- 1Ecology, Restoration Ecology and Landscape, Faculty of Agronomic Sciences, University of Lubumbashi (UNILU), Lubumbashi, Democratic Republic of Congo
- 2Crop Protection and Defense, Faculty of Agronomic Sciences, University of Lubumbashi (UNILU), Lubumbashi, Democratic Republic of Congo
Anthropogenic disturbances mainly involve the loss of habitats in tropical regions where there is also significant population growth. These disturbances also have an impact on the plant pollination service, which is struggling to be explored in the Lubumbashi region, where mining interests seem to take priority given the local connotations and the predominance of players within the sector. The present study focuses on an analysis of the pollination service and the interactions maintained between bees and their host plants, in a context of agricultural impetus through the practice of agroforestry, the benefits of which supposedly extend from improved yields to efforts to conserve global biodiversity. Subject to the sampling effort at the limits of the favorable periods, our results indicate a significant biodiversity of bees, unevenly distributed among the families Apidae, Halictidae and Megachilidae. The species Xylocopa albiceps, Nomia speciosana, X. olivaceae and Megachile torrida dominate the abundance ranks, while more restricted than general interactions between pollinators and their host plants are recorded.
1 Introduction
Food underproduction are recurring problems in the tropics (Bationo et al., 2006; FAO, 2016). Crop yield losses are partly attributable to the deficiency of plant pollination suppliers. These insects are in turn limited by the lack of food resources, but even more so by the loss of suitable habitat (Buchmann et al., 1996; Kearns et al., 1998). Incorporating trees into degraded plant formations can improve pollination services by providing floral resources, nesting sites and protection from adverse weather (Kelly and Elle, 2020). These factors suggest that the integration of trees and hedgerows could be as effective a method of pollinator conservation as other pollinator management systems (Donkersley, 2019). By creating a more welcoming environment for wild bees and other pollinators, agroforestry systems support agricultural productivity while helping to maintain ecological health and promote a sustainable environment (Pfiffner and Müller, 2016).
It is evident that pollinating insects, such as bees, have a crucial link in the regulation of biodiversity through their role in maintaining ecosystem function. This service is contingent upon the diversity of floricultural plants, which serve as food resources. Some research reports have highlighted the co-evolution between pollinators and flowering plants since the earliest stages of their divergence. In this mutually beneficial symbiosis, bees obtain nectar and pollen, while plants gain assured sexual reproduction (Buchmann et al., 1996; Ollerton et al., 2011).
It’s now well known that not all bees exhibit the same feeding behavior when it comes to choosing the host plants associated with them. There are generalist bees (Polylectics), just as there are specialists affiliated to a specific botanical family (Monolectics), but also Oligolectics who prefer a well-defined genus (Pekkarinen, 1997; Ritchie et al., 2016; Cane, 2021). It is imperative to analyze the intrinsic relationship between pollinators and their associated flora. Knowledge of their distribution according to these functional traits makes it possible to assess the vulnerability of their livelihoods. Consequently, this knowledge contributes to the establishment of related conservation statuses.
The aim of this study is to analyze plant-pollinator interactions, with regard to the diversity of bees potentially favored by the practice of agroforestry on the perimeter. In addition, it will involve: i) characterizing the diversity of wild bees in the AFODEK perimeter and ii) analyzing the interactions between the diversity of wild bee fauna and that of flowering plants, and specifying the type of foraging behavior of the bees collected.
By examining these interactions and relationships, this research seeks to contribute valuable insights into the benefits of agroforestry for supporting pollinator diversity and enhancing ecosystem resilience, Despite the fact that this research was carried out during the dry season, which is characterized by a scarcity of floral resources for bees and other pollinating insects.
2 Materials and methods
2.1 Study area
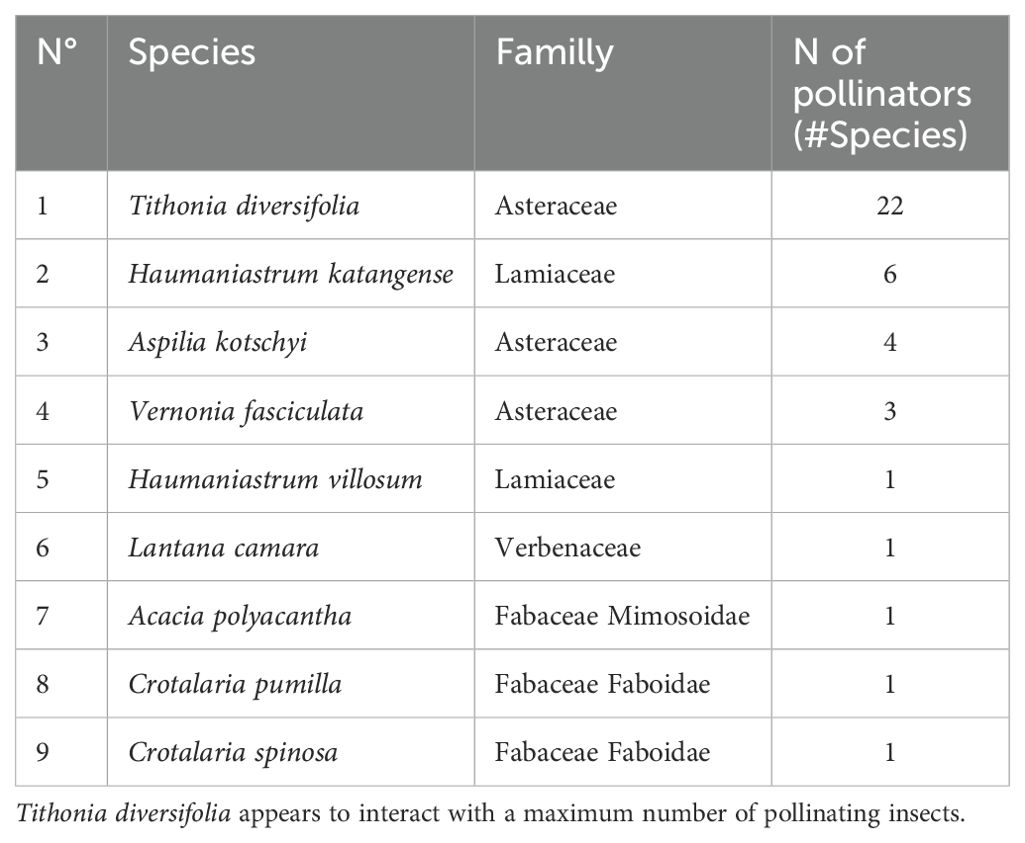
This work was carried out in the agroforestry area known as “Agro-forêts pour le développement de Kipushi” (AFODEK in abbreviation, Figure 1). This area is located on the outskirts of Lubumbashi, within the territory of Kipushi (Haut-Katanga, DRC). The project arose from the need for sustainable natural resource management alternatives in the face of forest dieback and food security issues.
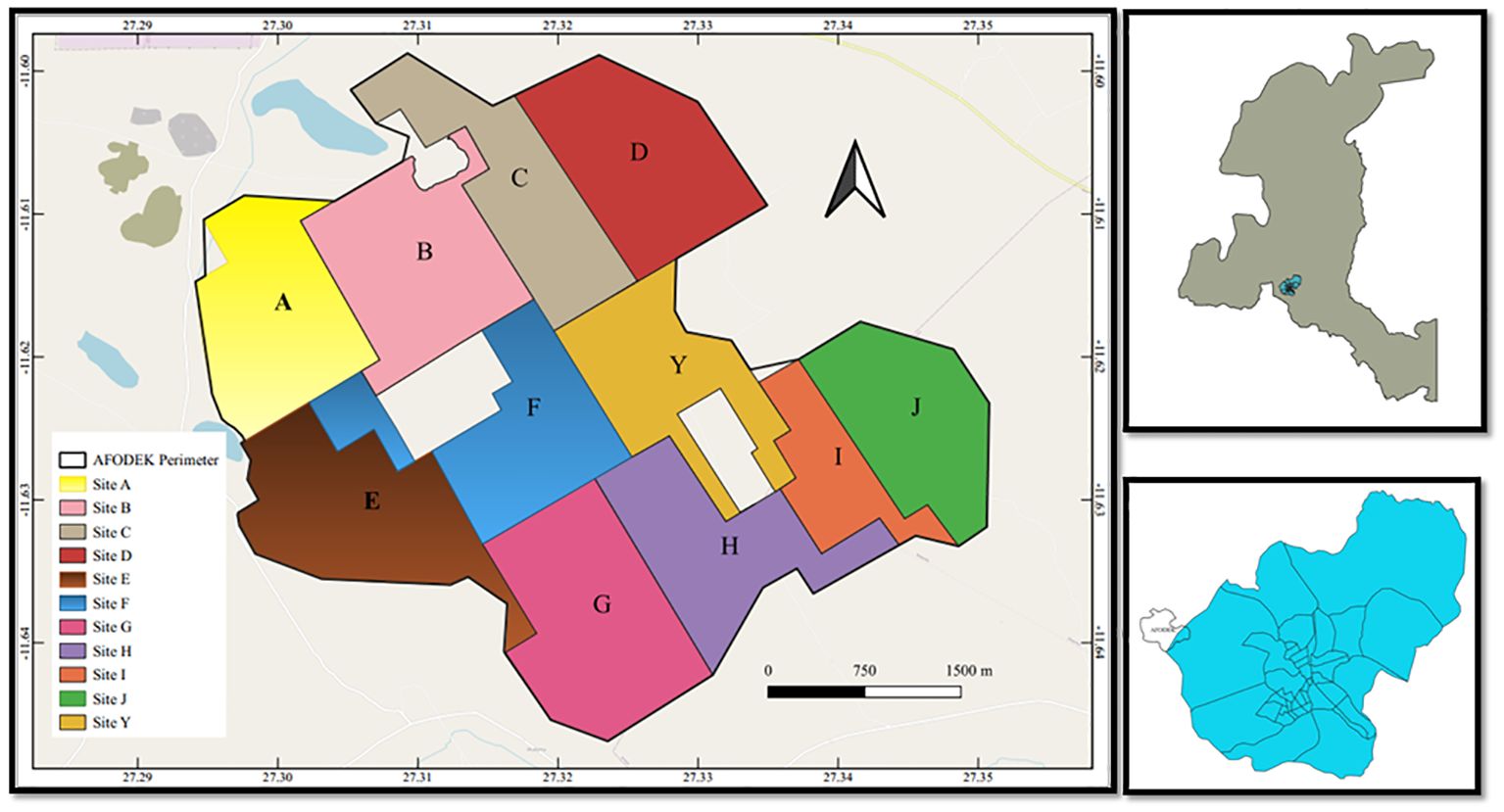
Figure 1. Study area. All sites are located within the same perimeter of land use (acacia plantation plots and agricultural fields). We subdivided the sites in accordance with the main axis of the perimeter.
The “Agro-forêts pour le développement de Kipushi” (AFODEK) project was set up with financial support from the European Union (DCI-FOOD/2012/294-526). This project was inspired by the experience of the Hanns Seidel Foundation in Mampu (Plateaux des Batékés, DRC). It ran from December 2012 to November 2017. Three organizations, GRET, the Belgian non-profit Nature+ and the Centre Promotionnel du Paysannat, worked together to develop a 2,000-hectare agroforestry area in a degraded savannah zone with low soil fertility.
The main aim of this initiative was to reduce deforestation and improve food security in Haut-Katanga by using agroforestry for the sustainable production of charcoal. As a result, more than 350 hectares of Acacia auriculiformis agroforestry plantations were established in 2017 as part of the AFODEK project. In addition, an NGO called CAPAK was set up to represent the 133 agroforestry farmer families and manage the agroforestry zone by helping farmers to implement agroforestry in accordance with the technical itinerary (Boldrini et al., 2017).
Each participant in the project is given 12 hectares of land on which to grow food crops by planting trees (A. auriculiformis). The synergy of these woody plants will greatly benefit soil fertilization, as well as charcoal production in the medium term.
2.2 Sample preparation and taxonomic identification of material
The biological materials consisted of specimens of pollinating insects, mainly wild bees, and the host plants on which these pollinating insects gathered. Bees are insects of the Hymenoptera order (Michener, 2007; Eardley et al., 2010). The pollinating insects in this study concerns all wild bee species, including a few specimens of honeybees (Apis mellifera) captured accidentally. The second type of biological material analyzed was the plant community, which was essentially herbaceous. Most of these are weeds that grow on the edges of fields and in fallow fields.
The specimens collected were pinned, labeled, identified and encoded in a database that reproduces the capture site and the specific nomenclature.
Sampling started at the beginning of 26 April 2021, which corresponds to the transition period between the rainy season and the dry season in the Lubumbashi region, as defined by Köppen (Bultot, 1954). Unfortunately, this does not correspond to the period of maximum flowering, as the majority of flowering plants fruit gradually.
Sampling consisted of capturing bees according to the vigorous sampling protocol, using swath nets with free 60-centimetre transects and 9 colored cups (3 white, 3 blue and 3 yellow) which were placed at each morning pass and collected in the afternoon at the end of the site visit (Westphal et al., 2008, see also Vereecken et al., 2021; Figure 2). The host plants were identified in the field, or brought back to the Lubumbashi herbarium for a more accurate comparison with the flora of Central Africa.
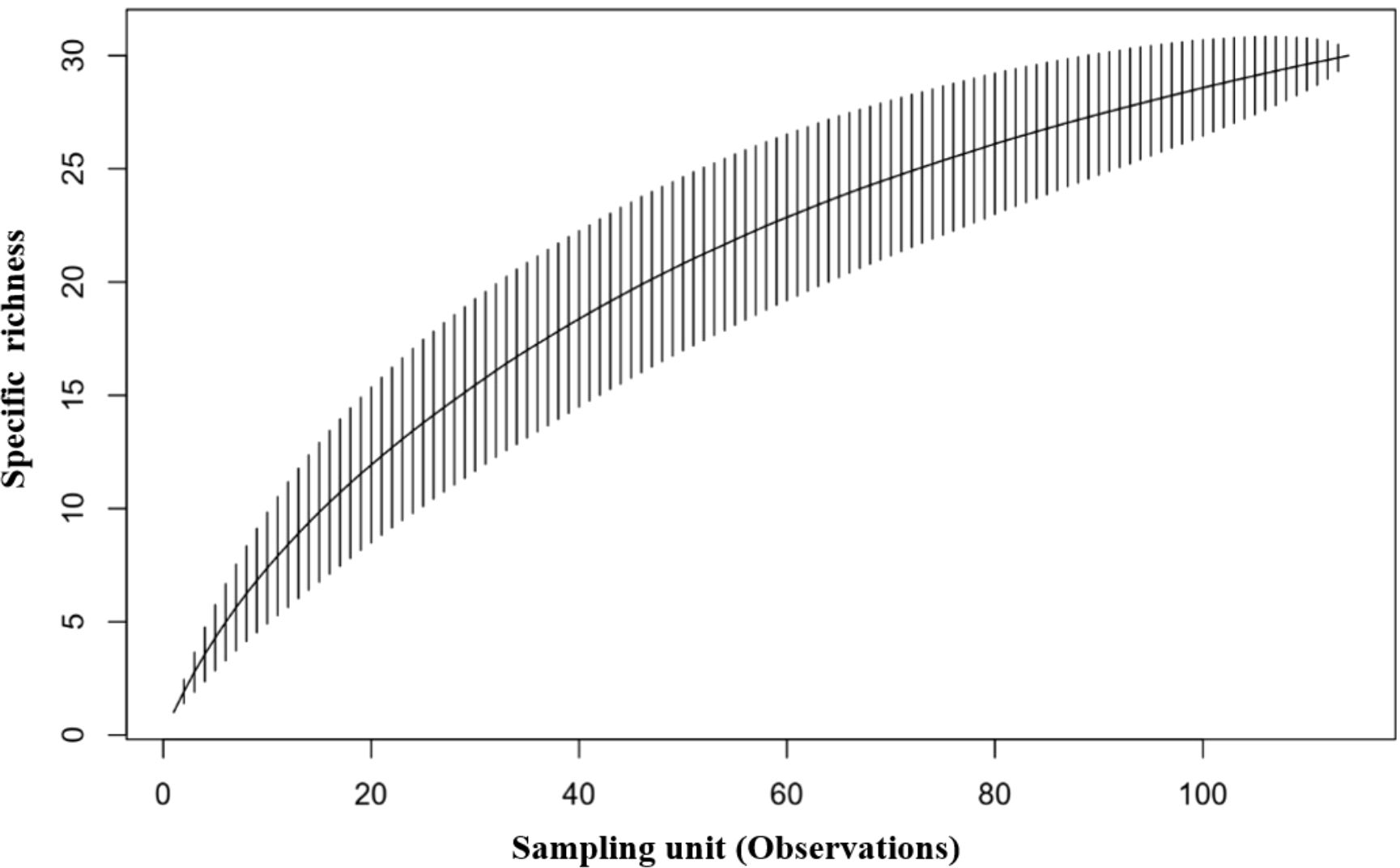
Figure 2. Pollinator species accumulation curve for the AFODEK perimeter. Observations reach a plateau at 30 species, indicating a considerable sampling effort.
As the AFODEK perimeter was subdivided into sites, the sampling plan was oriented in relation to each of them, so that each site was sampled in such a way as to detect its wild bee fauna and associated host flora.
As for the active trap, this involves ambushing insects by moving them down with a net, either in full flight or placed on a flower.
The taxonomic determination of the bee specimens collected refers to the key to the genera and sub-genera of bees in sub-Saharan Africa (Eardley et al., 2009). As several specimens of regional bees could not be identified at the species level, some of them were classified as “morpho-species” on the basis of morphological similarities within identical genera. Nevertheless, we had a quick comparison with some materials from Royal Museum for central Africa (RMCA, Belgium) and online repertories to identify bee species (Eardley et al., 2010; Ascher and Pickering, 2020; Tshibungu et al., 2023).
2.3 Analysis of pollinators diversity and their interactions with the local flora
The data relating to the pollinating insect fauna were processed in RStudio1.1. 463, so as to bring out the accumulation curve, by going through the “reshape2”, “vegan” and “BiodiversityR” packages (Gotelli and Colwell, 2001; Wickham, 2017; Oksanen, 2019). The “reshape2” package, through the “dcast” and “melt” functions, formed the basis of the statistical processing. The (di-)similarity was first analyzed by grouping the sites using non-metric multidimensional scaling. The (di-)similarity was first analyzed by grouping the sites using non-metric multidimensional scaling. We then used ANOSIM analysis to obtain information on the similarity of species diversity within the perimeter. The diversity of insect pollinators was thus calculated using the Shannon, Simpson and Pielou indices (Borcard et al., 2018).
3 Results
3.1 Characterization of the diversity of pollinating insects in the perimeter of the AFODEK project
We sampled a total of 114 specimens of pollinating insects, mainly wild bees, across the 11 sites that make up the AFODEK perimeter. The accumulative curve obtained for the whole group shows that the sampling effort was significant, reaching a maximum of 30 species (Figure 2).
In terms of specific rank abundance, there are a maximum of 11 top species accounting for ¾ of the dataset, while the top four species account for just over half (51.8%) of overall abundance. These include X. albiceps, Nomia speciosana, Xylocopa olivaceae and Megachile torrida.
With regard to the sampling effort, all specimens of the order Hymenoptera were collected, belonging to 3 families of bees (Apidae, Megachilidae and Halictidae) and 1 family of wasps (Crabonidae).
The diversity of the insect pollinators varies from one site to another (Figure 3). According to the Shannon index, it is highest at site Y with 20 species out of a total of 66 specimens, while the lowest diversity is recorded at site E with 2 species out of 4 specimens. The Simpson index show that 89.1% of the time a new species will be found at location Y. The probability of encountering a new species is also high at sites C and F, with almost ¾ of the observations. Finally, it is lower at site E with a proportion of 37.5%, i.e. just over 1/3 probability of observing a new species.
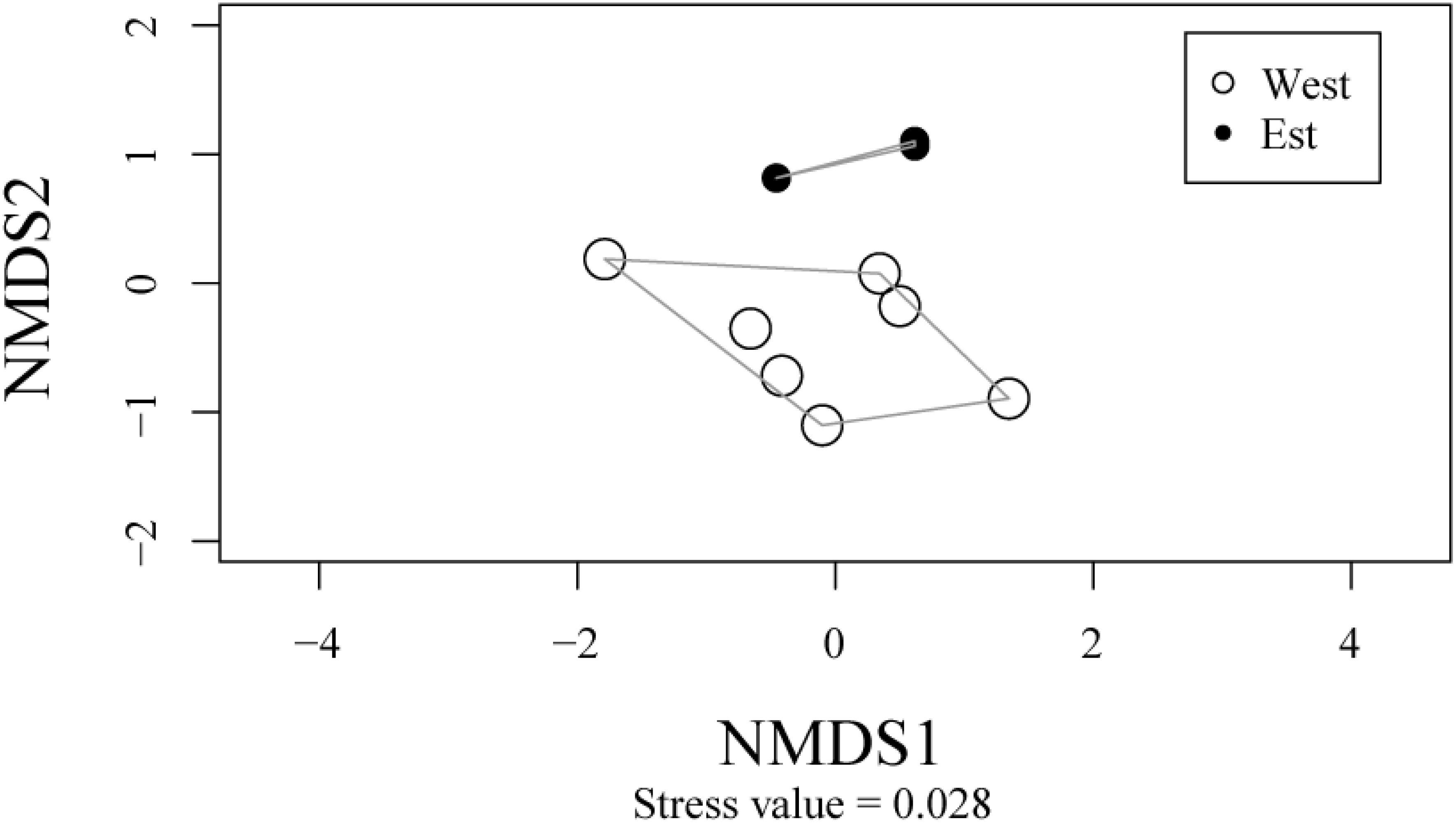
Figure 3. Groups of sites with respect to specific pollinator assemblages. NMDS analysis confirms the separation between sites east and west of the AFODEK perimeter, some of which are almost superimposed by similarity.
The Pielou index shows maximum equitability at sites A, B, D and I, and relatively low equitability at sites C, E, F, H, J, and Y, where the abundance of one or more species dominates the total of pollinating insects.
The ANOSIM analysis showed no significant difference between the sites (ANOSIM: 0.38; p = 0.02). Except for some specific features observed in some of the sites, such as site Y, which has a high specific variability, the different sites within the AFODEK perimeter have a rather similar specific pollinator composition. The analysis of the specific composition of the bees was a validation of the spatial distribution of the sites as perceived within the perimeter (Figure 4). There was a clustering of 3 sites to the east and 7 to the west, with a stress value indicating a good fit (stress < 0.2) of the model. In general, the dispersal of the sites appears to be significant, although the remoteness of most of them is emphasized. The exceptions are the pairs of sites E and H in the west, and I and Y in the east, whose similar composition imposes a quasi-superposition on the graph. In terms of specific differences between sites, the observed overall replacement (βsør) seems to be due more to the replacement of species between sites than to their turnover (Figure 3).
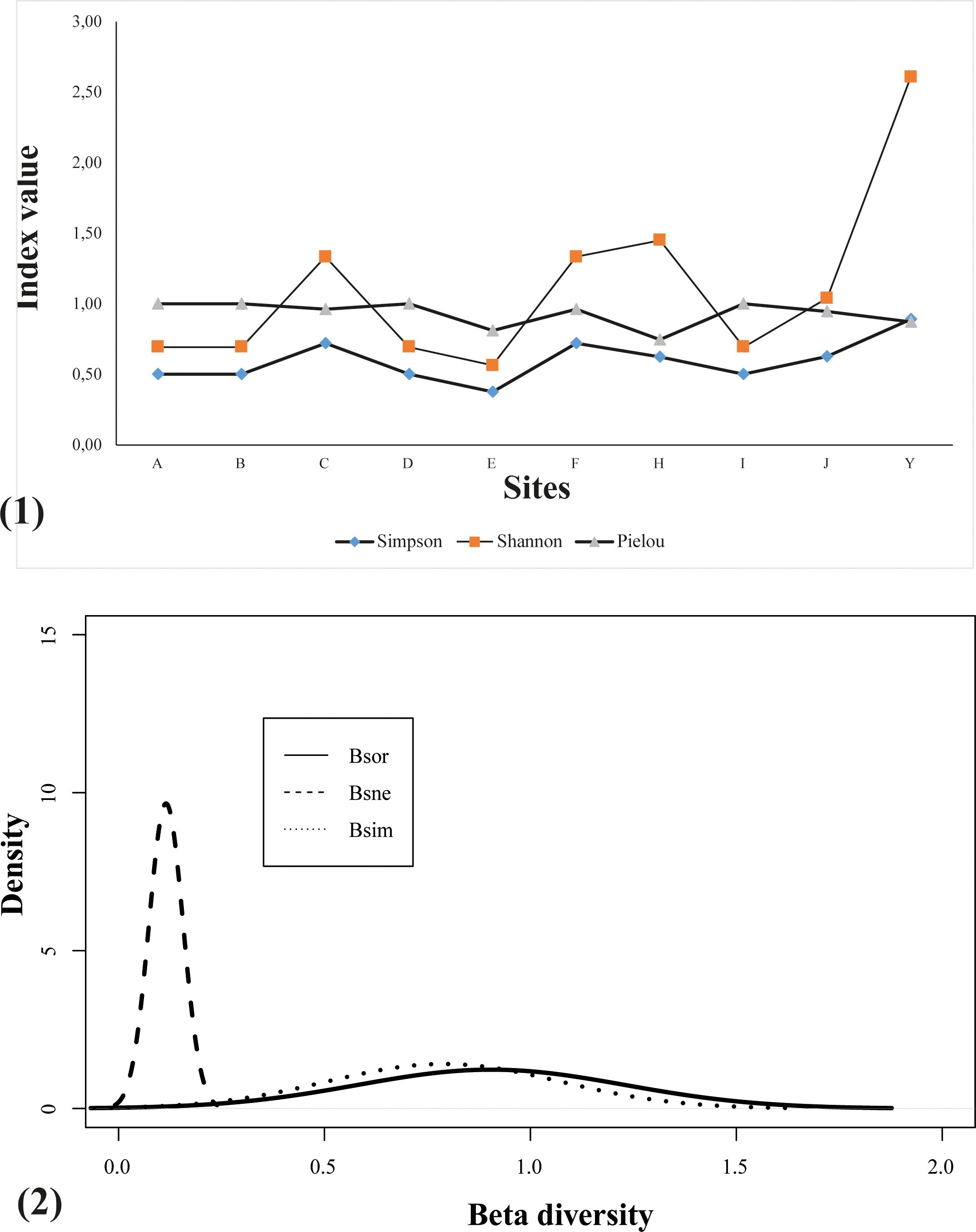
Figure 4. Insect pollinator diversity indices for the AFODEK perimeter. (1) Diversity within each site is relatively high, with a higher probability of observing a new species at site Y and a low specific evenness at site E. (2) Overall substitution is better explained by species replacement between sites. The metric βsør represents a measure of total dissimilarity, and its partitioning into its two major components: (i) species replacement among sampling units, i.e., turnover: βsim; and (ii) species loss/gain, i.e., nestedness: βsne; following the formula βsør =βsim + βsne (Baselga, 2010; Legendre, 2014).
3.2 Pollinator-plant interactions and typology of foraging behavior in the AFODEK perimeter
All the bees collected were reported on 9 plant species, divided into 4 botanical families (Asteraceae, Fabaceae Faboidae and Mimosoidae, Lamiaceae and Verbenaceae) (Table 1).
Of all the host plants recorded, the highest interaction score went to Tithonia diversifolia, which was associated with 22 species of pollinator identified, followed by Haumaniastrum robertii with 6 species, Aspilia kotschyi with 4 species, Vernonia fasciculata with 3 species and finally, Haumaniastrum villosum, Lantana camara and Acacia polyacantha, each with one pollinator species sampled (Figure 5).
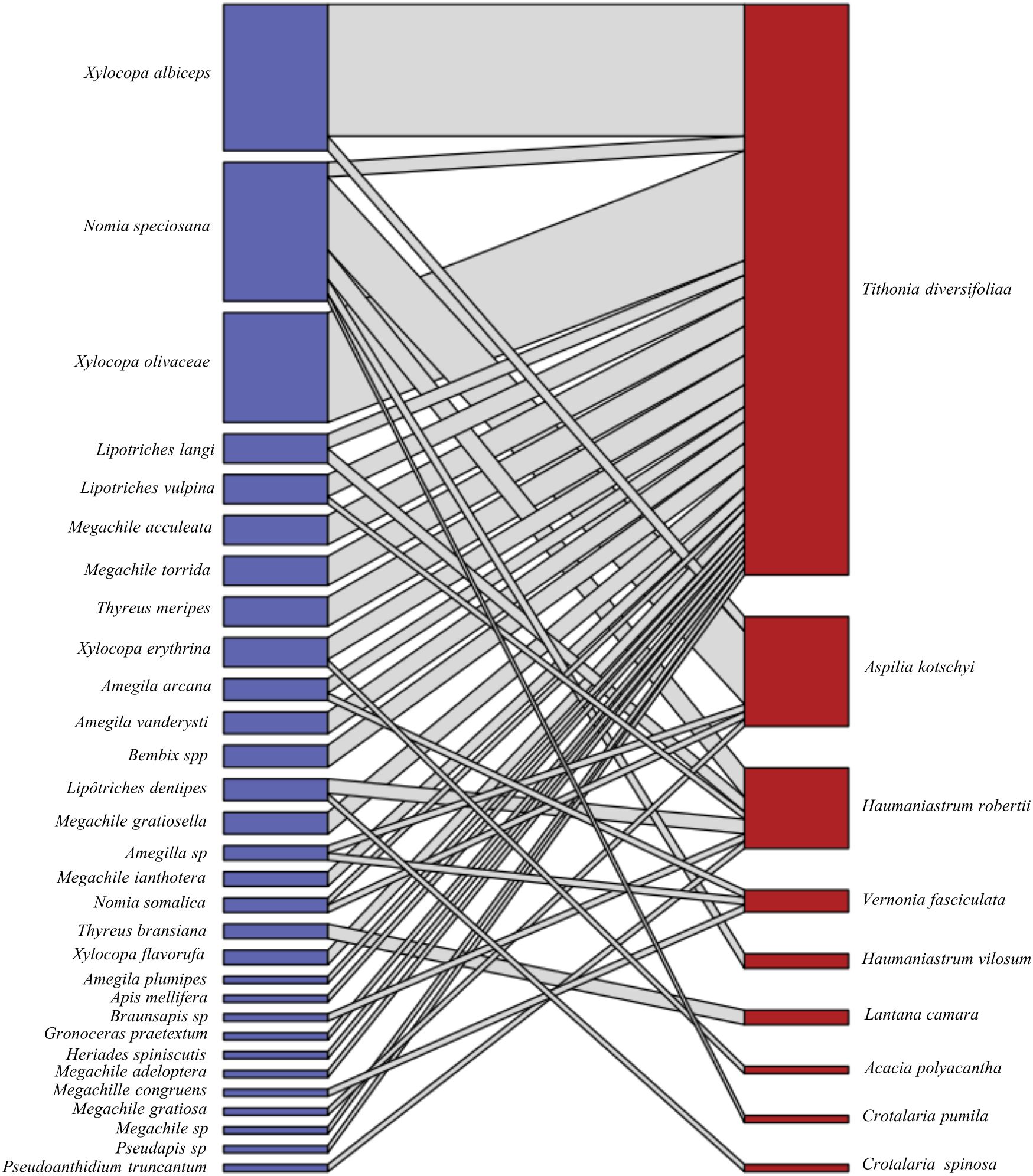
Figure 5. Bipartite pollinator-plant network of 30 pollinating insect species (blue boxes) and pollen from 9 host plants (red boxes–on top) across the AFODEK perimeter. The links between crops and pollinators are represented by grey lines, the number of which reflects the intensity of interactions, while the width of the nodes is proportional to interaction strength.
In terms of the specialization of pollinating insects, N. speciosana appears to be the most generalist bee species, with 5 different host plants. In view of the foraging behavior of the pollinating insects, it would appear that these pollinators are distributed as follows (Table 2). On the basis of the interaction analyses, it appears to exist a low connectivity (0.15) within the network, which justifies the low number of observed interactions compared to the number that could potentially be expected, and low nestedness (13.72), with the minority of pollinator species interacting within the few niche components supported by generalist species. Finally, a vulnerability of about 1.67 was recorded, which corresponds to an average of about 2 pollinating species per host plant.
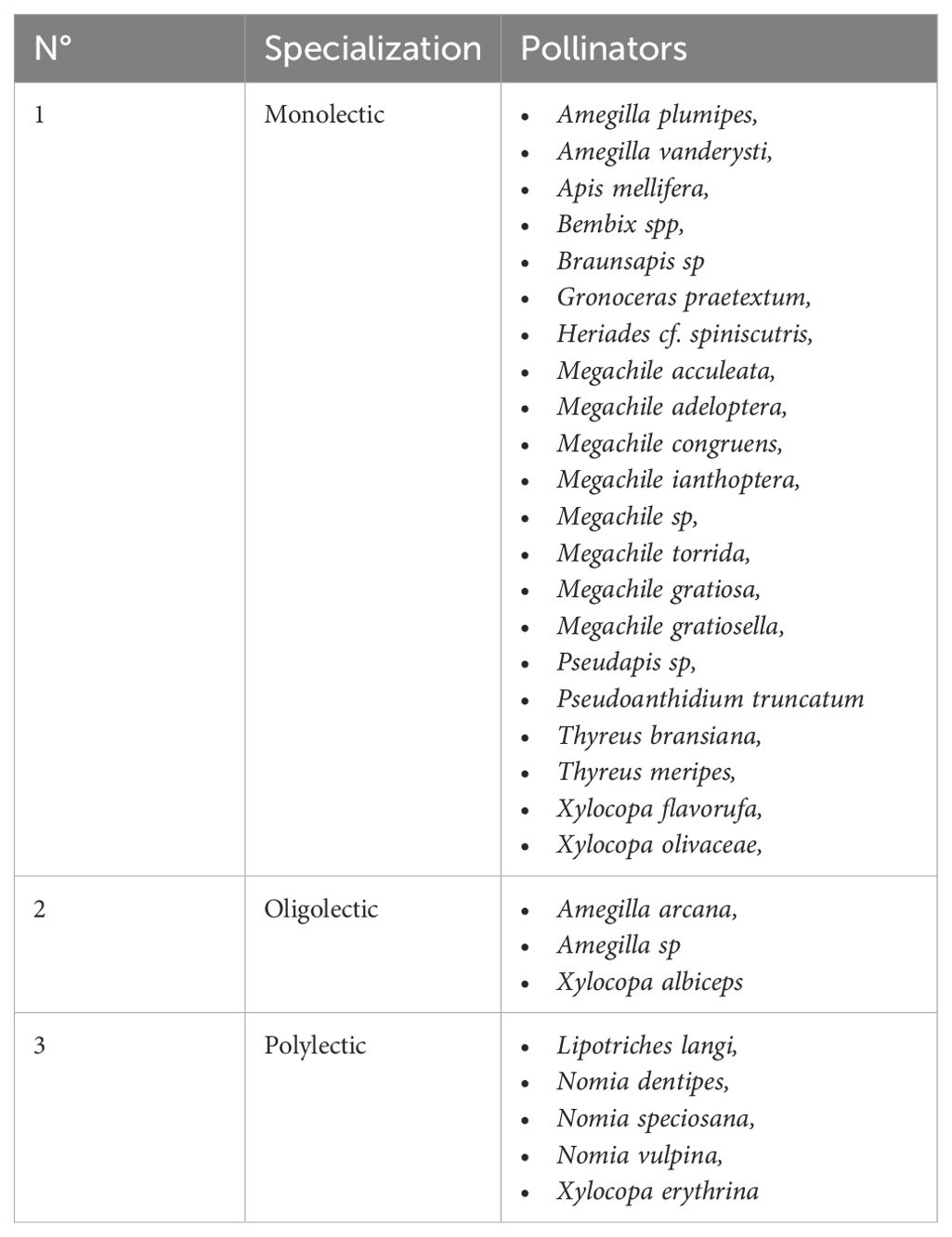
Table 2. Foraging behavior of the pollinating insects; there are more species with restricted interactions than there are generalists.
4 Discussion
4.1 Diversity of pollinating insects in the AFODEK perimeter
The results obtained in terms of pollinator diversity indicate a high level of diversity, provided that each site was sampled twice during the study period. In fact, the agroforestry perimeter extends over 2000 ha, with a total of 30 species recorded, suggesting a significant potential for diversity (Lomolino and Weiser, 2001; He and Legendre, 2002). As sampling was carried out beyond the favorable period, the level of sampling could be justified by the temporal and progressive homogenization of flowering, as floristic composition has an impact on wild bee diversity (Potts et al., 2003; Carrié, 2016). The present study revealed a considerable diversity of bees foraging on T. diversifolia flowers, extending beyond the period when several plants appeared to share pollinating insects. This appears to be an example of competitive effects, as described by Paul et al. (2024).
Of all pollinator species, carpenter bees belonging to X. albiceps (n = 20) and X. olivaceae (n = 15) were the most abundant. This dominance of carpenter bees on the perimeter seems to support the benefit of introducing trees for more nesting sites and floral resources. Carpenter bee nests in dead tree stems, digging tunnels for egg laying (Hurd, 1958; Vicidomini, 1996; Keasar, 2010). In addition to a wide range of food resources, tolerance to high temperatures, etc., carpenter bees also require a wide availability of nesting niches (Steffan-Dewenter et al., 2006; Keasar, 2010; Tarakini et al., 2021).
In addition to these carpenter bees, N. speciosana (n = 19), a common bee in the region, and M. torrida (n = 4) complete the ranks of abundant pollinators in the area.
It is worth noting that diversity at site Y may correspond to the abundance of bees collected, although distributional evenness is low. However, the diversity at site Y appears to be consistent as there is still a relatively high probability of finding a new species after each observation.
4.2 Interaction network and foraging typology of pollinating insects
The interaction network obtained in this study shows that certain plants, such as T. diversifolia, have a more complex interaction network involving most of the pollinating insects observed than others. Furthermore, T. diversifolia appears to be more competitive than the majority of native plants, which remain naive to this exotic species and even divert pollination suppliers, not to mention the subtlety of its presence in habitats (Shackleton et al., 2019; Rai et al., 2023). This species’ invasiveness was illustrated by its colonization of urban roadsides in Lubumbashi region, to disadvantage of native plants (Sikuzani et al., 2018). The floral phenology of this species, which dominates the majority of native plants and is maintained well beyond the flowering season of most native plants, would partly justify its attraction to some pollinators.
The pattern of interactions observed in this study seems to confirm the preferential relationships between pollinators and their host plants, in accordance with the order of specialization within pollination syndromes. Indeed, according to Fontaine et al. (2006), generalist pollinators interact primarily with specialist plants, just as generalists interact with each other. By analyzing plant-pollinator relationships, this network of interactions outlines the foraging behavior of these insects, with the majority of generalists considered to be “polylectic”, as they visit different flowers with no precise taxonomic link (Ritchie et al., 2016). The most common generalists recorded in this study include N. speciosana, Lipotriches vulpina, L. langi, L. dentipes and Xylocopa erythrina. Alongside the generalist pollinators are the specialists, in order of importance. These are “oligolectics”, which limit pollen collection to a particular floral taxon, while “monolectics” specialize in a single genus or species, although the latter remains controversial (Cane, 2021).
From the above, the scarcity of oligolectic and monolectic pollinators in the agroforestry perimeter could be related to floral phenology, while polylectic insects would have had to adapt to new hosts, thus broadening their food resource spectrum (Biella et al., 2019; Janovský and Štenc, 2023). It could also be that the apparently weak interactions of specialist pollinators are the result of food resource availability relationships. In this respect, specialist pollinators have been shown to benefit from the presence of pollen close to their nesting sites, while generalists are subject to competition for food resources (Buchmann et al., 1996; Paton, 1996; Wojcik, 2021).
Somehow this implies a change of foraging behavior of other pollinating insects. This was also observed at all sites within the AFODEK perimeter, particularly at sites C, D, E, F and J, where we observed strong pollination activity by A. mellifera, which foraged on most of the plant species encountered, in the case of Haumaniastrum katangense, a native plant species and indicator of copper in the soil (Ilunga Kabeya et al., 2018). Clearly, at a time of year when there is a marked lack of floral resources, this observation indicates an impact on the survival of solitary bee populations. The change in foraging behavior of certain pollinators that have proved to be generalists is a function of the phenophase that characterizes plant formations in the Haut-Katanga region. The causal agent is bushfire, and the dry season is punctuated and severe, lasting 6 months. Local bees, such as A. mellifera, need to be flexible in their feeding behavior.
4.3 Impact of agroforestry on the conservation of insect pollinator diversity in the AFODEK perimeter
With regard to the contribution of agroforestry to plant and animal biodiversity, it should be noted that A. auriculiformis is one of the host plants involved in the conservation of pollinator biodiversity, given the size of the AFODEK perimeter. It seems clear that the application of agroforestry practices has a great impact in promoting the diversity of floral resources in this formerly degraded area (Boldrini et al., 2017).
Indeed, the degraded landscape that preceded the introduction of agroforestry may not have been conducive to pollinator diversity, as floristic decline increased (Simanonok and Burkle, 2019). Evidence of the interdependence between pollinators and flowering plants gives way to considering a positive influence of agroforestry on increased floristic diversity and associated pollinator fauna (Jose, 2012; Bentrup et al., 2021; Udawatta et al., 2021; Santos et al., 2022).
The results obtained in this study regarding the interaction network support the existence of an inextricable link between pollinator species richness and surrounding flora diversity. Kearns et al. (1998) consider that the state of relative degradation of a site is an essential determinant of insect pollinator diversity. Thus, the proliferation of agroforestry plots within the perimeter and the introduction of new plant species is likely to promote a demographic explosion of these insect pollinator populations, which will subsequently benefit perimeter agriculture (Ulyshen et al., 2023).
It should also be noted that the restoration of woody cover is accompanied by the availability of nesting sites for pollinators in all their diversity (Brown et al., 2020). Clearly, carpenter bees in this perimeter find shelter from the elements in addition to reproductive lodges (Pfiffner and Müller, 2016). As A. auriculiformis trees can be harvested for charcoal after a decade, they promote a diversity of nesting sites for a variety of pollinating insect behaviors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
PMK: Formal analysis, Investigation, Methodology, Writing – original draft. ATN: Conceptualization, Formal analysis, Methodology, Software, Writing – review & editing. MNS: Supervision, Writing – review & editing. DB: Writing – review & editing, Validation
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The collection and publication of this article benefited from the partial financial support of M. Kasongo Mulumba Gaby Batoka.
Acknowledgments
We are grateful to the staff of the NGO CAPAK for allowing us to conduct this research in the AFODEK perimeter. Marcel Shawanga, the GRET officer responsible for liaison with CAPAK, deserves special thanks. Special thanks to M. Kasongo Mulumba Gaby Batoka for his financial support. We’d also like to thank Steven Ipo Wats’okla for his assistance with sampling and John Banza Mukalay for his proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ascher J. S., Pickering J. (2020).Discover Life bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). Available online at: http://www.discoverlife.org/mp/20q?guide=Apoidea_species (Accessed February 20, 2024).
Baselga A. (2010). Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134–143. doi: 101111/j.1466-8238.2009.00490.x
Bationo A., Hartemink A., Lungu O., Naimi M., Okoth P., Smaling E., et al. (2006). African soils: their productivity and profitability of fertilizer use. African Fertilizer Summit. Abuja, Nigeria, 9–13 June 2006. Available online at: https://edepot.wur.nl/26759 (Accessed 24 January 2025).
Bentrup G., Hopwood J., Adamson N. L., Powers R., Vaughan M. (2021). The role of temperate agroforestry practices in supporting pollinators. Agroforestry Ecosystem Serv. 275–304. doi: 10.1007/978-3-030-80060-4_11
Biella P., Akter A., Ollerton J., Tarrant S., Janeček Š., Jersáková J., et al. (2019). Experimental loss of generalist plants reveals alterations in plant-pollinator interactions and a constrained flexibility of foraging. Sci. Rep. 9 (1), 7376. doi: 10.1038/s41598-019-43553-4
Boldrini S., Bracke C., Daïnou K., Vermeulen C., Fétiveau J., Shutcha N. M., et al. (2017). Guide technique: plantation agroforestière d’Acacia auriculiformis dans le Haut-Katanga. Gembloux, Belgique: Les Presses agronomiques de Gembloux. Available online at: https://orbi.uliege.be/handle/2268/213989 (Accessed June 28, 2021).
Borcard D., Gillet F., Legendre P. (2018). Community Diversity, Numerical Ecology with R 2011 Use R! (Berlin: Springer), pg. 369–411. doi: 10.1007/978-3-319-71404-2
Brown J., Barton P. S., Cunningham S. A. (2020). Flower visitation and land cover associations of above ground-and below ground-nesting native bees in an agricultural region of south-east Australia. Agriculture Ecosyst. Environ. 295, 106895. doi: 10.1016/j.agee.2020.106895
Buchmann S. L., Mirocha P., Nabhan G. (1996). “The forgotten pollinators,” in Shearwater Books, Coverlo, California (Island Press, Washington, D.C), 320 pp.
Bultot F. (1954). Saisons et périodes seches et pluvieuse au Congo belge et au Rwanda-Urundi. Publ. Bur. Climatol 11, 90.
Cane J. H. (2021). A brief review of monolecty in bees and benefits of a broadened definition. Apidologie 52, 17–22. doi: 10.1007/s13592-020-00785-y
Carrié R. (2016). Hétérogénéité des paysages et des pratiques agricoles: effets sur la diversité des abeilles sauvages et la pollinisation. École doctorale Sciences écologiques, vétérinaires, agronomiques et bioingénieries (Toulouse): Université de Toulouse, 250p.
Donkersley P. (2019). Trees for bees. Agriculture Ecosyst. Environ. 270–271, 79–83. doi: 10.1016/j.agee.2018.10.024
Eardley C., Kuhlmann M., Pauly A. (2009). Les genres et sous-genres d’abeilles de l’Afrique subsaharienne. Abc Taxa 9, 1–144.
Eardley C., Kuhlmann M., Pauly A. (2010). The Bee Genera and Subgenera of sub-Saharan Africa. Abc Taxa 7, 1–38. doi: 10.2317/0022-8567-84.1.83
FAO (2016). État des ressources en sols du monde Résumé technique (Rome: Organisation des nations unies pour l’alimentation et l’agriculture), 79p.
Fontaine C., Meriguet J., Loreau M., Dajoz I. (2006). La diversité des interactions plantes-pollinisateurs: un pré-requis indispensable à la stabilité des écosystèmes. M/S: Med. Sci. 22, 817–819. doi: 10.1051/medsci/20062210817
Gotelli N. J., Colwell R. K. (2001). Quantifying biodiversity: procedures and pitfalls in measurement and comparison of species richness. Ecol. Lett. 4, 379–391. doi: 10.1046/j.1461-0248.2001.00230.x
He F., Legendre P. (2002). Species diversity patterns derived from species–area models. Ecology 83, 1185–1198. doi: 10.1890/0012-9658(2002)083[1185:SDPDFS]2.0.CO;2
Hurd P. D. (1958). Observations on the nesting habits of some new world carpenter bees with remarks on their importance in the problem of species formation (Hymenoptera: Apoidea). Ann. entomological Soc. America 51, 365–375. doi: 10.1093/aesa/51.4.365
Ilunga Kabeya F., Pongrac P., Lange B., Faucon M.-P., van Elteren J. T., Šala M., et al. (2018). Tolerance and accumulation of cobalt in three species of Haumaniastrum and the influence of copper. Environ. Exp. Bot. 149, 27–33. doi: 10.1016/j.envexpbot.2018.01.018
Janovský Z., Štenc J. (2023). Pollinator community and generalisation of pollinator spectra changes with plant niche width and local dominance. Funct. Ecol. 37, 2967–2976. doi: 10.1111/1365-2435.14439
Jose S. (2012). Agroforestry for conserving and enhancing biodiversity. Agroforestry Syst. 85, 1–8. doi: 10.1007/s10457-012-9517-5
Kearns C. A., Inouye D. W., Waser N. M. (1998). Endangered mutualisms: the conservation of plant"Pollinator interactions. Annu. Rev. Ecol. Systematics 29, 83–112. doi: 10.1146/annurev.ecolsys.29.1.83
Keasar T. (2010). Large carpenter bees as agricultural pollinators. Psyche: A J. Entomology 2010, 1–7. doi: 10.1155/2010/927463
Kelly T., Elle E. (2020). Effects of community composition on plant–pollinator interaction networks across a spatial gradient of oak-savanna habitats. Oecologia 193, 211–223. doi: 10.1007/s00442-020-04661-5
Legendre P. (2014). Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 23, 1324–1334. doi: 101111/geb.12207
Lomolino, Weiser (2001). Towards a more general species–area relationship: diversity on all islands, great and small. J. Biogeography 28, 431–445. doi: 10.1046/j.1365-2699.2001.00550.x
Oksanen J. (2019). vegan: ecological diversity: processed with vegan 2.5-6 in R version 3.6.1. pp8–p11.
Ollerton J., Winfree R., Tarrant S. (2011). How many flowering plants are pollinated by animals? Oikos 120, 321–326. doi: 10.1111/j.1600-0706.2010.18644.x
Paton D. C. (1996). Overview of feral and managed honeybees in Australia: Distribution, abundance, extent of interactions with native biota, evidence of impacts and future research (Australian Nature Conservation Agency).
Paul S., Roy R., Singha T., Debbarma P., Datta B. K. (2024). Foraging behavior and pollination efficiency of generalist floral visitors of Leucas aspera (Willd.) Link (Lamiaceae). J. Asia-Pacific Biodiversity. 17 (4), 644-652 doi: 10.1016/j.japb.2024.04.015
Pekkarinen A. (1997). Oligolectic bee species in Northern Europe (Hymenoptera, Apoidea). Entomologica Fennica, 8( 4): 205-214.Rodrigues, J.J.S., Brown Jr., K.S., Ruszczyk, A., (1993). Resources and conservation of Neotropical butterflies in urban forest fragments. Biol. Conserv. 64, 3–9. doi: 10.33338/ef.83945
Pfiffner L., Müller A. (2016). Abeilles sauvages et pollinisation. Institut de recherche de l'agiculture biologique (FiBL). Available online at: https://shop.fibl.org/fr/publication/c/biodiversite/p/1646-abeilles-sauvages.html
Potts S. G., Vulliamy B., Dafni A., Ne’eman G., Willmer P. (2003). Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84, 2628–2642. doi: 10.1890/02-0136
Rai P. K., Lee S. S., Bhardwaj N., Kim K.-H. (2023). The environmental, socio-economic, and health effects of invasive alien plants: Review on Tithonia diversifolia (Hemsl.) A. Gray in Asteraceae. South Afr. J. Bot. 162, 461–480. doi: 10.1016/j.sajb.2023.09.038
Ritchie A. D., Ruppel R., Jha S. (2016). Generalist behavior describes pollen foraging for perceived oligolectic and polylectic bees. Environ. Entomology 45, 909–919. doi: 10.1093/ee/nvw032
Santos M., Cajaiba R. L., Bastos R., Gonzalez D., Petrescu Bakış A.-L., Ferreira D., et al. (2022). Why do agroforestry systems enhance biodiversity? Evidence from habitat amount hypothesis predictions. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.630151
Shackleton R. T., Nunda W., Beale T., Van Wilgen B. W., Witt A. B. (2019). Distribution of invasive alien Tithonia (Asteraceae) species in eastern and southern Africa and the socioecological impacts of T. diversifolia in Zambia. Bothalia-African Biodiversity & Conservation. 49 (1), 1–11.
Sikuzani Y. U., André M., Mahy G., Kaleba S. C., Malaisse F., Kankumbi F. M., et al. (2018). Interprétation paysagère du processus d’urbanisation à Lubumbashi: Dynamique de la structure spatiale et suivi des indicateurs écologiques entre 2002 et 2008 (Vol. 281) (Gembloux, Belgium: Presses Agronomiques de Gembloux).
Simanonok M. P., Burkle L. A. (2019). Nesting success of wood-cavity-nesting bees declines with increasing time since wildfire. Ecol. Evol. 9, 12436–12445. doi: 10.1002/ece3.5657
Steffan-Dewenter I., Klein A. M., Gaebele V., Alfert T., Tscharntke T. (2006). Bee diversity and plant-pollinator interactions in fragmented landscapes. Specialization generalization plant-pollinator Interact., 387–410.
Tarakini G., Chemura A., Tarakini T., Musundire R. (2021). Drivers of diversity and community structure of bees in an agroecological region of Zimbabwe. Ecol. Evol. 11, 6415–6426. doi: 10.1002/ece3.7492
Tshibungu N. A., Pauly A., Dorchin A., Vereecken N. J. (2023). The Megachilidae (Hymenoptera, Apoidea, Apiformes) of the Democratic Republic of Congo curated at the Royal Museum for Central Africa (RMCA, Belgium). Zootaxa 5392, 1–103. doi: 10.11646/zootaxa.5392.1.1
Udawatta R. P., Rankoth L. M., Jose S. (2021). Agroforestry for biodiversity conservation. Agroforestry Ecosystem Serv., 245–274. doi: 10.1007/978-3-030-80060-4_10/
Ulyshen M., Urban-Mead K. R., Dorey J. B., Rivers J. W. (2023). Forests are critically important to global pollinator diversity and enhance pollination in adjacent crops. Biol. Rev. 98, 1118–1141. doi: 10.1111/brv.12947
Vereecken N. J., Weekers T., Marshall L., D’Haeseleer J., Cuypers M., Pauly A., et al. (2021). Five years of citizen science and standardised field surveys in an informal urban green space reveal a threatened Eden for wild bees in Brussels, Belgium. Insect Conserv. Diversity 14, 868–876. doi: 10.1111/icad.12514
Vicidomini S. (1996). Biology ofXylocopa violacea(Hymenoptera): In-nest ethology. Ital. J. Zoology 63, 237–242. doi: 10.1080/11250009609356139
Westphal C., Bommarco R., Carré G. (2008). Measuring bee diversity in different european habitats and biogeographical regions. Ecol. Monogr. 78, 653–671. doi: 10.1890/07-1292.1
Wickham H. (2017). reshape2: Flexibly Reshape Data: A Reboot of the Reshape Package [dataset]. In CRAN: Contributed Packages. The R Foundation. doi: 10.32614/cran.package.reshape2
Keywords: diversity, pollinators, flora, interaction, AFODEK
Citation: Makolo Kasongo P, Tshibungu Nkulu A, Ngoy Shutcha M and Bugeme DM (2025) Characterization of the diversity of pollinating insects and their interactions with the flora of the “Agro-forêts pour le développement de Kipushi” perimeter. Front. Bee Sci. 2:1394670. doi: 10.3389/frbee.2024.1394670
Received: 01 March 2024; Accepted: 30 October 2024;
Published: 06 March 2025.
Edited by:
Connal Eardley, North-West University, South AfricaReviewed by:
Hannah J. Penn, Agricultural Research Service (USDA), United StatesEstela Santos, Universidad de la República, Uruguay
Copyright © 2025 Makolo Kasongo, Tshibungu Nkulu, Ngoy Shutcha and Bugeme. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierre Makolo Kasongo, cGllcnJla2Fzb25nbzEwQGdtYWlsLmNvbQ==
 Pierre Makolo Kasongo
Pierre Makolo Kasongo Alain Tshibungu Nkulu1,2
Alain Tshibungu Nkulu1,2