- United States Department of Agriculture Agricultural Research Service (USDA-ARS) Bee Research Laboratory, Beltsville, MD, United States
Honey bees are managed by humans on all continents except Antarctica, leading to an exceptional database of colony growth and survival. Honey bee colony losses in the United States are approximately 50% annually, and losses in other countries range from 10% to 60%. These losses reflect chemical, climatic, and nutritional stresses alongside immense pressure from diverse parasites and pathogens. The combination of RNA viruses and parasitic mites that vector these viruses plays a primary role in colony losses. Here, we discuss virus infection with and without mite vectors, bee defenses, colony vulnerabilities, and the roles of managed beekeeping in mitigating and aggravating the impacts of Varroa mites and viral disease.
1 Introduction
As a concentrated resource, honey bee colonies are beset by diverse parasites and pathogens (Boncristiani et al., 2020). Among these, honey bee viruses are ancestral and are maintained thanks to horizontal and vertical transmission routes inherent to crowded social settings. Deformed wing virus (DWV) in the family Iflaviridae is a common virus in honey bee colonies. DWV was named for developmental pathologies that can occur when levels are high during the formation and differentiation of imaginal wing discs (de Miranda and Genersch, 2010). Honey bees have evolved defenses that mitigate the effects of viral transmission and fitness costs, including immune responses at the individual level (Steinmann et al., 2015) and social traits such as hygienic removal of diseased nestmates (Wagoner et al., 2019) and behavioral task shifts that minimize contact between bees most prone to high infection and susceptible nestmates (Geffre et al., 2020). These defenses and the entire system were greatly destabilized when the western honey bee, Apis mellifera, was first parasitized by the ectoparasitic mite Varroa destructor roughly one century ago. At this moment, the disease system transitioned from communicable infection transmitted host-to-host to a complex vector-borne system where pathogens move freely among mites and bees. With help from the beekeeping trade, Varroa mites have steadily moved across the globe, catching up with A. mellifera in virtually every part of this host’s current range (Chapman et al., 2023). The arrival and proliferation of Varroa in honey bee populations almost inevitably leads to wide-scale colony losses and pathologies (Steinhauer et al., 2018). These losses reflect, in large part, the effective vectoring of DWV among bees by Varroa (Bowen-Walker et al., 1999), a known factor in colony mortality (e.g., Dainat et al., 2012). The spread of Varroa as a novel vector has both increased DWV prevalence worldwide and helped shape the evolution of different DWV variants (Martin et al., 2012; Wilfert et al., 2016; Grindrod et al., 2021; Hasegawa et al., 2023; Doublet et al., 2024). Here, we contrast the direct impacts of Varroa mites while feeding on their hosts with the far more damaging role these mites play as vectors of disease. We will focus on the DWV group since this species is most prevalent and impactful, resulting in abundant insightful research.
2 Individual disease
As an intracellular pathogen, DWV is, by definition, a disease of individuals. Virus replication depends on host organelles and proteins, and viral impacts happen at the individual level. While wing pathologies are rare, honey bees emerging with overt DWV infection show high viral loads in the brain and nervous tissues (Yue and Genersch, 2005), while individual bees with covert infections have been shown to have memory deficits (Tang et al., 2021), reduced brood rearing (Zanni et al., 2018), and poor foraging success (Benaets et al., 2017). Hosts also respond as individuals to viral infection, mounting immune responses that likely play some role in mitigating disease impacts (Brutscher et al., 2017). Similarly, Varroa parasitism happens at the individual level, with mites exerting a physiological cost on developing bees by damaging and consuming tissues just below the bee cuticle (Ramsey et al., 2019), and by consuming hemolymph of both developing and adult bees (Han et al., 2024). As in viral responses, parasitized bees can mount deterrents to mite feeding, including melanization of feeding sites, although these deterrents themselves seem to be weakened by mite saliva (Richards et al., 2011) and/or microbial allies (Kanbar and Engels, 2005). DWV might itself be one such ally since DWV presence could, at least conditionally, affect the abilities of bees to mount cellular defenses at mite feeding sites (Gregory et al., 2005; Yang and Cox-Foster, 2005; Fang et al., 2022). Interestingly, mites with high levels of DWV kill their adult bee hosts quickly, while similar feedings by mites with low viral loads impart a low relative risk of death. The latter bees can develop infections and share viruses while remaining asymptomatic (Lamas et al., 2023), putting additional colony members at risk.
3 Colony dynamics and communicable infection
Viral transmission between bees is complex, involving both host-host and vector-borne transmission. DWV is transmitted between bee hosts vertically and horizontally and is very much driven by the social and reproductive systems of honey bee colonies. DWV is transmitted vertically by queens to their offspring (Amiri et al., 2018), potentially infecting hundreds of individual colony members daily. The source of these transmitted viruses can be the queen herself or her infected mates (Chen et al., 2006; Yue et al., 2007). Horizontal transmission occurs via behaviors ranging from food sharing (Lamas et al., 2024b) to cannibalism of infected nestmates (Posada-Florez et al., 2021). Finally, the shared enclosed colony structure of honey bees can itself be a reservoir of viruses and other pathogens (Schittny et al., 2020). All of these transmission routes impact honey bees, but current research suggests that their impacts on colony health pale in the face of vector-driven transmission by parasitic Varroa mites.
Varroa parasitism has historically been studied as a phenomenon of developing bees, with great attention paid to the invasion of brood cells and parasitism of late-instar larvae and pupae. In fact, while mites reproduce only on developing bees, the majority of host contacts involve adult bees. Between reproductive bouts, Varroa are mobile, actively switching from one adult host to another in order to feed (Figure 1). Thus, the mite population in a colony is always significantly lower than the number of parasitized bees (Lamas et al., 2023). Varroa parasitism is not constant, seasonally shifting across age and sex cohorts at the colony level (Lamas, 2022). Varroa form highly aggregated distributions on adult male (drone) bees early in the season. Co-infestation on bee hosts is an efficient strategy for pathogen transfer between vectors, allowing naive mites to become infectious vectors (Lamas et al., 2024b).
While drones are arguably a preferred food source, the more numerous worker bees are better candidates for virus maintenance within the colony. Maintenance hosts are important for pathogen persistence, and such worker bees have been shown to harbor high levels of DWV infection while remaining asymptomatic and interactive with naive nestmates. Worker bees are excellent candidates as maintenance hosts as they share nutrients, and hence potentially viruses, with every life stage in the colony, from larvae to adult workers, drones, and queens. Some worker hosts are especially susceptible to virus infection, later acting as highly infectious agents in their colony. These so-called super-spreaders have a disproportionate impact on infection, and although they appear as outliers, they are regular features of epidemics (Stein, 2011). Infectious hosts increase the risk of infection to susceptible nestmates and the naive Varroa that feed upon them (Lamas et al., 2024b). In laboratory assays, adult cannibals of infectious pupae subsequently infect their nestmates and the mites that feed on these newly infectious bees also become infectious (Posada-Florez et al., 2021; Lamas et al., 2024b). Superspreaders may also present themselves as vectors. In other vector-borne systems, the vector biting rate is a key parameter in disease spread (Garrett-Jones and Shidrawi, 1969). A small number of vectors will have a disproportionate effect on disease transmission dynamics, as the most frequent host switchers are more likely to acquire and then transmit a pathogen (Cooper et al., 2019). Varroa exhibit similar features as they rapidly move from one adult bee to another in order to feed. On average, mites switch hosts once every two and a half days, but a smaller number of mites switch at much higher frequencies, parasitizing far more hosts than their slower switching conspecifics (Lamas et al., 2023). Unlike most disease systems, this triad between mites, bees, and viruses leads to an intense combination of both vector-borne and social transmission.
Virus dynamics continue at the level of apiaries, which have stocking densities ranging from one to hundreds of adjacent colonies. Within apiaries, bees tend to drift into adjacent colonies, bringing their mites and/or viruses. Intriguingly, virus-infected bees seem to be more readily accepted as drifters into adjacent colonies, following changes in cuticular hydrocarbons that mask their ‘alien’ chemical profiles (Geffre et al., 2020). This is perhaps reflective of a pathogen manipulation of its host. Even more potently, diseased colonies in a state of weakness can be robbed by healthier colonies for honey and pollen, at which point raiding colonies readily acquire mites and/or viruses (Peck and Seeley, 2019). Bees living in collapsing colonies also regularly disperse and seek entry into distant colonies (Kulhanek et al., 2021).
4 Seasonal variation: the drone-to-worker shift
Varroa are highly attracted to drones when they are plentiful during the spring mating season. In fact, when drones are prevalent inside a colony, the absolute parasite burden is high on drones and low on workers. While drones attract this parasitism within their colony, they are unlikely candidates to infect their worker nestmates with viruses since they do not engage in colony maintenance tasks and receive but do not provide food donations during trophallactic events. Infected drones nonetheless pose a risk to the community of colonies surrounding them through their mating.
Honey bee colonies reproduce by fissioning in the spring and early summer, expelling the existing queen with more than half of the workforce while raising replacement queens who will mate on the wing with multiple drones. Drone production peaks in spring in preparation for these fission events. Given the great attractiveness of developing and adult drones to mites, drones are hotbeds of viral infection and hence excellent candidates for vertical transmission of DWV during mating (Amiri et al., 2016). Queens that harbor infectious levels of DWV in their ovaries lay eggs coated with high levels of DWV, potentially abetting the long-term persistence of the virus through numerous rounds of egg production (Amiri et al., 2018). Spring mite parasitism focused on drone bees drives vertical (venereal) transmission through queen mating to her offspring. These infections impact subsequent colonies, but not their birth colonies.
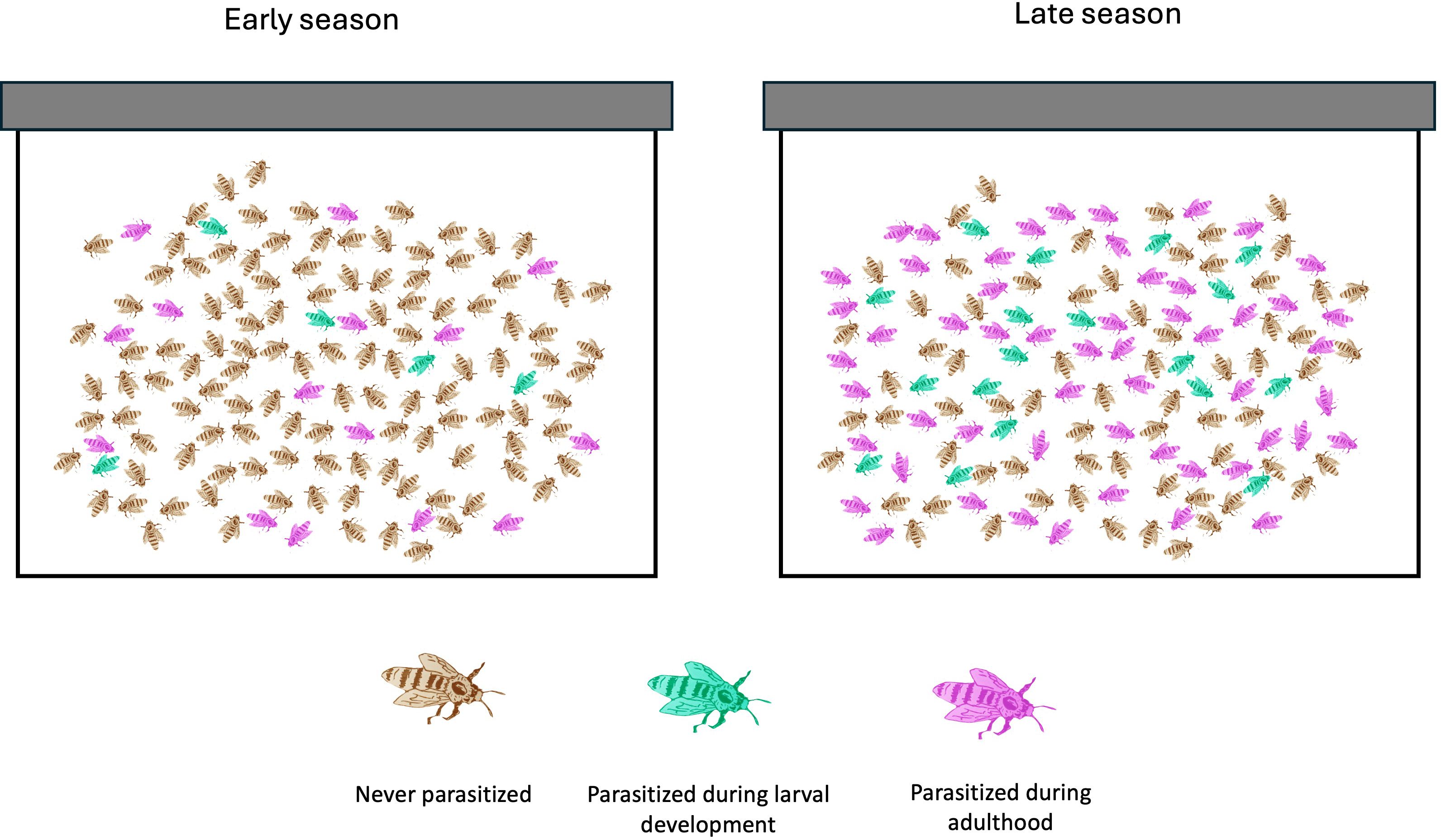
When drones become seasonally less abundant, mites originally on drones inevitably shift to the worker bee population, spreading disease to the critical workforce (Figure 2) While quantification of this shift is needed across apiaries, regions, and climates, movement of mites from heavily infested drones to workers likely has an impact on worker fitness and both bee-bee and mite-bee spread of viruses. Biting on worker bees becomes the defining feature of late-season infestation, when the absolute parasite burden significantly increases on the worker population. Invasions into worker brood significantly increase, as does parasitism of young worker bees. Due to the highly mobile nature of mites, upwards of 60% of worker bees can be parasitized in a little over a week. Given this dynamic, it is not surprising that DWV levels in worker bees and pupae are low early in the season when mite feedings predominate on drones and appreciate greatly in the late summer and fall when they are more frequent targets and when mite populations increase (Tentcheva et al., 2004; Francis et al., 2013; Diao et al., 2019). This increase in viral pressure is tied to increasing colony losses in fall and winter (Dainat et al., 2012).

Figure 2. Seasonal shift of mites from heavily parasitized drone adults to more numerous worker adults.
Vertical transmission pathways likely aid the long-term persistence of covert viral infections in honey bee populations (Chen et al., 2006; Peck and Seeley, 2019; Kulhanek et al., 2021). Beekeepers who allow individual colonies to develop heavy infestation in their dense apiaries may be benefiting mites and viruses, as there will always be new, healthy host colonies to accept them after their original host colony collapses.
5 Beekeeper interventions and the deadly triangle
Beekeepers are part of this complex pathogen-vector-host system, in that the interjection of husbandry and business decisions can have much influence over the persistence and dispersal of pathogens and vectors (Figure 3). Routine reproduction of colonies through the artificial splitting of colonies by beekeepers has enabled beekeepers to maintain a relatively consistent population of honey bee colonies in the United States despite high annual losses. Despite this consistency, the relationship between beekeepers and this system is anything but stable, and many beekeeping operations have high losses annually (Lamas et al., 2024a). At times, business decisions intrinsic to beekeeping are at odds with biosecurity and disease prevention. First, beekeepers keep tens if not hundreds of colonies in close contact in working apiaries. Beekeepers also routinely split colonies to recover from losses, promoting intra-operation dispersal of DWV and Varroa. At a larger scale, the sale of live colonies between operations promotes inter-operational dispersal at a continental scale. Infected and infectious colonies sold into new operations can bring pathogens into naive, susceptible populations. From the perspective of pathogens and parasites, which need to disperse in order to acquire new hosts, these management strategies and sales provide a crucial lending hand on behalf of mites and viruses.
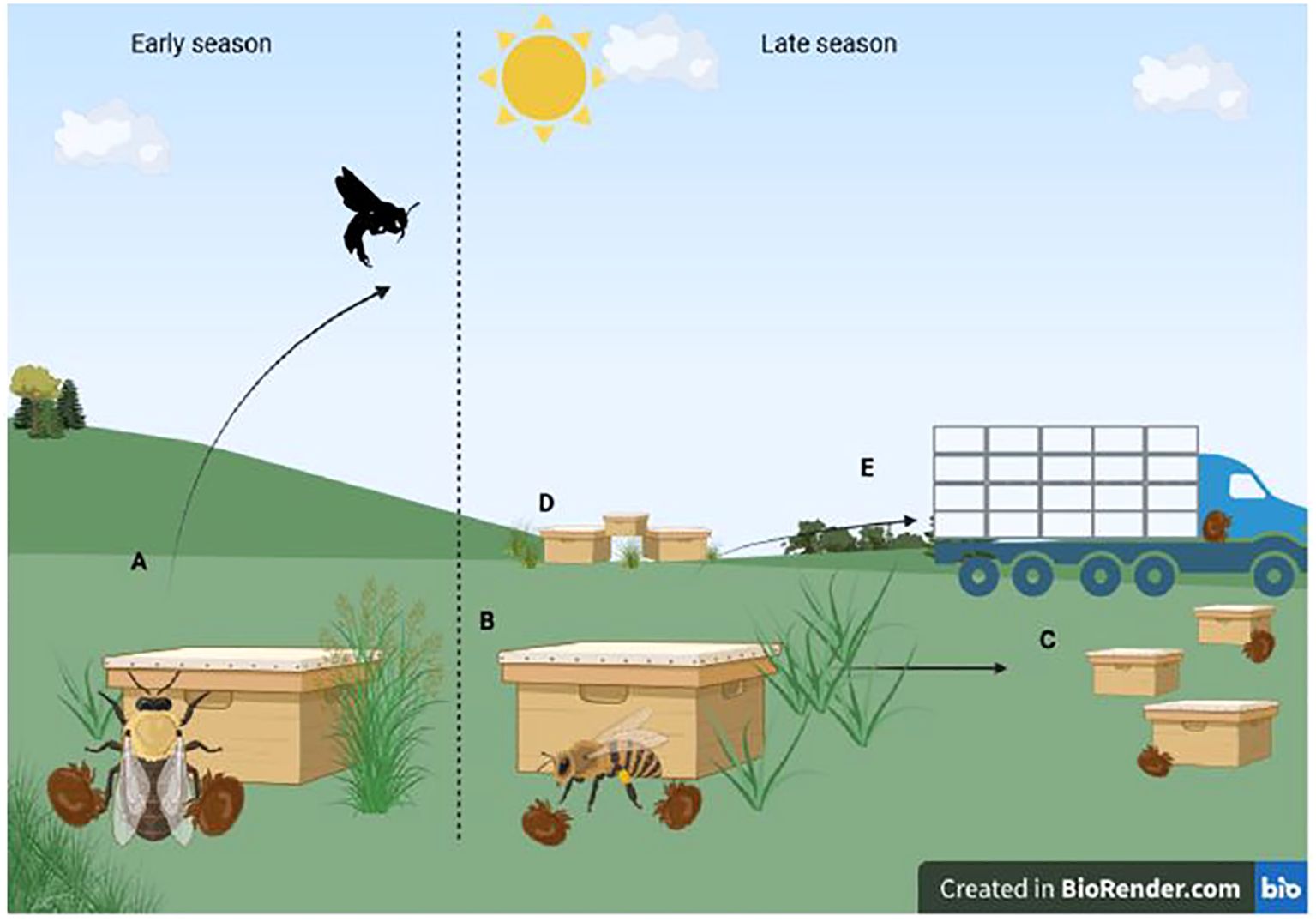
Figure 3. Multiple routes of movement by mites and vectors. (A) Drone infection and subsequent vertical transmission, (B) worker infection within hives, (C) Drift of workers between hives, (D) Diseased colonies as a local source of mites, (E) movement of diseased hives long distances by beekeepers. Source: Created with BioRender.com.
Recent suggestions that managing beehives in ways more consistent with natural colonies and populations (Seeley, 2017) have been taken to heart by some beekeepers as a strategy for disease control. Nevertheless, beekeepers in much of the world deploy chemical miticides to reduce vectoring and direct damage by mites, with treatments that have evolved for over 50 years. Heavy reliance on miticides has led to repetitive and rotating treatments, especially when, inevitably, mites evolve resistance (Haber et al., 2019). IPM treatment methods, including regular mite counts, can economize treatments and prolong the lifetimes of specific chemicals. Even when beekeepers successfully control Varroa populations, they can be left with circulating viruses among colony and apiary members (Locke et al., 2017). Given difficulties in monitoring both viruses and individual symptomatic bees, the first indication of high viral loads might be “dwindled” colonies that fail to grow or whose remaining bees are unable to thermoregulate or regenerate their lost adult bee population. When this occurs, beekeepers are left with economically non-viable units (Lamas et al., 2024a). More urgently, effective antiviral drugs could help release honey bees from some of the costs of persistent mite infection.
6 Hot topics and unknowns
This review highlights disease models for this complex pathogen-vector-host triad in light of ecological and economic importance. Many questions remain open in light of this triad and the protection of honey bee health. A few of the more pressing questions are below:
● Which current beekeeping strategies exacerbate parasites and viruses in colonies? For example, beekeepers split colonies for sale or to replace lost colonies, but splitting will inevitably aid in the artificial dispersal of mites and viruses. Which new practices would allow the movement of bees and re-use of equipment while reducing the risk of infection?
● What are the carrying capacities of landscapes with respect to unhealthy disease pressures?
● Varroa is a biological vector of some but not all DWV strains. How does this impact the triangle?
● More generally, roughly half of the known honey bee viruses seem to be vectored by Varroa; why are these distinct from other bee viruses?
● Continued work is needed to understand colony components that harbor and cause infection to new bees. How infectious are hive substrates? And brood food given to larvae?
● What are the roles of environmental stressors such as poor nutrition, sublethal pesticide exposures, and co-infection in increasing honey bee susceptibility to viruses?
● How can beekeepers reduce viral impacts with prevention, medication, or recovery?
Author contributions
ZL: Writing – original draft, Writing – review & editing. JE: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. ZL is supported by USDA-NIFA postdoctoral award 2023-67012-40247.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amiri E., Kryger P., Meixner M. D., Strand M. K., Tarpy D. R., Rueppell O. (2018). Quantitative patterns of vertical transmission of deformed wing virus in honey bees. PloS One 13, e0195283. doi: 10.1371/journal.pone.0195283
Amiri E., Meixner M. D., Kryger P. (2016). Deformed wing virus can be transmitted during natural mating in honey bees and infect the queens. Sci. Rep. 6, 33065. doi: 10.1038/srep33065
Benaets K., Van Geystelen A., Cardoen D., De Smet L., De Graaf D. C., Schoofs L., et al. (2017). Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proceedings of the Royal Society B: Biological Sciences 2017 284, 20162149. doi: 10.1098/rspb.2016.2149
Boncristiani H., Ellis J. D., Bustamante T., Graham J., Jack C., Kimmel C. B., et al. (2020). World honey bee health: the global distribution of western honey bee (Apis mellifera L.) pests and pathogens. Bee World 98, 2–6.
Bowen-Walker P. L., Martin S. J., Gunn A. (1999). The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invert. Pathol. 73, 101–106. doi: 10.1006/jipa.1998.4807
Brutscher L. M., Daughenbaugh K. F., Flenniken M. L. (2017). Virus and dsRNA-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 7. doi: 10.1038/s41598-017-06623-z
Chapman N. C., Colin T., Cook J., Da Silva C. R. B., Gloag R., Hogendoorn K., et al. (2023). The final frontier: Ecological and evolutionary dynamics of a global parasite invasion. Biol. Lett. 19. doi: 10.1098/rsbl.2022.0589
Chen Y., Evans J., Feldlaufer M. (2006). Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92, 152–159. doi: 10.1016/j.jip.2006.03.010
Cooper L., Kang S. Y., Bisanzio D., Maxwell K., Rodriguez-Barraquer I., Greenhouse B., et al. (2019). Pareto rules for malaria super-spreaders and super-spreading. Nat. Commun. 10, 3939. doi: 10.1038/s41467-019-11861-y
Dainat B., Evans J. D., Chen Y. P., Gauthier L., Neumann P. (2012). Dead or alive: Deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 78, 981–987. doi: 10.1128/AEM.06537-11
de Miranda J. R., Genersch E. (2010). Deformed wing virus. J. Invertebr. Pathol. 103, S48–S61. doi: 10.1016/j.jip.2009.06.012
Diao Q., Yang D., Zhao H., Deng S., Wang X., Hou C., et al. (2019). Prevalence and population genetics of the emerging honey bee pathogen DWV in Chinese apiculture. Sci. Rep. 9, 12042. doi: 10.1038/s41598-019-48618-y
Doublet V., Oddie M. A., Mondet F., Forsgren E., Dahle B., Furuseth-Hansen E., et al. (2024). Shift in virus composition in honeybees (Apis mellifera) following worldwide invasion by the parasitic mite and virus vector Varroa destructor. R. Soc. Open Sci. 11, 231529. doi: 10.1098/rsos.231529
Fang Y., Wubie A. J., Feng M., Ma C., Baer B., Li J. (2022). Larval exposure to parasitic Varroa destructor mites triggers specific immune responses in different honey bee castes and species. Mol. Cell. Proteomics 21, 100257. doi: 10.1016/j.mcpro.2022.100257
Francis R. M., Nielsen S. L., Kryger P. (2013). Varroa-virus interaction in collapsing honey bee colonies. PloS One 8, e57540. doi: 10.1371/journal.pone.0057540
Garrett-Jones C., Shidrawi G. R. (1969). Malaria vectorial capacity of a population of Anopheles Gambiae: an exercise in epidemiological entomology. Bull. World Health Organ 40, 531–545.
Geffre A. C., Gernat T., Harwood G. P., Jones B. M., Gysi D. M., Hamilton A. R., et al. (2020). Honey bee virus causes context-dependent changes in host social behavior. Proc. Natl. Acad. Sci. United States America 117, 10406–10413. doi: 10.1073/pnas.2002268117
Gregory P. G., Evans J. D., Rinderer T., de Guzman L. (2005). Conditional immune-gene suppression of honeybees parasitized by Varroa mites. J. Insect Sci. (Online) 5, 7. doi: 10.1093/jis/5.1.7
Grindrod I., Kevill J. L., Villalobos E. M., Schroeder D. C., Martin S. J. (2021). Ten years of deformed wing virus (Dwv) in hawaiian honey bees (Apis mellifera), the dominant dwv-a variant is potentially being replaced by variants with a dwv-b coding sequence. Viruses 13, 969. doi: 10.3390/v13060969
Haber A. I., Steinhauer N. A., Vanengelsdorp D. (2019). Use of chemical and nonchemical methods for the control of Varroa destructor (Acari: Varroidae) and associated winter colony losses in U.S. beekeeping operations. J. Econ. Entomol. 112, 1509–1525. doi: 10.1093/jee/toz088
Han B., Wu J., Wei Q., Liu F., Cui L., Rueppell O., et al. (2024). Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 15, 725. doi: 10.1038/s41467-024-44915-x
Hasegawa N., Techer M. A., Adjlane N., M.S. al-Hissnawi K., Beaurepaire A., Christmon K., et al. (2023). and A.S. Mikheyev, Evolutionarily diverse origins of deformed wing viruses in western honey bees. Proceedings of the National Academy of Sciences of the United States of America 2023 120, e2301258120. doi: 10.1073/pnas.2301258120
Kanbar G., Engels W. (2005). Communal use of integumental wounds in honey bee (Apis mellifera) pupae multiply infested by the ectoparasitic mite Varroa destructor. Genet. Mol. Res. 4, 465–472.
Kulhanek K., Garavito A., vanEngelsdorp D. (2021). Accelerated Varroa destructor population growth in honey bee (Apis mellifera) colonies is associated with visitation from non-natal bees. Sci. Rep. 16, e0245490. doi: 10.1038/s41598-021-86558-8
Lamas Z. S. (2022). Feeding behavior and distribution of Varroa destructor on adult bees of Apis mellifera. University of Maryland, College Park, MD.
Lamas Z. S., Chen Y. P., Evans J. D. (2024a). Case Report: Emerging losses of managed honey bee colonies. Biology 13, 117. doi: 10.3390/biology13020117
Lamas Z. S., Krichton M., Ryabov E. V., Hawthorne D. J., Evans J. D. (2024b). Susceptible and infectious states for both vector and host in a dynamic pathogen-vector-host system. Proc. R. Soc. B: Biol. Sci. 291. doi: 10.1098/rspb.2023.2293
Lamas Z. S., Solmaz S., Ryabov E. V., Mowery J., Heermann M., Sonenshine D., et al. (2023). Promiscuous feeding on multiple adult honey bee hosts amplifies the vectorial capacity of Varroa destructor. PloS Pathog. 19, e1011061. doi: 10.1371/journal.ppat.1011061
Locke B., Semberg E., Forsgren E., De Miranda J. R. (2017). Persistence of subclinical deformed wing virus infections in honeybees following Varroa mite removal and a bee population turnover. PloS One 12, e0180910. doi: 10.1371/journal.pone.0180910
Martin S. J., Highfield A. C., Brettell L., Villalobos E. M., Budge G. E., Powell M., et al. (2012). Global honey bee viral landscape altered by a parasitic mite. Science 336, 1304–1306. doi: 10.1126/science.1220941
Peck D. T., Seeley T. D. (2019). Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PloS One 14, e0218392. doi: 10.1371/journal.pone.0218392
Posada-Florez F., Lamas Z. S., Hawthorne D. J., Chen Y., Evans J. D., Ryabov E. V. (2021). Pupal cannibalism by worker honey bees contributes to the spread of deformed wing virus. Sci. Rep. 11, 8989. doi: 10.1038/s41598-021-88649-y
Ramsey S. D., Ochoa R., Bauchan G., Gulbronson C., Mowery J. D., Cohen A., et al. (2019). Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. United States America 116, 1792–1801. doi: 10.1073/pnas.1818371116
Richards E. H., Jones B., Bowman A. (2011). Salivary secretions from the honeybee mite, Varroa destructor: effects on insect haemocytes and preliminary biochemical characterization. Parasitology 138, 602–608. doi: 10.1017/S0031182011000072
Schittny D., Yañez O., Neumann P. (2020). Honey bee virus transmission via hive products. Vet. Sci. 7, 96. doi: 10.3390/vetsci7030096
Seeley T. (2017). Darwinian beekeeping: An evolutionary approach to apiculture. Am. Bee J. 157, 77–282.
Stein R. A. (2011). Super-spreaders in infectious diseases. Int. J. Infect. Dis. 15, e510–e513. doi: 10.1016/j.ijid.2010.06.020
Steinhauer N., Kulhanek K., Antúnez K., Human H., Chantawannakul P., Chauzat M. P., et al. (2018). Drivers of colony losses. Curr. Opin. Insect Sci. 26, 142–148. doi: 10.1016/j.cois.2018.02.004
Steinmann N., Corona M., Neumann P., Dainat B. (2015). Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PloS One 10, e0129956. doi: 10.1371/journal.pone.0129956
Tang C. K., Lin Y. H., Jiang J. A., Lu Y. H., Tsai C. H., Lin Y. C., et al. (2021). Real-time monitoring of deformed wing virus-infected bee foraging behavior following histone deacetylase inhibitor treatment. iScience 24, 103056. doi: 10.1016/j.isci.2021.103056
Tentcheva D., Gauthier L., Zappulla N., Dainat B., Cousserans F., Colin M. E., et al. (2004). Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 70, 7185–7191. doi: 10.1128/AEM.70.12.7185-7191.2004
Wagoner K., Spivak M., Hefetz A., Reams T., Rueppell O. (2019). Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Sci. Rep. 9. doi: 10.1093/jisesa/ieab064
Wilfert L., Long G., Leggett H. C., Schmid-Hempel P., Butlin R., Martin S. J., et al. (2016). Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351, 594–597. doi: 10.1126/science.aac9976
Yang X., Cox-Foster D. L. (2005). Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. United States America 102, 7470–7475. doi: 10.1073/pnas.0501860102
Yue C., Genersch E. (2005). RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86, 3419–3424. doi: 10.1099/vir.0.81401-0
Yue C., Schröder M., Gisder S., Genersch E. (2007). Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 88, 2329–2336. doi: 10.1099/vir.0.83101-0
Keywords: pollination, vector biology, host-parasite, pathology, virus
Citation: Lamas ZS and Evans JD (2024) Deadly triangle: honey bees, mites, and viruses. Front. Bee Sci. 2:1418667. doi: 10.3389/frbee.2024.1418667
Received: 16 April 2024; Accepted: 24 July 2024;
Published: 15 August 2024.
Edited by:
David De Jong, University of Sao Paulo, BrazilReviewed by:
Christian W. W. Pirk, University of Pretoria, South AfricaPeter Rosenkranz, University of Hohenheim, Germany
Copyright © 2024 Lamas and Evans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zachary S. Lamas, emFjbGFtYXNAZ21haWwuY29t; Jay D. Evans, amF5LmV2YW5zQHVzZGEuZ292
 Zachary S. Lamas*
Zachary S. Lamas* Jay D. Evans
Jay D. Evans