- Keystone Foundation, Nilgiris, Tamil Nadu, India
This paper provides insights into the ecology and conservation of Apis dorsata (Giant Honey Bee) which are integral to the ecology and livelihoods of those living in the Nilgiri Biosphere Reserve (NBR). Rather than directly comparing nest densities across regions and seasons, our study integrates scientific approaches with Indigenous knowledge to examine changes in nest locations, nesting behaviors, and seasonal patterns across three decades. Social surveys indicate that there has been a decline in wild colony populations over the past 10–20 years. This underscores the critical role of sacred sites and protected areas in conserving Apis dorsata populations. This research aims to inform future conservation strategies and policy frameworks, highlighting the value of interdisciplinary approaches in understanding and preserving wild bee species.
1 Introduction
Apis dorsata Fabricius, commonly known as the Giant Honey Bee, plays an integral role in the lives of Indigenous people and the forests of the Nilgiri Biosphere Reserve (NBR) (Keystone Foundation, 2007). Apis dorsata nests on single combs up to 150 cm wide, suspended from cliff faces or the branches of tall trees, often forming aggregations (Seeley et al., 1982). Distributed across South and Southeast Asia (Huang et al., 2022), Apis dorsata is found throughout India in sub-mountainous regions up to an altitude of 2000 m (Sharma and Rakesh, 2014). The species migrates locally in response to varying floral resources (Dyer and Seeley, 1994) and can rapidly expand its population size during mass flowering events (Itioka et al., 2001). Local migration has been recorded in the NBR (Leo, 2008; Roy et al., 2011; personal observation) and the Kalakad-Mundanthurai Tiger Reserve (Davidar et al., 1993). Apis dorsata tends to visit about 27% of the flowering plants in the Nilgiris, which are also visited by other bee species (Thomas et al., 2009a), indicating lower levels of floral specialization.
Many Indigenous communities in the NBR possess traditional skills for safely collecting honey from Apis dorsata nests (Keystone Foundation, 2007). As an economically important species, it serves as a pollinator for crops, non-timber forest products (Crane, 1999; Neupane et al., 2006; Rehel et al., 2009), including honey and wax for local communities (Singh et al., 2017). Honey hunting is culturally significant for Indigenous communities and supports the preservation of localized technical and ecological knowledge (FAO et al., 2021). Despite this, land clearing and deforestation threaten these bees by reducing nesting sites and floral resources (FAO et al., 2021). Given its ecological, economic, and cultural significance, the observation and research of Apis dorsata should utilize both scientific approaches and Indigenous knowledge.
Since 1993, Keystone Foundation has worked on eco-development initiatives integrating conservation, enterprise, and livelihoods. Honey bees have been central to our programs, leading to extensive field research on their status in the wild (Nath et al., 2001; Thomas et al., 2009a, 2009; Padmavathy and Rehel, 2014). Collaborating with national and global academic and development agencies, we have conducted studies to estimate the populations of all Apis species, understand their habitat preferences, and record their seasonality. Field observations on Apis dorsata were conducted in protected areas, sacred cliffs within Indigenous ancestral domains, and other land use types in the NBR. Revisitation of some sites after a decade provided insights into the current situation. This paper reviews and discusses:
a. The various methods employed for observing Apis dorsata in the forests of the NBR.
b. Observed changes over a decade regarding Apis dorsata nests, nesting sites, and seasonality; and
c. Indigenous people’s perspectives on changes in the ecology of Apis dorsata in the NBR.
This paper combines key facets of work from across three decades at Keystone Foundation on the ecology and traditional knowledge of Apis dorsata in the NBR. It aims to inform policy on wild bees and lay the groundwork for further field observations on Apis dorsata. Additionally, it highlights the depth of Indigenous knowledge of Apis dorsata leading to a profound connection regarding the role of culture often missing in conservation and protection efforts.
1.1 Study area
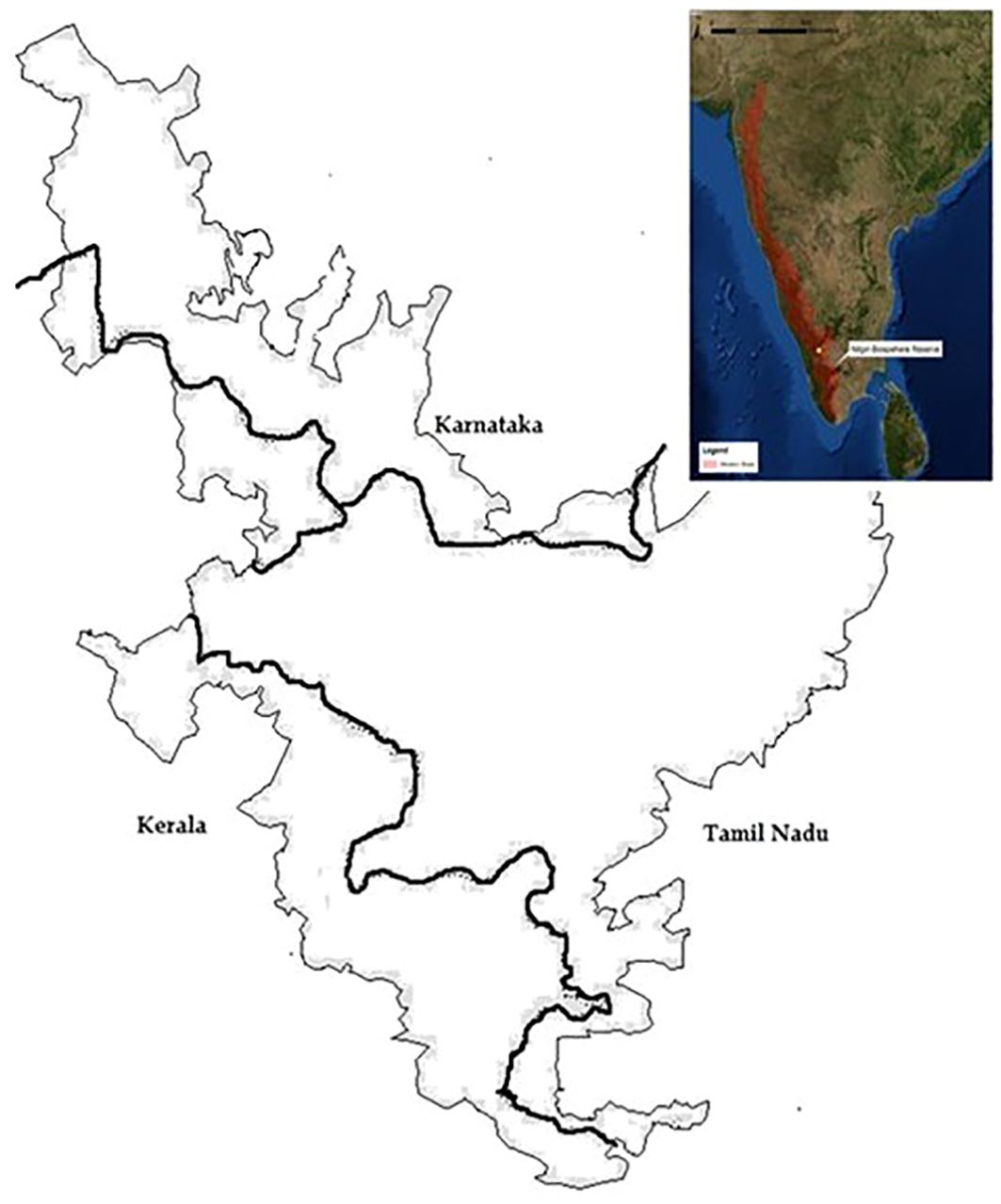
The NBR forms part of the Western Ghats Mountain chain in the Indian peninsula (5520 km²; 10° 45’ to 12° N latitude and 76° to 77° 15’ E longitude), spanning the states of Karnataka, Kerala, and Tamil Nadu. The reserve encompasses five protected areas and many reserved forests (Figure 1). The altitude ranges from 250 to 2650 m above sea level, with rainfall intensity varying across topographic and altitudinal gradients (Lengereke et al., 1989). The NBR has a wealth of biological and cultural diversity (Hockings, 1989). The region consists of Evergreen, Semi Evergreen, Moist, Dry, and Montane (shola) tropical forests. The NBR is rich in plant diversity, with four thousand flowering species recorded across the reserve. Out of the four thousand plant species, ~150 angiosperms and 150 vertebrates out of 700 species are endemic to the NBR (Daniels, 1996). It is home to Indigenous communities whose traditional livelihoods and foodways depend on non-timber forest products, such as the Todas, Irulas, Kurumbas, Kattunaickens, Cholanaickens, Mudugas, Paniyas, and many other Indigenous communities (Keystone Foundation, 2007).
2 Methods
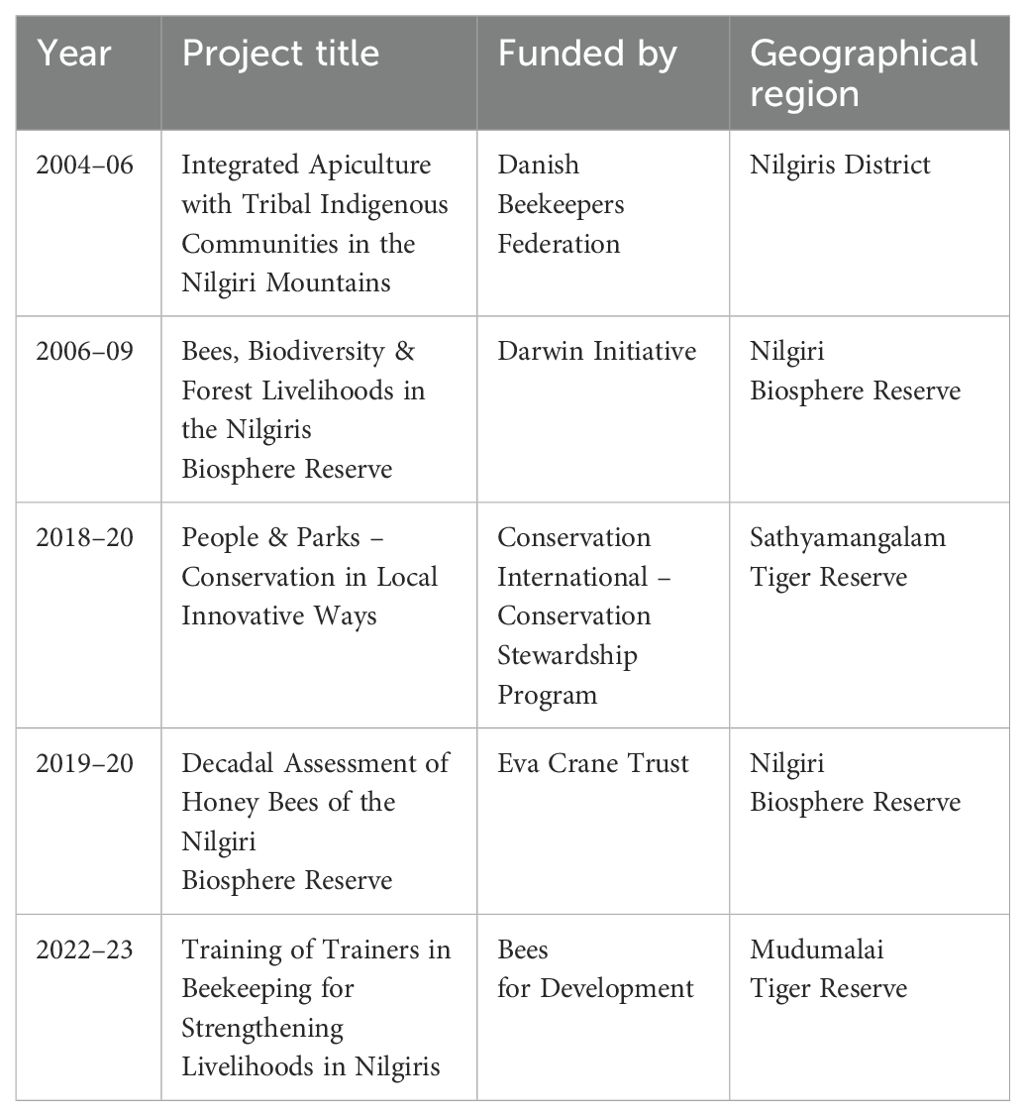
Keystone Foundation conducted a comprehensive review of research on Apis dorsata, examining funded and non-funded efforts to estimate Apis dorsata presence and locations. For donor-supported projects, we reviewed final and mid-term reports and project proposals (Table 1). For non-funded efforts, we conducted discussions among authors and reviews of internal publications. Maps showing locations of honey hunting cliffs and nesting trees were key outputs of these social surveys. Data sets on honey quality monitoring, available from 1998 to the present, were excluded as they do not reflect Apis dorsata nests or population sizes. Instead, we focused on estimating nesting sites and seasonality of Apis dorsata colonies. We identified the challenges and limitations of each method to synthesize lessons learned. The various methods employed during the survey were:
2.1 Colony survey methods
2.1.1 Transect method
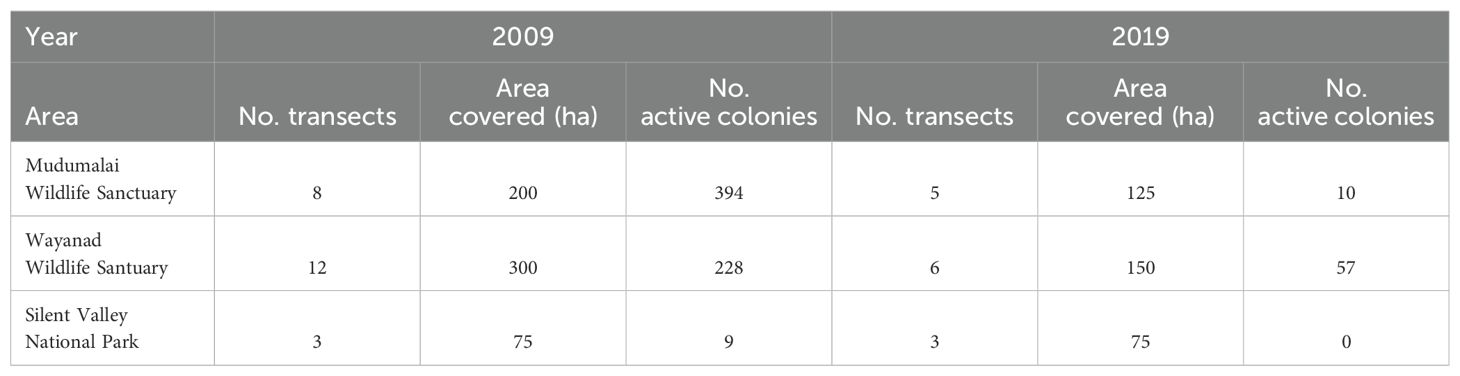
A survey was conducted during the dry season (January–June) of 2009 in protected areas within the NBR to assess the status of bee colonies in the wild. To estimate the status of bee colonies, bee nests were counted, tree species were identified, and bee activity was recorded. The number of transects in each vegetation type was decided based on the patch size. Detailed notes were taken on the nesting substrate. In the case of cliffs, estimates of height, location, and vegetation cover were recorded. Nesting trees were identified and the phenological condition and height were recorded. Transects measuring 5 km × 50 m (l×b) were laid, and 50 such transects were covered. Revisits to the same sites occurred in 2019 following the same methods to estimate the status of Apis dorsata honey bees. Due to permission limits, Mudumalai Wildlife Sanctuary was revisited in July 2019, and Wayanad Wildlife Sanctuary and Siley Valley National Park were revisited in December 2019. In Mudumalai, eight transects were placed over 200 ha in 2009, and five transects over 124 ha in 2019. In Wayanad, 12 transects were placed over 300 ha in 2009, and 6 transects over 150 ha in 2019. In Silent Valley, three transects were placed over 75 ha in 2009, and three transects over 75 ha in 2019. All transects were stratified by vegetation types, with colonies being located and marked using a GPS. Details on the nest, number of colonies, and dominant flowering species were recorded. All colonies were visible and monitored using binoculars.
2.1.2 Quadrant methods
100 plots of 10 m × 10 m dimension covering an areal of one ha were placed in two study sites. The plots were randomly placed in the forests at different distances and directions, and all plants were inventoried. With the help of local Indigenous honey hunters, an intensive search for Apis dorsata nests was carried out in each plot.
2.1.3 Point observation
Apis dorsata on cliffs were observed for the arrival of colonies. Observations were done once every month and colony counts were recorded along with the phenology of flowering species near the cliff. Observations commenced in 2022 and are ongoing. These cliffs are sacred in local communities and honey is not extracted from these colonies.
2.2 Perception survey
To document perceptions of changes observed by Indigenous communities, we interviewed 26 honey hunting groups in Coonoor, Kotagiri, Dhimbham, and Sigur regions, specializing in Apis dorsata honey collection from cliffs and nesting trees. Interviews were conducted using a semi-structured questionnaire regarding their perceptions of Apis dorsata populations, reasons for perceived changes, and sacred honeybee sites. Those interviewed were well known to Keystone Foundation and have been part of the honey enterprise supported by the Foundation.
A structured survey of honey bees was undertaken in 2004, although the project focused on apiculture reconnaissance-type surveys to understand the presence and seasonality of Apis dorsata colonies. This was followed by a rigorous scientific project funded by the Darwin Initiative, allowing experimentation with field methods such as transects, point observations, and plots. Over time, honey hunters were trained as para-ecologists, collecting and analyzing observations on Apis dorsata’s presence in their forests as part of a conservation stewardship project. The methods mainly involved point observations over 12 months.
3 Results
3.1 Field methods for survey of Apis dorsata in the forests of the NBR
Field surveys and interactions with honey hunters have been ongoing since Keystone Foundation’s establishment in 1993. A resurvey of transects from 2007–09 in 2019 provided insights into habitat status and highlighted the challenges of resurveys, especially regarding access and permit issues. Table 2 summarizes the projects, research goals, and main findings. These surveys in forest areas serve as a good baseline for further research.
3.2 Apis dorsata population field observations and Indigenous people’s perceptions
Between 2007–09, two large projects with rigorous methods helped set a baseline experiment for counting colonies in the wild. After a decade, a 2019 resurvey of some Darwin sites faced difficulties due to stricter protected area rules and short-term funding, leading to non-overlapping survey times and non-comparable data. Additionally, many routes walked in 2007–09 were no longer accessible due to increased invasive cover. Due to these limitations, this paper highlights diverse methods to assess nest distribution and seasonal behavior rather than a comparative or qualitative population analysis. However, the 2019 resurvey helped reassess forest transects and realistically plan for the future. Surveys also allowed for interactions with local Indigenous communities that engage with bees.
In 2007–09, 60 transects covered 1500 ha in the NBR, identifying 394 Apis dorsata colonies in Mudumalai forests. In 2019, 14 transects covered 350 ha, locating 10 combs with bees and 254 combs without bees. The survey in July 2019 had fewer sightings of inhabited combs, likely due to bee migration and monsoon onset. During this season, observed bees were either resting swarms or preparing to migrate. Table 3 compares Apis dorsata transects over a decade. The main limitation was conducting surveys in different seasons: 2007–09 surveys were from April to June, while the 2019 survey was in December, relying on abandoned combs to estimate colony numbers.
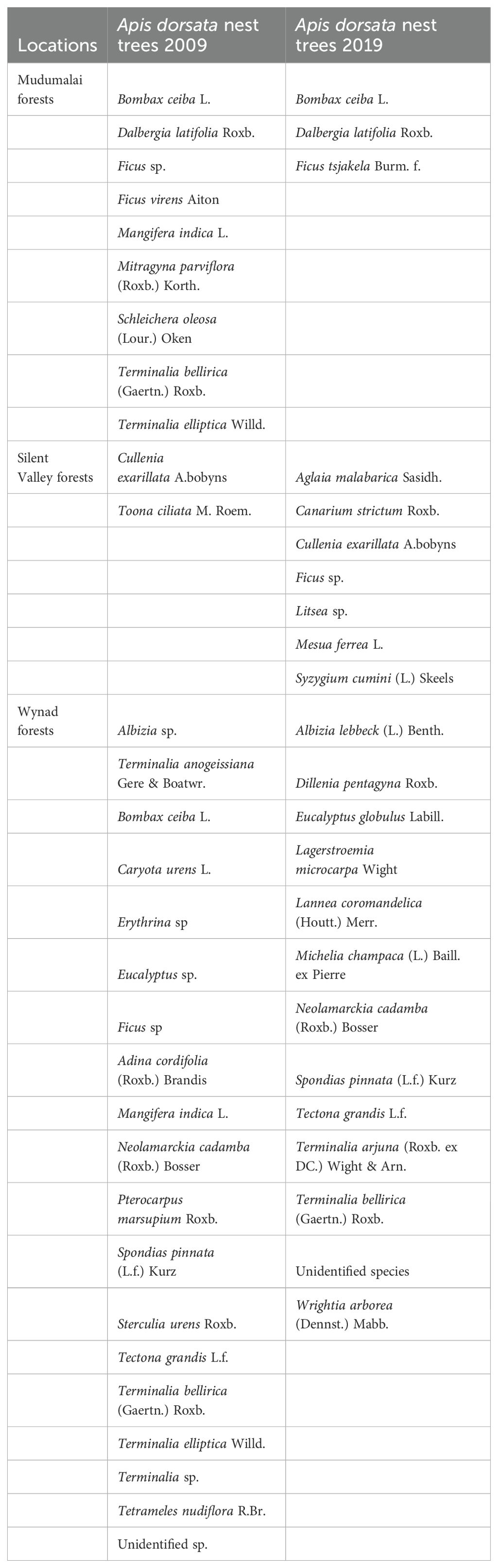
In the Wayanad Wildlife Sanctuary, no significant change was observed in the number of nests from 2009 to 2019. In Silent Valley National Park, no nests with bees were found, but most nesting trees were still intact. The Wayanad and Mudumalai forests, ranging from moist to dry deciduous, are designated Wildlife Sanctuaries with high state protection, and the adjacent landscapes provide vast habitats for large mammals including tigers and elephants. Mudumalai, now a tiger reserve, receives the utmost protection under national laws. However, Wayanad forests face fragmentation and human settlements, with Eucalyptus trees, introduced by the Forest Department, serving as crucial nesting sites for Apis dorsata bees. The decadal assessment also considered Apis dorsata nesting trees. Table 4 lists the tree species observed in the two research surveys: 30 species in 2007–09 and 23 in 2019. The shorter transect length in 2019 makes comparisons difficult.
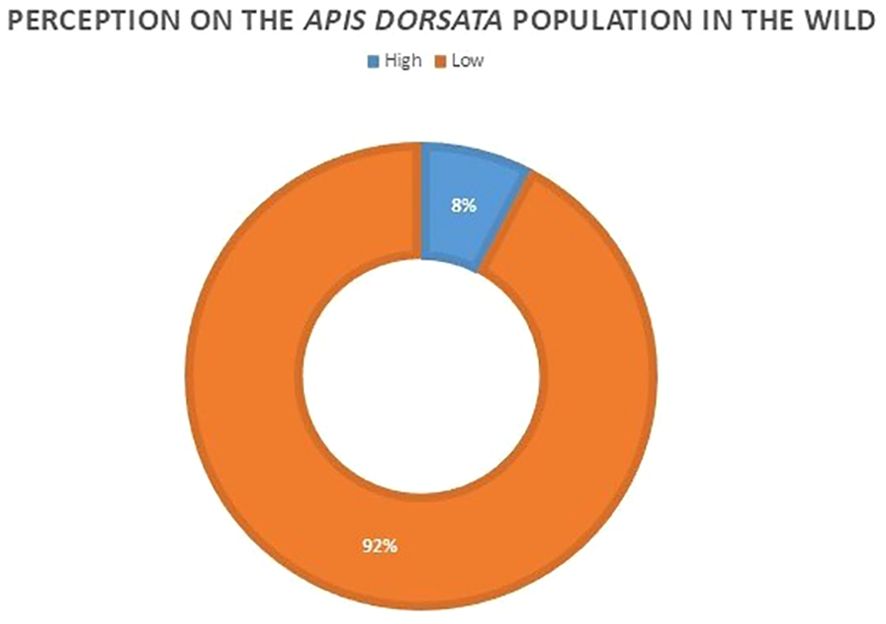
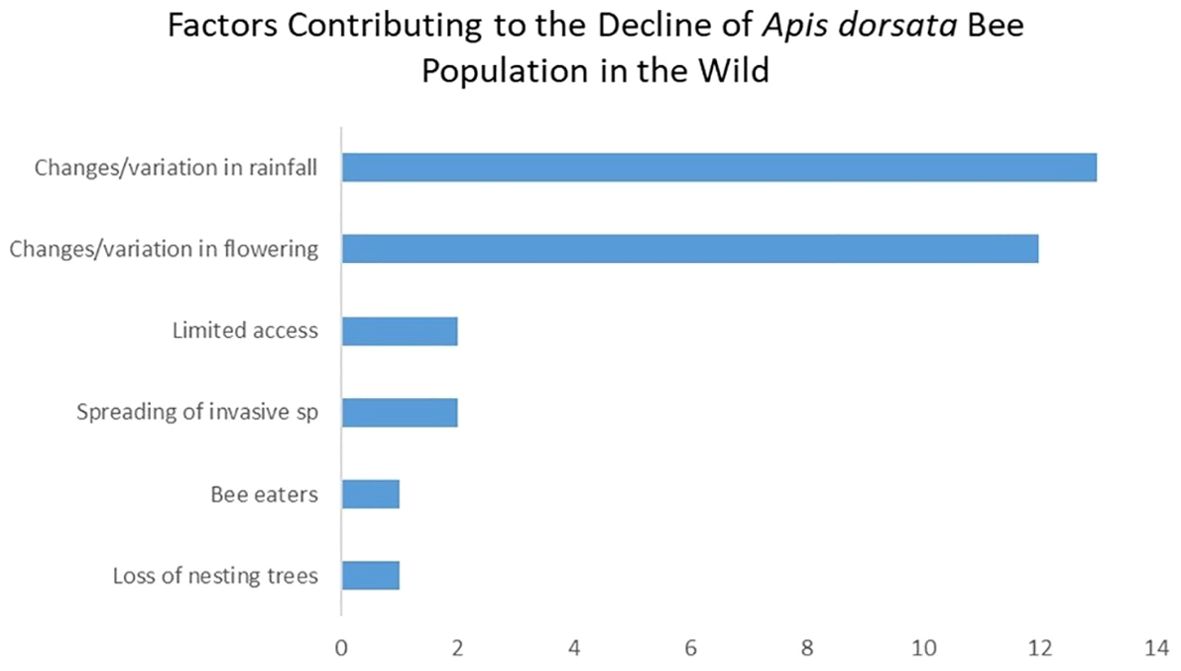
In social surveys, 24 of our 26 respondents believed there has been a decline in wild colony populations over the past 10–20 years (Figure 2). Figure 3 shows the causes for the low population, highlighting alterations and delays in flowering patterns due to unpredictable rainfall, reducing honey production. The use of chemicals in farming, the spread of invasive species resulting in reduced native plant foraging spaces, and the loss of nesting trees were also identified as contributing factors. Conversely, two respondents believed the number of colonies is rising due to limited wild honey collection in recent years and noted a decrease in honey harvested.
3.3 Sacred sites for Apis dorsata in the NBR
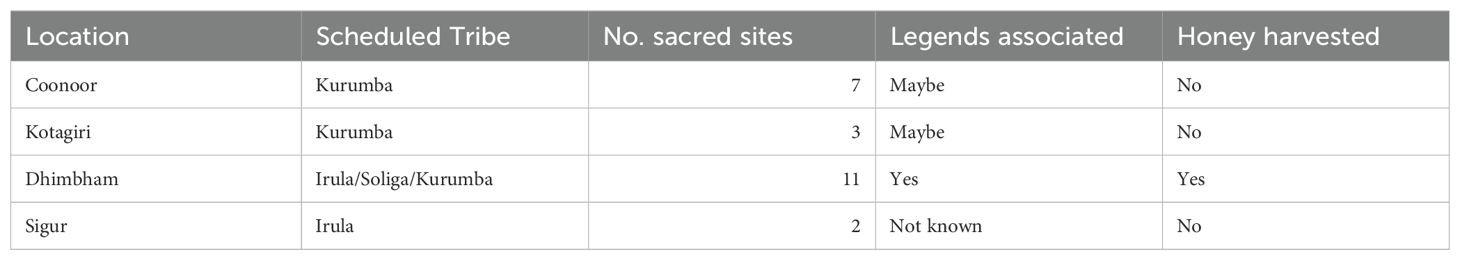
Honey hunting communities have many legends and lores passed down from generation to generation about sacred honey hunting cliffs which are the nesting sites of the Apis dorsata bees. In rapid surveys with honey hunters in three locations, these cliffs are considered ancient habitats of Apis dorsata bees. These preserved sacred sites still exist (Table 5), with access often restricted to specific families and individuals. Honey harvesting is permitted from some of them, especially in the Dhimbham region. In some areas, such as Sigur, the elders are unable to recall the legends associated with the cliffs. However, the elders we spoke to were able to name them and remember their locations (Table 6). We aim to continue preserving Indigenous knowledge of honey hunting sites to further understand and document traditional knowledge and associated practices.
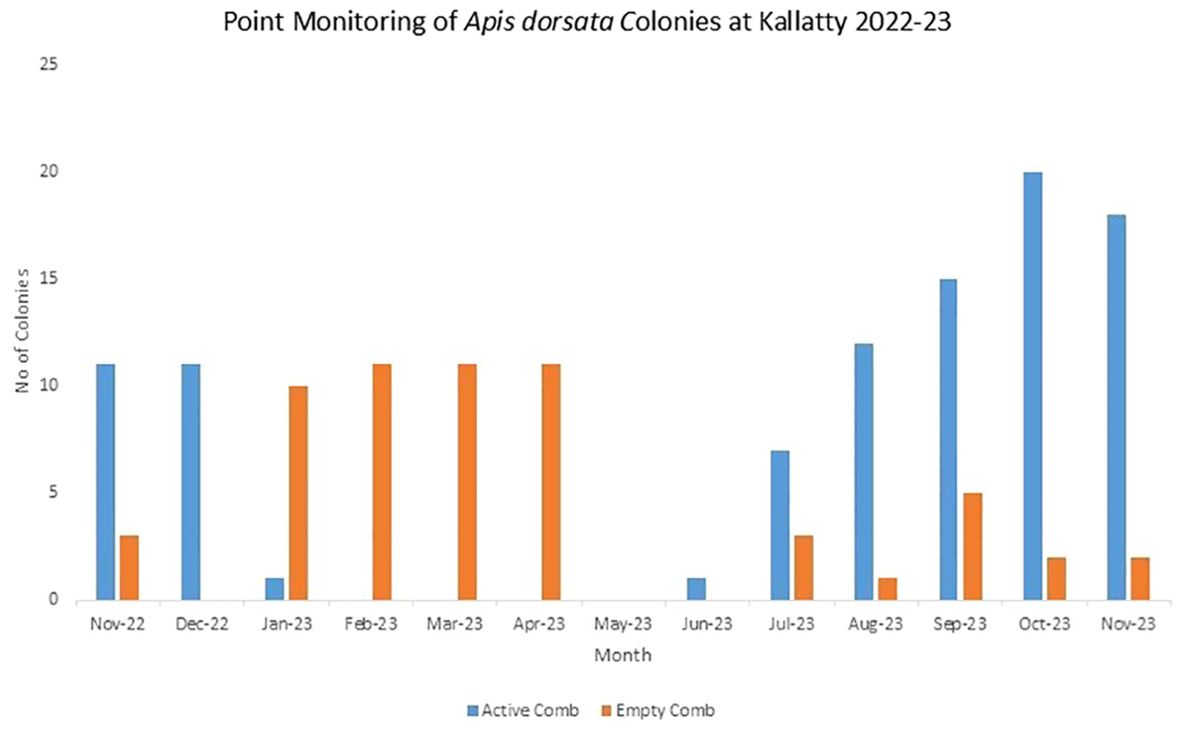
At one of the sacred sites, point observations were undertaken (Kalhatty, Nilgiris), indicating the arrival of bees from July onwards. This pattern is peculiar to this cliff, as bees usually migrate from January onwards. The flowering of Dahlia imperialis correlates with the arrival of the bees, providing forage. Figure 4 traces the arrival and departure of Apis dorsata bees at the Kallatty Cliff over 12 months.
4 Discussion
Researchers have adopted survey and interview methods (Bookhout, 1996; Tongco, 2007) to find Apis dorsata nests by following paths known by honey hunters (Nagir et al., 2016; Thomas et al., 2009b). In this study, transect methods coupled with the guidance of Indigenous individuals proved to be an optimal approach as it significantly facilitated the identification and location of Apis dorsata colonies. The quadrat method was more effective in locating cavity-dwelling species, including Apis cerana, Apis florea, and stingless bees. Studies from Maros forest, Indonesia show that the colony abundance of Apis dorsata binghami, generally found in June and July and less in December to March, varies due to migratory behavior (Nagir et al., 2016). Observations from northern Thailand support the idea that migration allows colonies to track seasonally varying resources in different regions (Dyer and Seeley, 1994). Field surveys conducted in southern Karnataka show that colonies were highest (839) during winter followed by rainy (807) and summer (761) seasons (Basavarajappa and Raghunandan, 2013). Observations in Nepal demonstrate that colonies of Apis dorsata decrease with increasing average temperature and precipitation and decreasing humidity (Devkota et al., 2022). In winter, the climate is characterized by moderate temperature and humidity with fair floral resources. The bees migrate during the rainy and summer seasons due to varying temperatures and uneven distribution of foraging sources. There are also significant linkages between flowering and bee abundance (Potts et al., 2003). Apis dorsata exhibits local migration patterns in direct response to the availability of floral resources in Southeast Asia and demonstrates the ability to multiply rapidly during flowering events (Itioka et al., 2001).
The observation at the cliff at Kalhatty shows a similar pattern of colonies during the winter season. People in the vicinity have observed that the bees fly toward the direction of Masinagudi. A study on the annual migratory pattern of Apis dorsata from 1984 to 2012 in the semiarid region of northwest India (Hisar Agricultural University campus) reported that the arrival of the bees started in October coinciding with the flowering of pigeon peas and stayed till mid-May (Sihag, 2014). Bees at the cliff at Kalhatty arrive earlier in July than at Hisar, coinciding with Dahlia flowering in the vicinity of the cliff. Sihag (2014) reported a decline in the number of colonies, decreasing to 44 (2012) from 104 (1984) resulting from a reduction in the number of nesting trees, impacting bee migration. One study on population status in urban areas shows a decrease in the number of colonies during the study period in 1987–89 and 2013–14 from 72 colonies to 7 with development activities contributing to the decline in the number of colonies (Venkatesh, 2014). Deforestation deprives bees of their nesting sites such as hollow trees for cavity-nesting bees and strong, high branches for Apis dorsata. Intensified agriculture threatens native honey bee populations by depleting floral resources and nesting sites in large-scale monocultural agro-systems (Guerin, 2020). FAO et al. (2021) reports that native bees face significant threats such as loss of nesting and foraging habitats due to land clearing and agriculture, the proliferation of exotic plant species, and the impacts of climate change. Climate change can affect honey bees at various levels, directly influencing their behavior and physiology (Winston, 1987). Several examples have highlighted the delicate balance between hosts and parasites, demonstrating that even minor climate changes can affect the establishment of invasive species at the edge of honey bees’ distribution range (Le Conte and Navajas, 2008).
Perception surveys in understanding the status of wild honey bees show that populations of wild honeybees, specifically the genus Apis, with a focus on Apis cerana (the Asian bee) and Apis florea (the little bee), have been consistently decreasing over the past thirty years. A spatial study in the Philippines showed that the nesting trees dropped from 1988 to 2015 (NDVI value 0.64 to 0.41 respectively), and the decreasing vegetation cover limited the presence of wild honey bees (Matias et al., 2018). The widespread application of pesticides is the predominant cause of this decline (Bhattacharyya et al., 2017). However, Apis dorsata may be more capable of withstanding pesticide-induced stress than Apis cerana (Chakrabarti et al., 2015).
5 Conclusion
This paper presents a synthesis of various methods employed to estimate wild colonies of Apis dorsata, exploring the limitations and challenges encountered. The surveys conducted in the forests of the NBR provide a baseline for further work on this species. Our approach emphasizes understanding changes in nest distributions and seasonal behaviors rather than direct comparisons of nest densities across different regions and seasons. The surveys’ timing posed limitations, and efforts to resurvey sites faced access issues and funding constraints. Social surveys with Indigenous honey hunters highlighted observations of changes in Apis dorsata populations and the perceived causes of these changes. This research suggests integrating scientific methods with traditional knowledge to understand the ecology and conservation needs of Apis dorsata in the NBR.
The importance of sacred sites for Apis dorsata conservation cannot be overstated. These sites protected by Indigenous beliefs and practices serve as crucial habitats for the species. The connection between sacred sites and Apis dorsata populations underscores the need for conservation strategies that respect and incorporate Indigenous knowledge and practices. This interdisciplinary approach, blending ecological research with traditional knowledge, can enhance conservation efforts and ensure the long-term survival of Apis dorsata in the NBR.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
SR: Data curation, Formal Analysis, Supervision, Writing – original draft. MB: Investigation, Data curation, Writing – original draft. JT: Data curation, Investigation, Writing – original draft. PR: Investigation, Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We extend our sincerest appreciation to Anita Varghese for her invaluable contribution in meticulously reviewing this paper. Her insightful feedback and expertise greatly enhanced the quality and clarity of our work. We also want to acknowledge the indispensable contributions of Sumin George Thomas, P. Chandran, R. Rajendran, and community members for their valuable insights and participation. We also thank Sophie Minoosh Bernstein for assistance with edits and proofreading, improving the quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Basavarajappa S., Raghunandan K. S. (2013). Colony status of Asian giant honeybee, Apis dorsata Fabricius in Southern Karnataka, India. Afr. J. Agric. Res. 8, 680–689. doi: 10.5897/ajar12.2169
Bhattacharyya M., Acharya S. K., Chakraborty S. K. (2017). Pollinators Unknown: People’s perception of native bees in an agrarian district of West Bengal, India, and its implication in conservation. Trop. Conserv. Sci. 10. doi: 10.1177/1940082917725440
Bookhout T. A. (1996). Research and Management Techniques For Wildlife And Habitats (Kansas (US: Allen Press Inc).
Chakrabarti P., Santanu R., Sagartirtha S., Barbara S., Parthiba B. (2015). Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 46, 107–129. doi: 10.1007/s13592-014-0308-z
Crane E. (1999). The World History of Bee Keeping and Honey Hunting (1st ed.). (Routledge: Taylor & Francis). doi: 10.4324/9780203819937
Daniels R. J. R. (1996). The Nilgiri Biosphere Reserve: A Review of Conservation Status with Recommendations for a Holistic Approach to Management (Paris: South-South Cooperation Programme, UNESCO).
Davidar P., Devy M. S., Ganesh T., Krishnan R. (1993). “Relationships between plants and pollinators in a wet evergreen forest in the Southern Western Ghats, India,” in Pollination in the Tropics. Eds. Veeresh G. K., Uma Shaanker R., Ganeshaiah K. N. (IUSSI, India), 325–334.
Devkota K., Fernando dos Santos C., Raguse-Quadros M., Blochtein B. (2022). Influence of seasonal weather variables and habitat type on numbers of colonies of the giant honey bee in Nepal. Apidologie 53. doi: 10.1007/s13592-022-00912-x
Dyer F. C., Seeley T. (1994). Colony migration in the tropical honey bee Apis dorsata F. (Hymenoptera: Apidae). Insectes Sociaux 41, 129–140. doi: 10.1007/bf01240473
FAO, IZSLT, Apimondia, CAAS (2021). Good beekeeping practices for sustainable apiculture FAO Animal Production and Health Guidelines No. 25 (Rome).
Guerin E. (2020). Native honey bees of Southeast Asia and conservation challenges: Heinrich böll foundation: Southeast Asia. Heinrich Böll Foundation | Southeast Asia. Available at: https://th.boell.org/en/2020/02/13/native-honey-bees-southeast-asia-and-conservation-challenges (Accessed 03 December 2024).
Hockings P. (1989). Blue Mountains: the Ethnography and Biogeography of a South Indian Region. Delhi: Oxford University Press.
Huang M., Hughes A. C., Xu C., Miao B., Gao J., Peng Y. (2022). Mapping the changing distribution of two important pollinating giant honeybees across 21000 years. Global Ecol. Conserv. 39, e02282. doi: 10.1016/j.gecco.2022.e02282
Itioka T., Inoue T., Kaliang H., Kato M., Nagmitsu T., Momose K., et al. (2001). Six year population fluctuation of the giant honey bee Apis dorsata (Hymenoptera: Apidae) in a tropical lowland dipterocarp forest in Sarawak. Ann. Entomological Soc. America 94, 545–549. doi: 10.1603/0013-8746(2001)094[0545:SYPFOT]2.0.CO;2
Keystone Foundation (2007). Honey Trails in the Blue Mountains: Ecology, People and Livelihood in the Nilgiri Biosphere Reserve, India. 1st ed. Kotagiri, Tamil Nadu, Nilgiris, India: Keystone Foundation, pp. 96, 100.
Le Conte Y., Navajas M. (2008). Climate change: impact on honey bee populations and diseases. Rev. Sci. Tech. Of. Int. Epiz. 27, 499–510.
Lengereke H. J. von, Blasco F., Hockings P. (eds.) (1989). The Nilgiri Environment in Blue Mountains: the Ethnography and Biogeography of a South Indian Region. New Delhi: Oxford University Press.
Leo R. (2008). Nature conservation is a thread well woven through forest beekeeping. Bees Dev. J. 87, 8. Available at: https://issuu.com/beesfd/docs/87_bfdj_jun2008/s/14154160
Matias D. M. S., Borgemeister C., Von Wehrden H. (2018). Ecological changes and local knowledge in a giant honey bee (Apis dorsata F.) hunting community in Palawan, Philippines. AMBIO 47, 924–934. doi: 10.1007/s13280-018-1038-7
Nagir M. T., Atmwidi T., Kahona S. (2016). The distribution and nest-site preference of Apis binghami at Maros Forest, South Sulawesi, Indonesia. J. Insect Biodiversity 4, 1–14. doi: 10.12976/jib/2016.4.23
Nath S., Roy P., John M., Leo R. (2001). Survey on Honey Hunters and Bee Keepers of Tamil Nadu (Keystone Foundation). Available at: https://drive.google.com/file/d/1aPxWBk64kDOvYTbrJk63E1cRgeN5J5lu/view?usp=sharing&usp=embed_facebook (accessed January 06, 2024).
Neupane K., Dhakal D., Thapa R., Gautam D. M. (2006). Foraging Preference of Giant Honeybee, Apis dorsata F., to Selected Horticultural Crops. J. Inst. Agric. Anim. Sci. 27, 87–92. doi: 10.3126/jiaas.v27i0.700
Padmavathy S., Rehel S. M. (2014). Bee plants of Apis dorsata during winter season from Coonoor Region, Nilgiri, Tamil Nadu, India. J. Academia Ind. Res. 2, 570–572. Available at: http://jairjp.com/MARCH%202014/10%20PADMAVATHY.pdf
Potts S. G., Vulliamy B., Dafni A., Ne’eman G., Willmer P. G. (2003). Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84, 2628–2642. doi: 10.1890/02-0136
Rehel M. S., Varghese A., Bradbear N., Davidar P., Robert S., Roy P., et al. (2009). Benefits of biotic pollination for non-timber forest products and cultivated plants. Conserv. Soc. 7, 213–219. doi: 10.4103/0972-4923.64732
Roy P., Leo R., Thomas S. G., Varghese A., Sharma K., Prasad S., et al. (2011). Nesting requirements of the rock bee Apis dorsata in the Nilgiri Biosphere Reserve, India. Trop. Ecol. 52, 285–291. doi: 10.1017/S026646740900621XP
Seeley T. D., Seeley R. H., Akratanakul P. (1982). Colony Défense strategies of the honeybees in Thailand. Ecol. Monogr. 52, 43–63. doi: 10.2307/2937344
Sharma D., Rakesh K. G. (2014). “Management of Asian honeybees,” in Beekeeping for Poverty Alleviation and Livelihood Security (Springer, Dordrecht), 205–245.
Sihag R. C. (2014). Phenology of migration and decline in colony numbers and crop hosts of giant honeybee (Apis dorsata F.) in semiarid environment of Northwest India. J. Insects, 2014, 1–9. doi: 10.1155/2014/639467
Singh V., Verma D. K., Chauhan D. (2017). “Beekeeping Technology and Honey Processing: Emerging Entrepreneurship for Rural Areas,” in Engineering Interventions in Foods and Plants (Apple Academic Press).
Thomas S. G., Rehel M. S., Varghese A., Davidar P., Potts S. P. (2009a). Social bees and food plant associations in the Nilgiri Biosphere Reserve, India. Trop. Ecol. 50, 79–88. Available at: https://www.researchgate.net/publication/237584631.
Thomas S. G., Varghese A., Roy P., Bradbear N., Potts S. G., Davidar P. (2009b). Characteristics of trees used as nest sites by Apis dorsata (Hymenoptera, Apidae) in the Nilgiri Biosphere Reserve. India. J. Trop. Ecol. 25, 559–562. doi: 10.1017/S026646740900621X
Tongco M. D. C. (2007). Purposive sampling as a tool for informant selection. Ethnobotany Res. Appl. 5, 147–158. doi: 10.17348/era.5.0.147-158
Venkatesh G. (2014). Study on population status in relation to urban development in few selected nesting site of rock bee colonies, Apis. Int. J. Sci. Res. Publications 4. Available at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=5860884ecb8ef754c71a4b74bfe6a22822925322
Keywords: Apis dorsata, wild bees, population estimates, Indigenous knowledge, sacred cliffs, Nilgiri Biosphere Reserve (NBR)
Citation: Rehel SM, Basavegowda M, Thankiyan J and Roy P (2024) Socio-ecological surveys of Apis dorsata in the mountains of the Nilgiri Biosphere Reserve, Western Ghats, India. Front. Bee Sci. 2:1385640. doi: 10.3389/frbee.2024.1385640
Received: 13 February 2024; Accepted: 25 November 2024;
Published: 12 December 2024.
Edited by:
Gard W. Otis, University of Guelph, CanadaReviewed by:
Surendra Raj Joshi, International Centre for Integrated Mountain Development, NepalHuu Chinh Phung, Mountain Bee Development Center, Vietnam
Copyright © 2024 Rehel, Basavegowda, Thankiyan and Roy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiny Mariam Rehel, c2hpbnlAa2V5c3RvbmUtZm91bmRhdGlvbi5vcmc=
 Shiny Mariam Rehel
Shiny Mariam Rehel Mahadesha Basavegowda
Mahadesha Basavegowda