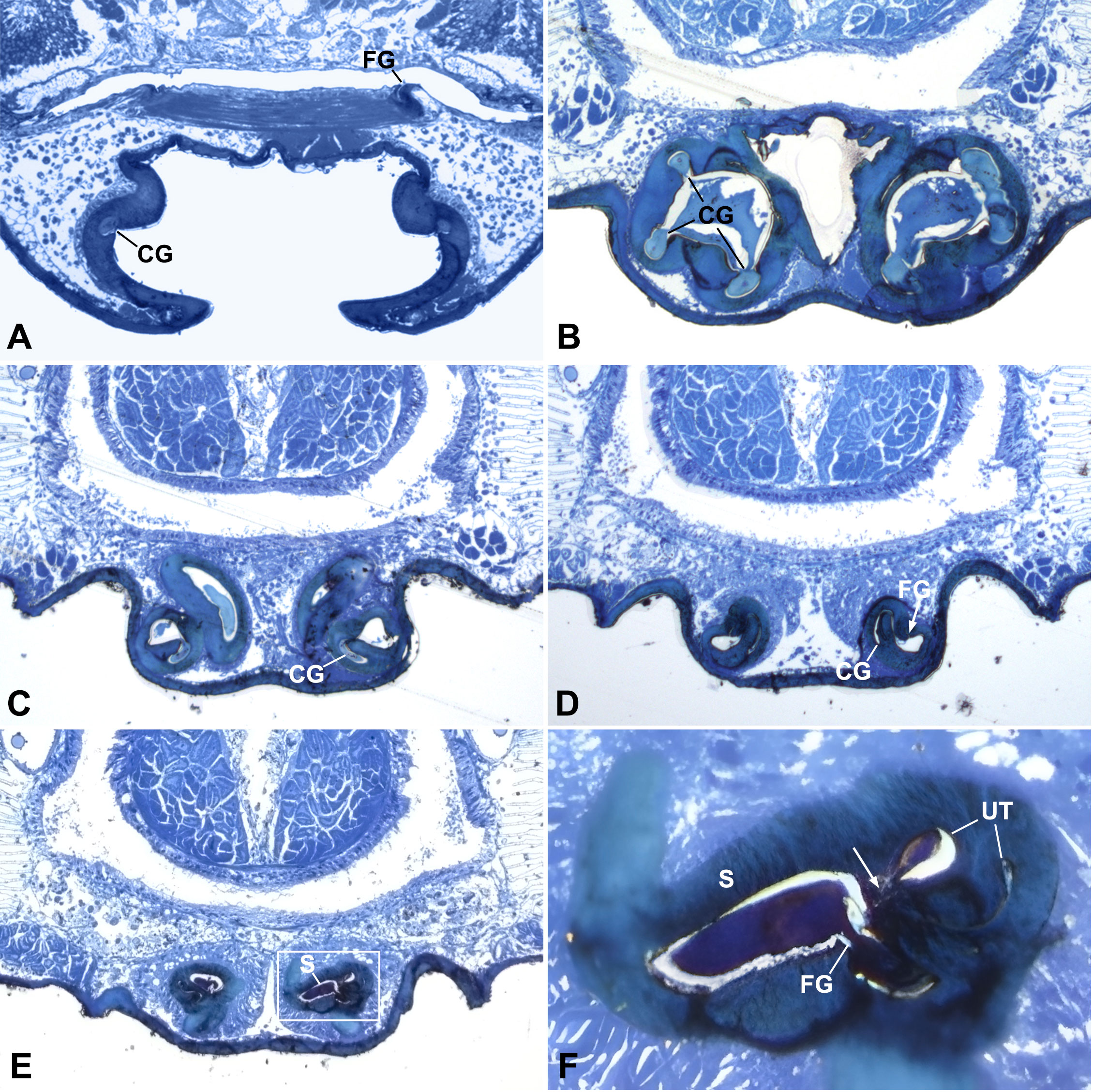
Figure 2 Cross sections of epigynum of Neriene emphana. (A), section above CO; (B), section cross atrium, shows secretion plug in CG and AT. (C), section cross starting point of U-tract, shows wide chamber and plug material inside; (D), section cross top depression of atrium, arrow points to FG opening; (E), section cross spermathecal openings; (F), detail of (E) shows narrow ascending lumen and wide descending lumen of U-tract, arrow points to spermathecal entrance. Section positions indicated in Figures 1A, B. AT, atrium; CG, copulatory groove; FG, fertilization groove; S, spermatheca; UT, U-tract.
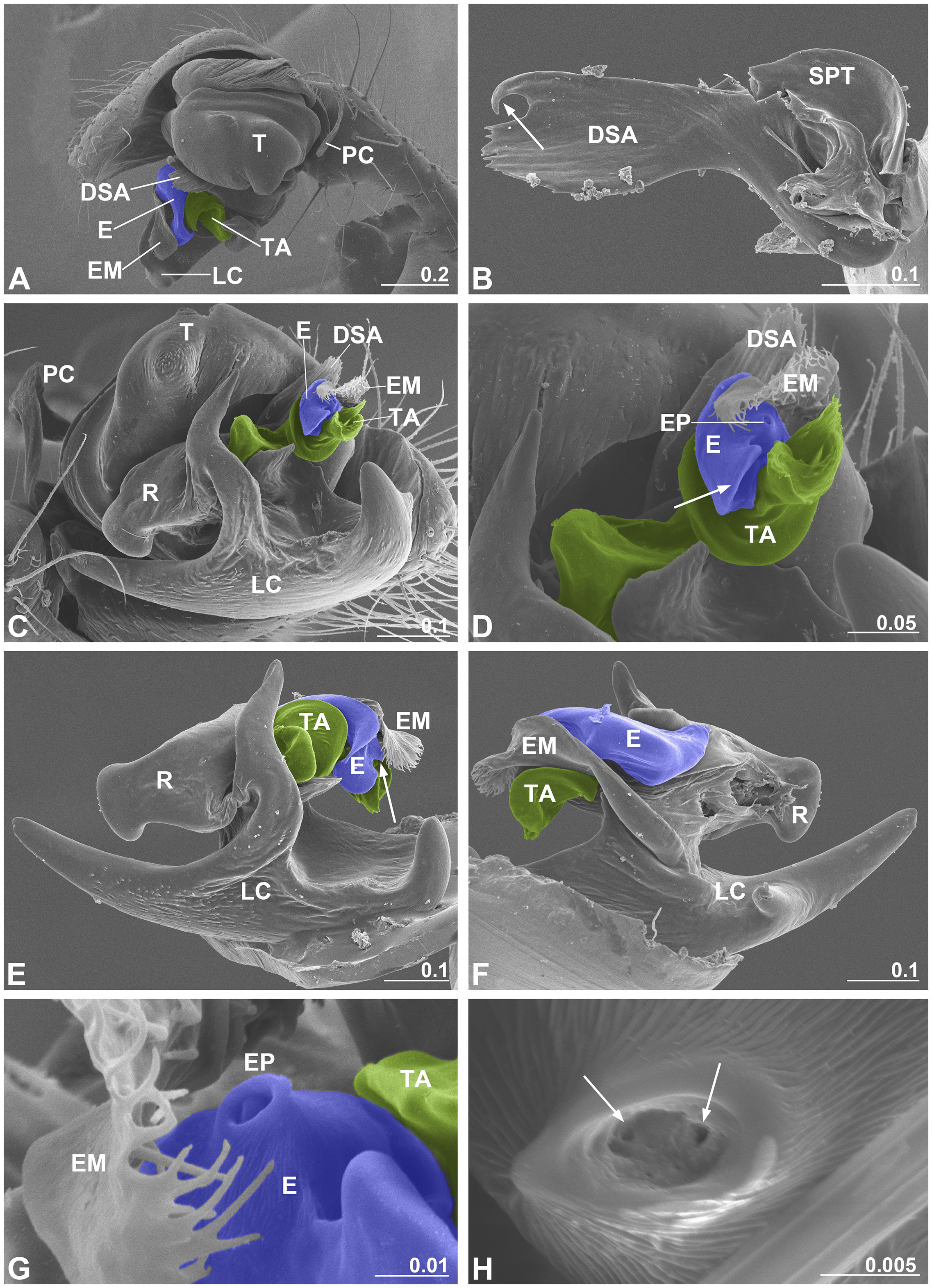
Figure 3 Male palp of Neriene emphana. (A), retrolateral view; (B), DSA, arrow points to pit hook; (C), ventral view, shows embolic division; (D), detail of C, shows screw-shaped TA, arrow points to embolus appendage; (E), embolic division, ventral view, arrow points to EP; (F), embolic division, dorsal view; (G), detail of EP; (H), distal pore of EP, arrows show two openings inside. Colors: blue represents embolus; green, TA. DSA, distal suprategular apophysis; E, embolus; EM, embolus membrane; EP, embolus proper; LC, lamella characteristica; PC, paracymbium; R, radix; SPT, suprategulum; TA, terminal apophysis. Scale bar: mm.
We collected mature and penultimate individuals of N. emphana from vegetation in Songshan National Nature Reserve, Yanqing, Beijing, in June 2015 to 2018. A total of 127 females were collected, 41 of them with a male on their web (referred as paired females hereafter), including four females that were mating with a male when collected, and others alone on the web (referred as single females hereafter). Mating trials in the field involved the single females before collection (n = 13). All specimens were collected into individual plastic tubes (diameter 15 mm, length 60 mm), then shifted to a container (diameter 120 mm, high 100 mm), and placed individually over night for web weaving. Among them, 27 females (including 16 paired, 11 single) molted before mating trials and thus were presumed virgins. Twenty virgins and other 52 females with unknown mating history were used in mating trials in the laboratory. Others were used for morphological study of genitalia and mating plug examination. All spiders were preserved in 75% ethanol before examination.
We used a Leica MZ205A stereomicroscope to examine the specimens. For morphological study by light microscopy (LM) and scanning electron microscopy (SEM), male palps and female epigyna were treated by SIGMA Pancreatin LP 1750 enzyme complex to digest the non-chitinous tissue and cleared by an ultrasonic cleaner (Álvarez-Padilla and Hormiga, 2008). The semi-thin histological serial sections (1μm, HSS) were made of the spider opisthosoma to check whether there are any internal structures that may close the spermathecal entrances and slits of copulatory grooves. Field pictures were captured on an iPhone 6 plus. LM pictures were collected using a Leica DFC 500 camera. SEM images were taken on a Hitachi S-3400N scanning electron microscope (SEM) at China Agriculture University. The semi-thin (1μm) historical serial sections (HSS) were performed using a LECAL EM UC6 microtome with a glass knife and stained with toluidine blue (1%) in an aqueous borax solution (1%) at approximately 90°C for 1-4 minutes. All HSS pictures were collected using a Leica DM5500 microscope with a Leica DFC 500 camera.
The males were introduced onto the female’s web in the field (n = 13) and in individual containers in the lab (n = 72). If copulation did not start in 60 minutes, we treated it as female reject, but for the presumed virgins more time was allowed. Eventually, 27 females mated with a male in container trials, either completely or incompletely (Tables 1, S1). We observed 9 complete copulations from when a male was introduced to a female web to the end of copulation when the male and female separated and the male no longer returning. To check for mating plugs formed at different stages, we terminated 17 copulations, after the first time the male left the female’s web (n = 7), after the second time the male left the female’s web (n = 5), and six palpal applications after the second time the male returned (n = 5), respectively, and in one case copulation was accidently interrupted. In addition, four copulations in the field were artificially interrupted. We introduced a second male to those females having experienced a completed copulation to test whether female N. emphana practice multiple copulations. Video records were taken by iPhone 6 plus from the time a male was introduced onto the female’s web to when the male left.
All analyses of mating behaviors were based on recorded videos. Times of male palpal insertion and wetting were counted for each palpal application. Three phases of copulation were recognized according to significant changes on the rhythm of palpal application. Actions of male and female in the three phases were compared; mean times of insertion and wetting for palpal applications were calculated and plotted, respectively. One-way ANOVA and multiple comparisons based on TukeyHSD algorithms (R Core Team, 2013) were applied to test the significant differences among the three phases. All statistical analyses were performed in R (version 3.5.3).
Epigyna of females involved in mating trials, including those females mating at the time when being collected (n = 4), those that accepted male mating in container (n = 26), and those that rejected the male in field (n = 13) or in container (n = 28 out of 45), as well as additional 38 females, were only examined for mating plugs by SEM. Plug occurrences and plug types were compared among the females of different conditions: whether the females are paired or single when collected, presumed virgin or unknown mating history, and those having copulation interrupted at different phases. The epigyna of the presumed virgins and confirmed mated females (whether completely or interrupted) were directly dried out and mounted for SEM examinations. To check whether amorphous plugs can be destroyed or removed by physical and chemical treatments, we treated 16 epigyna of those females rejecting males in trials by SIGMA Pancreatin LP 1750 enzyme complex, the other 25 females were saved as control. Additional 12 epigyna of females that did not undergo mating trials and the one female whose copulation was artificially interrupted in the field were cleared by an ultrasonic cleaner, two by both enzyme and ultrasonic cleaner, and another 24 as control.
The epigynum of N. emphana has the epigynal plate ventrally depressed, thereby forming an atrium, with two additional pits anterior to the atrium and a stretcher with a shallow pit protruding from the posterior margin of the atrium (Figure 1A). The paired groove-like epigynal tracts, copulatory and fertilization grooves spiral together, forming paired cones, with paired spermathecae lodged at their tops (Figure 1B), and groove slits opening on the inner surface of the atrium and copulatory openings located at each side within the atrium (Figure 1C). Copulatory grooves spiral upwards from the copulatory openings to the spermathecae, forming a U-shaped tract (U-tract hereafter) before entering the spermatheca. The weaker fertilization grooves spiral from the spermathecae downwards to the posterior margin of the atrium, then loop to extend anteriorly into the epigastric furrow along the dorsal surface of the epigynum. The sclerotized groove slits spiral along the atrial inner surface to shape the cone-like atrium (Figure 1C). HSS pictures show that copulatory grooves are C-shaped in cross section, with a break open to the atrium (Figure 2B). The chambers of U-tract are asymmetric, with narrow ascending lumen and wide descending lumen (Figures 2E, F). The plug material lodged in the copulatory groove does not enter the narrow ascending lumen of the U-tract (Figures 2C, D). Furthermore, the plug material that fills the copulatory groove chambers and the atrium differs from that deposited in the spermathecae (Figures 2B–D). We did not find any structure that would close the spermathecal entrances and copulatory grooves.
As is typical for linyphiid spiders, the N. emphana male palp is characterized by a complex embolic division with sclerites that interact with the female epigynal elements during copulation (Figure 3). Ventrally, lamella characteristica is a large plate with several projections (Figure 3C). The radix accepts the sperm duct coming from the suprategulum and transmits it to the embolus (Figure 3F). The embolus has a pointed proper with two openings included within the proper pore, covered by the embolic membrane arising from the membranous area connecting the radix and the suprategulum of the male palp, and a large appendage that extends out distally (Figures 3C–H). The screw-shaped terminal apophysis (Figures 3C, D) matches the cone-shaped epigynal atrium (Figure 1C), and the distal suprategular apophysis is modified as a small pit hook (Figure 3B).
85 females were subjected to mating trials (13 in the field, 72 in individual containers). All the males introduced onto female webs actively tried to approach the females and attempted mating. However, only 27 females in containers, including 20 presumed virgins, accepted male mating. Female N. emphana usually stays below her sheet web also when mating, but may escape to the scaffold to avoid male mating approaches (Figure S1D). During copulation process a male continuously performed palpal applications, except for two pauses when the male left for palpal induction. In the whole process the female readily collaborated. Copulations in nine trials were complete, five of them were video recorded entirely, one of the five videos was chosen as working video. Other trials were interruped at different stages (Table 1; for details see Tables S1, S2).
Most copulations commenced within 30 min (5.72 min, mean = 25.08 min, range = 1.75–114.00 min, n = 27, Table 1), two presumed virgins were allowed more time to start (Table S1). Males and females mounted into copula to resume palpal applications that lasted about 95 min (mean = 85.44 min, range = 45.00–139.10 min, n = 24). Typically, two pauses ensued for palpal induction (first 4.15 min, mean = 4.62 min, range = 3.80–6.07 min, n = 5; second 2.40 min, mean = 2.42 min, range = 1.98–2.97 min, n = 5). During the interval between the two pauses, the male returned to the female’s web and resumed palpal applications (2.84 min, mean = 2.30 min, range = 1.29–3.71 min, n = 5). After the second return of the male, copulation again took a relatively long time (86.61 min, mean = 63.51 min, range = 49.12–86.61 min, n = 5). The whole process lasted about three hours (191.81 min, mean = 178.04 min, range = 145.38–235.41 min, n = 9). 30 min after a completed copulation, we introduced another male onto the female’s web (n = 9), of which only one female accepted the second male, but the mating only lasted 37 min, then the male moved on.
Males performed palpal applications alternating the palps that lock to the female’s epigynum (Video S1). Each palpal application contributed to insemination of the female epigynal tract by a series of palpal actions: inserting the palp, the basal haematodocha expanding and compressing, and withdrawing the palp. After the palpal embolus proper inserted into the female copulatory opening, the basal haematodocha expanded. The consequential palpal bulb rotation facilitated palpal elements to lock with the epigynum. In particular, the male palpal terminal apophysis locked with the epigynal atrium thereby providing support for the compressing of the expanded basal haematodocha. A powerful compression allowed for ejaculation into the copulatory groove. When deflating, the male palp became loose again and while the palpal elements would return to their original position, the embolus was withdrawn from the copulatory opening. After that the palp was bent to the male’s mouthparts. Each insemination was followed by palpal wetting (i.e. the male using mouthparts to lubricate his organ) before the male employed the other palp for another copulation bout. By the end of copulation and after leaving the female, the male usually continued to wet his used palp.
In a single case a male inseminated using one palp, but failed with the other. When compressing, the expanded basal haematodocha of the failed palp would suddenly collapse, and the male palp decoupled from the epigynum without successful ejaculation. After two or three attempts, the male switched to the other palp (Video S2). This male nonetheless performed a complete copulation, the whole process lasting 218.90 min (No. 8, Table S1).
During the two pauses, a male ran off the female’s web to build a triangular sperm web with a few silken threads (Video S3; Figure S1C), deposited a drop of liquid on its upper surface, then returned to its underside to alternately charge the palps before returning to the female’s web to resume palpal applications.
The rhythm of male palpal application changed through the copulation process (Figures 4, 5). The times used for insertion and wetting of each palpal application varied after the two pauses of palpal induction, and were not synchronous (Tables 1, S2). In the first section before the first pause, a male performed palpal applications fast and smoothly for both palpal insertion and wetting (insertion: mean = 2.65s, range = 0.56–11.87s, n = 1292; wetting: mean = 1.28s, range = 0.12–11.10s, n = 1292; Video S1). After the male returned from the first palpal induction, the palpal applications remained smooth (Video S4) with similar insertion time (mean = 2.46s, rang = 1.34–4.34s, n = 24) and significantly longer wetting (mean = 3.39s, rang = 1.34–5.35s, n = 23, p < 0.001). Such a rhythm was kept for several palpal applications also subsequent to the second papal induction (insertion: mean = 2.90s, range = 1.46–4.22s, n = 14; wetting: mean = 2.76s, range = 1.68–4.31s, n = 14). Then the insertion times increased significantly, and the wetting times remained longer (insertion: mean = 27.94s, range = 4.03–48.62s, n = 172, p < 0.001; wetting: mean = 3.50s, range = 0.69–8.25s, n = 197).
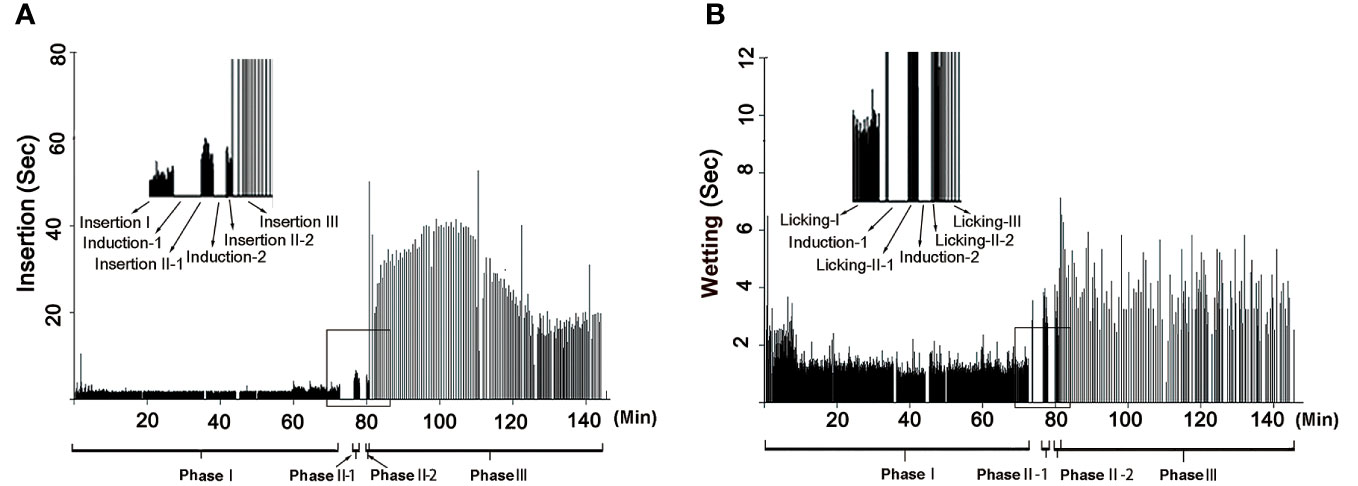
Figure 4 Rhythm of copulation in Neriene emphana. (A), insertion, each bar represents an insertion; (B), wetting, each bar represents a wetting. Induction-I and Induction-II refer to the two pauses when the male left for palpal induction; Phase II = Phase II-1 + Phase II-2.
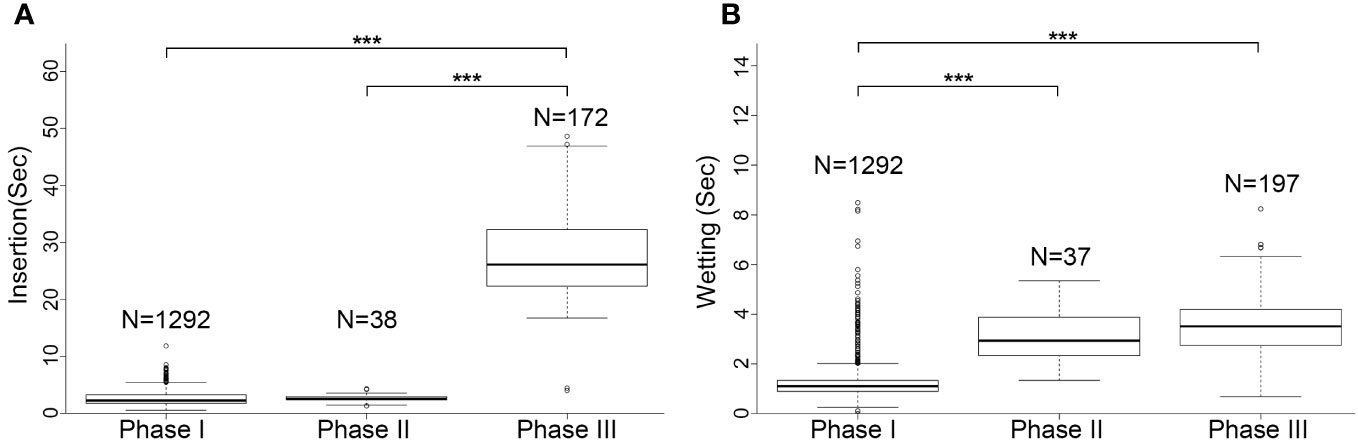
Figure 5 Comparison of palpal applications among three copulation phases in Neriene emphana. (A), insertion; (B), wetting. ***P < 0.001.
Based on the significant variation in the rhythm of palpal application, we divided the copulatory process into three phases (Figure 4; Table 1): Phase I refers to the copulation process from the first palpal application to the first pause when the male left for palpal induction. Palpal applications in this phase are smooth and fast, with short insertion and wetting times for each palpal application (Video S1). Phase II includes two sections: the interval between the two pauses (II-1) and a short period from the resumed palpal application after the second induction to the insertion time drastically increasing (II-2). As a short transition, Phase II only includes a few palpal applications, which are marked by short, smooth insertion and long wetting (Video S4). Phase III starts from the time of insertion drastically increasing to the male finally leaving the female. Palpal applications in this phase have significantly longer insertion and longer wetting than those in Phase I; the palpal movements of inserting, compressing and withdrawing became less predictable, with pauses. We also observed that the female’s abdomen trembled with the male’s action and a special pose of the male having both palps simultaneously rise up against the epigynum (Video S5).
All mating plugs in N. emphana are amorphous and span the copulatory openings, groove slits and the inner surface of the atrium (Figure 1). Their different appearances and textures suggest that the plug materials differ in thickness, viscosity and liquidity. Three plug types were recognized in this species: sperm plug (SP), secretion plug (SCP) and secretion plug of intermediate type (SCP-I). Sperm plugs are formed largely by spilled sperm liquid that floods the atrium and dries out, leaving a layer of sperm granules to cover the inner surface of the atrium, thereby sealing copulatory groove slits and copulatory openings simultaneously (Figures 1D, 1G). That the shape of sperm plugs adjusts to the shape of the atrium suggests the sperm liquid to be of good fluidity. The remaining sperm scabs are fragile, judging from their easy manipulation. Conversely, secretion plugs refer to hardened secretions lodged in the copulatory groove slits (Figure 1E). These plug materials extend along the copulatory groove slits (Figure 2B) from copulatory openings to spermathecal entrances. These secretions largely retain their original shape when manipulated, which indicates their greater density and viscosity. The hardened secretion plugs are durable and are difficult to be removed without severe damage to the epigynal tracts. Finally, secretion plugs of intermediate type (Figure 1F) are formed by thick secretions that fill the epigynal atrium and copulatory openings with a flat surface. The materials forming them are thicker than sperm liquid, but have less surface tension and better fluidity than those of secretion plugs. In epigyna of females that have laid eggs, sperm granules, inflated and broken in halves, overlap with secretion plugs (Figure 1H).
Of the three mating plug types, secretion plugs were the most common in N. emphana. They were detected not only in the epigynal atria of all females that had undergone complete copulation (n = 8, the other one lost), but also in all the females that had rejected male mating (n = 41, both in the field (n = 13), and the lab (n = 28 out of 45, the other 17 not checked), as well as in all the mature females randomly sampled in the field (n = 31 out of 38, the other seven are presumed virgins or juveniles). All secretion plugs remained intact even when treated enzymatically (n = 16), mechanically (n = 12), or both (n = 2). Sperm plugs were found in the females with interrupted copulation by the end of Phase I (n = 6, the other one juvenile; Table 1). Secretion plugs of intermediate type were found in those females whose copulations had been interrupted in Phase II-1 (n = 4, the other one lost) and Phase II-2 (n = 5). In several cases, the epigynal tracts were plugged on one side only (n = 3), or were plugged with two different plug types (n = 1). Surprisingly, a female whose partner successfully mated with one palp but not the other, had secretion plugs in both sides. Of the five females whose mating had been interrupted in the field (n = 4) and the lab (n = 1), two had secretion plugs, two had sperm plugs, and one was plug free (treated by ultrasonic cleaning). None of the control, virgin females, were plugged (n = 4).
Prior work on spider plug biology has shown that the phenomenon is widespread, and that plugs’ paternity protection function depends on whether or not they completely block the female tract (Masumoto, 1993; Uhl et al., 2010; Sentenská et al., 2015). Our study adds to this understanding by the finding that amorphous mating plugs do not only differ in the plug materials but also in plug origin and function. In this study we recognize three types of amorphous mating plugs in a sheet web spider N. emphana. They are formed by different materials and through different behavioral processes. Their common property is that they all derive from male materials and are deposited within epigynal tracts during copulation. We demonstrate that sperm plugs are scabs of sperm liquid overflown from copulatory groove slits, and are thus a side product of sperm transfer. On the other hand, secretion plugs are produced via male and female collaborative actions and represent a final product of completed copulations. Although secretion plugs in N. emphana might well impose physical obstacles to subsequent mating, thus serving paternity protection, the driving force behind their formation is more likely to be sperm protection.
We interpret the following evidence to suggest that secretion plugs serve as a sperm protection mechanism in N. emphana. The HSS images reveal no internal structure that might close the spermathecal entrances (Figure 2E). Secretion plugs extending through copulatory groove slits to the U-tracts do reach the spermathecal entrances as predicted by the sperm protection hypothesis (Figures 1B–E), and such blockage might take on the functionality to prevent sperm leakage and desiccation after copulation, as well as to prevent the sperm backflow from spermathecal entrances. The U-tracts with narrowed ascending lumens located in front of the spermathecae (Figure 2F) cannot close the spermathecal entrances but might serve as an obstacle to prevent the thick secretions from being over pushed into the spermatheca under the high pumping pressure during insemination. At the same time, these solid mating plugs lodged at spermathecal entrances can present obstacles to subsequent mating, which is usually used as evidence for the mating strategy hypothesis. However, these secretion plugs present qualities that go beyond a simple mating obstacle.
Our mating trial data reveal that the formation of secretion plugs is an obligate part of copulation in N. emphana. While the obvious goal of male palpal applications is to deposit sperm in spermathecae (van Helsdingen, 1965; Knoflach, 2004), our results show that sperm transfer in N. emphana only takes place in the first half of the copulation process and that all completed copulations result in the formation of secretion plugs. During the long copulation process, the basal haematodocha rhythmically expands and compresses (Videos S1, S4, S5) and thus male materials are transferred throughout this time period, except during the two pauses for palpal induction. Variation in mating rhythms and the three different types of mating plugs generated suggest that male materials transferred in the three phases are different (Figures 1, 4). According to Michalik and Lipke (2013), the spilled granules formed by the end of Phase I are spermatozoa in the transfer form, i.e. inactive sperm encapsulated by a sheath (Figure 1G). The bifid granules on the epigyna of the females having laid eggs, on the other hand, should be the spermatozoa in the released form (Figure 1H). Although one or more types of secretions are transferred with sperm fluid (Michalik and Uhl, 2005; Michalik, 2009; Michalik and Lipke, 2013), the plug textures suggest that the materials of those finally formed secretion plugs are more viscous with higher surface tension (Figure 1E) than those of the two intermediate products, sperm plugs and the secretion plugs of the intermediate type (Figures 1D, F). Therefore, the tasks of male palpal applications in the three phases are different: during Phase I, a male is devoted to sperm transfer; in Phase III he transfers materials to form secretion plugs; and Phase II is a transition stage. Since the thickness and viscosity of male materials increased after the two pauses, the male palpal induction should be responsible for filling the male palps with a material in addition to the regular “sperm induction” (Gerhardt, 1923; van Helsdingen, 1965; Knoflach, 2004; Michalik et al., 2010; Foelix, 2011). The two openings included within the terminal pore of the male palpal embolus (Figure 4H) suggest that secretion plugs consist of two components transferred separately. We hypothesize that two components are combined, forming viscous secretions after ejaculation to avoid blocking the fine duct in the embolus. Similar phenomena are also found in other spiders (Suhm et al., 1996; Uhl et al., 2014; Sentenská et al., 2015).
The formation of secretion plugs in N. emphana is an energetically costly and time-consuming work. SEM images show that their plug materials extend along the spiral slits of copulatory grooves from copulatory openings to the spermathecae (Figure 1E). The palpal applications in Phase III are less smooth than those in Phase I, with frequent pauses, and are accompanied with female abdomen trembling and a special pose of the male having both palps simultaneously rise up against the epigynum (Video S5). It seems that male engagements (pumping into the epigynal tracts and withdrawals of the palp) are not without difficulty, judging from the need to also use the other palp for assistance. Thus, the insertion of each palpal application in Phase III is significantly longer than those in Phases I and II (Figures 4, 5; Videos S1, S4, S5). Including the two pauses of male palpal induction, it takes nearly half the total copulation time to form secretion plugs (Figure 4; Tables 1, S2).
Secretion plugs in N. emphana are produced under the collaboration of both sexes. Both sperm plugs and secretion plugs are formed by male materials spilled from copulatory groove slits(Figure 1), but their formation processes are different. The low viscosity of sperm liquid enables it to easily spill out from groove slits under the pumping pressure and from copulatory openings due to the pressure changes when the male palpal embolus is withdrawn. We thus interpret the sperm plug in N. emphana to be a side product of sperm transfer. Similar sperm plugs with sperm-like granules are often detected in copulatory openings in many spiders (e.g., SEM images in Álvarez-Padilla and Hormiga, 2011). In comparison, secretion plugs are neither formed by chance, nor are they side products. During copulation in N. emphana, successful ejaculations are under the collaboration of both sexes. A “lock-and-key” mechanism between the male palpal terminal apophysis and epigynal atrium (Figures 1C, 3D; see also van Helsdingen, 1969) locks together the male palp with the female epigynum. This tight locking provides stable support to the male when compressing his expanded basal haematodocha, facilitating ejaculation and at the same time preventing decoupling of the relatively short palpal embolus proper and the copulatory opening. Just like sperm, the secretion plug materials also come from the male palp. Comparing to sperm transfer, however, it is even harder to pump the thick secretions into copulatory openings and further push them to block the spermathecal entrances (Video S5). This process thus would be inconceivable without female’s cooperation.
We interpret all the above as corroborating evidence for secretion plugs in N. emphana serving sperm protection. This is not to suggest that N. emphana secretion plugs do not impede female remating. First, the thick plug secretions filled in copulatory grooves would block the way of sperm entering the spermathecae (Figure 2B), since males N. emphana transfer sperm by pumping, rather than directly delivering sperm into the spermathecae. Second, the hardened secretion plugs have changed the shape of the epigynal atrium (Figures 1C, E), that breaks its strict match to male palpal terminal apophysis, therefore sperm might not be successfully transferred into copulatory grooves. The explanation of why one male inseminated one side only but his female partner had both epigynal tracts plugged is that the female must have already been unilaterally plugged before the mating trial (Video S2). Although only observed in one copulation, unilateral mating plugs are not uncommon judging from 3 out of 99 cases in this study. Nevertheless, lodging such thick plug materials at copulatory openings, or any parts of copulatory grooves would efficiently impede female remating in both ways; only pushing them to get to spermathecal entrances, even though costly as we described above, the plugs might function as a sperm protection mechanism. This reinforces our interpretation that the presence of secretion plugs in N. emphana does impose mating physical obstacles, but their target is to block spermathecal entrance.
The mating strategy hypothesis, either male or female using mating plugs to block unwanted copulations, theoretically builds on the assumption of female polyandry. Thus, in natural or experimental populations of polyandrous spiders, the female plugging frequencies, high or low, should be less than 100% (e.g., Kuntner et al., 2009b; Kuntner et al., 2012; Sentenská et al., 2015; Sentenská et al., 2018; Uhl and Busch, 2009). While this is difficult to measure in nature, not all spiders are polyandrous (van Helsdingen, 1965; Pollard and Jackson, 1982; Huber, 1993; Foelix, 2011; Wu et al., 2018). Because secretion plugs examined in this study remain intact after being treated by enzyme (n =16) and ultrasonic cleaner (n =12) or both (n = 2), this indicates they are solid enough to serve as efficient mating obstacles, although we cannot rule out the possibility of males displacing/dissolving them. The formation of secretion plugs is an obligate outcome of each completed copulation in N. emphana and all mature females in the field had secretion plugs. Given the above evidence, mated and plugged females may generally have little chance for remating, unless one of the paired tracts remains plug free (Snow et al., 2006). Our results thus suggest that the formation of secretion plugs in N. emphana targets to block spermathecal entrances, however, once present, they also prevent females from remating.
In conclusion, while secretion plugs may prevent females from subsequent mating, their more obvious function in N. emphana seems to be sperm protection. Our study provides the first support for the sperm protection hypothesis. However, this conclusion derived from the study in N. emphana may not simply generalize to other spiders due to its peculiar genital morphology and special mating system. Considering the known variation in the architecture of epigynal tracts and multiple origins of plug materials, mating plugs probably have different functions across the diversity of spiders.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
ST and LT contributed to conception and design of the study. ST conducted behavior experiments. HJ and QW conducted morphological analyses. ST and YZ performed statistical analysis. ST and LT wrote the first draft. MK and LT contributed critically to writing of the manuscript. All authors contributed to manuscript revision, and have read and approved the submitted version.
This research was funded by the National Natural Sciences Foundation of China (grant Nos. 31572244 and 31872188). MK was supported by the Slovenian Research and Innovation Agency (grants P1-0255 and J1-50015).
We thank Wei Chen, Zifu Zhao and Qingchen Xia for their help in field work and lab experiments. Addressing comments by four reviewers has improved our narrative substantially.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author MK declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frchs.2023.1211418/full#supplementary-material
Aisenberg A., Barrantes G. (2011). Sexual behavior, cannibalism, and mating plugs as sticky traps in the orb weaver spider Leucauge argyra (Tetragnathidae). Naturwissenschaften 98, 605–613. doi: 10.1007/s00114-011-0807-y
Aisenberg A., Eberhard W. G. (2009). Female cooperation in plug formation in a spider: Effects of male copulatory courtship. Behav. Ecol. 20, 1236–1241. doi: 10.1093/beheco/arp117
Álvarez-Padilla F., Hormiga G. (2008). A protocol for digesting internal soft tissues and mounting spiders for scanning electron microscopy. J. Arachnol. 35, 538–542. doi: 10.1636/Sh06-55.1
Álvarez-Padilla F., Hormiga G. (2011). Morphological and phylogenetic atlas of the orb-weaving spider family Tetragnathidae (Araneae: Araneoidea). Zool. J. Linn. Soc. 162, 713–879. doi: 10.1111/j.1096-3642.2011.00692.x
Berendonck B., Greven H. (2002). Morphology of female and male genitalia of Latrodectus revivensis Shulov 1948 (Araneae, Theridiidae) with regard to sperm priority patterns. Eds. Toft S., Scharff N. European Arachnology 2000: Proceedings of the 19th European Colloquium of Arachnology (Aarhus University Press), 157–167.
Eberhard W. G., Huber B. A. (1998). Courtship, copulation, and sperm transfer in Leucauge mariana (Aaraneae, Tetragnathidae) with implications for higher classification. J. Arachnol. 26, 342–368. doi: 10.2307/3706241
Eberhard W. G. (2004). Male-female conflict and genitalia: failure to confirm predictions in insects and spiders. Biol. Rev. Camb. Philos. Soc. 79, 121–186. doi: 10.1017/S1464793103006237
Eberhard W. G., Huber G. A. (2010). Spider genitalia: Precise maneuvers with a numb structure in a complex lock. In: The evolution of primary sexual characters in animals Eds. Leonard L. J., Córdoba-Aguilar A. (Oxford: Oxford University Press). pp. 249–284.
Fromhage L., Schneider J. M. (2006). Emasculation to plug up females: The significance of pedipalp damage in Nephila fenestrata. Behav. Ecol. 17, 353–357. doi: 10.1093/beheco/arj037
Gerhardt U. (1923). Weitere sexualbiologische Untersuchungen an Spinnen. Arch. Naturgesch 89 (A), 1–225.
Hormiga G. (2000). Higher level phylogenetics of erigonine spiders (Araneae, Linyphiidae, Erigoninae). Smithson. Contrib. to Zool. 609, 1–160. doi: 10.5479/si.00810282.609
Huber B. A. (1993). Genital mechanics and sexual selection in the spider nesticus cellulanus (Araneae, nesticidae). Can. J. Zool. 71, 2437–2447. doi: 10.1139/z93-340
Huber B. A. (1995). The retrolateral tibial apophysis in spiders-shaped by sexual selection? Zool. J. Linn. Soc. 113, 151–163. doi: 10.1111/j.1096-3642.1995.tb00931.x
Huber B. A. (2005). Sexual selection research on spiders: progress and biases. Biol. Rev. Camb. Philos. Soc. 80, 363–385. doi: 10.1017/S1464793104006700
Jackson R. R. (1980). The mating strategy of Phidippus johnsoni (Araneae, Salticidae): II. Sperm competition and the function of copulation. J. Arachnol. 8, 217–240.
Knoflach B. (1998). Mating in Theridion varians Hahn and related species (Araneae: Theridiidae). J. Nat. Hist. 32, 545–604. doi: 10.1080/00222939800770301
Knoflach B. (2004). “Diversity in the copulatory behaviour of comb-footed spiders (Araneae, Theridiidae),” in Diversität und Biologie von Web spinnen, Skorpionen und anderen Spinnentieren, vol. 12 . Ed. Thaler K. (Oberö'sterreichisches Landesmuseum, Linz), 161–256.
Kuntner M., Coddington J. A., Schneider J. M. (2009a). Intersexual arms race? Genital coevolution in nephilid spiders (Araneae, Nephilidae). Evol. (N. Y). 63, 1451–1463. doi: 10.1111/j.1558-5646.2009.00634.x
Kuntner M., Gregorič M., Zhang S., Kralj-Fišer S., Li D. (2012). Mating plugs in polyandrous giants: Which sex produces them, when, how and why? PloS One 7 (7), e40939. doi: 10.1371/journal.pone.0040939
Kuntner M., Kralj-Fišer S., Schneider J. M., Li D. (2009b). Mate plugging via genital mutilation in nephilid spiders: An evolutionary hypothesis. J. Zool. 277, 257–266.
Masumoto T. (1993). The effect of the copulatory plug in the funnel-web spider, agelena limbata (Araneae: agelenidae). Ecol. Res. 21, 55–59. doi: 10.1007/BF02347491
Michalik P. (2009). The male genital system of spiders (Arachnida, Araneae) with notes on the fine structure of seminal secretions. Contrib. to Nat. Hist. 12, 959–972. doi: 10.1186/1742-9994-2-12
Michalik P., Knoflach B., Thaler K., Alberti G. (2010). Live for the moment-adaptations in the male genital system of a sexually cannibalistic spider (Theridiidae, Araneae). Tissue Cell. 42, 32–36. doi: 10.1016/j.tice.2009.06.004
Michalik P., Lipke E. (2013). “Male reproductive system of spiders,” in Spider Ecophysiology Nentwig W. (Berlin Heidelberg: Springer-Verlag), 29–39. doi: 10.1007/978-3-642-33989-9_13
Miller J. A. (2007). Review of Erigonine spider genera in the Neotropics (Araneae: Linyphiidae, Erigoninae). Zool. J. Linn. Soc. 149, 1–263. doi: 10.1111/j.1096-3642.2007.00233.x
Nessler S. H., Uhl G., Schneider J. M. (2007). Genital damage in the orb-web spider Argiope bruennichi (Araneae: Araneidae) increases paternity success. Behav. Ecol. 18, 174–181. doi: 10.1093/beheco/arl074
Pollard S.D., Jackson R.R. (1982). The biology of Clubiona cambridgei (Araneae, Clubionidae): interspecific interactions. N. Z. J. Ecol. 5, 44–50.
Ramírez M. (2014). The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bull. Am. Museum Nat. Hist. 390, 1–374. doi: 10.1206/821.1
R Core Team (2013). R: A language and environment for statistical computing (Vienna: R Foundation for Statistical Computing).
Sentenská L., Pekár S., Lipke E., Michalik P., Uhl G. (2015). Female control of mate plugging in a female-cannibalistic spider (Micaria sociabilis). BMC Evol. Biol. 15 (18), 1–12. doi: 10.1186/s12862-014-0278-9
Sentenská L., Pekár S., Uhl G. (2018). Deposition, removal and production site of the amorphous mating plug in the spider Philodromus cespitum. Sci. Nat. 105 (50), 1–13.
Snow L. S. E., Abdel-Mesih A., Andrade M. C. B. (2006). Broken copulatory organs are low-cost adaptations to sperm competition in redback spiders. Ethology 112, 379–389. doi: 10.1111/j.1439-0310.2006.01163.x
Snow L. S. E., Andrade M. C. B. (2005). Multiple sperm storage organs facilitate female control of paternity. Proc. Biol. Sci. 272, 1139–1144. doi: 10.1098/rspb.2005.3088
Suhm M., Thaler K., Alberti G. (1996). Glands in the male palpal organ and the origin of the mating plug in Amaurobius species (Araneae: Amaurobiidae). Zool. Anz. 234, 191–199.
Uhl G., Busch M. (2009). Securing paternity: Mating plugs in the dwarf spider Oedothorax retusus (Araneae: Erigoninae). Biol. J. Linn. Soc 96, 574–583. doi: 10.1111/j.1095-8312.2008.01165.x
Uhl G., Kunz K., Vöcking O., Lipke E. (2014). A spider mating plug: Origin and constraints of production. Biol. J. Linn. Soc. 113, 345–354. doi: 10.1111/bij.12359
Uhl G., Nessler S. H., Schneider J. M. (2010). Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica 138, 75–104. doi: 10.1007/s10709-009-9388-5
van Helsdingen P. J. (1965). Sexual behaviour of Lepthyphantes leprosus (Ohlert) (Araneida, Linyphiidae), with notes on the function of the genital organs. Zool. Meded. 41, 15–42. doi: 10.1007/s10709-009-9388-5
van Helsdingen P. J. (1969). A reclassification of the species of Linyphia latreille based on the functioning of the genitalia (Araneida, Linyphiidae), Part I. Linyphia Latreille Neriene Blackwall. Zool. Verh. 105, 1–302.
Wu Q., Wen L., Chen J., Li D., Jiao X. (2018). Experimental evidence for the genetic benefits of female mate choice in the monandrous wolf spider Pardosa astrigera. Anim. Behav. 144, 87–93. doi: 10.1016/j.anbehav.2018.08.009
Keywords: entelegyne spermatheca, mating behavior, mating strategy, sperm protection mechanism, mating plug, secretion plug, sperm plug
Citation: Tian S, Jiang H, Zhan Y, Wu Q, Kuntner M and Tu L (2023) The function of mating plugs in the spider Neriene emphana: mating strategy or sperm protection? Front. Arachn. Sci. 2:1211418. doi: 10.3389/frchs.2023.1211418
Received: 24 April 2023; Accepted: 03 November 2023;
Published: 28 November 2023.
Edited by:
Jason E. Bond, University of California, Davis, United StatesReviewed by:
María Del Carmen Viera, University of the Republic, UruguayCopyright © 2023 Tian, Jiang, Zhan, Wu, Kuntner and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihong Tu, dHVsaEBjbnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.