- 1School of Environmental Sciences, University of Guelph, Guelph, ON, Canada
- 2Institute of Bee Health, Vetsuisse Faculty, University of Bern and Agroscope, Bern, Switzerland
- 3CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan, China
- 4Department of Zoology, Himalayan University, Itanagar, Arunachal Pradesh, India
- 5Trivedi Schools of Biosciences, Ashoka University, Sonipat, Haryana, India
- 6Department of Entomology, University of Poonch Rawalakot, Rawalakot, Pakistan
- 7Department of Zoology, Women University of Azad Jammu and Kashmir, Bagh, Pakistan
- 8Mountain Bee Development JSC, Hanoi, Vietnam
- 9Department of Biology and Center of Excellence in Entomology, Faculty of Science, Chulalongkorn University, Bangkok, Thailand
- 10Department of Biomedical Science and Environmental Biology, Kaohsiung Medical University, Kaohsiung, Taiwan
- 11Department of Entomology, China Agricultural University, Beijing, China
- 12Department of International Relations and IT, University of Veterinary Science, Nay Pyi Taw, Myanmar
- 13Prithu Technical College, Lamahi, Nepal
- 14Faculty of Agriculture, Agriculture and Forestry University, Rampur, Chitwan, Nepal
Introduction: Apis laboriosa, the Himalayan giant honeybee, inhabits the foothills of Himalaya and neighboring mountainous regions. Here we revise its distribution in light of recent reports and discoveries, review the ecozones it inhabits, and reassess its likely distribution through species distribution modeling.
Methods: We revised the range map for A. laboriosa by mapping locality records from various sources: refereed research publications, museum specimens, records with identifiable images of bees in publicly available databases, personal observations of the authors, and photos/videos and their coordinates submitted to the authors by honey-hunters, beekeepers, and extension workers. We then used that map to determine the ecozones in which the species occurs. The geographical coordinates of the data localities were used to estimate the potential suitable areas for the bee with MaxEnt modeling.
Results: Our research filled in several previously identified gaps in the distribution of A. laboriosa: in western Nepal; mountainous regions of Myanmar, northwestern Thailand, and northern Laos; several river valleys in Xizang and Yunnan, China; and northeastern Pakistan. Over most of its range this bee species primarily occupies subtropical broadleaf forests with strong Himalayan affinities. However, in the western part of its range it extends into zones dominated by conifers. The sites where A. laboriosa has been recorded closely match the predicted range of the species. Two variables, mean temperature of the coldest quarter and temperature seasonality, contributed most (76%) to the species distribution model.
Discussion: Apis laboriosa has a more extensive distribution in the foothills of the Himalaya and neighboring mountainous regions than has been previously recognized. The range now extends from longitude 74.4°–105.9°E, a linear distance of 3300 km, and from latitude 19.2°N–34.8°N. We have documented nesting on tree branches in northern Vietnam. Future research is warranted on its elevational migrations along river valleys, population differentiation, and ecological role as a pollinator in the different ecological zones it inhabits.
1 Introduction
“Distribution maps are among the most fundamental and historically informative data of any biogeographic study.”
Parenti and Ebach (2009), Comparative Biogeography
The Himalayan giant honey bee, Apis laboriosa Smith, 1871, is an inhabitant of mountainous regions of Asia, from northern India to northern Vietnam (Kitnya et al., 2020; Huang et al., 2022). It is a significant pollinator and a major source of honey in high-elevation regions of Asia (Batra, 1996; Gupta, 2014; Gogoi et al., 2017). The species was first collected and described from Yunnan, China (Moore et al., 1871), following which it was largely ignored until Maa (1953) resurrected it as a species, Megapis laboriosa, and provided a taxonomic description and key for identification. It again faded into obscurity until Sakagami et al. (1980) provided details for a number of distinct characters that clearly distinguish it from its sister species of mainland Asia, Apis dorsata Fabricius, 1793 (e.g., in workers, the color of thoracic hairs and gastral tergites, size of ocelli, ocellar platform, ocellocular distance, malar length, and number of sting barbs; see Figures 1A, B). Recently, genetic and morphometric analyses from several sites of sympatry in Arunachal Pradesh, India, confirmed that A. laboriosa and A. dorsata are distinct species (Kitnya et al., 2022). Classical morphological taxonomy (Kitnya et al., 2024) and genetic analyses (e.g., Arias and Sheppard, 2005; Raffiudin and Crozier, 2007; Lo et al., 2010; Kitnya et al., 2022; Bhatta et al., 2024) also differentiate these two species throughout their ranges. The approximate ranges of A. laboriosa and A. dorsata are depicted in Figure 2 of Huang et al. (2022).
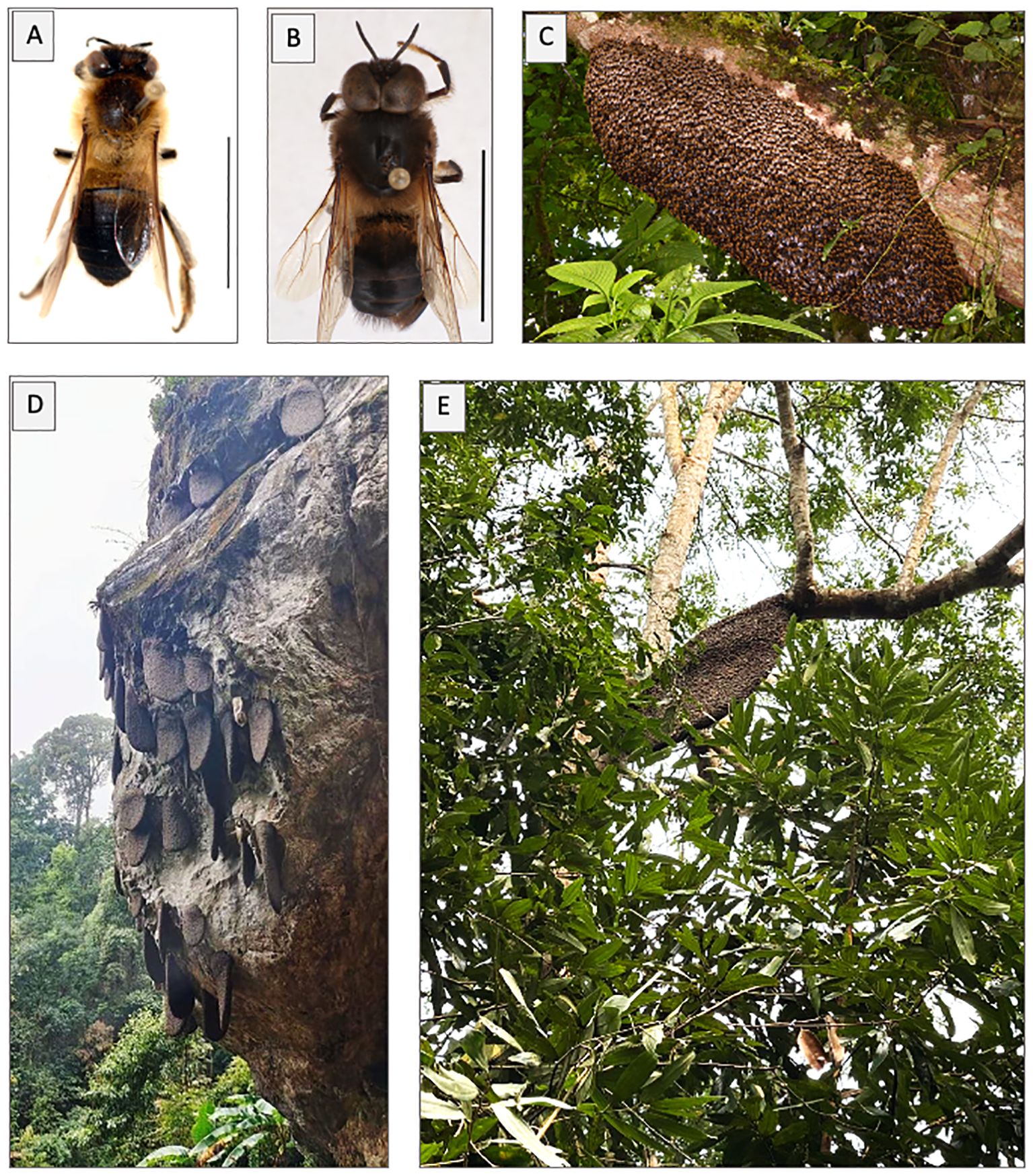
Figure 1 Habitus of worker (A) and drone (B) of Apis laboriosa; scale bar: 1 cm; specimens collected by B. A. Underwood, Kaski District, Nepal, 1,675 m elevation (specimens in the Cornell University Insect Collection). (C) Wintering swarm (bivouc) on a tree branch 2–3 m above ground in Yen Bai Province, Vietnam, at 1,200 m elevation; photo taken on 20 November 2022 by Eugene Popov. (D) Typical aggregation of nests on a cliff in Dien Bien Province, Vietnam; photo taken by Lo Van Anh of Son La Province. (E) Apis laboriosa nest constructed on a tree branch in Van Ban District, Lao Cai Province; photo taken by C. H. Phung.
Gradually, through accumulation of specimens, increasing amounts of research, and observations by naturalists, the extensive distribution of A. laboriosa has been elucidated. Maa (1953) described its range as limited to “India (Sikkim; Assam); China (W. Yunnan). Probably also occurring in N. Burma.” Sakagami et al. (1980) created the first range maps for A. laboriosa, depicting it as a resident from central Nepal to Yunnan, China. Batra (1996) reported it from mountain valleys of Uttarakhand, India, ca. 600 km northwest of where it was known to occur in Nepal. Its distribution was further extended northward to Sichuan Province, China, and eastward to northern Laos (Otis, 1996) and at about the same time even further eastward to northwestern Vietnam (Trung et al., 1996). Gogoi et al. (2017) provided a generalized range map that showed it extending southward in the mountains near the border between northeastern India and western Myanmar. An updated distribution map that integrated information from the literature, iNaturalist reports, museum collections, and the authors’ personal observations added numerous localities in Bhutan, northeastern India, and northern Vietnam, and confirmed that it occurs in the Naga Hills of northeastern India and Chin Hills of west central Myanmar (Kitnya et al., 2020). Most recently, Huang et al. (2022) added several localities in China, Nepal, and Myanmar.
Kitnya et al. (2020) used rainfall and elevation patterns to suggest a number of regions where A. laboriosa was likely to occur but had not been documented. These included the Pir Panjal Range at the border between India and Pakistan; western Nepal; eastern Myanmar and possibly as far south as Thailand; and river valleys that extend into the eastern edge of the Hengduan Mountains of China. Subsequently, a species distribution model was generated for A. laboriosa by Huang et al. (2022). Species distribution modeling (SDM; also referred to by other names such as ecological niche modeling) allows one to predict the complete range of a species based on the environmental characteristics of sites where it has been documented to occur (Guisan et al., 2017). The range estimated by Huang et al. (2022) echoed most of the same regions predicted by Kitnya et al. (2020), as well as sites in northeastern Vietnam and a region on the Tibetan Plateau. We sought additional locality information from numerous sources to address gaps in the distribution of the species and resolve the discrepancies between these two studies.
Here, we report several new discoveries that significantly extend the range of Apis laboriosa further to the east, south, and west. Our SDM, based on the expanded database of confirmed geographic locations of the bee, yielded new insights into the potential distribution of the species. New localities along river valleys in Xizang, China, provide hints about its seasonal migrations. Evaluation of the biotic characteristics of the ecoregions (Olson et al., 2001; Dinerstein et al., 2017) and the climatic zones it inhabits (Geiger, 1961; Kottek et al., 2006) may be instructive in predicting other locations where this species does and does not occur.
2 Methods
To create our revised range map for Apis laboriosa, we used the Quantum Information Geographic System (Version 3.32.2-Lima; QGIS.org) for mapping locality records from various sources. To create the base map, ESRI terrain and topographic maps were obtained through QuickMapServices, a function available in QGIS. A Raster Analysis of Hillshades was performed on the Digital Elevation Model (DEM) with 2.5 m resolution, obtained from Worldclim (https://www.worldclim.org/data/bioclim.html). Terrain and Hillshade were set at “Multiply blending mode,” with brightness set at −50 and 45 respectively and contrast set to 0 and −20 respectively, keeping the transparency format at the default setting. The geographic coordinates for bee localities were imported in comma delimited format (.csv), with a separate layer for each data source category.
The lowermost layer of bee data consists of the records with coordinates reported in the Supplementary Data File to Kitnya et al. (2020). We have eliminated several of their records that lacked a source or for which the geographic coordinates had previously been approximated, resulting in 339 records, and we corrected one site in SE Nepal that previously had been entered incorrectly.
The second layer depicts the localities used by Huang et al. (2022) for species distribution modeling, excluding the records already reported by Kitnya et al. (2020) and those reported in GBIF (2023) and iNaturalist (2023a, 2023b) (explained below).
In the third layer we have presented records obtained from the Global Biodiversity Information Facility (GBIF). GBIF (2023) reported occurrences of A. laboriosa in 8 datasets; we reviewed all of those (up to 3 November, 2023). Because records with coordinates from the Snow Entomological Museum, University of Kansas, and the US National Museum had been included by Kitnya et al. (2020) and most other GBIF records either lack coordinates or identifiable photos of the bees, we only obtained five new records from GBIF, all from the citizen science database Observation.org (Observation.org, 2023). Following the example of Dorey et al. (2023), we also checked the Symbiota Collections of Arthropods Network (SCAN, 2023); it contained only the records for specimens housed in the Snow Entomological Museum which had been retrieved previously from the GBIF database. iDigBio (2023) lacked records for this species. Data for specimens housed in the Natural History Museum London were included by Kitnya et al. (2020). There were no specimens in the American Museum of Natural History, the Chicago Field Museum, or the Bishop Museum (Hawaii).
In the fourth layer, we have presented records from three citizen-science databases that contain identifiable images of bees in searches of A. laboriosa or Indicator xanthanotus Blyth, 1842, the yellow-rumped honeyguide that is intimately associated with nests of the bee (Cronin and Sherman, 1976): iNaturalist (iNaturalist, 2023a, 2023b; all posted records for the species were reviewed between 28–30 October, 2023), the Bhutan Biodiversity Portal (BBP, 2023), and Macaulay Library (2023); sightings with photographs reported to eBird) (the latter two websites were both reviewed on 7 November, 2023). Photographic records that show abandoned honeycombs without identifiable bees were excluded. One additional locality was obtained from specimens housed in the insect collection of the Institute of Ecology and Biological Resources, Hanoi, Vietnam (L. T. P. Nguyen, pers. comm.). After removing the records previously extracted from these websites and included by Kitnya et al. (2020), we found 61 verifiable postings.
We searched the Web of Science under the topic “Apis laboriosa” on 11 November, 2023, and reviewed all 18 articles published subsequent to November, 2019, the cut-off date for articles included by Kitnya et al. (2020). Four articles contained information that allowed us to identify localities that were sufficiently precise to be included in our analysis. Localities reported in Vietnam by Long et al. (2012) were not added because it was apparent that some of their identifications of A. laboriosa and A. dorsata were incorrect. The localities from literature sources were plotted in the fifth layer.
The 6th layer shows 39 new localities where A. laboriosa was observed by the authors or it was reported to them by reliable sources (e.g., honey-hunters, extension workers) who included videos or photographs with identifiable images of bees. (For examples of honey collection from A. laboriosa, refer to Channel Kham Pha (2019), Rediff (2019), and Valli and Summers, 1988. Links to additional videos of honey-hunting are cited in Supplementary Data File 1).
The uppermost 7th layer contains 16 localities representing observations that seem credible but are not supported by specimens, photographs, or videos.
To better understand the biotic communities inhabited by A. laboriosa, we reviewed the ecoregions established by Olson et al. (2001) and Wikramanayake et al. (2002) and Köppen-Geiger climatic zones (Geiger, 1961; Kottek et al., 2006; Karki et al., 2016) that are known to be inhabited by A. laboriosa.
Finally, we followed the methodology of Huang et al. (2022) to simulate the potential suitable areas for A. laboriosa under current climate conditions, based on our expanded data set. We used maximum entropy modeling, MaxEnt, as it performs well with presence only data (Fithian and Hastie, 2013; Phillips et al., 2017). To summarize, we removed duplicate presence data within 5 km in R with the ENMwizard R package (https://github.com/HemingNM/ENMwizard) to prevent the model from overfitting (Kramer-SChadt et al., 2013; Boria et al., 2014), resulting in 325 locality records. To make our results comparable with the previous models of Huang et al. (2022), we downloaded the same eight climate variables (Bio2, Bio3, Bio4, Bio11, Bio12, Bio13, Bio18, Bio19, in 30 seconds, from WorldClim 2.1; https://www.worldclim.org/data/worldclim21.html) and the same six soil layers (https://soilgrids.org/) that they selected. We then applied the same model parameters (for the analysis based only on climate variables: regularization multiplier = 1.5, feature class = LQHPT; with soil variables included: regularization multiplier = 0.5, feature class = LQ) and used Pearson correlations. We then calculated the continuous Boyce index through the ecospat package in R and used it to evaluate the accuracy of the modeling results (Hirzel et al., 2006; Leroy et al., 2018). A Boyce index of 1 indicates that the predicted distribution perfectly matches the distribution of presences in the evaluation dataset; a value of zero indicates no difference from a random model. The results were imported into ArcGIS 10.2 for analysis, and the ten-percentile training presence threshold was used to identify suitable area for the species (Nazeri et al., 2012) using the reclassify tool. (Huang et al., 2022 provided full details of our methodology.)
The geographical terminology of Liu et al. (2022) for the Pan-Tibetan Highland region has been adopted.
3 Results
Figure 2 depicts the revised geographic range of Apis laboriosa with respect to mountainous regions of Asia. (Refer to Supplementary Data File 1 for details of the localities included in the map.) It was created using data from refereed research publications, museum specimens, records with identifiable images of bees in publicly available databases, personal observations of the authors, and photos/videos and their coordinates submitted to the authors by honey-hunters, beekeepers, and extension workers. This map extends the distribution in three cardinal directions—east, south, and west—and fills in several previous gaps.
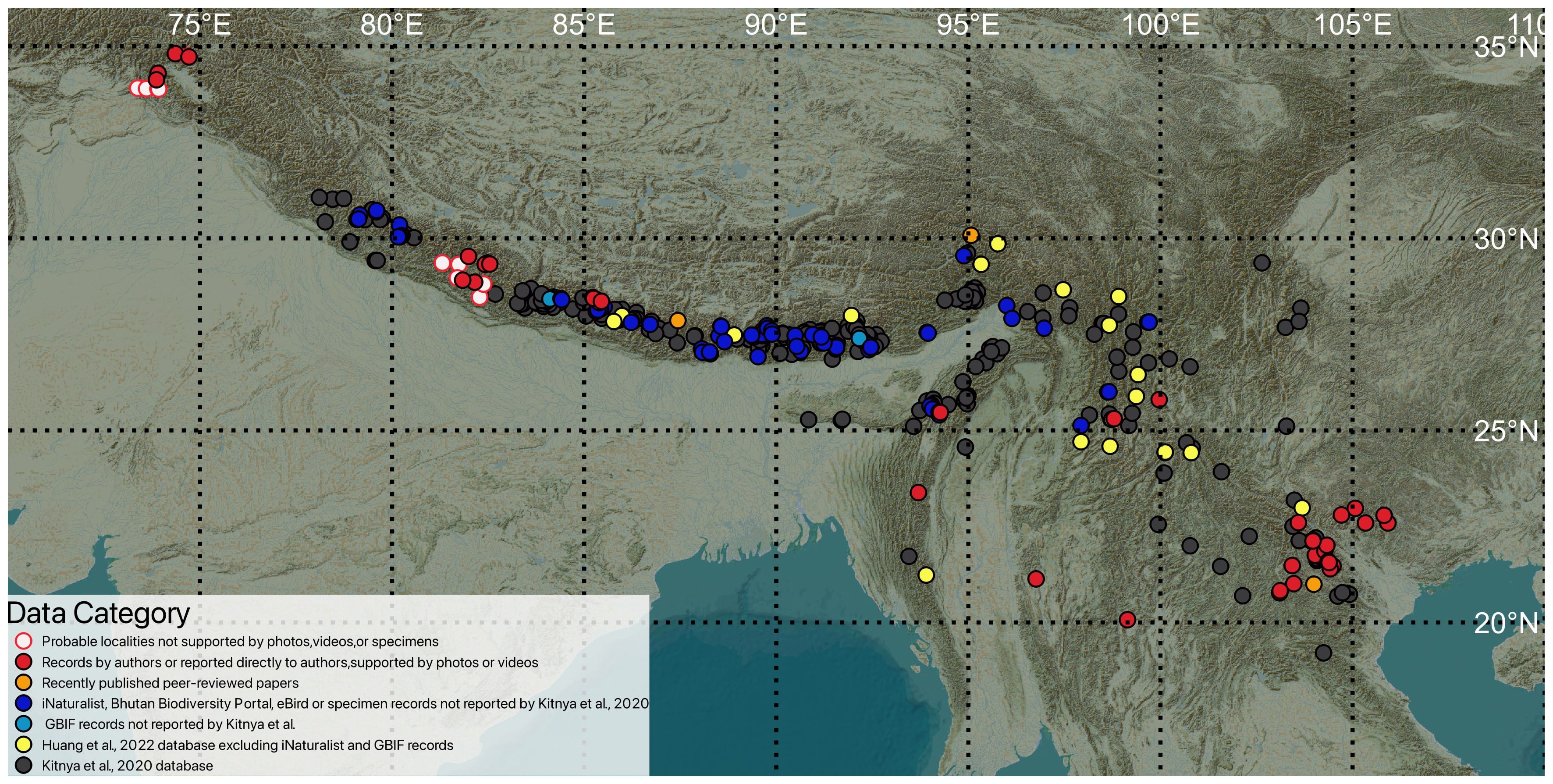
Figure 2 Revised distribution of Apis laboriosa. The colors of the dots reflect the various sources of locality information as indicated in the key at the lower left. Solid circles depict confirmed localities; open circles indicate probable localities based on credible reports that lack confirming photos, videos, or specimens.
In the east, in Vietnam and eastern Laos, the number of known localities was more than doubled, with the most easterly location documented to date being in Cao Bang Province, Vietnam (22.6°N, 105.9°E; Pham et al., 2023). M. Kato (pers. comm.) confirmed one site (Site 4) where he and his associates observed A. laboriosa in Laos (Kato et al., 2020).
Interestingly, although most nests in Vietnam are aggregated on cliffs (Figure 1D), in some regions a higher proportion of nests are constructed on tree branches (Figure 1E; Channel Kham Pha, 2019).
Across China, a number of new records add to the many already reported from there. Of note are the observations along several river valleys that extend northward into Xizang and Yunnan Provinces. For example, author X. Zhou and his students recently collected specimens from several sites in Jilong County, Xizang, near the border of Nepal, that lie within the Kirong Tsangpo (the Trishuli River in Nepal) drainage. Two nearly 50-year-old specimens in the National Zoological Museum of China were also collected in this same region. Cao et al. (2023) collected samples from several villages in Rikaze, Xizang, in the watershed of Phung Chu (the upper reaches of the Arun River) that extends northward from eastern Nepal. Further to the east, Huang et al. (2022) reported on specimens housed in the National Zoological Museum of China that were collected in the upper regions of the Brahmaputra River: one along the Yarlung Zangbo River (in Motuozhen, Medog County), and another near Bomé along the Palong Zhangbo River, a tributary to the Yarlung Zhangbo, both in Nyingche Prefecture, Xizang. Cao et al. (2023) analyzed specimens collected nearby in the valley of the Palong Zhangbo River. J. S. Xu (pers. comm.) confirmed the coordinates of the two locations from which mtDNA genomes were sequenced (Tang et al., 2023). In Yunnan, Yang et al. (2015) reported the species from Deqin County (Shengpingzhen), near the Lancang River (upper Mekong River; included in Huang et al., 2022). We did not learn of any recent records from Sichuan Province.
In India, seven new records from Sikkim and West Bengal States (iNaturalist, 2023a, b) confirm the presence of the bee in that region (Kitnya et al., 2020). There were also a number of new records in Uttarakhand and North East India.
In western Nepal, we have documented the occurrence of A. laboriosa in four districts of Karnali Province. S. Joshi (pers. comm.) shared his personal knowledge of A. laboriosa from several communities in Bajura and Bajhang Districts of Sudurpashchim Province; one, the rural community of Surma, is only 50 km from the border with Uttarakhand, India. Unfortunately, the locations of these observations were imprecise and could not be included in Figure 2. We also learned from agricultural extension agents, honey sellers, and honey hunters of an additional three districts that we were unable to verify (e.g., Kalikot, Salyan and West Rukum Districts, Karnali Prov.); these are depicted with open circles in Figure 2.
Several remarkable new records come from the southern and western edges of the range of the species. In extreme northwestern Thailand, A. laboriosa was independently observed foraging and nesting at the highest point in Doi Pha Hom Pok National Park, by nature photographers (iNaturalist, 2023a) and park personnel (Voraphab et al., 2024). Subsequent to us obtaining the data for our analyses, another photograph of A. laboriosa was taken in Thailand 10 km west of Doi Pha Hom Pok, at 1,470 m elevation (iNaturalist, 2023a). In central Myanmar, bees collecting fluids from soil were photographed northwest of Taunggyi, in central Myanmar (21.1°N, 96.8°E), near mountains that exceed 2,000 m in elevation at the western edge of the Shan Plateau. Y.Q. Peng collected the species at 3,022 m on Mount Victoria (Natma Taung), Chin State, western Myanmar (21.2°N, 93.9°E) (Huang et al., 2022).
Most surprisingly, we have confirmed that A. laboriosa inhabits the Neelum Valley, a region in northern Azad Jammu and Kashmir, Pakistan (AJK-P) dominated by coniferous trees (latitude 34.8°N). Active colonies were observed in Taobat at 2,750 m elevation and foragers were observed on red and white clover (Trifolium spp.) in both Taobat and Arang Kel. Foragers and aggregations of nests were also observed in Leepa and along the Neelum Jhelum River, respectively. The giant honey bees reported by Khan et al. (2014) from Murree, Pakistan, at ca. 2,000 m, were likely A. laboriosa that were incorrectly reported as A. dorsata. We have included these probable localities as open circles on Figure 2 (details are available in the Supplementary Data File).
Our review of the ecoregions of Asia (Wikramanayake et al., 2002) indicates that A. laboriosa primarily inhabits regions dominated by broadleaf trees, including the Tropical and Subtropical Moist Broadleaf Forests Biome in several ecoregions (Northern Indochina Subtropical Forests, Chin Hills–Arakan Yoma Montane Rain Forests, Meghalaya Subtropical Forests, Eastern Himalayan Broadleaf Forests, Himalayan Subtropical Broadleaf Forests, Western Himalayan Broadleaf Forests). All of these ecoregions exhibit strong Himalayan biogeographic affinities (e.g., a predominance of oaks and numerous rhododendrons) and have moist to wet climates. Additionally, some localities in the western part of its range lie within the Himalayan Subtropical Pine Forests ecoregion and possibly Western Himalayan Subalpine Conifer Forests (e.g., at Rara Lake, Nepal, and the Neelum Valley, AJK-Pakistan).
Over most of its range, from Yunnan, China, to Uttarakhand, India, and southward along the Arakan Mountains, A. laboriosa inhabits the Cwb (warm temperate, winter dry, warm summer) climate zone (Kottek et al., 2006). In contrast, in northern Vietnam and Laos the occupied climate zone is Cwa (warm temperate, dry winter, hot summer) and Cfa (warm temperate, fully humid, hot summer). In most parts of AJK-Pakistan, the inhabited regions are classified as Cfb (warm temperate, fully humid, warm summer), but the Neelum Valley is classified as Dfb (snow, fully humid, warm summer) (Geiger, 1961). These classifications are averages for the regions inhabited by the bee; in mountainous areas, microclimates can differ considerably over short distances due to local differences in elevation and rainfall.
The distribution of the bee predicted by our species distribution model closely matches confirmed localities of A. laboriosa (Figure 3). In our analysis based on 8 climate variables, the Boyce index, a parameter designed to evaluate environmental suitability predicted by SDMs, was 0.75 which compares with the Boyce index of 0.79 determined by Huang et al. (2022). When we added six soil variables to the analysis, the Boyce index value was higher, 0.87 (similar to 0.84 of Huang et al., 2022), indicating high predictive accuracy. As seen in Supplementary Table S2 (Supplementary Data Files), two climate variables, Bio11 (mean temperature of the coldest quarter) and Bio4 (temperature seasonality) together contributed most (76%) to the model. The next two most important variables related to soil conditions: total nitrogen (8.6%) and soil organic carbon stock (6.7%). Notable additions to the predicted range from our analysis compared to that of Huang et al. (2022) include an extensive region of northern Laos and Vietnam that extends southeastward to Ha Tinh and Quang Binh Provinces; additional sites within the Shan Plateau of eastern Myanmar; the entire Arakan Mountain range along the border of India and Myanmar south to Natma Taung; several river valleys that extend into Xizang, China; and Songpan County in the northwest corner of Sichuan Province, China. Three regions eliminated from the distribution predicted by Huang et al. (2022) were southeastern Nepal and adjacent portions of West Bengal, India; a region north of Himalaya on the Tibetan Plateau; and portions of Himachal Pradesh, India.
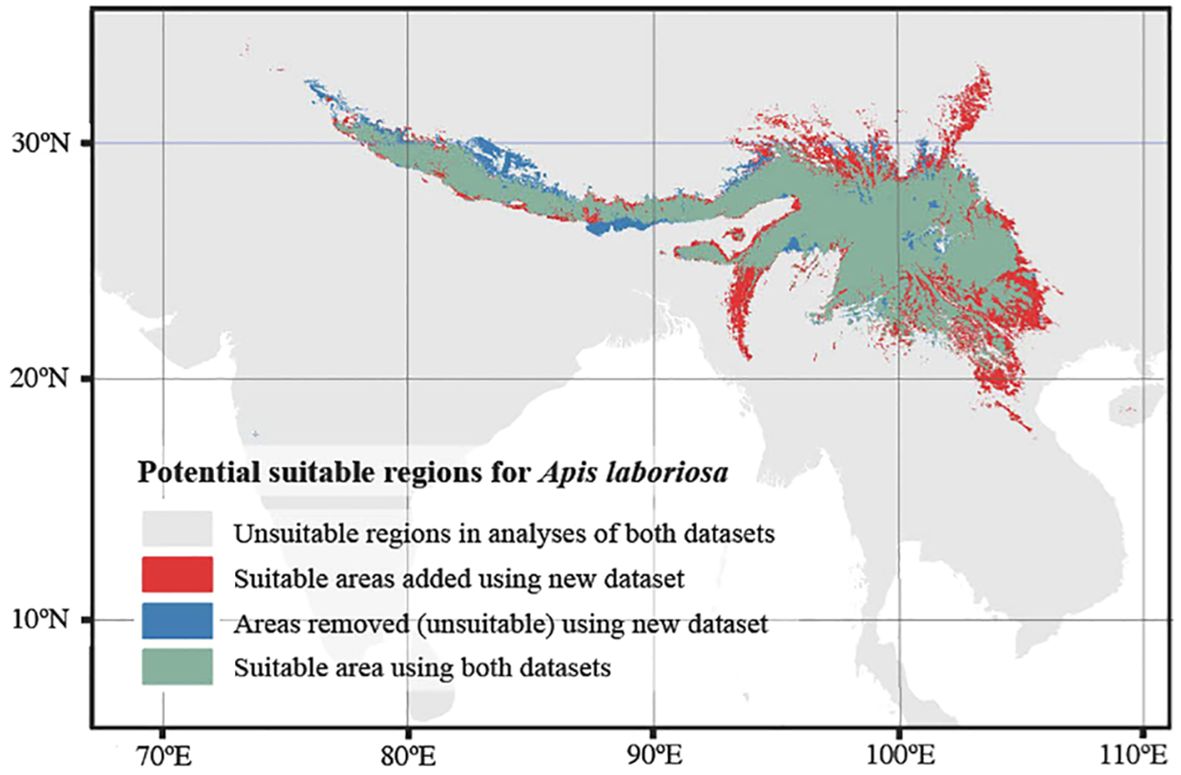
Figure 3 Map depicting the predicted distribution of Apis laboriosa, generated with MaxEnt species distribution modeling by Huang et al. (2022) and this study. Regions shown in olive green were predicted to be suitable in both analyses. Our new analysis detected substantial additional regions where A. laboriosa is predicted to occur (in red) and those that the new analysis no longer estimates are suitable (in blue).
4 Discussion
Our compilation of records provides the most up-to-date and comprehensive database for occurrences of Apis laboriosa (Supplementary Data File). We have indicated the sources of information under “Mapping Category” should others want to exclude some types of data from future analyses. New records are conservative, in that for us to include them they either represent observations of the authors who are familiar with the species or were accompanied by a photo or video with identifiable bees, specimens, or publicly accessible DNA sequences.
We report here many new localities for A. laboriosa in northern Vietnam and several in Laos, more than one quarter of which were at elevations <1,000 m, including what appears to be a combless wintering swarm (Figure 1C; see Underwood, 1990) (Figure 2). This species is widespread in the highlands of northern Vietnam, where we report for the first time that solitary nests are regularly constructed on tree branches, in some regions more frequently than on cliffs (C. H. Phung, unpublished data). The few records from Laos indicate that it remains poorly documented there. The new records we report, coupled with predictions from our species distribution modelling (Figure 3), suggest that this species is likely much more widely distributed in northern Laos and possibly further south in Vietnam than has been documented. The mountains of northern Vietnam and Laos are the southeasternmost extension of the Himalaya Range (Sterling et al., 2006) and exhibit considerable Himalayan biogeographic affinities (e.g., Spitzer et al., 1993; Bain and Truong, 2004; Sterling et al., 2006; Bakalin et al., 2018, 2023) that undoubtedly influence the success of the bee there. In contrast, further south in the Central Highland region of Vietnam, despite some Himalayan influences in the flora (e.g., Vuong and Sridith, 2016; Wu et al., 2023), several searches by author C. H. Phung and his associates for A. laboriosa at elevations >1,000 m in provinces south of 16.5°N latitude (Gia Lai, Kon Tum, Lam Dong, Quang Nam, Thua Thien Hue) have failed to detect this species (C. H. Phung, pers. obs.). This may be a consequence of the habitats having unsuitable flora and/or climate. Alternatively, it may reflect the failure of A. laboriosa to colonize the southern highlands due to a lack of connectivity with populations in the northern highlands caused by the broad region between 16.5–18.7°N latitude where elevations do not exceed 700 m.
We have now confirmed the species from several districts in western Nepal. An apparent gap in its distribution in that part of Nepal was discussed by Kitnya et al. (2020). Considering the new records (both confirmed and tentative), that gap likely represented a lack of exploration in that remote part of the country. As predicted by SDM (Huang et al., 2022; this study), this species probably occurs along the entire southern edge of the Himalaya in Nepal in a band of subtropical broadleaf forests (Wikramanayake et al., 2002) with Cwb climate (Karki et al., 2016), with extensions into landscapes at higher elevations dominated by conifers. The relatively dry environment of western Nepal (Karki et al., 2016) may cause its occurrence there to be patchy. Additional confirmations through well documented observations and collections are warranted.
Both Kitnya et al. (2020) and Huang et al. (2022) predicted the occurrence of A. laboriosa along river valleys that extend into the Tibetan Plateau and Hengduan Mountains of China. This prediction is supported by observations of bees in the watersheds of the Trishuli River/Kirong Tsangpo, the Arun River/Phung Chu, the Brahmaputra River/Yarlung Zangbo/Tsangpo, the Mekong River/Lancang, and the Dadu River (a tributary of the Yangtze River). Despite being surrounded by high mountains, the valleys at lower elevations along these rivers experience a subtropical climate dominated by broadleaf forests (Ni, 2000). A. laboriosa likely also occurs along the Salween River/Nu Jiang, the Jinsha River/Jinsha Jiang (the upstream portion of the Yangtze River), and the Yalong River (a major tributary of the Yangtze). Our SDM suggests the environmental conditions are suitable for this species to occur further upstream along these rivers than has been documented. It is not clear if it is a permanent inhabitant of these river valleys or if swarms migrate into them seasonally, as suggested by Underwood (1990) and Kitnya et al. (2020). Research on seasonal altitudinal migrations of this species is long overdue.
In western Myanmar, A. laboriosa occurs at high elevations of Natma Taung (Mt. Victoria), within the Chin Hills-Arakan Yoma Montane Rain Forests ecoregion (Wikramanayake et al., 2002). Cloud forests above 2,000 m on the mountain are dominated by Himalayan tree taxa and have a distinctly Palearctic temperate flora (Wikramanayake et al., 2002). The Purvanchal and Arakan mountain ranges extend continuously from the Himalaya southward through northeastern India and western Myanmar, providing contiguous habitat and a corridor for bee dispersal along the N/S axis of the mountains within a region predicted to be suitable by our SDM modeling. Considering all these factors, the presence of A. laboriosa on Natma Taung was anticipated.
Discoveries of A. laboriosa in central Myanmar and northwestern Thailand extend its distribution southward. These localities are far (ca. 350 km and 240 km respectively) from the closest known populations in Yunnan, China. Kitnya et al. (2020) and Huang et al. (2022) predicted that the species may occur on Doi Pha Hom Pok, Thailand (Kitnya et al., 2020; Huang et al., 2022. It was subsequently confirmed there (iNaturalist, 2023a; Voraphab et al., 2024), and our species distribution model predicts that the environmental conditions there are suitable for it. On the Shan Plateau of eastern Myanmar, relatively large areas exceed 1,000 m in elevation (see map provided by Evers and Taft, 2016), the approximate elevation at which the species was photographed in Pindaya Township, Shan State. The climate in this region is classified as “temperate, dry winter, hot summer” (Cwa) (Geiger, 1961; Kottek et al., 2006). Scattered over this region at higher elevations, the climatic zone is “temperate, dry winter, warm summer (Cwb) and more suitable for the bee. Apis laboriosa likely inhabits some of these high elevation sites on the Shan Plateau (Figure 3). It may even occur as far south as Nattaung (latitude 18.8°N), the highest mountain (2,623 m) of the Karen Hills, and the high elevation region surrounding that mountain, although our SDM did not predict its occurrence there.
As predicted by Kitnya et al. (2020), A. laboriosa has been recently observed in the Pir Panjal Range, ca. 530–630 km northwest of the closest known populations in Uttarakhand, India. Bees were observed in three regions of AJK-Pakistan by authors U. A. A. Sheik and A. H. Faiz who are very familiar with Apis dorsata, the species with which it would be most readily confused. They identified A. laboriosa in the Neelum Valley, Leepa Valley, and Jhelum River Valley on the basis of coloration of bees that were observed both foraging on flowers and nesting in aggregations on rock cliffs. In the Neelum Valley foragers were observed in September at high elevations (e.g., >2,500 m). Unfortunately, supporting specimens and photographs are currently lacking. The climate of the sites in the upper Neelum Valley where the bees have been reported spans zones Cfb (temperate, no dry season, warm summer), Dfb (cold, no dry season, warm summer), and Dsb (cold, dry summer, warm summer) (Geiger, 1961). Interestingly, some of these sites are dominated by conifers (e.g., Ecoregion 31: Himalayan subtropical pine forests) rather than broadleaf evergreen forests (Wikramanayake et al., 2002), suggestive that the niche of the species is broader than indicated by the predominantly broadleaf forests it usually inhabits elsewhere. An earlier report of Apis dorsata at three sites at ca. 2,000 m elevation near Murree, Pakistan (Khan et al., 2014; specimens not retained, K. A. Khan, pers. comm.) likely involved misidentifications of A. laboriosa. We have depicted unconfirmed sites with open circles on our revised distribution map (Figure 2) in the hope that others will be stimulated to confirm the occurrence of this disjunct population and study its biology in the Pir Panjal Mountain Range.
The SDM of Huang et al. (2022) predicted most of the new sites in which A. laboriosa has now been documented, showing the general utility of this research tool. However, it failed to detect the Central Myanmar and AJK-Pakistan localities, both now predicted in our revised SDM. Conversely, the SDM of Huang et al. (2022) did predict its occurrence on the southwestern Tibetan Plateau (between ca. 81.0–85.3°E longitude; Huang et al., 2022), for example in the upper basin of the Yarlung Zangbo River in Zhongba and Saga Counties, Xizang. This is a semi-arid region dominated by grasslands, with mean annual temperature <0°C (Wang et al., 2020) and climate over most of this region classified as “polar, tundra” (ET), with small portions of the region classified as “snow, winter dry, warm summer (Dwb)” and “snow, winter dry, cool summer” (Dwc) (Kottek et al., 2006). Our new SDM does not predict the occurrence of A. laboriosa in this region. Two sites on the Tibetan Plateau reported by Kitnya et al. (2020), the geographic coordinates of which had been estimated from general descriptions of their location, were removed from the current data set (Supplementary Data File). The environmental conditions at those two sites may have negatively influenced the species distribution modelling of Huang et al. (2022).
Our analysis shows that Apis laboriosa occurs from northeastern Vietnam to AJK-Pakistan, a linear east–west distance of ca. 3300 km, and from Sichuan, China, and northern AJK-Pakistan in the north to northern Thailand and Nghe An Province, Vietnam, in the south. It is a regular inhabitant of high elevation, moist evergreen broadleaf forests with strong Himalayan floral influences. In the western and northern portions of its range it extends into habitats dominated by conifers. We anticipate that A. laboriosa will eventually be confirmed in appropriate habitats/climates of: (1) the mountains of Himachal Pradesh and (2) Jammu in regions administered by India; (3) the sites we have provisionally shown, as well as the Murree Hills and the Galis in regions administered by Pakistan; (4) additional sites in an elevational band with appropriate climate and habitat across western Nepal; (5) more extensively in Sichuan Province, the eastern edge of the Hengduan Mountains, and along several river valleys in China; (6) at numerous sites in northern Laos, particularly in the northeast; and (7) scattered over the Shan Hills of eastern Myanmar, potentially as far southward as the Karen Hills. We hope that this reassessment of the range of Apis laboriosa, coupled with hints of ecological differences over this large region, will inspire more detailed studies of its ecology, population genetics, migratory behavior along river systems, and role as a pollinator within agroecosystems (e.g., Gautam et al., 2022) and natural communities (e.g., Kato et al., 2020).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
GO: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing, Investigation, Visualization. M-JH: Data analysis, Writing – review & editing, Validation. NK: Conceptualization, Visualization, Writing – review & editing. US: Investigation, Writing – review & editing. AF: Investigation, Writing – review & editing. CP: Investigation, Writing – review & editing. NW: Investigation, Writing – review & editing. Y-QP: Investigation, Writing – review & editing. XZ: Investigation, Writing – review & editing. HO: Investigation, Writing – review & editing. NA: Investigation, Writing – review & editing. KD: Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Y-QP received support for field work from Yunnan Fundamental Research Projects (grant No. 202201BC070001). Gard Otis provided personal funds for US and his students to conduct field work in the Neelum Valley, AJK-Pakistan.
Acknowledgments
We are indebted to numerous honey-hunters and beekeepers who shared locations, photographs and videos of A. laboriosa nesting sites. Steve Paiero, University of Guelph, assisted with the photographs of worker and drone specimens. Eugene Popov kindly allowed us to use his photo of a wintering swarm posted to iNaturalist. Similarly, honeyhunter Lo Van Anh gave us his permission to include his photo of an aggregation of bee nests. Prof. Chaodong Zhu and his team (Zeqing Niu, Qingtao Wu) from the Institute of Zoology, Chinese Academy of Sciences led the collection trip in 2023 in Jilong, Xizang, China, and kindly shared sample information included in this paper. We thank Itsarapong Voraphab (Thailand Dept. of National Parks, Wildlife and Plant Conservation), and Chawatat Thanoosing, Nontawat Chatthanabun, Pakorn Nalinrachatakan, Prapun Traiyasut, and Chawakorn Kunsete (Chulalongkorn University, Bangkok) for their contributions to the discovery of A. laboriosa nests in northern Thailand. Bilal Abdulah, Umer Ghaffar and Wajahat conducted field work that confirmed the species in the Neelum Valley, AJK-Pakistan. Surendra Joshi kindly shared information related to far-western Nepal.
In memoriam
Gard Otis dedicates this paper to Makhdzir Mardan (1953–2022). Makhdzir first introduced Gard to giant honeybees (Apis dorsata) in Malaysia in 1986, a profound experience that stimulated his interest in Asian honey bees that continues to the present. Throughout his career, Makhdzir championed research on and conservation of giant honey bees.
Conflict of interest
CP was employed by Mountain Bee Development JSC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2024.1374852/full#supplementary-material
References
Arias M. C., Sheppard W. S. (2005). Phylogenetic relationships of honey bees (Hymenoptera: Apinae: Apini) inferred from nuclear and mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 37, 25–35. doi: 10.1016/j.ympev.2005.02.017
Bain R. H., Truong N. Q. (2004). Herpetofaunal diversity of Ha Giang province in Northeastern Vietnam, with descriptions of two new species. Am. Mus. Novit. 2004, 1–42. doi: 10.1206/0003-0082(2004)453<0001:HDOHGP>2.0.CO;2
Bakalin V. A., Klimova K. G., Nguyen V., Nguyen H. M., Bakalin D. A., Choi S. S. (2023). Liverwort and hornwort flora of Hoang Lien National Park and the adjacent areas (Vietnam, IndoChina). Plants 12, 1841. doi: 10.3390/plants12091841
Bakalin V. A., Nguyen V. S., Borovichev E. A. (2018). New liverwort records for Vietnam. J. Bryol. 40, 68–73. doi: 10.1080/03736687.2017.1393140
Batra S. (1996). Biology of Apis laboriosa Smith, a pollinator of apples at high altitude in the great Himalaya range of Garhwal, India (Hymenoptera: Apidae). J. Kans. Entomol. Soc 69, 177–181.
BBP (2023). Bhutan Biodiversity Portal. Available online at: https://biodiversity.bt/ (Accessed November 7, 2023).
Bhatta C. P., Sazonj S. C., Smith D. R. (2024). Phylogeography of the giant honey bees based on mitochondrial gene sequences. Front. Bee Sci. 2, 1401851. doi: 10.3389/frbee.2024.1401851
Boria R. A., Olson L. E., Goodman S. M., Anderson R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275, 73–77. doi: 10.1016/j.ecolmodel.2013.12.012
Cao L., Dai Z., Tan H., Zheng H., Wang Y., Chen J., et al. (2023). Population structure, demographic history, and adaptation of giant honeybees in China revealed by population genomic data. Genome Biol. Evol. 15, evad025. doi: 10.1093/gbe/evad025
Channel Kham Pha (2019). Trinh phục tổ ong khoái đen siêu khủng. Available online at: https://www.youtube.com/watch?v=VLVuUwcEdog (Accessed April 2, 2024).
Cronin E. W. Jr, Sherman P. W. (1976). A resource-based mating system: the orange-rumped honeyguide. Living Bird 15, 5–32. Available at: https://www.biodiversitylibrary.org/item/278217#page/17/mode/1up.
Dinerstein E., Olson D., Joshi A., Vynne C., Burgess N. D., Wikramanayake E., et al. (2017). An ecoregion-based approach to protecting half the terrestrial realm. BioScience 67, 534–545. doi: 10.1093/biosci/bix014
Dorey J. B., Fischer E. E., Chesshire P. R., Nava-Bolaños A., O’Reilly R. L., Bossert S., et al. (2023). A globally synthesized and flagged bee occurrence dataset and cleaning workflow. Sci. Data 10, 747. doi: 10.1038/s41597–023-02626-w
Evers M., Taft L. (2016). A review of current and possible future human–water dynamics in Myanmar’s river basins. Hydrol. Earth Syst. Sci. 20, 4913–4928. doi: 10.5194/hess-20-4913-2016
Fithian W., Hastie T. (2013). Finite-sample equivalence in statistical models for presence-only data. Ann. Appl. Stat. 7, 1917–1939. doi: 10.1214/13-AOAS667
Gautam R. K., Shuyi G., Uniyal V. P. (2022). Comparative foraging behavior and pollination efficiency of Apis laboriosa S. and Apis cerana F. on black mustard (Brassica nigra L.) in western Himalaya, India. Curr. Sci. 122, 840–845. doi: 10.18520/cs/v122/i7/840-845
GBIF (2023). Global Biodiversity Information Facility. Available online at: https://www.gbif.org/ (Accessed November 3, 2023).
Geiger R. (1961). Überarbeitete Neuausgabe von Geiger, R.: Köppen-Geiger/Klima der Erde. (Wandkarte 1:16 Mill.) (Gotha, Germany: Klett-Perthes). Available at: https://koeppen-geiger.vu.wein.ac.at/present.htm.
Gogoi H., Tayeng M., Taba M. (2017). Pan-Himalayan high altitude endemic cliff bee, Apis laborisa Smith (Hymenoptera: Apidae): a review. Proc. Zool. Soc 72, 3–12. doi: 10.1007/s12595-017-0234-y
Guisan A., Thuiller W., Zimmermann N. E. (2017). Habitat suitability and distribution models: with applications in R (Cambridge: Cambridge University Press).
Gupta R. K. (2014). “Taxonomy and distribution of different honeybee species,” in Beekeeping for poverty alleviation and livelihood security. Technical aspects of beekeeping, vol. 1. Eds. Gupta R. K., Reybroek W., van Veen J. W., Gupta A. (Springer, Dordrecht), 63–103. doi: 10.1007/978-94-017-9199-1_2
Hirzel A. H., Lelay G., Helfer V., Randin C., Guisan A. (2006). Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 199, 142–152. doi: 10.1016/j.ecolmodel.2006.05.017
Huang M.-J., Hughes A. C., Xu Z.-Y., Miao B.-G., Gao J., Peng Y.-Q. (2022). Mapping the changing distribution of two important pollinating giant honey bees across 21000 years. Glob. Ecol. Conserv. 39, e02282. doi: 10.1016/j.gecco.2022.e02282
iDigBio (2023). Integrated Digitized Biocollections. Available online at: https://www.idigbio.org/portal (Accessed November 3, 2023).
iNaturalist (2023a). Himalayan giant honey bee (Apis laboriosa). Available online at: https://www.inaturalist.org/taxa/574869-Apis-laboriosa (Accessed October 28–30, 2023).
iNaturalist (2023b). Yellow-rumped honeyguide (Indicator xanthonotus). Available online at: https://www.inaturalist.org/taxa/17578-Indicator-xanthanotus (Accessed October 30, 2023).
Karki R., Talchabhadel R., Aalto J., Baidya S. J. (2016). New climatic classification of Nepal. Theor. Appl. Climatol. 125, 799–808. doi: 10.1007/s00704-015-1549-0
Kato M., Kawakita A., Gogo R., Okamoto T., Kobayashi C., Imada Y., et al. (2020). Community-level plant-pollinator interactions in a Paleotropical montane evergreen oak forest ecosystem. J. Nat. Hist. 54, 2125–2176. doi: 10.1080/00222933.2020.1837977
Khan K. A., Ansari M. J., Al-Ghamdi A., Sharma D., Ali H. (2014). Biodiversity and relative abundance of different honeybee species (Hymenoptera: Apidae) in Murree-Punjab, Pakistan. J. Ent. Zool. Stud. 2, 325–327. Available at: https://www.entomoljournal.com/vol2Issue4/27.1.html.
Kitnya N., Otis G. W., Brockmann A. (2024). Taxonomic revision and identification keys for the giant honey bees. Front. Bee 2, 1379952. doi: 10.3389/frbee.2024.1379952
Kitnya N., Otis G. W., Chakravorty J., Smith D. R., Brockmann A. (2022). Apis laboriosa confirmed by morphometric and genetic analyses of giant honey bees (Hymenoptera, Apidae) from sites of sympatry in Arunachal Pradesh, North East India. Apidologie 53, 47. doi: 10.3389/finsc.2023.1145158
Kitnya N., Prabhudev M. V., Bhatta C. P., Pham T. H., Nidup T., Megu K., et al. (2020). Geographical distribution of the giant honey bee Apis laboriosa Smith 1871 (Hymenoptera, Apidae, Apis). ZooKeys 951, 67–81. doi: 10.3897/zookeys.951.49855
Kottek M., Grieser J., Beck C., Rudolf B., Rubel F. (2006). World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 15, 259–263. doi: 10.1127/0941-2948/2006/0130
Kramer-Schadt S., Niedballa J., Pilgrim J. D., Schröder B., Lindenborn J., Reinfelder V., et al. (2013). The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 19, 1366–1379. doi: 10.1111/ddi.12096
Leroy B., Delsol R., Hugueny B., Meynard C. N., Barhoumi C., Barbet-Massin M., et al. (2018). Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 45, 1994–2002. doi: 10.1111/jbi.13402
Liu J., Milne R. I., Zhu G.-F., Spicer R. A., Wambulwa M. C., Wu Z.-Y., et al. (2022). Name and scale matter: clarifying the geography of Tibetan Plateau and adjacent mountain regions. Glob. Planet. Change 215, 103893. doi: 10.1016/j.gloplacha.2022.103893
Lo N., Gloag R. S., Anderson D. L., Oldroyd B. P. (2010). A molecular phylogeny of the genus Apis suggests that the giant honey bee of the Philippines, A. breviligula Maa, and the plains honey bee of southern India, A. indica Fabricius, are valid species. Syst. Entomol. 35, 226–233. doi: 10.1111/j.1365-3113.2009.00504.x
Long K. D., Hue L. X., Hoa D. T., Phong P. H. (2012). A preliminary study on bees (Hemenoptera: Apoidea: Apiformes) from northern and north central Vietnam. Tạp chí Sinh học 34, 419–426. doi: 10.15625/0866-7160/v34n4.2676
Maa T. C. (1953). An inquiry into the systematics of the tribus Apidini or honeybees (Hym.). Treubia 21, 525–640. Available at: https://archive.org/details/treubia-v21i3-2669.
Macaulay Library (2023). eBird, Macaulay Library, Cornell University, Ithaca, NY. Available online at: https://www.macaulaylibrary.org/ (Accessed November 7, 2023).
Moore F., Walker F., Smith F. (1871). Descriptions of some new insects collected by Dr. Anderson during the expedition to Yunnan. Proc. Zool. Soc Lond. 1871, 244–249. Available at: https://www.biodiversitylibrary.org/item/90542#page/16/mode/tup.
Nazeri M., Jusoff K., Madani N., Mahmud A. R., Bahman A. R., Kumar L. (2012). Predictive modeling and mapping of Malayan sun bear (Helarctos malayanus) distribution using maximum entropy. PloS One 7, e48104. doi: 10.1371/journal.pone.0048104
Ni J. (2000). A simulation of biomes on the Tibetan Plateau and their responses to global climate change. Mt. Res. Dev. 20, 80–89. doi: 10.1659/0276-4741(2000)020[0080:ASOBOT]2.0.CO;2
Observation.org (2023). Observation International (Aarlanderveen, The Netherlands). Available online at: https://observation.org (Accessed November 3, 2023).
Olson D. M., Dinerstein E., Wikramanayake E. D., Burgess N. D., Powell G. V., Underwood E. C., et al. (2001). Terrestrial ecoregions of the world: a new map of life on Earth. BioScience 51, 933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Otis G. W. (1996). Distributions of recently recognized species of honey bees (Hymenoptera: Apidae; Apis) in Asia. J. Kans. Entomol. Soc 69, 311–333. Available at: https://www.jstor.org/stable/25085727.
Parenti L., Ebach M. (2009). Comparative biogeography: discovering and classifying biogeographical patterns of a dynamic Earth (Berkeley, CA: University of California Press).
Pham H. D., Phung C. H., Bui D. T., Nguyen L. D., Nguyen T. T., Hand K. J., et al. (2023). Timing of drone flights and observations of other colony behaviors of Apis laboriosa in northern Vietnam. Apidologie 54, 35. doi: 10.1007/s13592-023-01014-y
Phillips S. J., Anderson R. P., Schapire R. E. (2017). Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. doi: 10.1016/j.ecolmodel.2005.03.026
Raffiudin R., Crozier R. H. (2007). Phylogenetic analysis of honey bee behavioral evolution. Mol. Phylogenet. Evol. 43, 543–552. doi: 10.1016/j.ympev.2006.10.013
Rediff (2019). Amazing honey hunters who risk their lives. Available online at: https://www.rediff.com/news/report/pix-amazing-honey-hunters-who-risk-their-lives/20190613.htm#google_vignette (Accessed April 2, 2024).
Sakagami S. F., Matsumura T., Ito K. (1980). Apis laboriosa in Himalaya, the little known world largest honeybee (Hymenoptera: Apidae). Insecta Matsumurana 19, 47–77. Available at: https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/9801/1/19_p47-77.pdf.
SCAN (2023). Symbiota Collections of Arthropods Network. Available online at: https://scan-bugs.org/portal/ (Accessed November 3, 2023).
Spitzer K., Novotny V., Tonner M., Leps J. (1993). Habitat preferences, distribution and seasonality of the butterflies (Lepidoptera: Papilionoidea) in a montane tropical rain-forest, Vietnam. J. Biogeogr. 20, 109–121. doi: 10.2307/2845744
Sterling E. J., Hurley M. M., Minh L. D. (2006). Vietnam: a natural history (New Haven, CT: Yale University Press).
Tang X.-Y., Yao Y.-X., Li Y.-H., Song H.-L., Luo R., Shi P., et al. (2023). Comparison of the mitochondrial genomes of three geographical strains of Apis laboriosa indicates high genetic diversity in the black giant honeybee (Hymenoptera: Apidae). Ecol. Evol. 12, e9782. doi: 10.1002/ece3.9782
Trung L. Q., Dung P. X., Ngan T. X. (1996). A scientific note on the first report of Apis laboriosa F. Smith 1871 in Vietnam. Apidologie 27, 487–488. doi: 10.1051/apido:19960608
Underwood B. A. (1990). Seasonal nesting cycle and migration patterns of the Himalayan honey bee Apis laboriosa. Natl. Geogr. Res. 6, 276–290.
Voraphab I., Chatthanabun N., Nalinrachatakan P., Thanoosing C., Traiyasut P., Kunsete C., et al. (2024). Discovery of the Himalayan giant honey bee, Apis laboriosa, in Thailand: a major range extension. Apidologie 55, 31. doi: 10.1007/s13592–024-01069–5
Vuong T. B., Sridith K. (2016). The phytogeographic note on the orchids flora of Vietnam: a case study from the Hon Ba Nature Reserve, Central Vietnam. Taiwania 61, 127–140. doi: 10.6165/tai.2016.61.127
Wang Z., Wu J., Niu B., He Y., Zu J., Li M., et al. (2020). Vegetation expansion on the Tibetan Plateau and its relationship with climate change. Remote Sens. 12, 4150. doi: 10.3390/rs12244150
Wikramanayake E., Dinerstein E., Loucks C. J., Morrison J., Lamoreaux J., McKnight M., et al. (2002). Terrestrial ecoregions of the Indo-Pacific: a conservation assessment (Washington D.C: Island Press).
Wu S. S., Jiang M. T., Miao J. L., Li M. H., Wang J. Y., Shen L. M., et al. (2023). Origin and diversification of a Himalayan orchid genus Pleione. Mol. Phylogenet. Evol. 184, 107797. doi: 10.1016/j.ympev.2023.107797
Yang J., Xu J.-X., Fan W.-B. (2015). Morphological description and geographical distribution of wild honeybees in China. J. Environ. Entomol. 37, 610–616. Available at: https://caod.oriprobe.com/articles/45121931/Morphological_description_and_geographical_distribution_of_wild_honeyb.htm.
Keywords: Apis laboriosa, Megapis, Pakistan, Myanmar, Thailand, ecoregion, range map, species distribution modeling
Citation: Otis GW, Huang M-J, Kitnya N, Sheikh UAA, Faiz AuH, Phung CH, Warrit N, Peng Y-Q, Zhou X, Oo HM, Acharya N and Devkota K (2024) The distribution of Apis laboriosa revisited: range extensions, biogeographic affinities, and species distribution modelling. Front. Bee Sci. 2:1374852. doi: 10.3389/frbee.2024.1374852
Received: 22 January 2024; Accepted: 11 June 2024;
Published: 22 July 2024.
Edited by:
Xesús Feás, Academy of Veterinary Sciences of Galicia, SpainReviewed by:
Petar Hristov, Bulgarian Academy of Sciences, BulgariaMichael Lattorff, University of KwaZulu-Natal, South Africa
Copyright © 2024 Otis, Huang, Kitnya, Sheikh, Faiz, Phung, Warrit, Peng, Zhou, Oo, Acharya and Devkota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gard W. Otis, Z290aXNAdW9ndWVscGguY2E=
†Retired
 Gard W. Otis
Gard W. Otis Man-Juan Huang3
Man-Juan Huang3 Nyaton Kitnya
Nyaton Kitnya Abu ul Hassan Faiz
Abu ul Hassan Faiz Chinh H. Phung
Chinh H. Phung Yan-Qiong Peng
Yan-Qiong Peng Xin Zhou
Xin Zhou Hlaing Min Oo
Hlaing Min Oo Kedar Devkota
Kedar Devkota