- 1Department of Biology, IPB University, Bogor, Indonesia
- 2Department of Conservation of Forest and Ecotourism, IPB University, Bogor, Indonesia
- 3Study Program of Agrotechnology, University of Jambi, Jambi, Indonesia
- 4Study Program of Forestry, University of Jambi, Jambi, Indonesia
- 5Department of Palynology and Climate Dynamics, Georg-August-Universität Göttingen, Göttingen, Germany
Introduction: Apis dorsata, the common bee pollinator in tropical forests, is experiencing a population decrease due to several anthropogenic factors that lead to land cover changes and habitat loss. Land cover changes may alter their resource supply and foraging behavior. Our study aimed to determine foraging behavior and botanical origin using pollen of A. dorsata honey in two land cover types: plantationdominated landscape (PL) in Kampar (Riau) and forest-agriculture-dominated landscape (FL) in Kerinci (Jambi) Sumatra, Indonesia.
Methods: We observed two colonies of A. dorsata flight direction and flight activities in each land cover from 9 am–3pm. Honey was harvested from both nests of A. dorsata and the pollen in the honey was analyzed using acetolysis procedure. Vegetation analysis in both locations was conducted based on the flight directions of the giant honey bees.
Results: The foraging data of A. dorsata showed a difference in the total number of bees between these two land cover types. The number of bees flying out and returning to the nest was higher in Kerinci than in Kampar, while high morning foraging activities were recorded in both land cover types. Furthermore, the foraging activity of the colonies in the PL landscape, i.e., flying out and returning to the nest with and without pollen, decreased at noon. The palynological results of the honey showed that in the PL landscape, pollen diversity was very low and mainly consisted of Elaeis gueneensis pollen (97%). Meanwhile, pollen types and concentrations were much higher in the FL than in the PL.
Discussion: This result suggests that A. dorsata exhibits a more varied foraging behavior in a diverse and heterogeneous landscape in Kerinci compared to a plantation-dominated habitat in Kampar.
1 Introduction
Insect pollination is one of the most important keys for ecosystem services (Bartholomée and Lavorel, 2019). Bees, in particular, are efficient pollinators due to their ability to carry more pollen than all non-bee taxa (Bernauer et al., 2022). The giant honey bee, Apis dorsata Fabricius 1793, is one of the most common pollinators found in subtropical Asia, i.e., China (Sakagami et al., 1980) and Nepal (Thapa, 2001), to the tropical Asia, covers India (Reddy, 1980), Thailand (Wongsiri et al., 1996), Sri Lanka (Koeniger and Koeniger, 1980), Philippine (Ruttner, 1988), including Indonesia (Ruttner, 1988; Nagir et al., 2016; Dyahastuti et al., 2022; Zahara et al., 2022; Kahono et al., 2023). Apis dorsata colonies have an essential ecological role in the ecosystem as pollinators for crops and natural plant communities (Rattanawannee et al., 2023). Almost 40 plant species were known to interact with A. dorsata in Pakistan (Sajjad et al., 2017). They also pollinate at least 15 plant species in Malaysia (Momose et al., 1998), six and 17 plant species in Thailand (Suwannapong et al., 2013; Stewart et al., 2018), and eight plant species in Indonesia (Bramasta et al., 2023). Considering the large number of workers per colony, hairy body of workers, generalized visitation pattern, floral constancy, and higher flower visitation rate make them effective pollinators (Layek et al., 2023). They also have a high flight range and efficient communication when foraging (Ruttner, 1988). Apis dorsata is important for honey hunters in Indonesia as the bees produce economically valuable honey (Schouten et al., 2020).
This giant honey bee is also a keystone species in dipterocarp forests (Rattanawannee et al., 2023). Indonesian archipelagoes were dominated by tall dipterocarp trees of more than 60 m. These forests are characterized by seasonal flowering and the so-called “general flowering,” most canopy trees mass-flower within several months but only every 4-5 years (Appanah, 1993; Sakai et al., 1999). When these events occurred, one of the main flower visitors was found to be A. dorsata (Momose et al., 1998). Under natural conditions, in the highly diverse lowland tropical rainforest of Sumatra, A. dorsata can select more nutrient-rich flowers and change the target plant following seasonal changes or mass flowering events (Rosmarlinasiah et al., 2015). In Sumatra, the nesting tree of A. dorsata is known by local people as the Sialang tree, which refers to more than one tree species, such as Koompasia excelsa (Shwetha et al., 2023) and Gluta renghas (Gussuwana et al., 2015; Dyahastuti et al., 2022). However, since the mid-20th century, the rainforests in Sumatra, particularly in the lowland, have been logged on a large scale and heavily converted into monoculture plantations of acacia (Acacia sp.), rubber (Hevea brasiliensis) and oil palm (Elaeis guineensis) (Drescher et al., 2016).
Several studies have shown that the conversion of rainforest to a transformation system affects the foraging behavior of bee pollinators (Gervais et al., 2020; Pulungan et al., 2023). Due to the plantation-dominated landscape, limited support for tree nesting (personal observation), and nectarine flower trees, as shown in the pollen in the honey in this study, might have affected the low number of bee colonies that migrated to Kampar in the past years. Besides that, the high temperature in the studied area reached 40°C, which is unfavorable for this important pollinator in the forest. The high temperature of 40-45°C also triggered the bees in Bengal to migrate in May and June (Singh et al., 2007). In addition to the temperature, rainfall and wind velocity highly influence the migration of A. dorsata colonies to safe places (Abrol, 1992). An extensive haze from the forest fire occurred in Riau Sumatra in 2015 and increased the temperature (Lee et al., 2016; Kozan, 2019; Sze et al., 2019). Before 2015, each nesting tree in Kampar, Riau, was home to hundreds of A. dorsata (Hotma Barinah, personal communication). After the haze, only a few colonies of A. dorsata migrate to the same Sialang trees (Raffiudin, personal observation).
While an extensive rainforest conversion arises in Sumatra, how A. dorsata adapts to this land cover is still unknown. Therefore, more studies are needed to improve our understanding of the important role of ecological functions of bee pollinators linked with forest conversion in Indonesia. To understand the effects of land cover on the ecological behavior of A. dorsata, our study aimed to (1) investigate the foraging behavior of A. dorsata and (2) compare the floral composition of the honey produced by colonies of A. dorsata in two land cover types in the transformation systems of plantations landscape (PL) in Kampar (Riau) and the mixed forest-agriculture landscape (FL) in Kerinci (Jambi), Sumatra.
2 Materials and methods
2.1 Study sites
Two locations on the island of Sumatra were selected for the study, i.e., Kampar Regency, Riau Province, and Kerinci Regency, Jambi Province, Indonesia. The first location represented the plantation-dominated landscape (PL) (Figure 1A), while the second represented the forest-agroforest landscape (FL) (Figure 1B). Two colonies per location of A. dorsata nested in the Sialang tree (Gluta renghas) were studied.
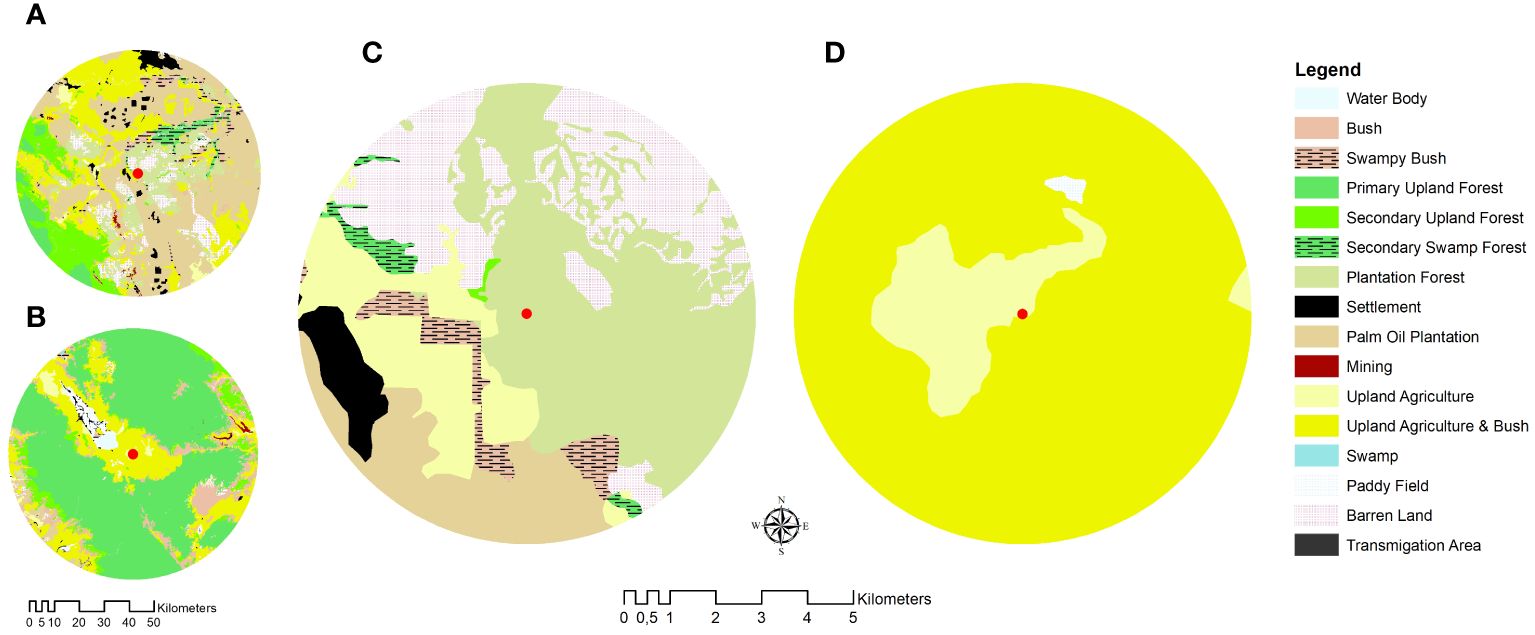
Figure 1 Map of study sites in (A) Kampar, Riau Province, represented the plantation-dominated landscape and (B) Kerinci, Jambi Province, represented the forest-agriculture-dominated landscape. Five km foraging range of A dorsata colonies in (C) Kampar and (D) Kerinci showing their nesting sites on the Sialang tree (red dot).
The Kampar site is located in the lowland area (36 m a.s.l) and is surrounded by the Eucalyptus and oil palm plantation (Figure 1A). The Kerinci Valley is located in the eastern part of the Kerinci Seblat National Park (KSNP) within the Barisan mountain range. The valley is surrounded by a montane rainforest (850 m a.s.l), farming crops such as hot pepper and coffee plantations (Figure 1B).
2.2 Observation of A. dorsata foraging behavior
The foraging behavior of two of A. dorsata colonies in Kampar and Kerinci was observed at their nesting tree in the rainy season of September-November 2017. We counted the number of bees flying out of the nest (FO), returning into the nest without pollen (RWoP), returning into the nest with pollen (RWP), and the flight directions (FD) from the tree house. The height of the tree house was around 20-30 m above the ground, which was the same height as the nest that we observed. The tree house is around 10 meters away from the nest of A. dorsata. We observed the A. dorsata flying out and returning with and without pollen at the bottom of the comb, approximately covering 20% of the area of the A. dorsata nest.
Observation of foraging activities was conducted from 9.00 am to 3.00 pm with 10-minute observation intervals in 4 days. The foraging time of A. dorsata is started in the early morning (Rattanawannee et al., 2023). However, for safety reasons, we collected foraging behavior data between 9.00 am and 3.00 pm.
2.3 Collection of honey samples
We collected honey from two colonies each in Kampar and Kerinci on the last observation day of A. dorsata foraging behavior. From each colony, we harvested 450 mL of honey samples for melissopalynological analysis. The wax that covered the honey part of the nest was sliced to release the honey. Honey was kept in a storage jar for further melissopalynological analyses.
2.4 Pollen analysis
Melissopalynological analyses were conducted on those four honey samples. Pollen in honey was extracted from 3 mL of honey (Louveaux et al., 1978). Each subsample consists of one tablet of Lycopodium clavatum spores to estimate pollen concentrations (Stockmarr, 1971). The method includes acetolysis to improve the visualization of diagnostic features, which is essential to pollen identification of tropical taxa (Erdtman, 1972). Additionally, untreated samples were checked for pollen taxa that did not preserve acetolysis (e.g., pollen grains from the Lauraceae family). Pollen was counted up to a total sum of at least 1200 pollen grains per subsample to obtain pollen spectra of the floral resources foraged by the bees in the period before collection (Louveaux et al., 1978). Pollen identification was then conducted based on morphological characteristics using the modern reference collection of pollen and spores from the University of Jambi (Indonesia) and the Georg-August University of Goettingen (Germany). This database includes ca. 130 pollen taxa and 45 spores from lowland and mountain rainforests, oil palm and rubber plantations, and coastal and peatland forests from Sumatra Island. Additionally, we used online pollen databases of Australasia Pollen and Spore Atlas (http://apsa.anu.edu.au/).
2.5 Vegetation analysis and index diversity
The transect direction for vegetation analysis was based on the fly direction of the bees. Eleven plots were made in Kampar and Kerinci, which comprise four subplots: (a) tree plots (20 m x 20 m), (b) pole plots (10 m x 10 m), (c) sapling plot (5 m x 5 m), and (d) seedling plot (2 m x 2 m).
In our attempt to determine the vegetation of the remnant forest in Kampar and Kerinci, we analyzed vegetation through the Importance Value Index (IVI), which shows the dominant plant species in a specific region. Based on the vegetation recorded, the Important Value Index was calculated on the sum of relative density (RD), relative frequency (RF), and relative dominance (RDo) for poles and trees. Meanwhile, seedlings and saplings were calculated based on relative density (RD) and relative frequency (RF) (Gonçalves et al., 2018). The Shannon diversity index (H’) was also calculated to determine plant diversity in Kampar and Kerinci based on recorded plant species from 11 plots (Shannon, 1948). All of the parameters in the vegetation analysis were calculated using the following formulas:
2.6 Statistical analysis
The correlation of environmental factors, i.e., temperature, humidity, and light intensity with the foraging activities of each A. dorsata colony in Kampar and Kerinci was performed using General Linear Models (GLM) with a Gaussian distribution in R package (R Core Team, 2018).
3 Results
3.1 Foraging behavior of Apis dorsata in Kampar and Kerinci
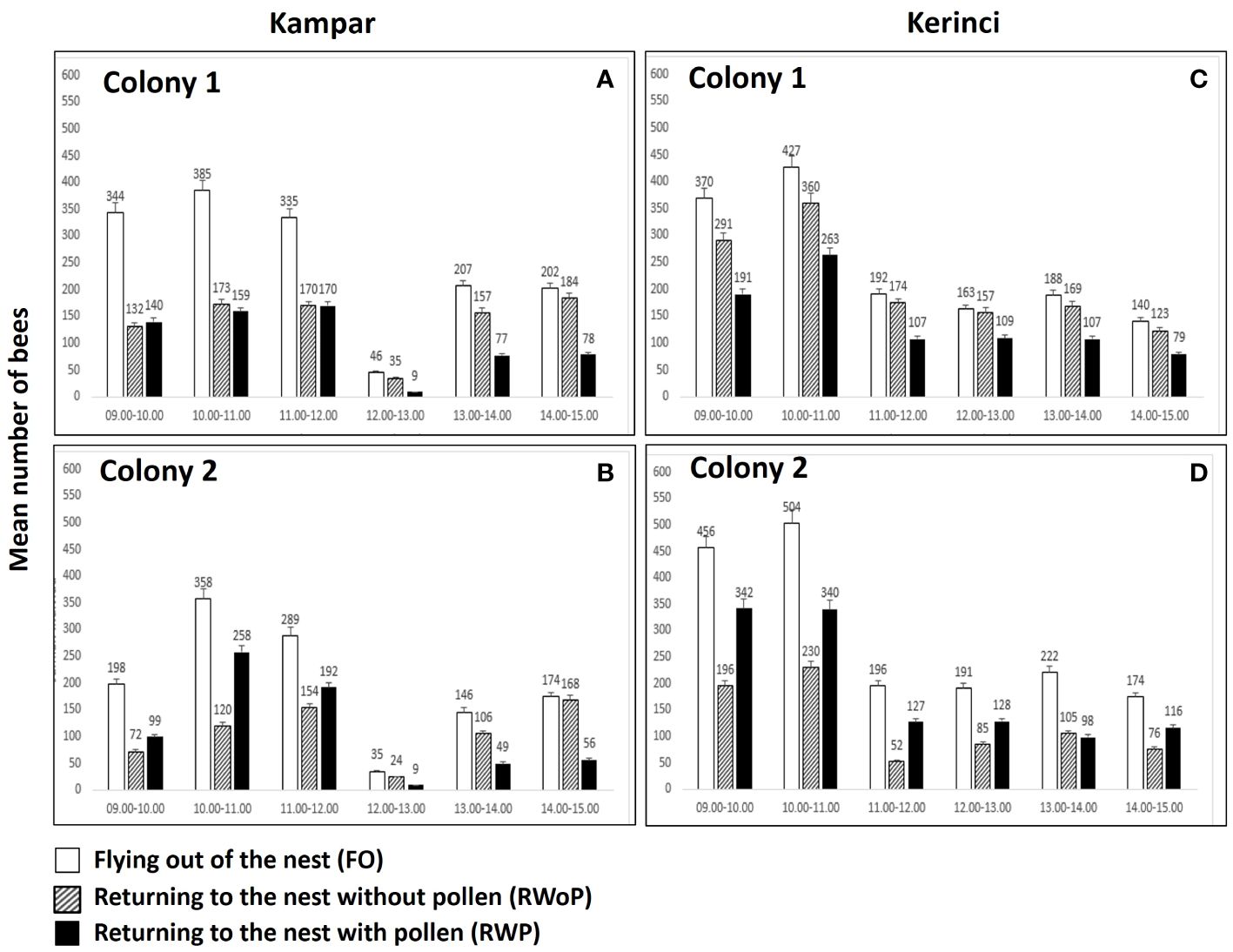
The foraging behavior of A. dorsata in Kampar showed that the two colonies peaked in their flight activity from 10:00 to 11:00 am with an average number of about 385 individuals in colony 1 (Figure 2A) and 198 individuals in colony 2 (Figure 2B). Foraging decreased from 12.00-13.00 h, with only 46 bees flying out in colony 1 (Figure 2A) and 35 bees in colony 2 in one hour (Figure 2B). Similarly, bee activities in Kerinci peaked from 10.00 h to 11.00 h with 427 individuals in colony 1 (Figure 2C) and 504 individuals in colony 2 (Figure 2D). Apis dorsata foraging activities in Kerinci decreased from ca. 11.00-12.00 h. However, the bee numbers in Kerinci are 3-4 times higher compared to Kampar, i.e., 192 bees flying out in colony 1 (Figure 2C) and 191 in colony 2 (Figure 2D). The individuals returning into the nest without pollen (RWoP: 360 and 263) and returning with pollen (RWP: 230, 340) in both colonies in Kerinci were two times larger compared to those in both colonies in Kampar (RWoP: 173, 150 and RWP: 120, 258) during the peak of foraging from 10.00-11.00 h (Figures 2A–D). Therefore, this study revealed that the land cover diversity affected the decrease in flight activities of this giant honey bee, flying out and returning to the nest with and without pollen.
The nesting tree of A. dorsata in Kampar was close to the large oil palm plantation. It was in a remnant forest surrounded by Eucalyptus and oil palm plantations (Figures 1A, 3A). The flight direction of forager bees during the peak foraging time of colonies 1 and 2 in Kampar was south and southwest, respectively (Figures 1C, 3C). On the other hand, the nesting tree of A. dorsata colonies in Kerinci was surrounded by montane rainforest vegetation and agricultural plantations (Figures 1B, 3B). Based on our observation, both A. dorsata colonies in Kerinci were flying out to the south and southeast directions from the nest tree (Figures 1D, 3D).
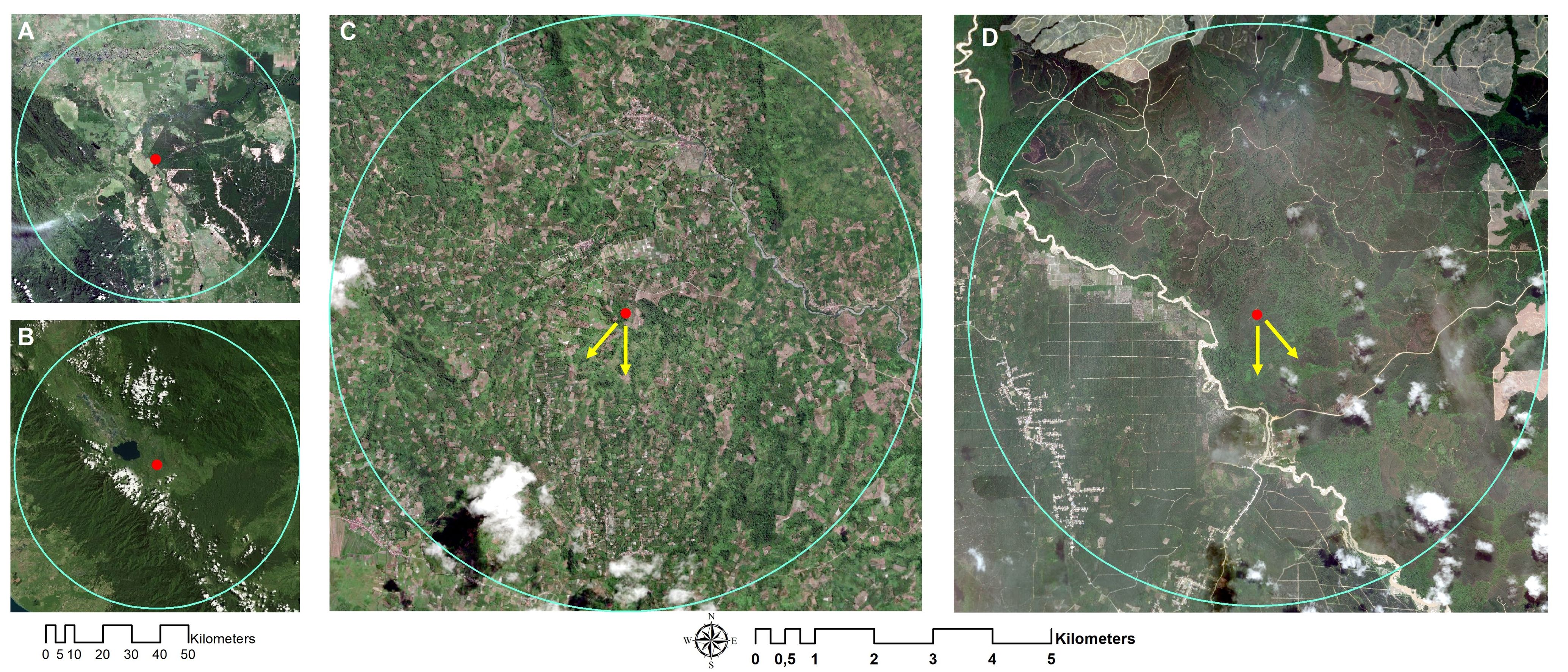
Figure 3 The position of A. dorsata nest in (A) Kampar and (B) Kerinci. The red dot indicates the nest position in the Sialang tree, and the yellow arrow indicates foraging directions in (C) Kampar: south and southwest and (D) Kerinci: south and southeast. Google Earth captured: January 2018.
The GLM analysis shows the correlation between A. dorsata foraging behavior and environmental factors such as temperature, humidity, and light intensity (Supplementary Table 1). It indicates that the foraging activities of A. dorsata in Kampar and Kerinci have similar trends, i.e., being negatively influenced by humidity and positively correlated with temperature and light intensity. However, these values were not significant, except for a few measurements.
3.2 Pollen analysis
Honey of A. dorsata from the landscape dominated by plantations in Kampar showed a low concentration of pollen, i.e., no more than 50.000 pollen grains/mL (Figure 4A). Colony 1 has low pollen concentration with an average of 11.502 grains/mL, while colony 2 has almost four times more pollen concentration. Only three pollen taxa are identified from the honey of A. dorsata in the Kampar site (Figures 4B, 5A, B). The pollen was dominated by 95-97% Elaeis guineensis, followed by 2-5% Myrtaceae and<0.5% Acacia type (Figures 6A–C).
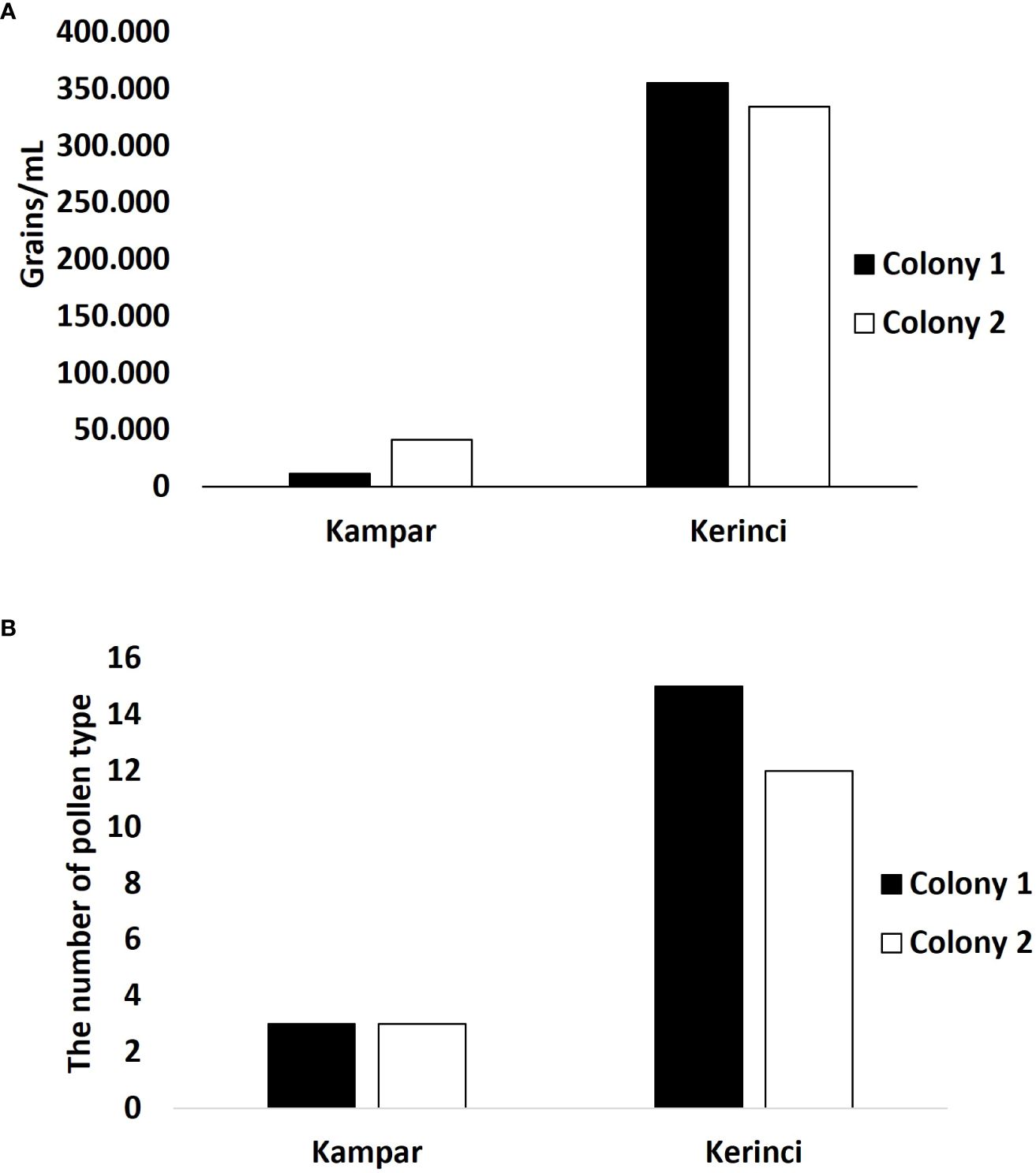
Figure 4 Pollen quantification and identification from A. dorsata honey from Kampar and Kerinci. (A) Pollen concentration and (B) The number of pollen types.
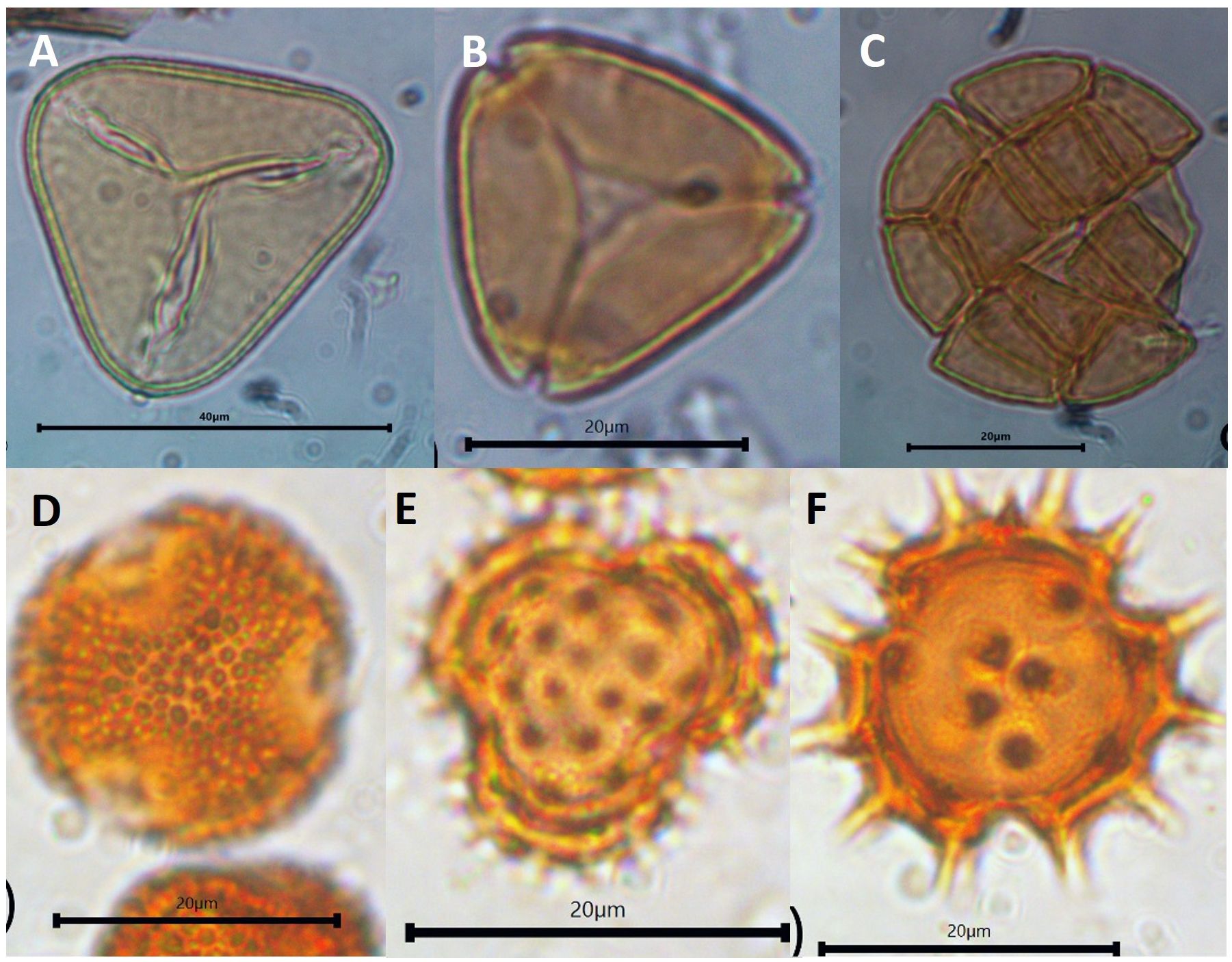
Figure 5 Morphology of dominant pollen contained in A. dorsata honey. (A) Elaeis guineensis, (B) Myrtaceae, and (C) Acacia found in the Kampar honey, (D) Ilex type, and Asteraceae (E, F) were dominant in Kerinci.
Pollen concentration in both bee colonies in the forest-agriculture dominant landscape in Kerinci was almost seven times higher than in Kampar (Figure 4A), which is reflected in the differences between the monoculture plantation area in Kampar and the agroforestry landscape in Kerinci. The number of pollen types in the honey samples from Kerinci was significantly higher than in Kampar, with 16 and 13 different pollen taxa found in colony one and colony 2 of A. dorsata, respectively (Figures 4B, 5C, D). Among those pollen types, Ilex type and Asteraceae pollen grains are co-dominant (Figures 5C, D, 6D–F).

Figure 6 Pollen composition and pollen percentage of A. dorsata honey from (A, B) Kampar and (C, D) Kerinci. Pollen percentages below 4% are not shown.
3.3 Vegetation analysis
A higher plant diversity was found in Kerinci, with a high 3.19 H’, compared to Kampar, with H’ 2.58 (Table 1). The vegetation analysis in Kampar showed that the dominant tree species is Artocarpus maingayi (Moraceae), with a 51% IVI value, while Alseodaphne sp. dominated the pole with a 42% IVI value. We found a very high dominance of Fabaceae in saplings and seedlings, with 114% and 107% IVI values, respectively (Supplementary Table 2).
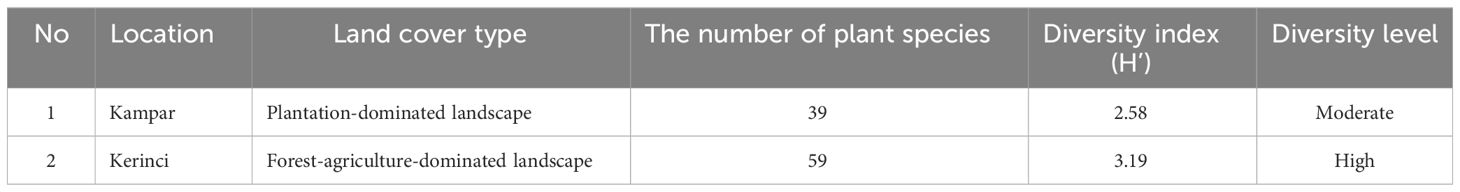
Table 1 Shannon diversity index of plant species surrounding nesting tree of A. dorsata in Kampar and Kerinci.
The dominant tree species in Kerinci is Ficus sp. (Moraceae), with a 56% IVI value, while pole vegetation was dominated by Knema cinerea (46%). Coffea sp. was dominated in sapling and seedling vegetations with 47% and 109% IVI values, respectively (Supplementary Table 3).
4 Discussion
This study attempted to answer the question of how A. dorsata adapts to different land covers. Overall, the lowland rainforests in Sumatra converted into plantations of oil palm, rubber, and acacia (Drescher et al., 2016). Our study revealed that the land cover transformation in Kampar affected A. dorsata behavior; the giant honey bee flew mainly to the oil palm plantation (Figure 3A) to reach the pollen, as shown in the melissopalynological results (Figures 6A, B). Even though the distance between the nest of A. dorsata was approximately 1.9 km to the nearest oil palm plantation (Figures 1C, 3C). This long-distance flight to collect the oil palm pollen was also shown by A. dorsata in Kampar Island flying across the strait to reach the oil palm plantation in Belitung Regency (Bramasta et al., 2023). Indeed, Apis dorsata can also have long-distance flights during migration across continents (Robinson, 2012, 2021). Oil palm pollen is the typical source of A. dorsata in Marang, Terengganu, Malaysia, even though it is in a tropical rainforest (Ibrahim et al., 2012). The experiment of A. dorsata foraging distance in Bangalore, India, showed a shorter distance according to the habitat. The bees preferred to forage in the garden with a distance of 800-900 m due to the various cultivated flowering plants (Young et al., 2021b). In accordance, A. mellifera foraged at a shorter distance during the major flowering seasons compared to the non-flowering seasons (Couvillon et al., 2015).
Our observations of two colonies of A. dorsata in each location of the landscape are nesting in the same aggregation of a tree. Although both colonies are foraging in the same direction, colonies within aggregation might not be related to mother and daughter colonies; this was shown in the A. dorsata aggregated colonies in the same tree in Assam, India. By using microsatellite DNA, Paar et al. (2004) revealed that the colonies apparently are not genetically related. Due to the high genetic differentiation among the bee colonies, the management of the conservation should be carefully taken, especially since these giant honey bees migrate to their home at the same site in the coming year (Paar et al., 2000).
Both A. dorsata colonies in the same aggregation in the plantation-dominated landscape (PL) in Kampar were dominated by oil palm pollen, although in low concentrations (Figure 4A). The low concentration also indicates a lack of pollen availability in the surrounding nest area, supported by a moderate plant diversity index (Table 1). Given the absence of a nectarine gland in oil palm (Silberbauer-Gottsberger, 1990), A. dorsata and other pollinators might suffer from the lack of important resources in the vicinity of the nest. The nectar resource in Kampar Riau remains unknown. However, it is possibly represented by the Myrtaceae species (Freitas et al., 2016) due to it being the second most abundant pollen grain found in the honey samples (Figures 6A, B). In the studied area of plantation-dominated landscape (Kampar), a hundred hectares of monoculture, Eucalyptus (Myrtaceae) is the nectar source. Myrtaceae is one of the nectar sources foraged by A. dorsata in West Bengal, India (Layek and Karmakar, 2018) and also in Kampak Island, Belitung, Indonesia (Bramasta et al., 2023).
We found that the pollen concentration of the honey from FL Kerinci was ten times higher compared to the PL in Kampar (Figure 4A). This finding was supported by the high foraging activity of A. dorsata in Kerinci (Figures 2C, D), which might have influenced the pollen concentration in honey. An experimental study of honey bee A. mellifera revealed that the amount and rate of pollen and nectar collection were positively correlated with the individual foraging experience of the bees (Klein et al., 2019). In addition, relationship analysis between the pollen contained in honey and vegetation types in the West Coast (WC) and Western Ghats (WG), India, revealed that floral resources also affected pollen concentration in honey (Hegde et al., 2023). Apis indica honey from the more diverse vegetation of the WG, showed a higher pollen concentration compared to those from the WC, which has less diverse vegetation (Hegde et al., 2023). In contrast, A. dorsata and A. florea honey from the less diverse vegetation of WC contained a higher pollen concentration compared to those from more diverse vegetation of WG. Thus, we suggest that the higher pollen concentration in A. dorsata honey from Kerinci could be shaped by two factors, i.e., different flower (pollen) resources and the foraging activities of the bees. This result was supported by a high number of pollen taxa found in the honey from forest-agriculture-dominated landscapes (FL) in Kerinci, which revealed more diversified pollen collected by the foraging honey bees with a total of sixteen pollen types (Figures 4B, 6C, D). Our result is concordant with the honey of A. dorsata produced in the more diverse vegetation of deciduous forests in Western Ghats, India, which contains a more diverse pollen type compared to the honey produced in less diverse vegetation in West Coast India (Hegde et al., 2023). No predominant pollen taxon was found in the FL Kerinci. Thus, our results in FL Kerinci suggest an admixture of open grassland and forest resources with the agriculture of coffee plantations surrounding the forest of the nesting trees of A. dorsata (Supplementary Table 3). We found the Coffee sp. pollen in a low percentage in A. dorsata honey in FL Kerinci (Figures 6C, D). Coffee flowers are also foraged by A. dorsata binghami in the agroecosystem near Lore Lindu National Park, Central Sulawesi (Klein et al., 2002). Besides A. dorsata, the flower of Coffee sp. was also pollinated by the native cavity-nesting Asian honey bee A. cerana (Saepudin, 2014; Sari and Putra, 2015). Apis dorsata might pollinate a lesser number of plant species compared to the other Asian honey bees, A. cerana and A. florea (Stewart et al., 2018). This is presumably due to the A. dorsata needs mass flowering plants despite the lower number of plant species. This giant honey bee needs much more pollen due to the larger colony size compared to A. cerana and A. florea (Stewart et al., 2018).
The maximum foraging activity of A. dorsata occurred during morning times in both landscapes, while the minimum activity occurred at noon (Figure 2). Foraging observation of A. dorsata on blooming Eucalyptus in South Gujarat, India, revealed the same phenomenon: the maximum and minimum foraging activity of A. dorsata occurred in the morning and during midday, respectively (Behera et al., 2018). Moreover, an extensive observation of A. dorsata foraging activity in Bangalore, India, revealed that the foraging peaks occurred during the morning (before 9 am) and evening twilight (after 5 pm) and still exhibit the foraging activity during the night time (Young et al., 2021a). The nocturnal activities of A. dorsata are supported by their higher eye sensitivity compared to A. cerana and A. florea (Somanathan et al., 2009). In addition, the flight activity of A. dorsata in forest-agriculture-dominated landscapes was three times higher than in plantation-dominated landscapes at noon (Figure 2). This is presumably due to the blooming flowering plants surrounding the nesting tree in FL Kerinci (Supplementary Table 3), which was also supported by the higher plant diversity in Kerinci compared to Kampar (Table 1). This phenomenon in Kerinci might be due to the high nectar-sugar concentration that positively influences the foraging activity of A. dorsata (Abrol, 1992).
Besides nectar-sugar concentration, our result revealed that the foraging activity of A. dorsata was also positively affected by temperature and light intensity and negatively affected by humidity. The same pattern of environmental influence also occurred in the foraging time of A. dorsata in India (Abrol, 1992). On the other hand, we found a lack of blooming flowering plants in the Kampar location due to the high domination of Fabaceae seedlings surrounding the nesting tree. The high domination of Fabaceae seedlings represented by the highest IVI value in Kampar (Supplementary Table 2) is presumably due to the high usage of Fabaceae plants for rehabilitation of the transformed land in Indonesia, including Sumatra (Wiryono et al., 2022).
In our study, A. dorsata in Kerinci migrates in March, and the highest number of migrations is in April-May (unpublished data). The blooming flowering plants are important for the survival of the bees, thus attracting them to migrate to their previous nesting site (Paar et al., 2004). The migration of A. dorsata is from October to June, which depends on the source of nectar flowering plants (Singh et al., 2007). While in Borneo, A. dorsata migrate after one-month peak flowering (Itioka et al., 2001). Different flowerings in certain seasons were found in West Bengal, India, attracting giant bees to migrate (Layek and Karmakar, 2018). In summer, the predominant pollen type that attracts the most is the blooming of Eucalyptus. In winter, Brassica sp. is predominant, followed by Borassus sp. in spring (Layek and Karmakar, 2018). The plant resources for A. dorsata in Mount Tinanggo Kolaka Southeast Sulawesi found a total of 237 types of flowering plants, and September is the flowering peak (Rosmarlinasiah et al., 2015).
Natural forests are dominant landscape habitats that provide nesting trees and food resources for honey bee species (A. dorsata, A. mellifera, and A. cerana). Based on our results, the composition of land cover in Kerinci, which is still dominated by natural forest, supported more pollen sources from different plant species (16 pollen types) for A. dorsata than the composition of plantation-dominated landscape in Kampar (3 pollen types) (Figure 6). A similar pattern also occurred in A. mellifera, where the forest landscape increased the diversity in honeybee diets, particularly trees, which were the dominant floral source of bee bread (Cannizaro et al., 2022). Apis cerana also collected more pollen types as a food diet in deciduous forests (16-28 pollen types) than in agricultural regions (9-16 pollen types) (Jhansi et al., 1994). Land cover transformation, such as natural forests to oil palm plantations, change pollination as the habitat function loss (Dislich et al., 2016) and the loss of biodiversity (Meijaard et al., 2018). Land cover change from forest to other land cover could change the diet of bees and might lead to loss of pollination services (Atmowidi et al., 2007; Pot et al., 2010) where honey bees are known as crucial pollinators (Kovács-Hostyánszki et al., 2018). A study on pollination services revealed that Apis dorsata has an essential role in compensating for stingless bee decline as critical pollinators during drought season in agricultural land in Bangalore, India (Mukherjee et al., 2019).
Our investigation concluded that the effect of land cover on the pollen diversity in the honey of A. dorsata related to pollen source with flight direction of the bees from a landscape perspective. The differences in environment can give empirical data to identify flower resources used by A. dorsata. Moreover, the information on floral resources used by A. dorsata is crucial for establishing a deterministic link between land cover, plant composition, and pollinator population development. The foraging activities of A. dorsata in the plantation-dominated landscape in Kampar showed low flight activities, particularly during the noon. The pollen results in the honey from Kampar show low diversity and concentration. While in the forest-agriculture-dominated landscape, this giant honey bee showed high flight activities. The pollen analysis from the honey harvested in this area revealed high pollen concentration and diversity of pollen types.
Although our research did not cover the natural habitat of A. dorsata, the result revealed a more diversified foraging behavior of A. dorsata in a more diverse heterogenetic landscape compared to the monoculture habitat. Our comprehensive study of foraging behavior, melissopalynology, and vegetation analysis in two land cover types proposes that enriching plant diversity around plantation habitats is needed to increase flower resources for A. dorsata. In this way, the sustainability of socioeconomic and ecological functions in plantation-dominated landscapes is expected to be enhanced.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the research method employed involved observation of the foraging behavior of honey bee Apis dorsata.
Author contributions
RR: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MD: Formal analysis, Investigation, Project administration, Writing – review & editing. RN: Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. TS: Formal analysis, Writing – original draft, Writing – review & editing. ND: Methodology, Writing – review & editing. ES: Formal analysis, Investigation, Visualization, Writing – review & editing. VA: Formal analysis, Investigation, Visualization, Writing – review & editing. RM: Formal analysis, Investigation, Visualization, Writing – review & editing. SB: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. CS: Formal analysis, Writing – review & editing. LP: Formal analysis, Methodology, Validation, Visualization, Writing – review & editing. WP: Resources, Writing – review & editing. TA: Supervision, Writing – review & editing. AS: Writing – review & editing. HB: Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by Collaborative Research Centre (CRC) 990: Ecological and Socioeconomic Functions of Tropical Lowland Rainforest Transformation Systems (EFFoRTS) (Sumatra, Indonesia) for the Access-Benefit-Sharing (ABS) funds under contract No: 09/IY3/SP/CRC/2017.
Acknowledgments
We expressed our high appreciation for the support during field works to Hotma Barinah from Watershed and Protected Forest Management Unit (BPDASHL) Indragiri Rokan, Riau, Bahrizon, the honey farmer in Kampar, Riau and the Head of Forest Agency (KPH) Kerinci, staffs, and honey farmer team in Kerinci, Jambi. We also thank Kuntadi, MSc.Agr and Dr. Sih Kahono for the valuable discussion of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frbee.2024.1366287/full#supplementary-material
Supplementary Table 1 | Correlation of A. dorsata foraging with environmental factors.
Supplementary Table 2 | Important Value Index of plant species in Kampar region.
Supplementary Table 3 | Important Value Index of plant species in Kerinci region.
References
Abrol D. P. (1992). Foraging honeybees in Apis cerana indica F. and A. dorsata F. (Hymenoptera: apidae)-activity and weather conditions. J. Ind. Inst. Sci. 72, 395–401.
Appanah S. (1993). Mass flowering of dipterocarp forests in the seasonal tropics. J. Biosci. 18, 457–474. doi: 10.1007/BF02703079
Atmowidi T., Buchori D., Manuwoto S., Suryobroto B., Hidayat P. (2007). Diversity of pollinator insects in relation to seed set of mustard (Brassica rapa L.: Cruciferae). HAYATI J. Biosci. 14, 155–161. doi: 10.4308/hjb.14.4.155
Bartholomée O., Lavorel S. (2019). Disentangling the diversity of definitions for the pollination ecosystem service and associated estimation methods. Ecol. Indic. 107, 105576. doi: 10.1016/j.ecolind.2019.105576
Behera L. K., Mehta A. A., Dholariya C. A., Patel S. M., Gunaga R. P. (2018). Foraging activity of Rockbee (Apis dorsata) on Eucalyptus: A promising MPTs in South Gujarat condition. J. Entomol. Zool. Stud. 6, 550–553.
Bernauer O. M., Tierney S. M., Cook J. M. (2022). Efficiency and effectiveness of native bees and honey bees as pollinators of apples in New South Wales orchards. Agric. Ecosyst. Environ. 337, 108063. doi: 10.1016/j.agee.2022.108063
Bramasta D., Qayim I., Djuita N. R., Raffiudin R., Putra R. E., Soesilohadi R. H., et al. (2023). Melissopalynology and vegetation analysis surrounding sunggau of giant honey bee Apis dorsata in Belitung Regency. HAYATI J. Biosci. 30, 1167–1174. doi: 10.4308/hjb.30.6.1167-1174
Cannizaro C., Keller A., Wilson R. S., Elliott B., Newis R., Ovah R., et al. (2022). Forest landscapes increase diversity of honeybee diets in the tropics. For. Ecol. Manage. 504, 119869. doi: 10.1016/j.foreco.2021.119869
Couvillon M. J., Riddell Pearce F. C., Accleton C., Fensome K. A., Quah S. K. L., Taylor E. L., et al. (2015). Honey bee foraging distance depends on month and forage type. Apidologie 46, 61–70. doi: 10.1007/s13592-014-0302-5
Dislich C., Keyel A. C., Salecker J., Kisel Y., Meyer K. M., Auliya M., et al. (2016). A review of the ecosystem functions in oil palm plantations, using forests as a reference system. Biol. Rev. 92, 1539–1569. doi: 10.1111/brv.12295
Drescher J., Rembold K., Allen K., Beckschäfer P., Buchori D., Clough Y., et al. (2016). Ecological and socioeconomic functions across tropical land use systems after rainforest conversion. Phil. Trans. R. Soc B. 371, 20150275. doi: 10.1098/rstb.2015.0275
Dyahastuti M., Raffiudin R., Widjaja M. C., Afriani N., Listyowati S. (2022). Flight activity before migration and pollen identification from honey of Apis dorsata in Kampar, Riau. Jurnal Sumber Daya Hayati. 8, 34–41. doi: 10.29244/jsdh.8.2
Freitas M. L., Dutra M. B., Bolini H. M. (2016). Sensory profile and acceptability for pitanga (Eugenia uniflora L.) nectar with different sweeteners. Food Sci. Technol. Int. 22, 720–731. doi: 10.1177/1082013215607077
Gervais A., Courtouis E., Fournier V., Belisle M. (2020). Landscape composition and local floral resources influence foraging behavior but not the size of Bombus impatiens Cresson (Hymenoptera: Apidae) workers. PloS One 15, e0234498. doi: 10.1371/journal.pone.0234498
Gonçalves F. M. P., Revermann R., Cachissapa M. J., Gomes A. L., Aidar M. P. M. (2018). Species diversity, population structure, and regeneration of woody species in fallows and mature stands of tropical woodlands of southeast Angola. J. For. Res. 29, 1569–1579. doi: 10.1007/s11676-018-0593-x
Gussuwana I., Yoza D., Mardhiansyah M. (2015). The characteristic of beehive trees and the preference of bee nesting in the beehive surround forest of Gunung Sahilan Village, Gunung Sahilan Distric, Kampar Regency, Riau Province. J.O.M 2, 1–8.
Hegde S., Sharathchandra K., Sridhar K. R. (2023). Honey-producing bee–pollen–vegetation relationships in the West Coast and Western Ghats of India. Palynology 47, 2127957. doi: 10.1080/01916122.2022.2127957
Ibrahim I. F., Balasundram S. K., Abdullah N. A. P., Alias M. S., Mardan M. (2012). Morphological characterization of pollen collected by Apis dorsata from a tropical rainforest. Int. J. Bot. 8, 96–106. doi: 10.3923/ijb.2012.96.106
Itioka T., Inoue T., Kaliang H., Kato M., Nagamitsu T., Momose K., et al. (2001). Six-year population fluctuation of the giant honey bee Apis dorsata (Hymenoptera: Apidae) in a tropical lowland dipterocarp forest in Sarawak. Ann. Entomol. Soc Am. 94, 545–549. doi: 10.1603/0013-8746(2001)094[0545:SYPFOT]2.0.CO;2
Jhansi P., Kalpana T. P., Ramanujam C. G. K. (1994). Pollen analysis of some Apis cerana Fabr honeys from Andhra Pradesh, India. Apidologie 25, 289–296. doi: 10.1051/apido:19940303
Kahono S., Peggie D., Lamerkabel J. S. A., Engel M. S. (2023). “Diversity, recent distribution, and nesting behavior of giant honeybees in Indonesia and their role in natural and agricultural ecosystems,” in Role of Giant Honeybees in Natural and Agricultural Ecosystems. Ed. Abrol D. P. (CRC Press, Boca Raton, FL), 292–304.
Klein A., Steffan-Dewenter I., Buchori D., Tscharntke T. (2002). Effects of land use intensity in tropical agroforestry systems on coffee flowering-visiting and trap nesting bees and wasp. Conserv. Biol. 16, 1003–1014. doi: 10.1046/j.1523-1739.2002.00499.x
Klein S., Pasquaretta C., He X. J., Perry C., Sovik E., Devaud J. M., et al. (2019). Honey bees increase their foraging performance and frequency of pollen trips through experience. Sci. Rep. 9, 6778. doi: 10.1038/s41598-019-42677-x
Koeniger N., Koeniger G. (1980). Observations and experiments on migration and dance communication of Apis dorsata in Sri Lanka. J. Apic. Res. 19, 21–34. doi: 10.1080/00218839.1980.11099994
Kovács-Hostyánszki A., Földesi R., Báldi A., Endrédi A., Jordán F. (2018). The vulnerability of plant-pollinator communities to honeybee decline: A comparative network analysis in different habitat types. Ecol. Indic. 97, 35–50. doi: 10.1016/j.ecolind.2018.09.047
Kozan O. (2019). Assessment of the health impacts of haze pollutants caused by peatland fires. Newslett. Towards Regen. Trop. Peatland Societies. 6, 1–4.
Layek U., Das N., Mondal R., Karmakar P. (2023). “Distribution, nesting biology, and floral preference of giant honeybee (Apis dorsata Fabricius) in Southern West Bengal, India,” in Role of Giant Honeybees in Natural and Agricultural Ecosystems. Ed. Abrol D. P. (CRC Press, Boca Raton, FL), 305–323.
Layek U., Karmakar P. (2018). Pollen analysis of Apis dorsata Fabricius honeys in Bankura and Paschim Medinipur districts, West Bengal. Grana 57, 298–310. doi: 10.1080/00173134.2017.1390604
Lee J. S. H., Jaafar Z., Tan A. K. J., Carrasco L. R., Ewing J. J., Bickford D. P., et al. (2016). Toward clearer skies: Challenges in regulating transboundary haze in Southeast Asia. Environ. Sci. Policy. 55, 87–95. doi: 10.1016/j.envsci.2015.09.008
Louveaux J., Maurizio A., Vorwohl G. (1978). Methods of melissopalynology. Bee World 59, 139–157. doi: 10.1080/0005772X.1978.11097714
Meijaard E., Garcia-Ulloa J., Sheil D., Wich S. A., Carlson K. M., Juffe-Bignoli D., et al. (2018). Oil palm and biodiversity: a situation analysis by the IUCN Oil palm Task Force. (Gland, Switzerland: International Union for Conservation of Nature and Natural Resources (IUCN)). doi: 10.2305/IUCN.CH.2018.11.en
Momose K., Yumoto T., Nagamitsu T., Kato M., Nagamasu H., Sakai S., et al. (1998). Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 85, 1477–1501. doi: 10.2307/2446404
Mukherjee R., Deb R., Devy S. M. (2019). Diversity matters: Effects of density compensation in pollination service during rainfall shift. Ecol. Evol. 29, 9701–9711. doi: 10.1002/ece3.5500
Nagir M. T., Atmowidi A., Kahono S. (2016). The distribution and nest-site preference of Apis dorsata binghami at Maros Forest, South Sulawesi, Indonesia. J. Insect Biodivers. 4, 1–14. doi: 10.12976/jib/2016.4.23
Paar J., Oldroyd B. P., Huettinger E., Kastberger G. (2004). Genetic structure of an Apis dorsata. J. Hered. 95, 119–126. doi: 10.1093/jhered/esh026
Paar J., Oldroyd B. P., Kastberger G. (2000). Giant honeybees return to their nest sites. Nature 406, 475. doi: 10.1038/35020196
Pot S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. (2010). Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evolut. 25, 345–353. doi: 10.1016/j.tree.2010.01.007
Pulungan Z. N., Priawandiputra W., Grass I., Li K., Robo R. J., Raffiudin R. (2023). Tropical lowland rainforest conversion to rubber monoculture affects flight activity and pollen resources of the stingless bees Tetragonula laeviceps (Smith). J. Entomol. Indones. 20, 88–100. doi: 10.5994/jei
Rattanawannee A., Rod-im P., Duangphakdee O. (2023). “Ecological services potential of Apis dorsata in Thailand,” in Role of Giant Honeybees in Natural and Agricultural Ecosystems. Ed. Abrol D. P. (CRC Press, Boca Raton, FL), 123–114.
Reddy C. C. (1980). Studies on the nesting behaviour of Apis dorsata F. Intl. Conf. Apic. Trop. Climate. 2, 391–397.
Robinson W. S. (2012). Migrating giant honey bees (Apis dorsata) congregate annually at Stopover Site in Thailand. PloS One 7, e44976. doi: 10.1371/journal.pone.0044976
Robinson W. S. (2021). Surfing the sweet wave: migrating giant honey bees (Hymenoptera: Apidae: Apis dorsata) display spatial and temporal fidelity to annual stopover site in Thailand. J. Insect Sci. 21, 1–12. doi: 10.1093/jisesa/ieab037
Rosmarlinasiah M. D., Paembonan S., Yusuf Y. (2015). Resource potential analysis of honey bee feed Apis dorsata in Mountain Tinanggo Kolaka. Int. J. Sci. Technol. Res. 4, 313–318.
Ruttner F. (1988). Biogeography and Taxonomy of Honeybees (Berlin: Spinger-Verlag). doi: 10.1007/978-3-642-72649-1
Saepudin R. (2014). Sustainability analysis and the effect of honeybee-coffee plantation integration model on improving the honey and coffee bean product. Jurnal Ilmiah Ilmu-Ilmu Peternakan. 17, 1–9. doi: 10.22437/jiiip.v17i1.2254
Sajjad A., Ali M., Saeed S. (2017). Yearlong association of Apis dorsata and Apis florea with flowering plants: planted forest vs. Agric. lands. Sociobiol. 64, 18–25. doi: 0.13102/sociobiology.v64i1.995
Sakagami S. F., Matsumura T., Ito K. (1980). Apis laboriosa in Himalaya, the little-known world largest honeybee (Hymenoptera: Apidae). Insecta Matsumurana. 19, 47–77.
Sakai S., Momose K., Yumoto T., Nagamitsu T., Nagamasu H., Hamid A. A., et al. (1999). Plant reproductive phenology over four years including an episode of general flowering in a lowland dipterocarp forest, Sarawak, Malaysia. Am. J. Bot. 86, 1414–1436. doi: 10.2307/2656924
Sari D. A., Putra R. E. (2015). Kajian karakter bunga Coffea arabica L. terkait dengan kemungkinan aplikasi lebah madu lokal sebagai agen penyerbuk. Jurnal Matematika Sains. 20, 1–5.
Schouten C., Lloyd D., Ansharyani I., Salminah M., Somerville D., Stimpson K. (2020). The role of honey hunting in supporting subsistence livelihoods in Sumbawa, Indonesia. Geogr. Res. 58, 64–76. doi: 10.1111/1745-5871.12380
Shannon C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423. doi: 10.1002/bltj.1948.27.issue-3
Shwetha B. V., Neethu T., Bharanth Kumar A. K., Bhat N. S. (2023). “Distribution and nest site preference of Apis dorsata Fabricius,” in Role of Giant Honeybees in Natural and Agricultural Ecosystems. Ed. Abrol D. P. (CRC Press, Boca Raton, FL), 158–169.
Singh R. P., Singh A. K., Singh R. P. (2007). The effect of the availability of bee forage plants and environmental conditions on the nesting of Apis dorsata Fabr. J. Apic. Res. 46, 276–281. doi: 10.1080/00218839.2007.11101408
Somanathan H., Warrant E. J., Borges R. M., Wallén R., Kelber A. (2009). Resolution and sensitivity of the eyes of the Asian honeybees Apis florea, Apis cerana and Apis dorsata. J. Exp. Biol. 212, 2448–2453. doi: 10.1242/jeb.031484
Stewart A. B., Sritongchuay T., Teartisup P., Kaewsomboon S., Bumrungsri S. (2018). Habitat and landscape factors influence pollinators in a tropical megacity, Bangkok, Thailand. PeerJ 6, e5335. doi: 10.7717/peerj.5335
Stockmarr J. (1971). Tablets with spores used in absolute pollen analysis. Pollen Spores 13, 615–621.
Suwannapong G., Maksong S., Yemor T., Junsuri N., Benbow M. E. (2013). Three species of native Thai honey bees exploit overlapping pollen resources: Identification of bee flora from pollen loads and midguts from Apis cerana, A. dorsata and A. florea. J. Apic. Res. 52, 196–201. doi: 10.3896/IBRA.1.52.5.05
Sze J. S., Jefferson, Lee. J. S. H. (2019). Evaluating the social and environmental factors behind the 2015 extreme fire event in Sumatra, Indonesia Environ. Res. Lett. 14, 015001. doi: 10.1088/1748-9326/aaee1d
Thapa R. (2001). The Himalayan giant honey bee and its role in ecotourism development in Nepal. Bee World 82, 139–141. doi: 10.1080/0005772X.2001.11099516
Wiryono, Lukman A. H., Nurliana S. (2022). The species diversity and composition of seedlings for degraded land rehabilitation in different phytogeographical regions in Indonesia. Biodiversitas 23, 5771–5781. doi: 10.13057/biodiv/d231130
Wongsiri S., Thapa R., Oldroyd B. P., Burgett D. M. (1996). A magic bee tree. Home to Apis dorsata Fab. Am. Bee J. 136, 796–799.
Young A. M., Kodabalagi S., Brockmann A., Dyer F. C. (2021a). A hard day’s night: Patterns in the diurnal and nocturnal foraging behavior of Apis dorsata across lunar cycles and seasons. PloS One 16, e0258604. doi: 10.1371/journal.pone.0258604
Young A. M., Kohl P. L., Rutschmann B., Steffen-Dewenter I., Brockmann A., Dyer F. C. (2021b). Temporal and spatial foraging pattern of three Asian honey bee species in Bangalore. India. Apidologie 52, 503–523. doi: 10.1007/s13592-020-00839-1
Keywords: forest-agriculture landscape, flight activity, flight directions, honey bee conservation, melissopalynology, plantation-dominated landscape, pollen diversity, vegetation analysis
Citation: Raffiudin R, Dyahastuti M, Nugraha R, Sayusti T, Djuita NR, Suwananda E, Allvioningrum V, Mardhony R, Biagioni S, Setyaningsih CA, Prasetyo LB, Priawandiputra W, Atmowidi T, Saad A and Behling H (2024) The effect of land cover on the foraging behavior and pollen in the honey of the giant bee Apis dorsata in Sumatra. Front. Bee Sci. 2:1366287. doi: 10.3389/frbee.2024.1366287
Received: 06 January 2024; Accepted: 19 March 2024;
Published: 05 April 2024.
Edited by:
Axel Brockmann, National Centre for Biological Sciences, IndiaReviewed by:
Bożena Denisow, University of Life Sciences of Lublin, PolandUjjwal Layek, Rampurhat College, India
Copyright © 2024 Raffiudin, Dyahastuti, Nugraha, Sayusti, Djuita, Suwananda, Allvioningrum, Mardhony, Biagioni, Setyaningsih, Prasetyo, Priawandiputra, Atmowidi, Saad and Behling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rika Raffiudin, cmlrYS5yYWZmaXVkaW5AYXBwcy5pcGIuYWMuaWQ=
 Rika Raffiudin
Rika Raffiudin Meis Dyahastuti1
Meis Dyahastuti1 Tiara Sayusti
Tiara Sayusti Erik Suwananda
Erik Suwananda Siria Biagioni
Siria Biagioni Windra Priawandiputra
Windra Priawandiputra Asmadi Saad
Asmadi Saad