- 1Section of Neuroscience & Clinical Pharmacology, Department of Biomedical Science, University of Cagliari, Cagliari, Italy
- 2Child & Adolescent Neuropsychiatric Unit “A. Cao” Paediatric Hospital, ASL Cagliari, Cagliari, Italy
- 3Unit of Clinical Psychiatry, University Hospital Agency of Cagliari, Cagliari, Italy
- 4Unit of Psychiatry, Department of Medical Science and Public Health, University of Cagliari, Cagliari, Italy
- 5Department of Pharmacology, Dalhousie University, Halifax, NS, Canada
Several studies suggest that children and adolescents with autism spectrum disorder (ASD) often present deficits in executive functions (EFs). The research on cold EF shows a high heterogeneity across different cohorts of patients as well as different study designs, while studies investigating hot EF and their relationship with different ASD phenotypes are still limited and related only to specific domains, although this concept could contribute to clarify the phenotypical variability by explaining the difficulties encountered by individuals with ASD in daily life, where stimuli are often emotionally charged. With the aim to identify specific neuropsychological profiles in children and adolescents with ASD without intellectual disability, we designed a study protocol comparing a clinical sample of individuals with ASD to aged-matched (10–17 years) typically developing controls (TDC) on a neuropsychological test battery investigating both “cold” and “hot” EF with the purpose of further investigating their relationships with ASD symptoms. Autonomic measures including heart rate, heart rate variability, skin conductance, and salivary cortisol were also recorded before/during/after the neuropsychological testing session. This paper describes the case–control study protocol named “Caratterizzazione NEuropsicologica del disturbo dello Spettro Autistico, senza Disabilità Intellettiva, CNeSA study,” its rationale, the specific outcome measures, and their implications for the clinical management of individuals with ASD and a precision medicine approach.
Introduction
Autism spectrum disorder (ASD) is a complex, lifelong, and multifactorial neurodevelopmental condition with onset in the first years of life. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM 5) (1) introduced the term “spectrum” to highlight the broad heterogeneity of etiologies, onsets, clinical entities, and prognostic trajectories. Furthermore, individuals affected by ASD present heterogeneous neuropsychological profiles, due to the combination of several genetic and environmental factors that complexify the diagnosis and tailored interventions (2).
Children and adolescents with ASD without intellectual disability (ID) can use compensatory strategies for dealing with their difficulties in social contexts thus improving their social functioning (3, 4). However, they maintain significant difficulties in grasping social cues and other intentions, and have limited intuitive judgment skills and more inflexible decision-making processes (5, 6) as well as a lower sensitivity to rewards rather than punishments (7). Furthermore, individuals with ASD without ID may present atypical emotional responses to a stimulus and high aversive motivation toward social stimuli (8) with consequent impairment in their social functioning during daily life. Even when they present correct moral judgment, individuals with ASD tend to estimate certain transgressions or unfair acts more seriously than those with typical development, showing they rely on more rigid moral criteria (9).
Several theories tried to explain the core of social functioning difficulties in individuals with ASD (10–12). The hypothesis of executive disfunction (13, 14) includes deficits in the following functions: attention; flexibility and set-shifting; planning; inhibitory control; generativity; and working memory (15). Early studies reported a relationship between set-shifting impairment and restricted and repetitive behaviors (16, 17) and difficulties in facing changing situations (14). Disorders in selective attention were associated with restricted and repetitive behaviors (18, 19) while deficits on working memory were associated with difficulties in social communication and interaction, as the ability to take others perspective, social reciprocity and initiation (20). Moreover, a lack of generativity could compromise the quality of communication, due to the difficulty in generating ideas relevant to the context of conversation with others (21). Despite these results, the research on executive dysfunction in ASD shows contrasting results for the high variability in study designs and cohorts of patients (22, 23).
Furthermore, an early impairment in multisensory and sensory–motor integration (24, 25) is widely observed in children with ASD.
In children with ASD, the severity of sensory symptoms and intelligence quotient (IQ) seem to predict motor abilities, suggesting a relationship between the sensory, motor, and cognitive domains (26). A severe complex sensory processing impairment also appears to impact their motor variability (27).
In a seminal study, children with ASD aged younger than 4 years performed fewer exploratory actions on objects within a naturalistic setting, or engaged in repetitive, primitive sensorimotor actions compared to their typically developing (TD) peers who instead were more prone to explore action possibilities during means-end tasks (28). Difficulties in both fine and gross motor abilities can limit infants’ active exploration of objects and their ability to discover means-end relationships (29, 30).
The sensorimotor difficulties are often associated with behavioral motor problems, such as stereotyped behavior and restricted interests. Other studies found the presence of a relationship between early motor delay and later communication delay in infants at risk for ASD (31, 32), with a cascading effect on all aspects of neurodevelopment, including detail perception, motor planning, social communication, and cognitive domains.
In the last decade, a distinction between “cool” and “hot” executive functions (EFs) (33) has been introduced with the aim to discriminate the purely cognitive processes from those elicited by affective stimuli or related to emotionally salient situations in which emotional and cognitive processes are integrated to generate a behavior (34, 35). The “hot” EFs could contribute to explain the difficulties encountered by individuals with ASD in daily life where stimuli are often charged emotionally.
The high difficulty in managing daily changes, unpleasant events, negative social interactions, and external sensory stimuli also leads individuals with ASD to experience greater stress than neurotypical individuals (36).
Several studies suggest that heart rate (HR), heart rate variability (HRV), electrodermal activity (EDA), and cortisol levels appear to be altered in the ASD population: children with ASD seem to present a chronic state of hyperarousal (37–39), a psychophysiological inflexibility to stimuli (e.g., appropriate vagal withdrawal to attention-demanding stimuli) (40), and greater physiological responses to threat (41). Although individuals with ASD present a greater lower overall regulation than their typically developing peers, the ASD patients without intellectual impairment demonstrate more autonomic flexibility and responsiveness to stimuli (42) than those with intellectually impaired ASD (39). Moreover, ASD in individuals without intellectual impairment exhibits more autonomic responsivity showing more variable cardiac responding to familiar vs unfamiliar social situations in comparison to TD controls (TDCs) who do not show changes across stimuli (42).
Other studies investigating stress levels by measuring the salivary cortisol also report higher cortisol levels in individuals with ASD compared to both younger autistic individuals and age-compared TDCs during social interactions with peers (43, 44).
Numerous studies report the presence of a relationship between autonomic nervous system (ANS) activity and social and communication functioning in individuals with ASD (45–48). Relative to TDCs, patients with ASD present greater electrodermal activity during feedback rewards and the greatest increases were more likely exhibited by children with more repetitive symptoms, reduced executive functions, and internalizing symptoms (46).
According to these notions, some studies support the hypothesis that stress, either acute or chronic, affects cognitive performances and the way in which individuals with ASD perceive, understand, and react to the social world (49, 50). Excessive levels of stress have been associated with poorer performance in short-term memory tasks, learning, and attention tasks (51, 52). It is not yet clear, however, whether the cognitive dysfunctions observed in individuals with ASD entails a greater susceptibility to stressful situations or, conversely, whether stress produces an influence on the performance of cognitive tasks.
Moreover, to date, the studies investigating both hot and cold EFs are still limited, and the research is often focused on singular domains.
Considering the current literature, further research is required to understand the neuropsychological characteristics of patients with ASD in both domains of hot and cold EFs and how they differ from TDCs. Exploring the relation between symptom phenotypes, neuropsychological functioning and autonomic nervous system response will enhance the current knowledge on the neurobiological mechanisms underlying the difficulties presented by individuals with ASD. This may allow the identification of cognitive and physiological pathways underlying the disorders and improve the intervention strategies to support patients' autonomy in daily life activities.
Furthermore, the assessment of physiological parameters in association with the behavioral performance could help the clinician to understand the way the individuals with ASD process the experiences related to hot and cold tasks.
With this purpose in mind, we designed the present protocol to compare a clinical sample of individuals with ASD to aged-matched TDCs to investigate their neuropsychological functioning and the relationships with ASD symptoms.
Methods and analysis
Study design
This is a monocentric study including two phases: a screening and clinical assessment visit (phase I); and a case–control study (phase II) comparing the neuropsychological and autonomic functioning profiles of children and adolescents with ASD without ID to age-matched TDCs.
Participants
Two cohorts of children and adolescents (aged 10–17 years and 10 months at screening visit) of both genders were enrolled in the study.
Group 1 (ASD group) included children and adolescents with a clinical diagnosis of ASD according to DSM 5 criteria without intellectual disability (IQ score ≥80).
Group 2 (TDC group) included typically developing children and adolescents without any psychopathology, matched to the ASD group for age, gender, and IQ.
Eligibility criteria
Inclusion criteria for all participants
For eligibility, participants from both groups had to be aged 10–17 years and 10 months at the screening visit with an IQ >80 measured with Wechsler IQ scales (Wechsler Intelligence Scale for Children—Fourth Edition, WISC-IV or Wechsler Adult Intelligence Scale-IV, WAIS-IV) administered within 2 years before enrollment into the study.
ASD group inclusion criteria
Participants in the ASD group had to comply with the following requirements:
• Diagnosis of ASD in accordance with the DSM 5 criteria formulated by a qualified clinician according to normal clinical practice;
• Drug-naïve for psychotropic medications or off any psychotropic medication [psychostimulants, antipsychotics, serotonin and norepinephrine reuptake inhibitors (SNRIs), mood stabilizers, or antidepressants] within the last 6 months before the screening visit;
• Signed informed consent and absent documents provided by the individual's parent/legal guardians and the patients;
• Participants meeting the criteria for co-morbid Attention Deficit and Hyperactivity Disorders (ADHD), Anxiety, or Post Traumatic Stress Disorder (PTSD) as well as language and motor disorders were not excluded from the study (as for the clinical judgment of the investigator).
TDC group inclusion criteria
The participants included in the TDC group had to comply with the following requirements:
• A total score on the Social Communication Questionnaire (SCQ) below the clinical range for ASD: ≤10;
• Drug-naïve for psychotropic medications;
• Signed informed consent and absent documents provided by the individual's parent/legal guardians and the TDCs.
Exclusion criteria ASD group
Potential eligible participants for the ASD group were excluded from participation in the study if they met the following criteria:
• IQ <80 (Wechsler IQ scales, within the last 2 years before enrollment);
• Presence of a primary DSM 5 diagnosis of schizophrenia-related disorders, schizophrenia, bipolar disorder, depression;
• Presence of any acute or unstable medical condition compromising the reliability of the study;
• The participant had any psychotropic medication (psychostimulants, antipsychotics, SNRIs, mood stabilizers, or antidepressants) within the last 6 months before the beginning of the study;
• Biological siblings of the participants were already included within the ASD group.
Exclusion criteria TDC group
Potential TDCs were not enrolled into the study if they met any of the following criteria:
• IQ <80 (Wechsler IQ scales, within the last 2 years before enrollment);
• The participant was treated with any psychotropic medication (psychostimulants, antipsychotics, SNRIs, mood stabilizers, or antidepressants);
• The presence of a primary DSM 5 diagnosis of ADHD, oppositional defiant disorder (ODD), conduct disorder (CD), or any other psychiatric condition;
• The presence of any acute or unstable medical condition compromising the reliability of the study.
Sample size calculation
To compare the performance between the ASD and TDC groups, a sample size of 40 ASD cases and 40 TDCs had a statistical power of >80% at the 0.05 level to detect group differences with a moderate effect size allowing for the inclusion of covariates (sex, site, children vs adolescents, IQ, co-morbidity with ADHD) (53).
Enrollment
The ASD group was composed of inpatients or outpatients or clinical referrals from community centers and their parents were informed about the study by the clinical team or the study investigators. TDCs were identified through other clinical departments or from ASD participants’ family members or classmates who wanted to participate in the study.
Before starting any procedures, the parents/legal guardian and the child/adolescent provided written informed consent and the assent for participating in the study.
All participants were free to withdraw from the study at any time, for any reason, and without any consequences to their clinical treatment. The investigator could decide to withdraw an individual from the study for urgent medical or psychiatric reasons and a new participant was recruited from the same group (ASD/TDC) and gender as the individual who withdrew from the study. Data collected until the point of withdrawal were collected and included in the final analysis.
Study procedures
This study is a non-interventional case–control study divided into two phases: the screening and clinical assessment visit (phase I); and the case–control study including neuropsychological testing and physiological measures collection (phase II). To reduce the fatigue effect on testing performance, the case–control phase was divided into 2 days (visits 0a and 0b) (Figure 1; see also Supplementary Material Table S1: study design).
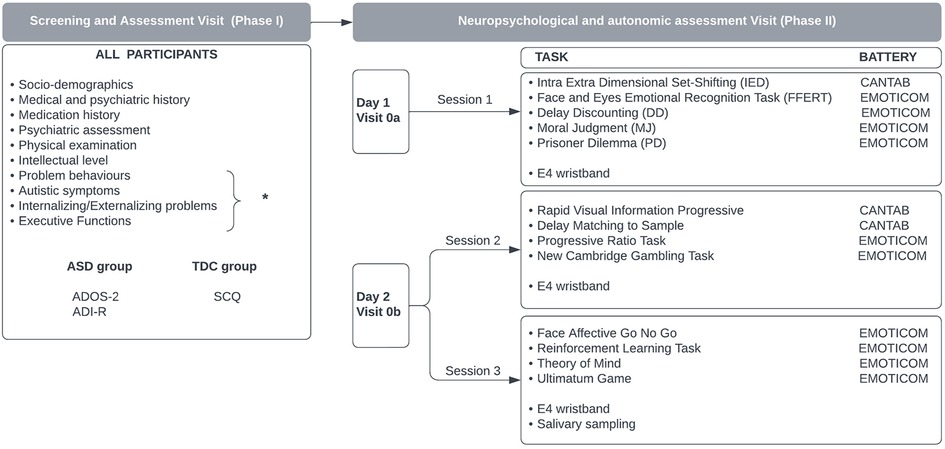
Figure 1. Study design. *The intellectual level is measured using Weschler scales (WISC-IV or WAIS-IV); behavioral problems are assessed with Kiddie-SADS-PL, CPRS-RS, NCBRF-TIQ, MOAS, ICU, C-GAS, and CGI; the assessment of autistic symptoms includes ADOS-2, SCQ, SRS-2, and EQ-40; internalizing/externalizing symptoms are evaluated with CBCL, TRF, and YSR; the assessment of executive functions includes the BRIEF questionnaire.
Screening and clinical assessment visit (phase I)
After receiving the signed informed consent and assent, sociodemographic information and data on medical, psychiatric, and pharmacological history of participants as well as medical history of relatives were collected for all participants and all criteria for enrollment were verified.
The screening session included the following (see Supplementary Material Table S2: clinical assessment; SM1: Description of screening and clinical instruments):
• WISC-IV (54) or WAIS-IV (55);
• Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime Version (Kiddie-Sads-PL) (56).
An evaluation of autistic symptoms was different among the two groups.
Children/adolescents with ASD underwent a detailed psychiatric assessment for autistic symptoms using the following principal instruments for the diagnosis of ASD:
• Autism Diagnostic Interview—Revised (ADI-R) (57);
• Autism Observational Scale—Second Edition (ADOS-2) (58).
Parents of the participants included in TDC group completed the SCQ (59). A score of 10 is used as the cutoff for excluding autism in the participant.
Further assessments included the following:
• Modified Overt Aggression Scale (MOAS) (60);
• The Nisonger Child Behaviour Rating Form (NCBR-TIQ) parent version (61);
• Clinical Global Impression-Severity (CGI-S) (62);
• Children's Global Assessment Scales (C-GAS) (63).
Participant forms:
• Self Report (YSR) (64);
Parent and teacher forms:
• Social Responsiveness Scale—Second Edition (SRS-2) (65);
• Child Behavior Checklist (CBCL), Teacher Report Form (TRF) (64);
• Conner's Parent Rating Scale-Revised (CPRS-RS) (66);
• Behavior Rating Inventory of Executive Function (BRIEF) (67);
• Empathy Quotient (EQ-40) (68);
• Inventory of Callous Unemotional Traits (ICU) (69).
Vital signs, body temperature, height, and weight
Vital signs (blood pressure, heart rate), body temperature, height, and weight were recorded; a physical examination was also performed.
Case–control study (phase II)
Within 1 month from the screening visit, each participant took part in the case–control study consisting of neuropsychological testing (Figure 1). The computerized test battery was split into three sessions lasting 3 hours in total to perform over 2 days (visits 0a and 0b) at a maximum interval of 1 week.
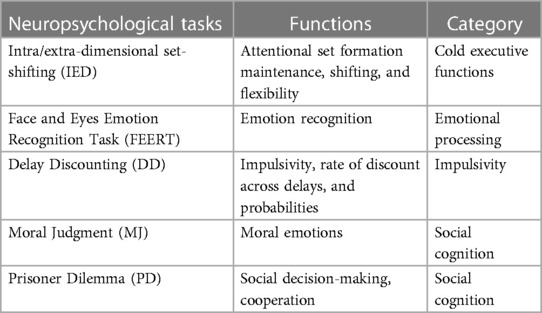
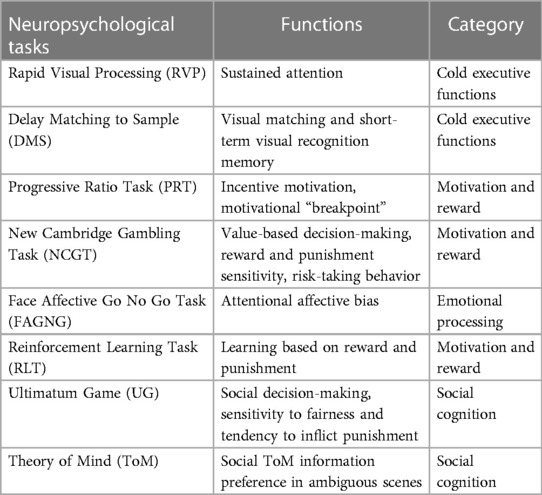
In the first of the 2 days (visit 0a), the first session of neuropsychological tests comprising five tasks was administered lasting approximately 50 min in total (Table 1); during the second day (visit 0b), the participants completed the second and third sessions, each consisting of four tasks (Table 2). The second and third sessions were separated by an interval of 45 min during which the participant rested.
During all three sessions, the autonomic parameters HR, HRV, and EDA were collected through the application of the E4 wristband worn by the participant 20 minutes before the start until the end of the session.
During visit 0b, two samples of saliva were collected from all participants: 5 min before the first testing session and immediately after the end of the second session, in order to detect salivary cortisol levels.
The neuropsychological test battery
In this study, a computerized battery of neuropsychological tasks was used with the aim of exploring both the “cold” and “hot” EFs. All tasks were administered using a touchscreen tablet (10.1-inch screen) and included tasks from the neuropsychological test batteries “Cambridge Neuropsychological Automated Battery” (CANTAB; Cambridge Cognition: RRID:SCR_003001 https://www.cambridgecognition.com/cantab/) (70) and the EMOTICOM battery (71).
Three different domains of cold EFs (the sustained attention, the acquisition and maintenance of rules, and the set-shifting abilities and the visual working memory) were evaluated using three tasks from the CANTAB battery.
The tasks chosen to evaluate the domains of hot EFs (emotional processing, motivation and reward, impulsivity and social cognition, social decision-making) were derived by the EMOTICOM battery. For a detailed description of the administered neuropsychological tasks, see Supplementary Material: SM2: Neuropsychological Assessment.
A screening test was first administered with the aim of making the individual familiar with the tablet and other test materials. Within each visit, the presentation order of the tests was randomized for each participant.
Physiological measures
The stress levels due to task administration were evaluated through physiological measures collection (Table 3).
Autonomic measures using Empatica E4.
During the neuropsychological assessment, task-related stress and arousal levels were assessed with the application of the Empatica E4 wristband recording the autonomic measures HR and HRV by a photoplethysmography technique, and EDA. The bracelet was placed on the wrist of the non-dominant hand of the participant. The recording started at least 5 min before the testing session, while the participant was at rest, to yield baseline, and continued during the performance of the whole neuropsychological test battery.
During the test administration, a “tag event” function was used to delimit the beginning and the end of each test with the purpose of comparing stress and arousal levels during different neuropsychological tasks. The tag event delimiting the end of the task does not correspond with the start of the next task. Data collected during the period between the end of a task and the beginning of the next were excluded from the analysis.
Collection of saliva cortisol samples
During the second day of neuropsychological testing (visit 0b), two samples of saliva were collected with a “passive drool” method. Each 0b visit was scheduled in the morning to collect a baseline sample in a time window between 8:00 and 9:30, 5 min before the start of the first testing session. The stress sample was collected at the end of the second session, depending on the time needed for the execution of all tasks. The time of both collections was reported in the patient's case report form (CRF) to consider the circadian trend of cortisol by age.
If the participant had trouble spitting, sugar- and flavor-free chewing gum were provided to assist salivation. They were asked to rinse their mouth with water and then waited approximately 1 minute before saliva collection.
All samples were centrifuged after collection and then frozen and stored at −20°C until assay. Cortisol levels were extracted by an external lab (San Raffaele Hospital, Milano, Italy).
A difference between salivary cortisol levels at baseline and at the end of administration was calculated, within and between the two groups.
Outcome measures
Primary outcome measures included the following: quantitative and qualitative behavioral measures evaluated through the neuropsychological test battery: measures of cold (sustained attention, attentional set formation maintenance, shifting, visual matching, and short-term visual recognition memory) and hot EFs (emotion recognition, attentional biased, reward-punishment sensitivity, moral judgment, cooperation, theory of mind, test completion, motivation). These were calculated using the following:
• response latency (reaction times);
• accuracy (number or proportion of errors).
The following physiological measures were recorded: heart rate; heart rate variability; skin conductance at rest and during the test performance; and salivary cortisol levels before and after testing session.
The secondary outcome measures were as follows: clinical measures to assess the association between autistic symptoms severity; co-morbidities; problem behaviors obtained by the administration of tests, interviews, rating scales, and questionnaires; and neuropsychological profile. These were obtained using the following:
• Screening questionnaires: SRS-2, CBCL, TRF, YSR, CPRS, BRIEF, ICU;
• Screening rating scales: MOAS and Nisonger;
• CGI-S, C-GAS.
Handling and storage of data and documents
Data were handled confidentially and anonymously: a different participant identification code was used to link the data to each individual. The key to the code was safeguarded by the investigators. Demographic data (name, address, etc.) and identification numbers were coupled in a file, which was saved on a password-protected PC, only accessible to the investigators. The handling of personal data complied with Personal Data Protection Acts.
A unique code was given to each collected salivary sample, consisting of the type of sample, the visit session and the date of collection, and a unique, consecutive number. The code was not based on the patients’ initials and birthdate.
Statistical analysis
The cognitive-behavioral measures for each task include mean reaction time, number or proportion of errors, and other quantitative measures.
The group differences will be evaluated using chi-square or one-way analysis of variance (ANOVA) tests in case of the data meeting the assumptions of normality and homogeneity of variance, while the effect of covariates will be explored using analysis of covariance (ANCOVA) and, thereafter, by determination of simple effects or interactions. Non-parametric tests (e.g., Mann–Whitney U test) or bootstrap-based non-parametric ANOVA will be used for variables that do not respect these assumptions. Simple and multiple and logistic regression models will be applied to the whole sample and to the ASD group. A mixed repeated-measures analysis will be used to compare the performance of ASD to the TD group within each task when appropriate.
Variables including age, gender, IQ, and co-morbidities such as ADHD, anxiety, and depression, will be also investigated as covariates.
Correlations, simple and multiple regressions, and ANCOVAs will be used to investigate the demographic, clinical, neuropsychological, and neurophysiological predictors to establish their role as moderating or modulating variables with symptom severity.
If, as expected, the raw cortisol values are positively skewed, they will be normalized using log transformation. To assess the group differences in cortisol variations, mixed repeated-measures ANOVAs will be performed with the group variable as a between-subjects factor and time (pre- and post-testing) as a within-subjects factor; the collection time of the samples will be used as a covariate.
One-way ANOVAs will be used for group comparisons of HR, HRV, and skin conductance means during the task performance. To quantify HR, HRV, and skin conductance responsiveness to the stress related to the performance during tasks, the change relative to baseline will be calculated. A clustering analysis will be also used to group individuals belonging to the whole sample in classes not defined a priori, according to autonomic characteristics.
Discussion and clinical implications
ASD is a condition characterized by impaired socioemotional skills and patterns of restricted and repetitive interests and behaviors lasting in general for a lifetime. Although patients with ASD without ID implement compensatory strategies to face difficulties, most of them experience negative experiences, such as social isolation, and have few opportunities for sociability (72, 73). When they make friends like their TD peers, compared to the latter they experience a poorer quality of friendship (74). The presence in ASD individuals of hyper moral behaviors, such as scolding classmates who break the school rules or trying to socialize with peers or monopolizing speeches, make individuals with ASD vulnerable to bullying episodes. Furthermore, their difficulties in recognizing social cues [e.g., difficulties understanding the communicative intent of gaze (75, 76)], the tendency to incorrectly interpret others’ behavior (77, 78) as well as difficulties in interpreting verbal communication (79, 80) compromise the identification of bullying episodes, leading them to experience uncomfortable emotions.
To date, published studies on the neuropsychological functioning of ASD show contrasting results due to methods issued without providing exhaustive answers in explaining the neuropsychological dysfunctions in the ASD population. Studies on social decision-making as cooperation indicated lower correct predictions of others’ moves compared with TDCs (81) and an improvement of this ability with age (82, 83). Moreover, for individuals with ASD, their cooperation relies on more rigid criteria that does not differ depending on the morality of the interacting partner (84). In contrast, other studies report similar cooperation behavior in autistic and non-autistic individuals (85). Autistic individuals appear to make less use of contextual cues and reported less emotion reaction to the scenarios described in vignettes (86, 87).
In addition, the studies on reward-based decision-making using the gambling task reported contrasting results. Whereas the study by Faja et al. (46) showed a similar pattern of gambling selection between individuals with ASD and TDCs, the study by Yechiam et al. showed that, unlike TDCs, individuals with ASD presented fewer advantageous choices and difficulties in developing and maintaining a congruent choice strategy switching from a deck to another (88).
Considering the lack of knowledge in the field and the high heterogeneity of the ASD, the present study will help to better define the neuropsychological and autonomic characteristics underpinning ASD in order to provide useful information to identify the underlying potential neuropsychological/physiological mechanisms behind the clinical symptoms. Although several therapeutic strategies have already been developed to help autistic patients in dealing with social and emotional difficulties, including video modeling (89, 90), emotional recognition training (91, 92), social stories (93), and social skill training (94, 95), our study could contribute to develop further targeted intervention strategies.
Targeted interventions should consider the importance of factors that typically contribute to the valuable perception of social situations, including emotions and motivation, the stimuli relevance, the reward and punishment sensibility, the attentional affective bias vs. stimuli, and the uncertainty valence. For example, individuals with autism can orient their attention toward a stimulus rather than another leading to dysfunctional decision-making thus compromising their social functioning and their autonomy development. The results of this study will help to provide the basis for developing more effective psycho-educational strategies aimed at improving patients’ autonomy and at enhancing their social skills.
Strengths and limitations of this study
The main strength of the present study protocol is to investigate, at the same time, both domains of hot and cold EFs as well as the autonomic functioning in a cohort of individuals with autism.
Another strength of this study is the wide neuropsychological and clinical characterization of the ASD sample within a narrow age range including different sources of information (parents, teachers, and the individuals themselves).
On the other hand, the co-morbidity with other neurodevelopmental disorders, such as ADHD or specific learning disorders, could represent a limitation of the study due to their influence on the task performances (e.g., tasks requiring reading words could be less suitable for patients with dyslexia, therefore requiring additional efforts; the length of tasks could influence the performance in children with hyperactivity or inattention symptoms). Moreover, the main limitation of this study is that the Emoticom tasks are not yet standardized in the pediatric population and the battery administered in this study is an “experimental” version.
Ethics and dissemination
The present study was approved from the local Ethical Commitee (Comitato Etico Indipendente) of Cagliari University Hospital on 28 March 2018 and conducted in accordance with the principles of the Declaration of Helsinki. Before starting any study procedure, all participants' parents/legal guardians, patients, and controls signed an informed consent and assent document, respectively, as provided for by the national law. The results of the study will be disseminated through peer-reviewed publications and at scientific conferences.
Conclusions
An integrative model of neuropsychological functioning in ASD would better explain the difficulties with ASD. An understanding of neuropsychological and autonomic functioning and the relation with ASD symptoms may lead to defining more effective intervention behavioral strategies that represent first-line therapy due to the poor responsivity to pharmacological treatments.
The assessment, which integrates both domains of cold and hot EFs, can provide insights into the executive deficits that hinder the development of the skills necessary in ecological social contexts.
Ethics statement
The studies involving human participants were reviewed and approved by the Local Ethical Committee, Cagliari University Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
FD, CB, and AZ designed the study protocol. FD wrote the manuscript. FD, CB, MM, and SC worked on the editing, added minor corrections, and supervised the writing. FD, CB, and JB contributed to the project administration. FD, CB, SC, and MM contributed to the revision of the manuscript. All authors have read and agreed to the published version of the manuscript. AZ prematurely passed away before the completion of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Trevor W. Robbins, Barbara J. Sahakian, Rebecca Elliott, and Amy Blend for developing and sharing the EMOTICOM neuropsychological test battery. The authors are also grateful to Caterina Medda, who was involved in the project management, and all the MDs who contributed to the recruitment phase and organization processes of the study. The authors also thank the parents/caregivers and their children who generously dedicated their time to participate in the study. Finally, the authors wish to express their profound gratitude toward Professor Alessandro Zuddas, without whom the CNeSA study would not have been carried out.
Conflict of interest
FD had collaborations as sub-investigator in clinical trials sponsored by Lundbeck and as an independent rater in clinical trials sponsored by Servier and Acadia. CB had collaborations within projects from the European Union (7th Framework Program) and as a sub-investigator in sponsored clinical trials by Lundbeck, Otsuka, Janssen Cilag, Angelini, and Acadia. JB had collaborations as a sub-investigator in sponsored clinical trials by Angelini and Servier. MM received honoraria from/has been a consultant for Angelini and Lundbeck. AZ served in an advisory or consultancy role for Angelini, EduPharma, Servier, Taked, and Acadia. He received conference support or speaker's fees from Angelini and Janssen. He was involved in clinical trials conducted by Angelini, Janssen, Lundbeck, Otsuka, Roche, Sevier, and Shire. He received royalties from Giunti OS and Oxford University Press. SC had collaborations within projects from the European Union (7th Framework Program) and as a sub-investigator in sponsored clinical trials by Lundbeck, Otsuka, Janssen Cilag, Angelini, and Acadia.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frcha.2023.1149244/full#supplementary-material.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association (2013).
2. Bhat S, Acharya UR, Adeli H, Bairy GM, Adeli A. Autism: cause factors, early diagnosis and therapies. Rev Neurosci. (2014) 25(6):841–50. doi: 10.1515/revneuro-2014-0056
3. Grossman JB, Klin A, Carter AS, Volkmar FR. Verbal bias in recognition of facial emotions in children with Asperger’s syndrome. Child Psychol Psychiatry. (2000) 41:369–79. doi: 10.1111/1469-7610.00621
4. Teunisse J-P, de Gelder B. Impaired categorical perception of facial expressions in high-functioning adolescents with autism. Child Neuropsychol. (2001) 7(1):1–14. doi: 10.1076/chin.7.1.1.3150
5. De Martino B, Harrison NA, Knafo S, Bird G, Dolan RJ. Explaining enhanced logical consistency during decision making in autism. J Neurosci. (2008) 28(42):10746–50. doi: 10.1523/JNEUROSCI.2895-08.2008
6. Mussey JL, Travers BG, Klinger MR. Decision-making skills in ASD. Performance on the Iowa gambling task. Autism Res. (2015) 8(1):105–11. doi: 10.1002/aur.1429
7. South M, Chamberline PD, Wingham S, Newton T, Le Couteur A, McConachie H, et al. Enhanced decision making and risk avoidance in high-functioning autism spectrum disorder. Neuropsychology. (2014) 28(2):222–8-8. doi: 10.1037/neu0000016
8. Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev Psychobiol. (2002) 40(3):213–25. doi: 10.1002/dev.10028
9. Fadda R, Parisi M, Ferretti L, Saba G, Foscoliano M, Salvago A, et al. Exploring the role of theory of mind in moral judgment: the case of children with autism spectrum disorder. Front Psychol. (2016) 7:523. doi: 10.3389/fpsyg.2016.00523
10. Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. (1985) 21(1):37–46. doi: 10.1016/0010-0277(85)90022-8
11. South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J Autism Dev Disord. (2005) 35(2):145–58. doi: 10.1007/s10803-004-1992-8
12. Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. (2006) 36(1):5–25. doi: 10.1007/s10803-005-0039-0
13. Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. (1996) 37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x
14. Hill EL. Evaluating the theory of executive dysfunction in autism. Dev Rev. (2004) 24(2):189–233. doi: 10.1016/j.dr.2004.01.001
15. Lai CLE, Lau Z, Lui SS, Lok E, Tam V, Chan Q, et al. Meta-analysis of neuropsychological measures of executive functioning in children and adolescent with high functioning autism spectrum disorder. Autism Res. (2017) 10(5):911–39. doi: 10.1002/aur.1723
16. South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. (2007) 11(5):437–51. doi: 10.1177/136236130707960
17. Mosconi M, Kay M, D’Cruz A, Seidenfeld A, Guter S, Stanford L, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychol Med. (2009) 39(9):1559–66. doi: 10.1017/S0033291708004984
18. Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learn Mem. (1997) 4:1–35. doi: 10.1101/lm.4.1.1
19. Turner M. Annotation: repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. (1999) 40:839–49. doi: 10.1111/1469-7610.00502
20. Pellicano E. Links between theory of mind and executive function in young children with autism: clues to developmental primacy. Dev Psychol. (2007) 43:974. doi: 10.1037/0012-1649.43.4.974
21. Bishop DVM, Norbury CF. Executive functions in children with communication impairments, in relation to autistic symptomatology—I: generativity. Autism. (2005) 9(1):7–27. doi: 10.1177/1362361305049027
22. Hill EL. Executive dysfunction in autism. Trends Cogni Sci. (2004) 8(1):26–32. doi: 10.1016/j.tics.2003.11.003
23. Geurts H, Sinzig J, Booth R, Happé F. Neuropsychological heterogeneity in executive functioning in autism spectrum disorders. Int J Dev Disabil. (2014) 60:155–62. doi: 10.1179/2047387714Y.0000000047
24. Purpura G, Costanzo V, Chericoni N, Puopolo Scattoni ML, Muratori F, et al. Bilateral patterns of repetitive movements in 6- to 12-month-old infants with autism spectrum disorders. Front Psychol. (2017) 8:1168. doi: 10.3389/fpsyg.2017.01168
25. Ozonoff S, Young GS, Rogers SJ. Gross motor development, movement abnormalities and early identification of autism. J Autism Dev Disord. (2008) 38:644–56. doi: 10.1007/s10803-007-0430-0
26. Surgent OJ, Walczak M, Zarzycki O, Ausderau K, Travers BG. IQ and sensory symptom severity best predict motor ability in children with and without autism spectrum disorder. J Autism Dev Disord. (2021) 51(1):243–54. doi: 10.1007/s10803-020-04536-x
27. Purpura G, Cerroni F, Carotenuto M, Nacinovich R, Tagliabue L. Behavioural differences in sensorimotor profiles: a comparison of preschool-aged children with sensory processing disorder and autism spectrum disorders. Children. (2022) 9:408. doi: 10.3390/children9030408
28. Lösche G. Sensorimotor and action development in autistic children from infancy to early childhood. J Child Psychol Psychiatry. (1990) 31(5):749–61. doi: 10.1111/j.1469-7610.1990.tb00815.x
29. Lobo MA, Galloway JC. Postural and object-oriented experiences advance early reaching, object exploration, and means-end behavior. Child Dev. (2008) 79(6):1869–90. doi: 10.1111/j.1467-8624.2008.01231.x
30. Srinivasan M, Bhat AN. Differences in means-end exploration between infants at risk for autism and typically developing infants in the first 15 months of life. Autism Res. (2022) 15(6):1156–78. doi: 10.1002/aur.2711
31. Bhat AN, Galloway JC, Landa RJ. Relationship between early motor delay and later communication delay in infants at risk for autism. Infant Behav Dev. (2012) 35:838–46. doi: 10.1016/j.infbeh.2012.07.019
32. Iverson JM. Early motor and communicative development in infants with an older sibling with autism spectrum disorder. J Speech Lang Hear Res. (2018) 61:2673–84. doi: 10.1044/2018_JSLHR-L-RSAUT-18-0035
33. Perone S, Almy B, Zelazo PD. Toward an understanding of the neural basis of executive function development. In: Gibb R, Kolb B, editors. The neurobiology of brain and behavioral development. London: Elsevier (2018). 291–314. doi: 10.1016/B978-0-12-804036-2.00011-X
34. Elliott R, Zahn R, Williams Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. (2011) 36:153–82. doi: 10.1038/npp.2010.77
35. Zelazo PD, Carlson SM. Hot and cool executive function in childhood and adolescence: development and plasticity. Child Dev Perspect. (2012) 6(4):354–60. doi: 10.1111/j.1750-8606.2012.00246.x
36. Gilott A, Standen PL. Levels of anxiety and sources of stress in adults with autism. J Intellect Disabil. (2007) 11(4):359–70. doi: 10.1177/1744629507083585
37. Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. J Autism Dev Disord. (2010) 40(3):358–70. doi: 10.1007/s10803-009-0884-3
38. Cohen DJ, Johnson WT. Cardiovascular correlates of attention in normal and psychiatrically disturbed children: blood pressure, peripheral blood flow, and peripheral vascular resistance. Arch Gen Psychiatry. (1977) 34(5):561. doi: 10.1001/archpsyc.1977.01770170071006
39. Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, et al. Cardiovascular arousal in individuals with autism. Focus Autism Other Dev Disabl. (2006) 21(2):100. doi: 10.1177/10883576060210020101
40. Porges SW. Peripheral and neurochemical parallels of psychopathology: a psychophysiological model relating autonomic imbalance to hyperactivity, psychopathy, and autism. Adv Child Dev Behav. (1976) 11:35–65. doi: 10.1016/S0065-2407(08)60094-4
41. Hutt C, Hutt SJ, Lee D, Ounsted C. Arousal and childhood autism. Nature. (1964) 204:908–9. doi: 10.1038/204908a0
42. Van Hecke AV, Lebow J, Bal E, Lamb D, Harden E, Kramer A, et al. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Dev. (2009) 80(4):1118–33. doi: 10.1111/j.1467-8624.2009.01320.x
43. Ogawa S, Lee YA, Yamaguchi Y, Shibata Y, Goto Y. Association of acute and chronic stress hormones with cognitive functions in autism spectrum disorder. Neuroscience. (2017) 343:229–39. doi: 10.1016/j.neuroscience.2016.12.003
44. Corbett BA, Schupp CW, Lanni KE. Comparing biobehavioural profiles across two social stress paradigms in children with and without autism spectrum disorders. Mol Autism. (2012) 3(1):13. doi: 10.1186/2040-2392-3-13
45. Romanczyk R, Gillis JM, Baron MG, Groden J, Groden G, Lipsitt LP. Autism and the physiology of stress and anxiety. In: Baron MG, Groden J, Groden G, Lipsitt L, editors. Stress and coping in autism. Oxford: Oxford University Press (2006). p. 183–204.
46. Faja S, Murias M, Beauchaine TP, Dawson G. Reward-based decision making and electrodermal responding by young children with autism spectrum disorders during a gambling task. Autism Res. (2013) 6(6):494–505. doi: 10.1002/aur.1307
47. Sheinkopf SJ, Neal-Beevers AR, Levine TP, Miller-Loncar C, Lester B. Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Res Treat. (2013) 2013:868396. doi: 10.1155/2013/868396
48. Neuhaus E, Bernier R, Beauchaine TP. Brief report: social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. J Autism Dev Disord. (2014) 44(3):730–7. doi: 10.1007/s10803-013-1923-7
49. Silberman S. First autistic presidential appointee speaks out. Retrieved from Wired Science (2010). Available at: http://www.wired.com/wiredscience/2010/10/exclusive-ari-neeman-qa/all/1. (Accessed October 6, 2010).
50. Patriquin MA, Hartwig EM, Friedman BH, Porges SW, Scarpa A. Autonomic response in autism spectrum disorder: relationship to social and cognitive functioning. Biol Psychol. (2019) 145:185–97. doi: 10.1016/j.biopsycho.2019.05.004
51. Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. (1999) 113:420–30. doi: 10.1037/0735-7044.113.3.420
52. Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. (2008) 33(5):643–53. doi: 10.1016/j.psyneunen.2008.02.004
53. Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE. Set-shifting in children with autism spectrum disorders reversal shifting deficits on the intradimensional/extradimensional shift test correlate with repetitive behaviors. Autism. (2009) 13(5):523–38. doi: 10.1177/1362361309335716
54. Wechsler D. Wechsler intelligence scale for children. 4th ed. San Antonio, TX: Pearson Education, Inc. (2003).
55. Wechsler D. Wechsler adult intelligence scale. 4th ed. San Antonio, TX: Pearson Education, Inc. (2008). Technical and Interpretive Manual.
56. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36(7):980–88. doi: 10.1097/00004583-199707000-00021
57. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. (1994) 24:659–85. doi: 10.1007/BF02172145
58. Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule (ADOS-2). 2nd ed. Los Angeles, CA: Western Psychological Corporation (2012).
59. Rutter M, Bailey A, Lord C. The social communication questionnaire manual. Los Angeles, CA: Western Psychological Services (2003).
60. Kay SR, Wolkenfeld F, Murrill LM. Profiles of aggression among psychiatric patients. J Nerv Ment Dis. (1998) 176(9):539–46. doi: 10.1097/00005053-198809000-00007
61. Aman M, Leone S, Lecavalier L, Park L, Buican B, Coury D. The Nisonger child behavior rating form: typical IQ version. Int Clin Psychopharmacol. (2008) 23(4):232–42. doi: 10.1097/yic.0b013e3282f94ad0
62. Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. revised (DHEW Publ. No. ADM 76-338). Rockville, MD: National Institute of Mental Health (1976) 217–222.
63. Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A Children’s Global Assessment Scale (CGAS). Arch Gen Psychiatry. (1983) 40(11):1228–31. doi: 10.1001/archpsyc.1983.01790100074010
64. Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: Department of Psychiatry, University of Vermont (1991).
65. Constantino JN, Gruber CP. Social responsiveness scale–second edition (SRS-2). Torrance, CA: Western Psychological Services (2012).
66. Conners KC, Nobile M. CRS-R Conners’ rating scales—revised: Manuale, Giunti O.S. Firenze: Organizzazioni Speciali (2007).
67. Gioia G, Isquith P, Guy S, Kenworthy L. BRIEF—behavior rating inventory of executive function. Odessa, FL: Professional Manual; Psychological Assessment Resources (2000). EQ-40.
68. Baron-Cohen S, Wheelwrigh S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. (2004) 34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00
69. Essau CA, Sasagawa S, Frick PJ. Callous-unemotional traits in a community sample of adolescents. Assessment. (2006) 13(4):454–69. doi: 10.1177/1073191106287354
70. Morris RG, Evenden JL, Sahakian BJ, Robbins TW. Computer-aided assessment of dementia: comparative studies of neuropsychological deficits in Alzheimer-type dementia and Parkinson’s disease. In: Stahl SM, Iversen SD, Goodman EC, editors. Cognitive neurochemistry. Oxford: Oxford University Press (1987). p. 21–36.
71. Bland AR, Roiser JP, Mehta MA, Schei T, Boland H, Campbell-Meiklejohn DK, et al. EMOTICOM: a neuropsychological test battery to evaluate emotion, motivation, impulsivity, and social cognition. Front Behav Neurosci. (2016) 10:25. doi: 10.3389/fnbeh.2016.00025
72. Bauminger N, Kasari C. Loneliness and friendship in high-functioning children with autism. Child Dev. (2000) 71(2):447–56. doi: 10.1111/1467-8624.00156
73. Chamberlain B, Kasari C, Rotheram-Fuller E. Involvement or isolation? The social networks of children with autism in regular classrooms. J Autism Dev Disord. (2007) 37(2):230–42. doi: 10.1007/s10803-006-0164-4
74. Mendelson JL, Gates JA, Lerner MD. Friendship in school-age boys with autism spectrum disorders: a meta-analytic summary and developmental, process-based model. Psychol Bull. (2016) 142(6):601–22. doi: 10.1037/bul0000041
75. Mundy P, Kim K, McIntyre N, Lerro L, Jarrold W. Brief report: joint attention and information processing in children with higher functioning autism spectrum disorders. J Autism Dev Disord. (2016) 46:2555–60. doi: 10.1007/s10803-016-2785-6
76. Vivanti G, McCormick C, Young GS, Abucayan F, Hatt N, Nadig A, et al. Intact and impaired mechanisms of action understanding in autism. Dev Psychol. (2011) 47(3):841–56. doi: 10.1037/a0023105
77. Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Are children with autism blind to the mentalistic significance of the eyes? Br J Dev Psychol. (1995) 13(4):379–98. doi: 10.1111/J.2044-835X.1995.TB00687.X
78. Senju A. Spontaneous theory of mind and its absence in autism spectrum disorders. Neuroscientist. (2012) 18(2):108–13. doi: 10.1177/1073858410397208
79. Jolliffe T, Baron-Cohen S. The strange stories test: a replication with high-functioning adults with autism of Asperger syndrome. J Autism Dev Disord. (1999) 29(5):395–406. doi: 10.1023/a:1023082928366
80. Vulchanova M, Saldaña D, Chahboun S, Vulchanov V. Figurative language processing in atypical populations: the ASD perspective. Front Hum Neurosci. (2015) 9:24. doi: 10.3389/fnhum.2015.00024
81. Mantas V, Pehlivanidis A, Papanikolaou K, Kotoula V, Papageorgiou C. Strategic decision making and prediction differences in autism. PeerJ. (2022) 10:e13328. doi: 10.7717/peerj.13328
82. Jin P, Wang Y, Li Y, Xiao Y, Li C, Qiu N, et al. The fair decision-making of children and adolescents with high-functioning autism spectrum disorder from the perspective of dual-process theories. BMC Psychiatry. (2020) 20(1):152. doi: 10.1186/s12888-020-02562-8
83. Kaartinen M, Puura K, Pispa P, Helminen M, Salmelin R, Pelkonen E, et al. Associations between cooperation, reactive aggression and social impairments among boys with autism spectrum disorder. Autism. (2019) 23(1):154–66. doi: 10.1177/1362361317726417
84. Li J, Zhu L, Gummerum M. The relationship between moral judgment and cooperation in children with high-functioning autism. Sci Rep. (2014) 4:1–6. doi: 10.1038/srep04314
85. Schmitz EA, Banerjee R, Pouw LBC, Stockmann L, Rieffe C. Better to be equal? Challenges to equality for cognitively able children with autism spectrum disorders in a social decision game. Autism. (2015) 19(2):178–86. doi: 10.1177/1362361313516547
86. Buon M, Dupoux E, Jacob P, Chaste P, Leboyer M, Zalla T. The role of causal and intentional judgments in moral reasoning in individuals with high functioning autism. J Autism Dev Disord. (2012) 43(2):458–70. doi: 10.1007/s10803-012-1588-7
87. Zalla T, Leboyer M. Judgment of intentionality and moral evaluation in individuals with high functioning autism. Rev Philos Psychol. (2011) 2(4):681–98. doi: 10.1007/s13164-011-0048-1
88. Yechiam E, Arshavsky O, Shamay-Tsoory SG, Yaniv S, Aharon J. Adapted to explore: reinforcement learning in autistic spectrum conditions. Brain Cogn. (2010) 72(2):317–24. doi: 10.1016/j.bandc.2009.10.005
89. Apple AL, Billingsley F, Schwartz IS. Effects of video modelling behaviors of children with high-functioning ASD. J Posit Behav Interv. (2005) 7(1):33–46. doi: 10.1177/10983007050070010401
90. Simpson A, Langone J, Ayres KM. Embedded video and computer based instruction to improve social skills for students with autism. Educ Train Dev Disabil. (2004) 39(3):240–52.
91. Webster PJ, Wang S, Li X. Posed vs. genuine facial emotion recognition and expression in autism and implications for intervention. Front Psychol. (2021) 12:1–9. doi: 10.3389/fpsyg.2021.653112
92. Berggren S, Fletcher-Watson S, Milenkovic N, Marschik PB, Bölte S, Jonsson U. Emotion recognition training in autism spectrum disorder: a systematic review of challenges related to generalizability. Dev Neurorehabil. (2018) 21:141–54. doi: 10.1080/17518423.2017.1305004
93. Thiemann K, Goldstein H. Effects of peer training and written text cueing on social communication of school-age children with pervasive developmental disorder. J Speech Lang Hear Res. (2004) 47(1):126–44. doi: 10.1044/1092-4388(2004/012)
94. Gilmore R, Ziviani J, Chatfield MD, Goodman S, Sakzewski L. Social skills groups training in adolescents with disabilities: a systematic review. Res Dev Disabil. (2022) 125:104218. doi: 10.1016/j.ridd.2022.104218
Keywords: autism spectrum disorder, neuropsychological functioning, autonomic functioning, control design, social cognition, hot executive functions, cold executive functions, study protocol
Citation: Donno F, Balia C, Boi J, Manchia M, Zuddas A and Carucci S (2023) Social and executive functioning in individuals with autism spectrum disorder without intellectual disability: The case–control study protocol of the CNeSA study. Front. Child Adolesc. Psychiatry 2:1149244. doi: 10.3389/frcha.2023.1149244
Received: 21 January 2023; Accepted: 15 March 2023;
Published: 21 April 2023.
Edited by:
David Coghill, The University of Melbourne, AustraliaReviewed by:
Giulia Purpura, University of Milano Bicocca, ItalyAna Moscoso, Hôpital Robert Debré, France
© 2023 Donno, Balia, Boi, Manchia, Zuddas and Carucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federica Donno ZmVkZXJpY2EuZG9ubm84N0BnbWFpbC5jb20=
†Deceased
Specialty Section: This article was submitted to Autism and Other Neurodevelopmental Disorders, a section of the journal Frontiers in Child and Adolescent Psychiatry
 Federica Donno
Federica Donno Carla Balia
Carla Balia Jessica Boi
Jessica Boi Mirko Manchia
Mirko Manchia Alessandro Zuddas
Alessandro Zuddas Sara Carucci
Sara Carucci